Abstract
The plasma membrane of all eukaryotic cells contain heterogeneous self organizing intrinsically unstable liquid ordered domains or lipid assemblies in which key signal transduction proteins are localized. These assemblies are classified as “lipid rafts” (10–200 nm), which are composed mostly of cholesterol and sphingolipid microdomains and therefore do not integrate well into the fluid phospholipid bilayers. In addition, caveolae represent a subtype of lipid raft macrodomain that form flask-shaped membrane invaginations containing structural proteins, i.e., caveolins. With respect to the diverse biological effects of long chain polyunsaturated fatty acids (PUFA), increasing evidence suggests that n-3 PUFA and perhaps conjugated fatty acids uniquely alter the basic properties of cell membranes. Because of its polyunsaturation, docosahexaenoic acid (DHA) and possibly conjugated linoleic acid (CLA) are sterically incompatible with sphingolipid and cholesterol and, therefore, appear to alter lipid raft behavior and protein function. This review examines the evidence indicating that dietary sources of n-3 PUFA can profoundly alter the biochemical make up of lipid rafts/caveolae microdomains, thereby influencing cell signaling, protein trafficking, and cell cytokinetics.
Keywords: membrane rafts, omega-3 fatty acids, conjugated fatty acids, microdomains
The balance between cell proliferation and apoptosis is critical to the maintenance of steady-state cell populations in the body. In general, dysregulation of this mechanism can disrupt homeostasis, resulting in clonal expansion, with the resultant over production of affected cells. The programmed induction of cell death also represents a mechanism by which inappropriately activated cells and cells possessing DNA damage can be deleted. It has now been clearly established that chronic inflammation can perturb cellular homeostasis and drive malignant transformation by progressively inhibiting apoptosis of target cell types, e.g., T-cells and epithelial cells (1,2). Hence, chemotherapeutic agents such as dietary long chain n-3 polyunsaturated fatty acids (PUFA) and possibly conjugated fatty acid species, which restore the normal proliferative and apoptotic pathways have the potential for effectively treating cancers that depend on aberrations of these pathways to stay alive (3). The following sections describe a mechanistic membrane-based model which may in part explain the pleiotropic chemoprotective properties of bioactive PUFA.
n-3 PUFA
Although beyond the scope of this review, a number of investigators have recently addressed the role of n-3 PUFA in suppressing chronic inflammation and cancer (4, 5, 6). With respect to mechanisms which functionally link the pleiotropic effects of bioactive dietary long chain n-3 PUFA, inflammation and cancer, examples include (i) metabolic interconversion into novel bioactive eicosanoids (7, 8), (ii) modulation of nuclear receptor activation, gene transcription and translation (9–12) (Figure 1), (iii) alteration of membrane phospholipid composition and functionality of self organizing lipid domains (13) (Figure 2), (iv) effects on protein trafficking, including cytosol-to-membrane translocation (4,14), and (v) interaction with short chain fatty acids to trigger lipid oxidation and intracellular Ca2+ compartmentalization (15,16).
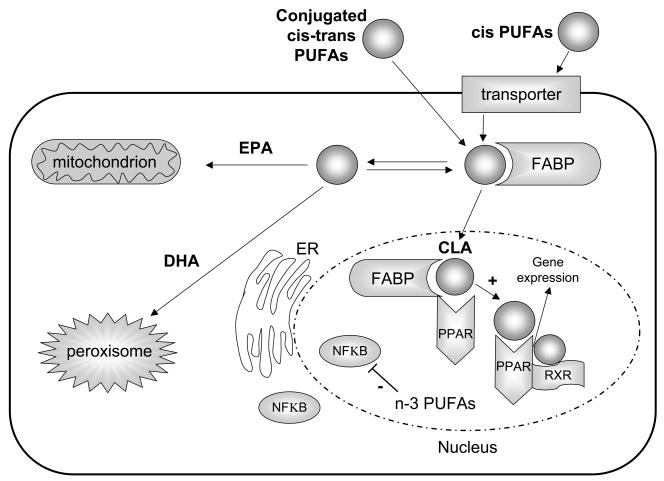
Figure 1. Nuclear receptor activation by conjugated linoleic acid.
ER, endoplasmic reticulum; FABP, fatty acid binding proteins (molecular chaperone); PPAR, peroxisome proliferators-activated receptors; RXR, retinoid X receptors. CLA transactivates PPAR nuclear receptors, n-3 PUFA suppress NF-kB activation. All membranes incorporporate EPA, DHA and conjugated PUFA to different degrees.
Figure 2. Putative membrane microdomain altering properties of n-3 PUFA and CLA.
Dietary DHA and CLA are incorporated into both the bulk phase of the plasma membrane as well as discrete heterogeneous cholesterol/sphingolipid-rich raft domains. This can alter plasma membrane organization of inner leaflets and the dynamic partitioning of transduction proteins, thereby modulating their function
The health benefits of long chain PUFA are diverse and nutritional studies continue to demonstrate important benefits from the consumption of n-3 PUFA (4,7,8,17–19). Since the use of a health claim on labels for foods containing n-3 PUFA has been approved, food companies are now mobilizing to incorporate these fatty acids into a range of novel commercial foods in order to provide for the wider public consumption of these bioactive compounds. Hence, it is now important to precisely determine how specific long chain PUFA modulate cell phenotype and reduce the risk of developing cancer and inflammatory disorders.
Conjugated Linoleic acid (CLA)
There is growing interest with regard to the use and commercial availability of conjugated positional and geometric isomers of PUFA, particularly conjugated dienoic isomers of linoleic acid (CLA). For example, in certain model systems, CLA is a powerful anti-cancer agent, capable of promoting growth arrest and apoptosis in tumor cells (20–22). In addition, CLA triggers adipose delipidation in rodent species (23–25), and although there has been very little published clinical research (26,27), recent preliminary evidence suggests that mixed isomer CLA supplementation can alter fat oxidation and energy expenditure in humans (28,29). However, the precise mechanism of action remains elusive. Since n-3 PUFA and CLA exhibit overlapping phenotypic properties, we propose a unifying molecular mechanism which may in part explain their protective effects.
Effect of bioactive PUFA on cell membranes
It is generally believed that the plasma membrane consists of a mosaic of functional microdomains that facilitate interactions between resident proteins and lipids (30,31). Visible examples of these include caveolae, flask shaped invaginations containing the structural protein caveolin-1 and many signal transduction proteins (32). In addition, morphologically heterogeneous featureless microdomains, consisting mostly of cholesterol and sphingolipids, unable to integrate well into the fluid phospholipid bilayers, exist as “lipid rafts” (33). Although, the existence of lipid rafts is still debated, new sophisticated imaging approaches have started to define cell surface nanoscale organization (31). Significantly, both cholesterol-dependent microdomains, analogous to lipid rafts, and non-raft signaling microdomains have been observed using electron microscopic imaging of 2D plasma membrane sheets (34). These studies have provided a template for further investigation into the effects of dietary PUFA on cell surface organization and cell cytokinetics, apoptosis and disease progression.
With respect to the biological effects of n-3 PUFA, increasing evidence suggests that docosahexaenoic acid (DHA) is a unique fatty acid because it significantly alters basic properties of cell membranes, including acyl acyl (ester linked fatty acid) chain order and fluidity, phase behavior, elastic compressibility, ion permeability, fusion, rapid flip-flop and resident protein function (35). In part, due to the number of cis double bonds, DHA is sterically incompatible with sphingolipid and cholesterol and, therefore, appears to alter lipid raft behavior (35). Interestingly, a number of studies have recently demonstrated that dietary n-3 PUFA are incorporated into diverse cell types and appear to uniquely modulate cell membrane microdomains (13, 36–39). Indeed, we recently demonstrated that n-3 PUFA feeding can markedly alter lipid/protein composition of mouse colonic caveolae microdomains, thereby selectively modulating the localization and function of caveolar proteins (13,14,37). In addition, we demonstrated that H-Ras and endothelial nitric oxide synthase (eNOS) are displaced from caveolae in n-3 PUFA-fed mice, which was associated with the suppression of Ras-dependent signaling. In contrast, localization of non-caveolae resident proteins, K-ras and clathrin, was not affected indicating selective displacement of acylated signaling proteins from caveolae by n-3 PUFA. Our findings highlight a novel modality by which n-3 PUFA influence membrane micro-organization, thereby modulating biological responses.
Using T-cell culture models, Stulnig and colleagues were the first to document the ability of PUFA enrichment to selectively modify the cytoplasmic layer of lipid rafts (40,41). In complimentary experiments, we investigated the effect of dietary n-3 PUFA on cholesterol/sphingolipid-rich plasma membrane microdomains (i.e., rafts) in mouse splenic T-cells (5,42,43). A very novel and unexpected outcome from this effort was the demonstration that dietary n-3 PUFA reduced (by ~45%) lipid raft sphingolipid content and altered raft fatty acid composition (36,44,45). Therefore, we hypothesized that PUFA classes (n-6 vs n-3) differentially modulate T-cell membrane microdomains, which is supported by recent studies indicating that stimulation-induced PKCθ translocation into T-cell lipid rafts is suppressed by dietary n-3 PUFA (36). In addition, in an attempt to further probe the effects of DHA on PKCθ effector pathway signaling, we have recently demonstrated that the diet modification of lipid rafts is associated with the suppression of NF-kB, AP-1 activation, IL-2 secretion and lymphoproliferation (36). With respect to lymphocyte subsets, recent studies indicate that the macromolecular complex organization in lipid rafts is distinct in nonpolarized, Th1 and Th2 polarized subsets (46). This would suggest that these subsets, i.e., regulators of cell-mediated immunity (Th1) and humoral immunity (Th2), would respond differently to dietary PUFA-induced perturbation. However, the ability of DHA to influence membrane raft-mediated signaling in polarized T-cells has not been determined to date.
There is cogent evidence indicating that lipid raft integrity is a prerequisite for optimized signaling between T-cells and antigen presenting cells (47,48). In addition, recent studies suggest that long chain PUFAs can block antigen presentation by interfering with lipid raft-dependent formation of the immunological synapse (38, 39, 49). Overall, these findings provide evidence indicating that dietary n-3 PUFA can profoundly alter the biochemical make up of cell membrane lipid rafts/caveolae microdomains, which may directly or indirectly influence membrane fusion and cell-cell signaling. Interestingly, only a single study to date has examined the effects of CLA with regard to lipid raft/caveolae composition. Huot and Ma (50) demonstrated that mixed CLA isomers are concentrated in caveolae phospholipids, resulting in the reduction of caveolae resident proteins, caveolin-1 and Her-2/neu, in MCF-7 breast cancer cells. Unfortunately, few studies to date have assessed the physical properties of CLA isomers (51). Ergo, future studies using purified CLA isomers are needed in order to elucidate how conjugated fatty acid structure affects membrane structure and function.
DHA alters the size and distribution of lipid rafts
In a proof of principle study, we sought to determine the effect of DHA on the size and distribution of lipid rafts in vivo (17). Using immunogold electron microscopy of plasma membrane sheets coupled with spatial point analysis, morphologically featureless microdomains were visualized in HeLa cells. Cells were transfected with green fluorescent protein truncated H-ras (GFP-tH), which is located exclusively to inner leaflet rafts, and subsequently incubated with DHA and control fatty acid, e.g., oleic acid (18:1 n-9) for 48 h. Univariate K-function analysis of GFP-tH (5 nm gold) revealed that the interparticle distance was significantly reduced by DHA treatment compared to control fatty acid, indicating that select PUFA can increase clustering of proteins in cholesterol-dependent microdomains (GFP-tH). whereas non raft microdomains are insensitive to DHA modulation. These novel findings suggest that the plasma membrane organization of inner leaflets is fundamentally altered by DHA-enrichment (Figure 2).
“Cholesterol-centric” view of membranes
Saturated fatty acids compared to PUFA have a preferential affinity for cholesterol. This relationship provides the basis for a lipid-driven mechanism for the lateral segregation of membrane elements into cholesterol-rich and -poor microdomains (35, 52–55). For example, unfavorable interaction between cholesterol and PUFA chains has been clearly demonstrated by the exclusion of cholesterol from dipolyunsaturated phosphatidylcholine (PC) membranes where it is forced to directly contact polyunsaturated chains. Studies using a variety of techniques including differential scanning calorimetry (56), 1H NMR and nuclear Overhauser enhancement spectroscopy with magic angle spinning (52), determination of partition coefficients (57), measurements of lateral compressibility (58), and fluorescence anisotropy (53,59), indicate that the poor affinity of DHA and perhaps CLA for cholesterol provides a lipid-driven mechanism for lateral phase separation of cholesterol-rich lipid microdomains from the surrounding bulk membrane. This could in principle alter the size, stability and distribution of cell surface lipid microdomains such as rafts. Indeed, growing evidence from model membrane studies suggest that the energetically less favorable interaction between cholesterol and PUFAs, especially DHA, promotes lateral phase segregation into sterol-poor/PUFA-rich and sterol-rich/saturated fatty acid-rich microdomains (52,53,57,60–62).
Huang and co-workers proposed the umbrella model to describe the solubility and condensing effect of cholesterol within membranes (63). According to this model, phospholipid head groups act as ‘umbrellas’ to prevent the energetically unfavorable contact of the non-polar part of cholesterol with interfacial water. This shielding will be less effective for DHA-containing phospholipids with a large molecular cross-sectional area, facilitating cholesterol precipitation at a lower concentration. In addition, this model allows for speculation that phosphatidylethanolamine (PE) with a smaller head group may enhance the DHA-associated reduction in shielding effects relative to PC. Consistent with this notion, unlike PC bilayers where a marked reduction in cholesterol solubility requires polyunsaturation at both sn-1 and sn-2 positions, DHA at sn-2 position with a saturated sn-1 chain is sufficient in PE to trigger cholesterol precipitation (64).
Conjugated n-3 PUFA
There is growing evidence that the combination of conjugated double bonds and n-3 PUFA may have enhanced chemoprotective properties. Recent studies using a number of model systems suggest that conjugated EPA (CEPA) and conjugated DHA (CDHA) suppress tumor growth (65,66), suppress topoisomerases (67), induce apoptosis (68,69), inhibit lipid accumulation (70) and have potential use as therapeutic dietary supplements for minimizing tumor angiogenesis (68,71). Typically, CEPA and CDHA are generated by alkaline isomerization, producing a mixture of isomers with conjugated double bonds, although small amounts are found in marine algae and seal oil. Since the exact structure of these novel fatty acid species has not been fully characterized, future studies are required to verify their safety and efficacy in humans. In addition, it remains to be determined whether CEPA and CDHA alter the size and distribution of cell surface microdomains.
Conclusion
A growing body of literature supports the contention that bioactive food components containing n-3 PUFA are important in suppressing chronic inflammation and cancer. Although the mechanism of EPA and DHA action is still not fully defined in molecular terms, it is becoming increasingly clear that n-3 PUFA alter cell membrane lipid microdomain composition, thereby favorably modulating the relay of extracellular signals from surface receptors to downstream signaling networks. Clearly, further studies are needed to clarify the nature of lipid rafts and the biological role of conjugated fatty acid species, including CLA, CEPA and CDHA families.
Acknowledgments
Financial support by NIH grants CA59034, CA129444, DK071707 and P30ES09106, USDA 2005-34402-16401 Designing Foods for Health, and the American Institute for Cancer Research is greatfully acknowledged. There are no conflicts of interest associated with this manuscript. Drs. Chapkin, Davidson, Fan and Lupton compiled data from collaborative experiments; Dr. McMurray evaluated the T-cell literature as well as contributions made from collaborative experiments. Dr. Patil provided input related to novel fatty acid sources.
References
- 1.Karin M. Nuclear factor-kB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 2.Rollins BJ. Inflammatory chemokines in cancer growth and progression. Eur J Cancer. 2006;42:760–767. doi: 10.1016/j.ejca.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Hong MY, Lupton JR, Morris JS, et al. Dietary fish oil reduces O6-methylguanine DNA adduct levels in rat colon in part by increasing apoptosis during tumor initiation. Cancer Epidemiol Biomarkers & Prev. 2000;9:819–826. [PubMed] [Google Scholar]
- 4.Chapkin RS, McMurray DN, Lupton JR. Colon cancer, fatty acids and anti-inflammatory compounds. Curr Opin Gastroenterol. 2007;23:48–54. doi: 10.1097/MOG.0b013e32801145d7. [DOI] [PubMed] [Google Scholar]
- 5.Fowler KH, Chapkin RS, McMurray DN. Effects of purified dietary n-3 ethyl esters on murine T-lymphocyte function. J Immunol. 1993;151:5186–5197. [PubMed] [Google Scholar]
- 6.Serhan CN, Yacoubian S, Yang R. Anti-inflammatory and proresolving lipid mediators. Annu Rev Pathol Mech Dis. 2008;3:279–312. doi: 10.1146/annurev.pathmechdis.3.121806.151409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ariel A, Serhan CN. Resolvins and protectins in the termination program of acute inflammation. Trends Immunol. 2007;28:176–183. doi: 10.1016/j.it.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Bazan NG. Omega-3 fatty acids, pro-inflammatory signaling and neuroprotection. Curr Opin Clin Nutr Metab Care. 2007;10:136–141. doi: 10.1097/MCO.0b013e32802b7030. [DOI] [PubMed] [Google Scholar]
- 9.deUrquiza AM, Liu S, Sjoberg M, et al. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science. 2000;290:2140–2144. doi: 10.1126/science.290.5499.2140. [DOI] [PubMed] [Google Scholar]
- 10.Fan YY, Spencer TE, Wang N, Moyer MP, Chapkin RS. Chemopreventive n-3 fatty acids activate RXRα in colonocytes. Carcinogenesis. 2003a;24:1541–1548. doi: 10.1093/carcin/bgg110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davidson LA, Nguyen DV, Hokanson RM, et al. Chemopreventive n-3 polyunsaturated fatty acids reprogram genetic signatures during colon cancer initiation and progression in the rat. Cancer Res. 2004;64:6797–6804. doi: 10.1158/0008-5472.CAN-04-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jump DB, Botolin D, Wang Y, Xu J, Christian B, Demeure O. Fatty acid regulation of hepatic gene transcription. J Nutr. 2005;135:2503–2506. doi: 10.1093/jn/135.11.2503. [DOI] [PubMed] [Google Scholar]
- 13.Ma DW, Seo J, Switzer KC, et al. n-3 PUFA and membrane microdomains: a new frontier in bioactive lipid research. J Nutr Biochem. 2004a;15:700–706. doi: 10.1016/j.jnutbio.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Seo J, Barhoumi R, Johnson AE, Lupton JR, Chapkin RS. Docosahexaenoic acid selectively inhibits plasma membrane targeting of lipidated proteins. FASEB J. 2006;20:770–772. doi: 10.1096/fj.05-4683fje. [DOI] [PubMed] [Google Scholar]
- 15.Kolar SS, Barhoumi R, Lupton JR, Chapkin RS. Docosahexaenoic acid and butyrate synergistically induce colonocyte apoptosis by enhancing mitochondrial Ca2+ accumulation. Cancer Res. 2007;67:5561–5568. doi: 10.1158/0008-5472.CAN-06-4716. [DOI] [PubMed] [Google Scholar]
- 16.Kolar SS, Barhoumi R, Callaway ES, et al. Synergy between docosahexaenoic acid and butyrate elicits p53-independent apoptosis via mitochondrial Ca2+ accumulation in colonocytes. Am J Physiol. doi: 10.1152/ajpgi.00312.2007. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chapkin RS, Wang N, Fan YY, Lupton JR, Prior IA. Docosahexaenoic acid alters the size and distribution of cell surface microdomains. Biochim Biophys Acta –Biomembranes. 2008;1778:466–471. doi: 10.1016/j.bbamem.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biscione F, Pignalberi C, Totteri A, Messina F, Altamura G. Cardiovascular effects of omega-3 free fatty acids. Curr Vasc Pharmacol. 2007;5:163–172. doi: 10.2174/157016107780368334. [DOI] [PubMed] [Google Scholar]
- 19.Harris WS, Poston WC, Haddock CK. Tissue n-3 and n-6 fatty acids and risk for coronary heart disease events. Atherosclerosis. 2007;193:1–10. doi: 10.1016/j.atherosclerosis.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Ou L, Ip C, Lisafeld B, Ip MM. Conjugated linoleic acid induces apoptosis of murine mammary tumor cells via Bcl-2 loss. Biochem Biophys Res Commun. 2007;356:1044–9. doi: 10.1016/j.bbrc.2007.03.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bozzo F, Bocca C, Colombatto S, Miglietta A. Antiproliferative effect of conjugated linoleic acid in caco-2 cells: Involvement of PPARγ and APC/β-catenin pathways. Chemico-Biol Interactions. 2007;169:110–121. doi: 10.1016/j.cbi.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Song HJ, Sneddon AA, Heys SD, Wahle KW. Induction of apoptosis and inhibition of NF-kappaB activation in human prostate cancer cells by the cis-9, trans-11 but not the trans-10, cis-12 isomer of conjugated linoleic acid. Prostate. 2006;66:839–846. doi: 10.1002/pros.20351. [DOI] [PubMed] [Google Scholar]
- 23.Chung S, Brown JM, Provo JN, Hopkins R, Mclntosh MK. Conjugated linoleic acid promotes human adipocyte insulin resistance through NF-kB dependent cytokine production. J Biol Chem. 2005;280:38445–38456. doi: 10.1074/jbc.M508159200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu LF, Purushotham A, Wendel AA, Belury MA. Combined effects of rosiglitazone and conjugated linoleic acid on adiposity, insulin sensitivity, and hepatic steatosis in high-fat-fed mice. Am J Physiol Liver Physiol. 2007;292:G1671–G1682. doi: 10.1152/ajpgi.00523.2006. [DOI] [PubMed] [Google Scholar]
- 25.Perez-Matute P, Marti A, Martinez JA, et al. Conjugated linoleic acid inhibits glucose metabolism, leptin and adiponectin-secretion in primary cultured rat adipocytes. Mol Cell Endocrinol. 2007;268:50–58. doi: 10.1016/j.mce.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 26.Navarro V, Fernandez-Quintela A, Churruca I, Portillo MP. The fat-lowering effect of conjugated linoleic acid: a comparison between animal and human studies. J Physiol Biochem. 2006;62:137–148. doi: 10.1007/BF03174074. [DOI] [PubMed] [Google Scholar]
- 27.Tricon S, Yaqoob P. Conjugated linoleic acid and human health: a critical evaluation of the evidence. Curr Opin Nutr Metab Care. 2006;9:105–110. doi: 10.1097/01.mco.0000214567.44568.fb. [DOI] [PubMed] [Google Scholar]
- 28.Close RN, Schoeller DA, Watras AC, Nora EH. Conjugated linoleic acid supplementation alters the 6-mo change in fat oxidation during sleep. Am J Clin Nutr. 2007;86:797–804. doi: 10.1093/ajcn/86.3.797. [DOI] [PubMed] [Google Scholar]
- 29.Whigham LD, Watras AC, Schoeller DA. Efficacy of conjugated linoleic acid for reducing fat mass: a meta-analysis in humans. Am J Clin Nutr. 2007;85:1203–1211. doi: 10.1093/ajcn/85.5.1203. [DOI] [PubMed] [Google Scholar]
- 30.Laude AJ, Prior IA. Plasma membrane microdomains: organization, function and Trafficking. Mol Membr Biol. 2004;21:193–205. doi: 10.1080/09687680410001700517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hancock JF. Lipid rafts: contentious only from simplistic standpoints. Nat Rev Mol Cell Biol. 2006;7:456–462. doi: 10.1038/nrm1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson RGW. The caveolae membrane system. Annu Rev Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- 33.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 34.Prior IA, Muncke C, Parton RG, Hancock JF. Direct visualization of Ras proteins in spatially distinct cell surface microdomains. J Cell Biol. 2003;160:165–170. doi: 10.1083/jcb.200209091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stillwell W, Wassall SR. Docosahexaenoic acid: membrane properties of a unique fatty acid, Chem. Phys Lipids. 2003;126:1–27. doi: 10.1016/s0009-3084(03)00101-4. [DOI] [PubMed] [Google Scholar]
- 36.Fan YY, Ly LH, Barhoumi R, McMurray DN, Chapkin RS. Dietary docosahexaenoic acid suppresses T-cell protein kinase C-theta lipid raft recruitment and interleukin-2 production. J Immunol. 2004;173:6151–6160. doi: 10.4049/jimmunol.173.10.6151. [DOI] [PubMed] [Google Scholar]
- 37.Ma DW, Seo J, Davidson LA, et al. n-3 PUFA alter caveolae lipid composition and resident protein localization in mouse colon. Faseb J. 2004b;18:1040–1042. doi: 10.1096/fj.03-1430fje. [DOI] [PubMed] [Google Scholar]
- 38.Zeyda M, Saemann MD, Stuhlmeier KM, et al. Polyunsaturated block dendritic cell activation and function independently of NF-kappaB activation. J Biol Chem. 2005;280:14293–14301. doi: 10.1074/jbc.M410000200. [DOI] [PubMed] [Google Scholar]
- 39.Geyeregger R, Zeyda M, Zlabinger GJ, Waldhausl W, Stulnig TM. Polyunsaturated fatty acids interfere with formation of the immunological synapse. J Leuk Biol. 2005;77:680–688. doi: 10.1189/jlb.1104687. [DOI] [PubMed] [Google Scholar]
- 40.Stulnig TM, Berger M, Sigmund T, Raederstorff D, Stockinner H, Waldhausl W. Polyunsaturated fatty acids inhibit T cell signal transduction by modification of detergent-insoluble membrane domains. J Cell Biol. 1998;143:637–644. doi: 10.1083/jcb.143.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stulnig TM, Huber J, Leitinger N, et al. Polyunsaturated eicosapentaenoic acid displaces proteins from membrane rafts by altering raft lipid composition. J Biol Chem. 2001;276:37335–37340. doi: 10.1074/jbc.M106193200. [DOI] [PubMed] [Google Scholar]
- 42.Fowler KH, McMurray DN, Fan YY, Aukema HM, Chapkin RS. Purified dietary n-3 polyunsaturated fatty acids alter diacylglycerol mass and molecular species composition in concanavalin A stimulated murine splenocytes. Biochim Biophys Acta. 1993;1210:89–96. doi: 10.1016/0005-2760(93)90053-c. [DOI] [PubMed] [Google Scholar]
- 43.Switzer KC, McMurray DN, Morris JS, Chapkin RS. Dietary n-3 polyunsaturated fatty acids selectively promote activation-induced cell death in T-lymphocytes. J Nutr. 2003;133:496–503. doi: 10.1093/jn/133.2.496. [DOI] [PubMed] [Google Scholar]
- 44.Fan YY, McMurray DN, Ly LH, Chapkin RS. Dietary (n-3) polyunsaturated fatty acids remodel mouse T-cell lipid rafts. J Nutr. 2003;133:1913–1920. doi: 10.1093/jn/133.6.1913. [DOI] [PubMed] [Google Scholar]
- 45.Switzer KC, Fan YY, Wang N, McMurray DM, Chapkin RS. Dietary n-3 polyunsaturated fatty acids promote activation-induced cell death in the Th1-polarized murine CD4+ T-cells. J. Lipid Res. 2004;45:1482–1492. doi: 10.1194/jlr.M400028-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leitenberg D, Balamuth F, Bottomly K. Changes in the T cell receptor macromolecular signaling complex and membrane microdomains during T cell development and activation. Sem Immunol. 2001;13:129–138. doi: 10.1006/smim.2000.0304. [DOI] [PubMed] [Google Scholar]
- 47.Gaus K, Chklovskaia E, St Groth B. Condensation of the plasma membrane at the site of T lymphocyte activation. J Cell Biol. 2005;171:121–131. doi: 10.1083/jcb.200505047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harder T, Rentero C, Zech T, Gaus K. Plasma membrane segregation during T cell activation: probing the order of domains. Curr Opin Immunol. 2007;19:470–475. doi: 10.1016/j.coi.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Shaikh SR, Edidin M. Immunosuppressive effects of polyunsaturated fatty acids on antigen presentation by human leukocyte antigen class I molecules. J Lipid Res. 2007;48:127–138. doi: 10.1194/jlr.M600365-JLR200. [DOI] [PubMed] [Google Scholar]
- 50.Huot PS, Ma WL. CLA incorporates into caveolae phospholipids and reduces caveolin-1 expression in MCF-7 cells. Submitted for publication. Presented at the 98th American Oil Chemists’ Society (AOCS) Annual Meeting; Quebec, Canada. May, 2007; AOCS Press; p. 81. [Google Scholar]
- 51.Yin JJ, Kramer JK, Yurawecz MP, Eynard AR, Mossoba MM, Yu L. Effects of conjugated linoleic acid (CLA) isomers on oxygen diffusion-concentration products in liposomes and phospholipids solutions. J Agric Food Chem. 2006;54:7287–7293. doi: 10.1021/jf0610918. [DOI] [PubMed] [Google Scholar]
- 52.Huster D, Arnold K, Gawrisch K. Influence of docosahexaenoic acid and cholesterol on lateral lipid organization in phospholipid mixtures. Biochemistry. 1998;37:17299–17308. doi: 10.1021/bi980078g. [DOI] [PubMed] [Google Scholar]
- 53.Mitchell DC, Litman BJ. Effect of cholesterol on molecular order and dynamics in highly polyunsaturated phospholipid bilayers. Biophys J. 1998;75:896–908. doi: 10.1016/S0006-3495(98)77578-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pasenkiewicz-Gierula M, Subczynski WK, Kusumi A. Influence of Phospholipid Unsaturation on the Cholesterol Distribution in Membranes. Biochimie. 1991;73:1311–1316. doi: 10.1016/0300-9084(91)90094-h. [DOI] [PubMed] [Google Scholar]
- 55.Niu SL, Litman BJ. Determination of membrane cholesterol partition coefficient using a lipid vesicle-cyclodextrin binary system: effect of phospholipid acyl chain unsaturation and headgroup composition. Biophys J. 2002;83:3408–3415. doi: 10.1016/S0006-3495(02)75340-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zerouga M, Jenski LJ, Stillwell W. Comparison of phosphatidylcholines containing one or two docosahexaenoic acyl chains on properties of phospholipid monolayers and bilayers. Biochim Biophys Acta. 1995;1236:266–272. doi: 10.1016/0005-2736(95)00058-b. [DOI] [PubMed] [Google Scholar]
- 57.Kariel N, Davidson E, Keough KM. Cholesterol does not remove the gel-liquid crystalline phase transition of phosphatidylcholines containing two polyenoic acyl chains. Biochim Biophys Acta. 1991;1062:70–76. doi: 10.1016/0005-2736(91)90336-7. [DOI] [PubMed] [Google Scholar]
- 58.Needham D, Nunn RS. Elastic deformation and failure of lipid bilayer membranes containing cholesterol. Biophys J. 1990;58:997–1009. doi: 10.1016/S0006-3495(90)82444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mitchell DC, Litman BJ. Molecular order and dynamics in bilayers consisting of highly polyunsaturated phospholipids. Biophys J. 1998;74:879–891. doi: 10.1016/S0006-3495(98)74011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brzustowicz MR, Cherezov V, Caffrey M, Stillwell W, Wassall SR. Molecular organization of cholesterol in polyunsaturated membranes: microdomain formation. Biophys J. 2002;82:285–298. doi: 10.1016/S0006-3495(02)75394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brzustowicz MR, Cherezov V, Zerouga M, Caffrey M, Stillwell W, Wassall SR. Controlling membrane cholesterol content. A role for polyunsaturated (docosahexaenoate) phospholipids. Biochemistry. 2002;41:12509–12519. doi: 10.1021/bi0262808. [DOI] [PubMed] [Google Scholar]
- 62.Pasenkiewicz-Gierula M, Subczynski WK, Kusumi A. Rotational diffusion of a steroid molecule in phosphatidylcholine-cholesterol membranes: fluid-phase microimmiscibility in unsaturated phosphatidylcholine-cholesterol membranes. Biochemistry. 1990;29:4059–4069. doi: 10.1021/bi00469a006. [DOI] [PubMed] [Google Scholar]
- 63.Huang J, Feigenson GW. A microscopic interaction model of maximum solubility of cholesterol in lipid bilayers. Biophys J. 1999;76:2142–2157. doi: 10.1016/S0006-3495(99)77369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shaikh SR, Cherezov V, Caffrey M, Stillwell W, Wassall SR. Interaction of cholesterol with a docosahexaenoic acid-containing phosphatidylethanolamine: trigger for microdomain/raft formation? Biochemistry. 2003;42:12028–12037. doi: 10.1021/bi034931+. [DOI] [PubMed] [Google Scholar]
- 65.Danbara N, Yuri T, Tsujita-Kyutoku M, et al. Conjugated docosahexaenoic acid is a potent inducer of cell cycle arrest and apoptosis and inhibits growth of Colo 201 human colon cancer cells. Nutr Cancer. 2004;50:71–79. doi: 10.1207/s15327914nc5001_10. [DOI] [PubMed] [Google Scholar]
- 66.Kimura Y, Sumiyoshi M. Antitumor and antimetastatic actions of eicosapentaenoic acid ethylester and its by-products formed during accelerated stability testing. Cancer Sci. 2005;96:441–450. doi: 10.1111/j.1349-7006.2005.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yonezawa Y, Tsuzuki T, Eitsuka T, et al. Inhibitory effect of conjugated eicosapentaenoic acid on human DNA topoisomerases I and II. Arch Biochem Biophys. 2005;435:197–206. doi: 10.1016/j.abb.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 68.Tsuzuki T, Tanaka K, Kuwahara S, Miyazawa T. Synthesis of the conjugated trienes 5E,7E,9E,14Z,17Z-eiocospentaenoic acid and 5Z,7E,9E,14Z,17Z-eiocsapentaenoic acid and their induction of apoptosis in DLD-1 colorectal adenocarcinoma cells. Lipids. 2005;40:147–154. doi: 10.1007/s11745-005-1369-1. [DOI] [PubMed] [Google Scholar]
- 69.Tsuzuki T, Kambe T, Shibata A, Kawakami Y, Nakagawa K, Miyazawa T. Conjugated EPA activates mutant p53 via lipid peroxidation and induces p53-dependent apoptosis in DLD-1 colorectal adenocarcinoma human cells. Biochim Biophys Acta. 2007;1771:20–30. doi: 10.1016/j.bbalip.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 70.Tsuzuki T, Kawakami Y, Nakagawa K, Miyazawa T. Conjugated docosahexaenoic acid inhibits lipid accumulation in rats. J Nutr Biochem. 2006;17:518–524. doi: 10.1016/j.jnutbio.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 71.Tsuzuki T, Shibata A, Kawakami Y, Nakagaya K, Miyazawa T. Anti-angiogenic effects of conjugated docosahexaenoic acid in vitro and in vivo. Biosci Biotechnol Biochem. 2007;71:1902–1910. doi: 10.1271/bbb.70114. [DOI] [PubMed] [Google Scholar]