Abstract
Seasonal changes in behavior and its underlying neural substrate are common across animal taxa. These changes are often triggered by steroid sex hormones. Song in seasonally breeding songbirds provides an excellent example of this phenomenon. In these species, dramatic seasonal changes mediated by testosterone and its metabolites occur in adult song behavior and in the neural circuitry controlling song. While song rate can quickly change in response to seasonal breeding cues, it is unknown how quickly other aspects of song change, particularly the stereotypy of song phonology and syntax. In this study we determined whether and how quickly song rate, phonology, and syntax change in response to breeding and non-breeding physiological cues. We asked these questions using Gambel’s white-crowned sparrows (Zonotrichia leucophrys gambelii), a closed-ended learner with well-characterized changes in the neural circuitry controlling song behavior. We exposed ten photosensitive sparrows to long-day photoperiod and implanted them with subcutaneous testosterone pellets (day 0) to simulate breeding conditions. We continuously recorded song and found that song rate increased quickly, reaching maximum around day 6. The stereotypy of song phonology changed more slowly, reaching maximum by day 10 or later. Song syntax changed minimally after day 6, the earliest time point examined. After 21 days, we transitioned five birds from breeding to non-breeding condition. Song rate declined precipitously. These results suggest that while song rate changes quickly, song phonology changes more slowly, generally following or in parallel with previously investigated changes in the neural substrate.
Keywords: Gambel’s white-crowned sparrow, Zonotrichia leucophrys gambelii, testosterone, birdsong, songbird, seasonal plasticity, photoperiod
INTRODUCTION
Steroid sex hormones modulate neural substrates to change behavior. These changes in behavior can sometimes happen within minutes of hormone exposure (Remage-Healey and Bass, 2006), but often they occur more slowly, over days, weeks, or even across seasons (Tramontin and Brenowitz, 2000). Hormone-induced changes in behavior and in the underlying neural substrate occur in all vertebrate taxa, but seasonally breeding songbirds provide a particularly useful model system for studying hormone-mediated plasticity in adult brain and behavior (Tramontin and Brenowitz, 2000; Ball et al., 2004; Brenowitz, 2004; Meitzen and Thompson, 2008). In these species, song behavior and its underlying neural substrate, the song control system, change dramatically between the breeding and non-breeding seasons. Early in the breeding season, longer day lengths and other environmental cues stimulate gonadal growth, which leads to an increase in plasma testosterone (T) level. Increased circulating T levels trigger structural and electrophysiological changes within the brain nuclei that regulate song behavior (Nottebohm, 1981; Ball et al., 2004; Brenowitz, 2004; Meitzen and Thompson, 2008); seasonal changes in auditory processing may also occur (Lucas et al., 2002; Del Negro et al., 2005; Lucas et al., 2007; Maney et al., 2008). Conversely, a sudden transition of birds from breeding to non-breeding conditions induces rapid regression of song control system nuclei within hours (Thompson et al., 2007).
Different aspects of song behavior change across seasons, and some aspects of song behavior have received much more attention than others. For instance, it is well established that song rate (i.e., the quantity of song) quickly changes with the onset of the breeding season (Wingfield and Farner, 1993; Catchpole and Slater, 1995). Seasonal changes in repertoire have also been studied, especially the incorporation of new syllables into canary song (Nottebohm et al., 1986, 1987; Voigt and Leitner, 2008). How quickly other aspects of song behavior change, and in particular, seasonal change in more subtle aspects of song quality such as phonology or syntax, are unknown.
This study asks whether and how quickly song rate, phonology, and syntax change in response to breeding and non-breeding cues. We chose to ask this question using the adult male Gambel’s white-crowned sparrow, a closed-ended learner (Marler and Tamura, 1964) in which the time course of seasonal-like change in the song control system is well-investigated (Tramontin et al., 2000; Thompson et al., 2007; Meitzen et al., 2007a). We exposed birds to photoperiod and plasma testosterone levels typical of the breeding season, and continuously recorded their song. Birds quickly began to sing, reaching maximum song rate approximately six days after initial exposure to breeding cues. The stereotypy of the phonological aspects of song changed more slowly, reaching maximum 10 days or longer after initial exposure. Song syntax changed minimally after day 6, the earliest time point examined. When breeding cues where removed, song rate dropped precipitously. These results suggest that while exposure to breeding conditions quickly changes song rate, other aspects of song behavior change more slowly.
METHODS
Animals
The Institutional Animal Care and Use Committee at the University of Washington approved all procedures used in this study. We collected 10 adult male Gambel’s white-crowned sparrows (Zonotrichia leucophrys gambelii) in eastern Washington during their autumnal migration. We housed the birds in outdoor aviaries for up to 30 weeks prior to placing them in indoor aviaries. Once indoors, they were exposed to a short-day photoperiod (SD; 8 h light: 16 h dark) for at least 10 weeks before use to ensure that they were photosensitive and therefore responsive to circulating plasma testosterone (T) and the long day photoperiod typical of their Alaskan breeding grounds (LD: 20 h light: 4 h dark). Birds kept on SD maintain regressed testes and song nuclei, a low intrinsic spontaneous firing rate in the robust nucleus of the arcopallium (RA; a pre-motor song control nucleus), and basal levels of T typical of the non-breeding season (Smith et al., 1995; Tramontin et al., 2000; Brenowitz, 2004; Park et al., 2005; Meitzen et al., 2007b). Food and water were available ad libitum throughout the experiment. During the initial 10 weeks of SD photoperiod exposure, birds were group housed in indoor cages and could see and hear the other birds housed in the same room.
Systemic Hormone and Photoperiod Manipulations
On Day 0 we implanted birds with a single subcutaneous Silastic capsule of T, shifted them to LD photoperiod, and housed them in individual sound isolation chambers (Industrial Acoustics). We made the capsules from Silastic tubing (i.d. 1.0 mm; o.d. 2.0 mm; length: 12 mm; VWR, West Chester, PA) filled with crystalline T (~0.017 g) as in Tramontin et al., (2003), rinsed them with ethanol, and soaked them overnight in 0.1 M phosphate-buffered saline (PBS) prior to implantation. We implanted birds housed on LD with T capsules because exposure of wild-caught birds to LD alone in the laboratory does not elevate circulating T levels into the physiological breeding range observed in wild Gambel’s white-crowned sparrows (Wingfield and Farner, 1978; Wingfield and Moore, 1987; Smith et al., 1995), and in order to reduce the variability of circulating T levels across individual birds. We note that circulating steroid hormone levels are not necessarily identical to those in the brain parenchyma, due to local neurosteroid synthesis (Schlinger and London, 2006).
On day 21 we removed the subcutaneous T capsules of five of the ten birds and then castrated them to determine how quickly the transition from breeding to non-breeding condition changes song behavior. To castrate birds we anesthetized them with isoflurane, made a small incision on the left side anterior to the caudal-most rib and dorsal to the uncinate process, and aspirated the testes. This procedure was completed within 10 min, and the animals recovered within minutes from anesthesia and were returned to their isolation chamber. We shifted the birds overnight to 14 h light:10 h dark photoperiod the same day as the T withdrawal (evening of day 21) and the next day shifted them to SD (evening of day 22; SD: 8 h light: 16 h dark). The intermediate photoperiod helped birds adjust to the reduction in available feeding time. We confirmed that castration was successful by examining the remains of the five birds for the presence of testes when they were sacrificed 20 days after T-withdrawal for a different study (Thompson et al., 2007).
Hormone Assay
We collected blood from each bird while exposed to LD photoperiod and implanted with a T capsule (5 birds measured on day 21; 5 birds measured between day 7 through 14, see Thompson et al. [2007]). In the five birds transitioned from breeding-like to nonbreeding-like conditions we collected blood on day 21 after the T capsule was removed. Blood from the alar vein in the wing was collected into a heparinized microhematocrit tube and stored on ice until centrifugation (within 1 hour). We harvested the plasma and stored it at −20°C for subsequent steroid radioimmunoassay (RIA). To measure circulating T we followed the RIA protocol of Tramontin et al. (2001), using a Coat-a-Count total testosterone RIA kit (Diagnostic Products Corp., Los Angeles, CA). The minimum detectable plasma T concentration was 0.2 ng/ml. One bird’s plasma T levels were not analyzed because not enough plasma was collected for an accurate assay.
Song Behavior Analysis
To analyze song behavior we individually housed birds in sound isolation chambers (Industrial Acoustics) immediately after T implantation (day 0) and continually recorded their vocalizations using Syrinx software (John Burt, www.syrinxpc.com). Following established protocols (Meitzen et al., 2007a), song was sampled at a rate of 22050 Hz, with trigger settings as follows: threshold above background trigger: 70%, 1000 msec minimum recording time, 500 msec pre-sound buffer, 1000 msec post-sound buffer.
Song rate
The rate of song production was measured by counting the total number of songs per day. Individual songs were defined for counting by the presence of a silent period of at least 500 msec or a call. We collected a total of 23,528 songs from ten birds over 27 days of recording.
Phonology
We further analyzed song on days 6, 8, 10 and 21. Day 6 was the earliest time point at which a sufficient number of songs for quantitative analysis were recorded. Day 21 was analyzed as song is maximally stereotyped at that point (Tramontin et al., 2000; Meitzen et al., 2007a), and song control nuclei have reached full breeding condition (Tramontin et al., 2000; Meitzen et al., 2007a). We analyzed song every two days after day 6 (i.e., day 8 and day 10), until song reached its maximum stereotypy. In 5 birds we measured song stereotypy on days 12, 14, 16 and 18, and found that stereotypy remained stable and maximal (data not shown).
We measured overall song phonological stereotypy using two different protocols. We first used Sound Analysis Pro software (Tchernichovski et al., 2000; ofer.sci.ccny.cuny.edu) to generate a “% Similarity” score. “% Similarity” generates a similarity score based on the acoustic features of two compared songs (Tchernichovski et al., 2000). For each bird, we made pairwise comparisons among 10 randomly selected songs from each day to generate a mean “% Similarity” score. The default Sound Analysis Pro settings were used.
We also assessed song variability (the inverse of song stereotypy), using the protocol initially described in Tramontin et al. (2000) and as modified by Meitzen et al. (2007a), to enable direct comparison to previous studies. We digitized ten randomly selected songs from each bird using Syrinx (the same songs analyzed with Sound Analysis Pro). We assessed a total of 16 different song attributes by measuring the duration of the entire song, and the minimum frequency, maximum frequency and duration of each of the five syllables of white-crowned song (Table 1). To determine whether groups differed in the stereotypy of the song attributes, we compared coefficients of variation (CV = SD/mean). For each song attribute, we calculated the mean value for each bird. We both averaged across birds to create a group mean for each song attribute and averaged each bird’s 16 attributes together to acquire an overall mean for that bird.
Table 1.
Song Structure and Variability Measures
| Time | Statistics | ||||
|---|---|---|---|---|---|
| Day 6 (n = 9) | Day 8 (n = 10) | Day 10 (n = 9) | Day 21 (n = 9)* | ANOVA | |
| Whole song duration (sec) | |||||
| Mean | 1.61 ± 0.09a | 1.87 ± 0.06b | 1.91 ± 0.09b | 1.89 ± 0.05b | F3,9 = 5.23, P = 0.006 |
| CV | 0.28 ± 0.04a | 0.16 ± 0.03b | 0.10 ± 0.04b | 0.10 ± 0.02b | F3,9 = 7.70, P < 0.001 |
|
| |||||
| Whistle duration (sec) | |||||
| Mean | 0.39 ± 0.01 | 0.42 ± 0.01 | 0.44 ± 0.02 | 0.43 ± 0.02 | F3,9 = 2.86, P > 0.05 |
| CV | 0.18 ± 0.04a | 0.10 ± 0.02a,b | 0.09 ± 0.02b | 0.07 ± 0.01b | F3,9 = 4.71, P = 0.011 |
|
| |||||
| Warble duration (sec) | |||||
| Mean | 0.38 ± 0.02 | 0.40 ± 0.02 | 0.38 ± 0.02 | 0.36 ± 0.02 | F3,9 = 3.00, P = 0.05 |
| CV | 0.18 ± 0.04 | 0.09 ± 0.02 | 0.11 ± 0.02 | 0.07 ± 0.02 | F3,9 = 2.95, P = 0.054 |
|
| |||||
| 1st buzz duration (sec) | |||||
| Mean | 0.23 ± 0.01a | 0.24 ± 0.02a | 0.24 ± 0.02a,b | 0.28 ± 0.01b | F3,9 = 3.92, P = 0.02 |
| CV | 0.17 ± 0.11 | 0.14 ± 0.03 | 0.09 ± 0.02 | 0.08 ± 0.01 | F3,9 = 2.18, P > 0.05 |
|
| |||||
| 2nd buzz duration (sec) | |||||
| Mean | 0.27 ± 0.01a | 0.30 ± 0.01a,b | 0.30 ± 0.01a,b | 0.32 ± 0.01b | F3,9 = 5.36, P = 0.005 |
| CV | 0.25 ± 0.11 | 0.09 ± 0.01 | 0.09 ± 0.01 | 0.08 ± 0.01 | F3,9 = 1.86, P > 0.05 |
|
| |||||
| 3rd buzz duration (sec) | |||||
| Mean | 0.40 ± 0.02 | 0.41 ± 0.02 | 0.39 ± 0.02 | 0.44 ± 0.03 | F3,9 = 1.74, P > 0.05 |
| CV | 0.16 ± 0.03 | 0.12 ± 0.02 | 0.13 ± 0.02 | 0.19 ± 0.04 | F3,9 = 1.07, P > 0.05 |
|
| |||||
| Whistle minimum frequency (kHz) | |||||
| Mean | 3.38 ± 0.12 | 3.38 ± 0.14 | 3.33 ± 0.14 | 3.30 ± 0.10 | F3,9 = 1.21, P > 0.05 |
| CV | 0.06 ± 0.03 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.01 | F3,9 = 3.90, P = 0.024 |
|
| |||||
| Whistle maximum frequency (kHz) | |||||
| Mean | 3.89 ± 0.10 | 3.91 ± 0.13 | 3.86 ± 0.12 | 3.80 ± 0.10 | F3,9 = 0.01, P > 0.05 |
| CV | 0.04 ± 0.02 | 0.03 ± 0.01 | 0.02 ± 0.00 | 0.03 ± 0.00 | F3,9 = 1.02, P > 0.05 |
|
| |||||
| Warble minimum frequency (kHz) | |||||
| Mean | 2.88 ± 0.10 | 2.89 ± 0.11 | 2.81 ± 0.10 | 2.90 ± 0.12 | F3,9 = 0.18, P > 0.05 |
| CV | 0.06 ± 0.02 | 0.04 ± 0.00 | 0.04 ± 0.01 | 0.03 ± 0.00 | F3,9 = 1.08, P > 0.05 |
|
| |||||
| Warble maximum frequency (kHz) | |||||
| Mean | 5.34 ± 0.09 | 5.31 ± 0.22 | 5.35 ± 0.25 | 5.25 ± 0.27 | F3,9 = 0.76, P > 0.05 |
| CV | 0.08 ± 0.02 | 0.06 ± 0.01 | 0.06 ± 0.02 | 0.04 ± 0.01 | F3,9 = 2.23, P > 0.05 |
|
| |||||
| 1st buzz minimum frequency (kHz) | |||||
| Mean | 4.08 ± 0.09 | 4.01 ± 0.08 | 3.98 ± 0.15 | 4.00 ± 0.06 | F3,9 = 1.38, P > 0.05 |
| CV | 0.05 ± 0.00 | 0.04 ± 0.00 | 0.03 ± 0.00 | 0.06 ± 0.03 | F3,9 = 0.13, P > 0.05 |
|
| |||||
| 1st buzz maximum frequency (kHz) | |||||
| Mean | 6.22 ± 0.07 | 6.18 ± 0.09 | 6.25 ± 0.08 | 6.05 ± 0.14 | F3,9 = 0.25, P > 0.05 |
| CV | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.05 ± 0.01 | F3,9 = 0.67, P > 0.05 |
|
| |||||
| 2nd buzz minimum frequency (kHz) | |||||
| Mean | 3.85 ± 0.06 | 3.79 ± 0.06 | 3.69 ± 0.12 | 3.82 ± 0.06 | F3,9 = 0.15, P > 0.05 |
| CV | 0.03 ± 0.01 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 | F3,9 = 0.42, P > 0.05 |
|
| |||||
| 2nd buzz maximum frequency (kHz) | |||||
| Mean | 5.33 ± 0.07 | 5.30 ± 0.04 | 5.33 ± 0.05 | 5.27 ± 0.08 | F3,9 = 1.32, P > 0.05 |
| CV | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.05 ± 0.02 | 0.04 ± 0.01 | F3,9 = 0.71, P > 0.05 |
|
| |||||
| 3rd buzz minimum frequency (kHz) | |||||
| Mean | 2.25 ± 0.01 | 2.16 ± 0.08 | 2.15 ± 0.10 | 2.24 ± 0.10 | F3,9 = 1.18, P > 0.05 |
| CV | 0.08 ± 0.01 | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.08 ± 0.03 | F3,9 = 1.64, P > 0.05 |
|
| |||||
| 3rd buzz maximum frequency (kHz) | |||||
| Mean | 6.91 ± 0.47 | 7.05 ± 0.36 | 7.13 ± 0.40 | 7.32 ± 0.48 | F3,9 = 1.86, P > 0.05 |
| CV | 0.10 ± 0.02 | 0.09 ± 0.01 | 0.06 ± 0.01 | 0.09 ± 0.01 | F3,9 = 0.30, P > 0.05 |
All values are mean ± SEM. CV = Coefficient of Variation (SD/mean). Different superscript letters denote significant differences across a row. Across a row, groups with the same letter do not significantly differ from one another. Lack of letters indicates that no significant differences were found.
N is 8 for 3rd buzz, Day 21.
We analyzed the spectral properties of individual syllables using Sound Analysis Pro. For each syllable, we measured four spectral properties: pitch (indicates the period of the sound, increasing with shorter periods), frequency modulation (FM; the mean slope of frequency contours), entropy (a measure of randomness; white noise has an entropy of 1 and a pure tone 0) and pitch goodness (a measure of periodicity; increasing with harmony; Tchernichovski et al., 2000; Sound Analysis Pro manual: http://ofer.sci.ccny.cuny.edu/Chapter_3b.htm). Using the same 10 randomly chosen digitized songs as the previous analysis, we calculated each property’s mean value per syllable for each bird, and then assessed stereotypy by calculating the CV. While other methods of analysis are available (discussed in Wu et al., 2008), for this study we favor the CV method because we are most interested in the stereotypy of individual syllables, and becuase the CV method can be directly compared with previous analyses (Tramontin et al., 2000; Meitzen et al., 2007b; this study).
Syntax
Since the “phonology” analyses do not measure syllable order, to quantify the stereotypy of song syntax we calculated internal linearity and consistency (Scharff and Nottebohm, 1991; Foster and Bottjer, 2001). We randomly selected 20 songs for each day of analysis. A small number of birds did not sing 20 songs on a particular day, so we analyzed the total number of songs available, but always a minimum of ten songs (number of birds with fewer than 20 songs; mean ± StdDev number of songs: day 6: two birds, 15.5 ± 2.1; day 8: two birds, 12.5 ± 3.5; day 10: one bird, 13 songs). Birds with fewer than 10 songs were not analyzed. Internal linearity measures the number of transitions between syllables, using the formula:
A “syllable” is defined as one of the individual components of white-crowned song (a whistle, a warble, or the first, second or third buzz; Fig 2) that is separated from another component by silence. A “non-stop transition” is a transition between syllables that does not come at the beginning or end of the song (Foster and Bottjer, 2001). An internal linearity score of 1 indicates a perfectly linear and stereotyped song. Consistency measures how frequently the bird sings different song variations, using the formula:
in which T(d) is the most frequent transition for each syllable, T(a) is the total number of transitions, and N is the total number of syllables. We counted end-stops as syllables, following Foster and Bottjer (2001). An “end-stop” is the beginning and end of a song, i.e., in song #ABCDE#, the “#A and E#” indicate end-stops. This analysis incorporates the variability of when a bird begins and ends song into the consistency score. A completely stereotyped song where the bird always sings syllables in the same order would have a consistency score of 1.
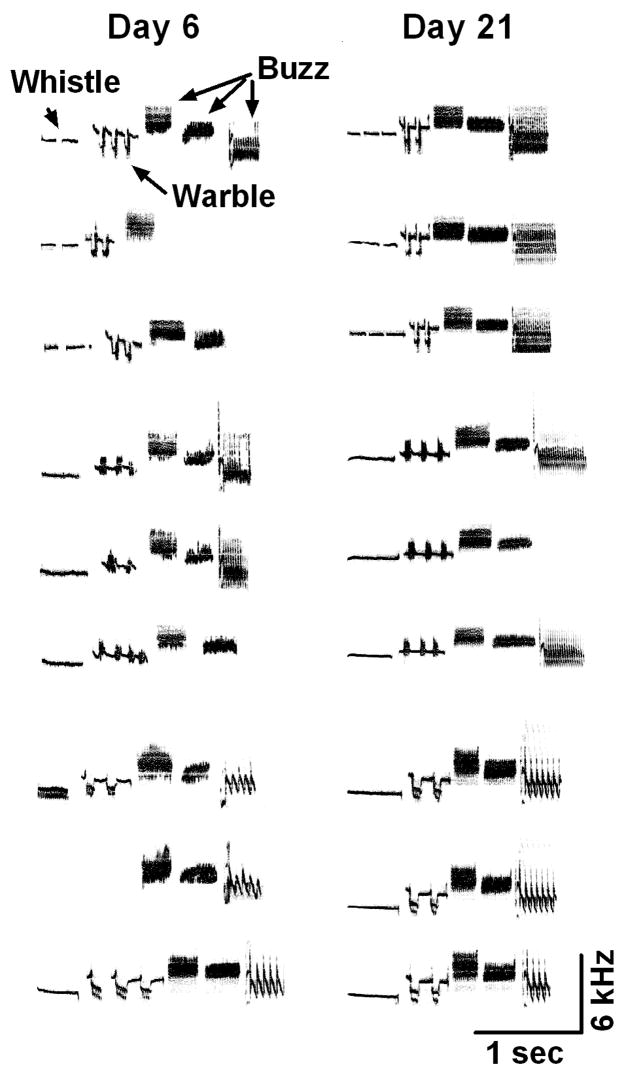
Figure 2.
Representative songs from day 6 and 21 of LD photoperiod and systemic T treatment. Song from three different birds is shown (top, middle and bottom, respectively). Day 21 song is more stereotyped compared with day 6 song.
We also analyzed song completeness, as neither internal linearity nor consistency directly measures how many syllables were sung per song. To quantify song completeness, we calculated percent complete songs, and the average number of syllables in both all songs and only incomplete songs. We defined a “complete song” as a song containing the maximum number of different syllables produced by the bird. In Gambel’s white-crowned sparrows this is most often five syllables. An “incomplete song” is a song containing fewer syllables than a complete song. We randomly selected 20 songs for each day of analysis, except in the cases noted above. To calculate percent complete songs, we divided the number of complete songs by the total number of songs for each bird. To analyze the extent to which a bird’s song was complete or incomplete, we averaged the number of syllables in all songs, and then in only incomplete songs.
Differences in song behavior between breeding and non-breeding conditions have been previously investigated in Gambel’s white-crowned sparrows in both the wild (DeWolfe et al., 1974; Austen and Handford, 1991; Smith et al., 1995) and in the laboratory (Smith et al., 1995; Tramontin et al., 2000; Meitzen et al., 2007a), but no prior study addressed the questions posed here. Specifically, previous studies in Gambel’s white-crowned sparrows did not determine how quickly changes in song rate, stereotypy and syntax occurred (DeWolfe et al., 1974; Austen and Handford, 1991; Smith et al., 1995; Tramontin et al., 2000; Meitzen et al., 2007a). Additionally, due to differences in focus, some previous studies limited analysis to particular song syllables (Smith et al., 1995; Tramontin et al., 2000), did not analyze song syntax (Smith et al., 1995; Tramontin et al., 2000; Meitzen et al., 2007a), song rate (DeWolfe et al., 1974; Austen and Handford, 1991), or the song stereotypy of individual birds across breeding conditions (DeWolfe et al., 1974; Austen and Handford, 1991; Smith et al., 1995). The current study was designed to address these issues.
Statistics
All comparisons were made with a one-way repeated measures ANOVA and a Tukey’s post-hoc test, with the exception of song rate data (Fig 1), where we also used a Dunnett’s post-hoc test with day 0 as the comparison, and fit a sigmoidal curve. Analyses were made using Prism 5.00 (Graphpad Software, La Jolla, CA) and SigmaStat 3.00 (SPSS, Chicago, IL).
Figure 1.
LD photoperiod and systemic T significantly increase song rate. Mean ± SEM is plotted. The number above each point indicates how many birds sang that day. The mean includes birds that did not sing. See Results for P values. A) The song rate of ten birds exposed to LD photoperiod and systemic T beginning on day 0. Song rates on day 2 through day 21 all significantly differed from day 0. Maximum average song rate was reached around day 6. B) The song rate of five birds in which systemic T was removed and photoperiod was decreased on day 21. Song rates on days 22 – 26 did not differ significantly when compared with day 0.
RESULTS
Plasma Hormone Levels
Silastic T pellets implanted subcutaneously on day 0 increased plasma levels of T into the physiological breeding range seen in wild male sparrows under breeding conditions (mean ± SEM [Standard error of the mean]: 11.1 ± 1.03 ng/mL, n=9. After the withdrawal of T and transition to short day (SD) photoperiod on day 21, levels of circulating T dropped to basal levels (mean ± SEM: 0.46 ± 0.08 ng/mL, n=4).
Song Rate
Song rate quickly increased after transition to breeding condition
LD photoperiod and systemic T rapidly increased song rate (Fig. 1A; averages reported in Figure 1 include birds that did not sing; F21,9 = 2.734, P < 0.001). Song rate significantly differed from day 0 by day 2 (P<0.05) with 4 out of 10 birds producing a mean song rate of 20.3 ± 12.7 songs (Fig 1A). Song rate remained significantly elevated on days 3 through 21 (P < 0.05 for all), but remained highly variable (Fig. 1). Both between subject and within subject variability was present, as the repeated measures pairing was significantly effective (F = 16.93, P<0.0001), but had a relatively low R squared value (0.382).
We performed two different analyses to determine when mean maximum song rate was achieved. We first analyzed song rate on day 0 through day 6, and on day 6 through 21, using a repeated measured ANOVA and Tukey’s post-hoc test to determine whether song rate changed significantly. Between day 0 and day 6, song rate changed significantly (F6,9 = 4.793, P < 0.001). We found that day 6 differed significantly from day 0, 1, and 2 (P =0.007, P =0.007, P =0.042, respectively), and that day 5 differed significantly from day 0 and 1 (P = 0.021 for both). These results indicate that song rate was still changing significantly between days 0 through 6. This is in contrast to day 6 and 21, where song rate did not change significantly (F15,9 = 0.995, P = 0.464). This analysis indicates that song rate reached a maximum around day 6.
We also assessed when average maximum song rate was reached by fitting a sigmoidal function to song rates from day 0 to day 21. The half-maximal value of the sigmoid was 3.49 ± 0.41 (R2 = 0.6808), indicating that maximum mean song rate was reached around day 6 or 7. This agreed well with the previous analysis.
Song rate rapidly decreased after transition to nonbreeding condition
In five birds, we withdrew systemic T and decreased photoperiod on day 21 to study how the transition from breeding to non-breeding condition affected song behavior. Song rate dropped precipitously (Fig. 1B); song rates on days 22 through 26 were not significantly different compared with day 0 (F5,4 = 0.91, P > 0.05). This reduction in song rate is similar to that reported in previous studies which found that captive birds exposed to SD photoperiod and not implanted systemically with T rarely sing (Tramontin et al., 1999; Tramontin et al., 2000). We verified this previous finding by continually recording two birds for four days before exposure to LD photoperiod and systemic T implantation on day 0, and one bird for two days before day 0; none of these three birds sang.
Song Phonology
LD photoperiod and systemic T implantation increased song stereotypy (Fig. 2). We quantified the stereotypy of song phonology and syntax on days 6, 8, 10 and 21. Day 6 was the earliest time point at which enough song was recorded to calculate phonological stereotypy. Song phonological stereotypy after transition to non-breeding conditions could not be assessed due to low song rates (Fig 1B).
Phonological stereotypy of the entire song
We analyzed overall song stereotypy by calculating the % similarity of an entire song within a day using Sound Analysis Pro (Fig 3A). This analysis indicates that song was significantly more stereotyped on days 8, 10 and 21 compared with day 6 (Fig. 3A; F3,9 = 8.89, P< 0.001; P < 0.01 for all). Song stereotypy did not significantly differ between days 8, 10, and 21 (P > 0.05 for all).
Figure 3.

LD photoperiod and systemic T significantly increase song phonological stereotypy. Horizontal line indicates the mean. Different letters denote significant differences; groups with the same letter do not differ significantly (see Results for P values). A) % Similarity for songs recorded from within day 6, 8, 10 and 21 of LD photoperiod and systemic T treatment, calculated using Sound Analysis Pro. Higher percentage values indicate higher within-day stereotypy. B) Mean coefficient of variation (CV; SD/mean; higher CV indicates less stereotypy) of each bird’s song across days. C) Comparison of mean CV values for individual song attributes across days. Each point represents the mean CV of one of the 16 song characteristics measured across 10 birds.
We also analyzed overall song stereotypy by measuring six temporal and ten spectral attributes of song (Table 1) and calculating their coefficients of variation (CV = StdDev/mean, higher CV indicates lower stereotypy). We first calculated an overall CV for each bird by averaging the CV values of its individual song attributes. As measured by calculating a mean CV for each bird, song was significantly more stereotyped on days 10 and 21 compared with day 6 (Fig. 3B; F3,9 = 4.88, P = 0.009; P < 0.03 for all), and song stereotypy on day 8 was not significantly different compared with day 6, day 10 or day 21 (Fig. 3B; P > 0.05 for all). Stereotypy on days 10 and 21 also did not differ from each other (Fig. 3B; P > 0.05 for both).
Phonological stereotypy of individual syllables
When stereotypy was examined by calculating the mean CV for each attribute across birds, overall song was significantly more stereotyped on days 8, 10 and 21 compared with day 6 (Figure 3C; F3,15 = 9.60, P < 0.0001; P < 0.05 for all). Two individual song characteristics significantly increased in stereotypy over the four days analyzed: whole song duration and whistle duration (Table 1). The duration of the whole song was significantly more stereotyped on days 8, 10 and 21 compared with day 6 (Table 1; F3,9 = 7.70, P < 0.001; P < 0.05 for all). The stereotypy of whistle duration followed a similar time scale, becoming significantly more stereotyped on days 10 and 21 compared with day 6 (Table 1; F3,9 = 4.71, P = 0.011; P < 0.05 for both).
The statistical analysis of the CV values of individual song characteristics across all four time points suffered from low power (Mean ± SEM power: 0.229 ± 0.067, n=16 with 4 groups each). Non-significant results presented in Table 1 should thus be interpreted cautiously. If the number of time points analyzed is reduced from four to two (days 6 and 21), five individual CV values differ significantly (P < 0.05 for all; data not shown), similar to the results of Tramontin et al. (2000), who found that seven individual CV values differed significantly across two time points (day 7 and 20).
The spectral stereotypy of individual syllables as measured using Sound Analysis Pro also increased significantly over time (Fig. 4, Table 2, Table 3). We measured four different spectral properties of each syllable (pitch, FM, entropy, and pitch goodness) on days 6, 8, 10 and 21, and calculated their CV (Table 2, Table 3). Seven of these values significantly increased in stereotypy over the time course measured: whistle pitch, whistle entropy, warble FM, warble entropy, 1st buzz pitch, 2nd buzz entropy, and 3rd buzz pitch goodness (Figure 4). Whistle pitch stereotypy was significantly more stereotyped on day 21 compared with day 6 (Fig 4A; F3,9 = 4.66, P = 0.011; P < 0.05). Whistle entropy followed a similar time course, with significantly increased stereotypy on day 21 compared with day 6 and 8 (Fig 4B; F3,9 = 4.65, P = 0.011; P < 0.05 for both). Warble FM stereotypy was increased significantly on day 10 and 21 compared with day 6 (Fig 4C; F3,9 = 5.12, P = 0.007; P < 0.05 for both), and warble entropy stereotypy on day 21 compared with day 6 (Fig 4D; F3,9 = 3.34, P = 0.037; P < 0.05). The buzz syllables showed fewer changes in stereotypy. The stereotypy of the first buzz’s pitch was increased significantly on day 21 compared with day 6 (Fig 4E; F3,9 = 3.90, P = 0.022; P < 0.05). Second buzz entropy stereotypy was significantly increased on day 21 compared with day 6 (Fig 4F; F3,9 = 4.14, P = 0.017; P < 0.05), and the stereotypy of the third buzz’s pitch goodness increased significantly on day 8 and 10 compared with day 6 (Fig 4G; F3,9 = 4.07, P = 0.019; P < 0.05 for both). Overall, the stereotypy of individual syllables was significantly increased around day 10 or later.
Figure 4.
LD photoperiod and systemic T significantly increased individual syllable phonological stereotypy. Each graph plots the mean CV per bird of one spectral syllable property across time. Horizontal line indicates the mean. Different letters denote significant differences; groups with the same letter do not significantly differ (see Results for P values). A) Whistle pitch. B) Whistle entropy. We note that entropy is negatively signed. C) Warble FM. D) Warble entropy. E) 1st buzz pitch. F) 2nd buzz entropy. G) 3rd buzz pitch goodness.
Table 2.
Spectral Properties, Warble and Whistle
| Time | Statistics | ||||
|---|---|---|---|---|---|
| Day 6 (n = 9) | Day 8 (n = 10) | Day 10 (n = 9) | Day 21 (n = 9) | ANOVA | |
| Whistle pitch (kHz) | |||||
| Mean | 3.34 ± 0.09 | 3.44 ± 0.15 | 3.42 ± 0.11 | 3.41 ± 0.11 | F3,9 = 1.22, P = 0.325 |
| CV | 0.11 ± 0.03a | 0.06 ± 0.02a,b | 0.04 ± 0.01a,b | 0.03 ± 0.01b | F3,9 = 4.66, P = 0.011 |
|
| |||||
| Whistle FM (degrees) | |||||
| Mean | 6.94 ± 0.40a | 5.65 ± 0.57a,b | 4.51 ± 0.47b,c | 3.75 ± 0.39c | F3,9 = 7.69, P < 0.001 |
| CV | 0.50 ± 0.08 | 0.41 ± 0.08 | 0.40 ± 0.08 | 0.32 ± 0.06 | F3,9 = 1.24, P = 0.318 |
|
| |||||
| Whistle entropy | |||||
| Mean | −5.68 ± 0.13a | −5.66 ± 0.12a | −5.82 ± 0.12a,b | −6.09± 0.05b | F3,9 = 5.49, P = 0.005 |
| CV | −0.06 ± 0.01a | −0.06 ± 0.02a | −0.04 ± 0.01a,b | −0.01± 0.01b | F3,9 = 4.65, P = 0.011 |
|
| |||||
| Whistle pitch goodness | |||||
| Mean | 34.57± 4.41a | 34.19± 5.28a | 28.20 ± 4.35a,b | 17.34±1.71b | F3,9 = 8.11, P < 0.001 |
| CV | 0.18 ± 0.04 | 0.09 ± 0.02 | 0.11 ± 0.02 | 0.07 ± 0.02 | F3,9 = 4.81, P = 0.010 |
|
| |||||
| Warble pitch (kHz) | |||||
| Mean | 3.56 ± 0.14a | 3.78 ± 0.11a,b | 3.74 ± 0.11a,b | 3.90 ± 0.09b | F3,9 = 4.41, P = 0.014 |
| CV | 0.11 ± 0.04 | 0.06 ± 0.02 | 0.05 ± 0.01 | 0.03 ± 0.00 | F3,9 = 2.12, P = 0.126 |
|
| |||||
| Warble FM (degrees) | |||||
| Mean | 25.67± 2.12a | 22.17 ± 2.06b | 22.16 ± 2.28b | 20.89±3.49b | F3,9 = 5.48, P = 0.005 |
| CV | 0.28 ± 0.06a | 0.16 ± 0.03a,b | 0.14 ± 0.02b | 0.10 ± 0.02b | F3,9 = 5.12, P = 0.007 |
|
| |||||
| Warble entropy | |||||
| Mean | −5.05 ± 0.15a | −5.28 ± 0.09a,b | −5.27 ± 0.13a,b | −5.56± 0.12b | F3,9 = 5.42, P = 0.006 |
| CV | −0.08 ± 0.02a | −0.04 ± 0.01a,b | −0.03 ± 0.01a,b | −0.02± 0.01b | F3,9 = 3.34, P = 0.037 |
|
| |||||
| Warble pitch goodness | |||||
| Mean | 80.87± 8.78a | 68.39 ± 6.32a,b | 69.58 ± 7.89a,b | 58.88±10.82b | F3,9 = 4.60, P = 0.012 |
| CV | 0.26 ± 0.05a | 0.14 ± 0.02b | 0.15 ± 0.02a,b | 0.13±0.01a,b | F3,9 = 3.94, P = 0.021 |
All values are mean ± SEM. CV = Coefficient of Variation (SD/mean). FM = frequency modulation. Entropy and pitch goodness are unitless. Different superscript letters denote significant differences across a row. Across a row, groups with the same letter do not significantly differ from one another. Lack of letters indicates that no significant differences were found.
Table 3.
Spectral Properties, Buzzes
| Time | Statistics | ||||
|---|---|---|---|---|---|
| Day 6 (n = 9) | Day 8 (n = 10) | Day 10 (n = 9) | Day 21 (n = 9)* | ANOVA | |
| 1st buzz pitch (kHz) | |||||
| Mean | 4.32 ± 0.22 | 4.61 ± 0.06 | 4.64 ± 0.07 | 4.80 ± 0.07 | F3,9 = 2.57, P = 0.079 |
| CV | 0.09 ± 0.02a | 0.06 ± 0.01a,b | 0.05 ± 0.01a,b | 0.02 ± 0.00b | F3,9 = 3.90 P = 0.022 |
|
| |||||
| 1st buzz FM (degrees) | |||||
| Mean | 49.21± 2.06a | 44.26 ± 2.11b | 41.43 ± 2.48b | 32.76±1.71c | F3,9 = 20.38, P < 0.001 |
| CV | 0.11 ± 0.01 | 0.14 ± 0.01 | 0.11 ± 0.01 | 0.14 ± 0.01 | F3,9 = 3.96, P = 0.021 |
|
| |||||
| 1st buzz entropy | |||||
| Mean | −4.33 ± 0.20a | −4.41 ± 0.14a | −4.56 ± 0.14a | −5.13± 0.07b | F3,9 = 6.26, P = 0.003 |
| CV | −0.11 ± 0.03 | −0.09 ± 0.01 | −0.06 ± 0.01 | −0.04 ± 0.01 | F3,9 = 2.64, P = 0.073 |
|
| |||||
| 1st buzz pitch goodness | |||||
| Mean | 148.47±4.71a | 128.70 ± 6.72b | 118.77 ± 8.65b | 90.51±8.65c | F3,9 = 21.26, P < 0.001 |
| CV | 0.19 ± 0.03 | 0.20 ± 0.02 | 0.14 ± 0.01 | 0.18 ± 0.03 | F3,9 = 2.05, P = 0.135 |
|
| |||||
| 2nd buzz pitch (kHz) | |||||
| Mean | 4.05 ± 0.17 | 4.27 ± 0.04 | 4.26 ± 0.05 | 4.45 ± 0.04 | F3,9 = 2.84, P = 0.060 |
| CV | 0.06 ± 0.02 | 0.05 ± 0.01 | 0.04 ± 0.00 | 0.02 ± 0.00 | F3,9 = 2.36, P = 0.098 |
|
| |||||
| 2nd buzz FM (degrees) | |||||
| Mean | 54.92 ± 1.49 | 54.09 ± 1.49 | 53.24 ± 1.29 | 51.06± 2.55 | F3,9 = 1.49, P = 0.245 |
| CV | 0.08 ± 0.02 | 0.08 ± 0.01 | 0.10 ± 0.01 | 0.09 ± 0.01 | F3,9 = 0.92, P = 0.445 |
|
| |||||
| 2nd buzz entropy | |||||
| Mean | −4.89 ± 0.18a | −5.12 ± 0.08a | −5.07 ± 0.11a | −5.62± 0.05b | F3,9 = 7.81, P < 0.001 |
| CV | −0.08 ± 0.02a | −0.06 ± 0.01a,b | −0.04 ± 0.01a,b | −0.02 ±0.01b | F3,9 = 4.14, P = 0.017 |
|
| |||||
| 2nd buzz pitch goodness | |||||
| Mean | 127.80±8.59a,b | 133.14 ± 6.98a | 125.24± 6.18a,b | 102.38±7.84b | F3,9 = 3.92, P = 0.02 |
| CV | −0.08 ± 0.02 | −0.06 ± 0.01 | −0.04 ± 0.01 | −0.02± 0.01 | F3,9 = 1.47, P = 0.248 |
|
| |||||
| 3rd buzz pitch (kHz) | |||||
| Mean | 3.21 ± 0.09 | 3.35 ± 0.07 | 3.32 ± 0.05 | 3.36 ± 0.11 | F3,9 = 1.81, P = 0.174 |
| CV | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.05 ± 0.01 | 0.06 ± 0.02 | F3,9 = 1.41, P = 0.266 |
|
| |||||
| 3rd buzz FM (degrees) | |||||
| Mean | 54.92 ± 3.19 | 55.59 ± 3.39 | 54.47 ± 3.32 | 54.64± 3.02 | F3,9 = 0.12, P = 0.945 |
| CV | 0.09 ± 0.04 | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.05 ± 0.01 | F3,9 = 0.69, P = 0.571 |
|
| |||||
| 3rd buzz entropy | |||||
| Mean | −4.34 ± 0.16 | −4.49 ± 0.16 | −4.59 ± 0.15 | −4.52 ± 0.11 | F3,9 = 1.23, P = 0.322 |
| CV | −0.14 ± 0.05 | −0.06 ± 0.01 | −0.05 ± 0.01 | −0.05 ± 0.01 | F3,9 = 2.79, P = 0.065 |
|
| |||||
| 3rd buzz pitch goodness | |||||
| Mean | 184.40±14.78 | 192.49 ± 19.88 | 193.68 ± 18.66 | 201.89±20.41 | F3,9 = 1.70, P = 0.196 |
| CV | 0.23 ± 0.04a | 0.11 ± 0.02b | 0.11 ± 0.02b | 0.13±0.03a,b | F3,9 = 4.07, P = 0.019 |
All values are mean ± SEM. CV = Coefficient of Variation (SD/mean). FM = frequency modulation. Entropy and pitch goodness are unitless. Different superscript letters denote significant differences across a row. Across a row, groups with the same letter do not significantly differ from one another. Lack of letters indicates that no significant differences were found.
N is 8 for 3rd buzz, Day 21.
Mean values of song attributes
The mean values of several phonological attributes of song differed significantly across time, including the durations of the first and second buzzes, which increased significantly over time (Fig. 5A; ANOVA: F3,9 = 3.92, P = 0.02 for first buzz; and ANOVA: F3,9 = 5.36, P = 0.005 for 2nd buzz). The duration of the first buzz was increased significantly on day 21 compared with day 6 (Fig. 5B; p=0.023) and day 8 (Fig. 5B; p=0.037). The duration of the second buzz was increased significantly on day 21 compared with day 6 (Fig. 5C; p=0.003).
Figure 5.

Buzz duration significantly increases in response to LD photoperiod and systemic T. Horizontal line indicates the mean. Different letters denote significant differences; groups with the same letter do not significantly differ (see Results for P values). A) Top: Example day 6 buzzes. Bottom: Example day 21 buzzes. All buzzes are from the same bird. B) Duration of first buzz. C) Duration of second buzz.
Several spectral properties of song also changed significantly across time. Whistle FM, entropy, and pitch goodness all decreased significantly over time (Table 2; see table for statistics). Similar changes occurred with the warble and first buzz, with FM, entropy and pitch goodness decreasing over time (Table 2; Table 3; see table for statistics). Warble pitch also increased significantly (Table 2; see table for statistics). The second buzz underwent fewer changes, with entropy decreasing over time (Table 3; see table for statistics), and pitch goodness significantly increasing from day 6 on day 8, and then significantly decreasing by day 21 (Table 3; see table for statistics). The third buzz showed no changes in mean spectral properties (Table 3; see table for statistics).
Song Syntax
Stereotypy of song syntax changed minimally across days 6 and 21
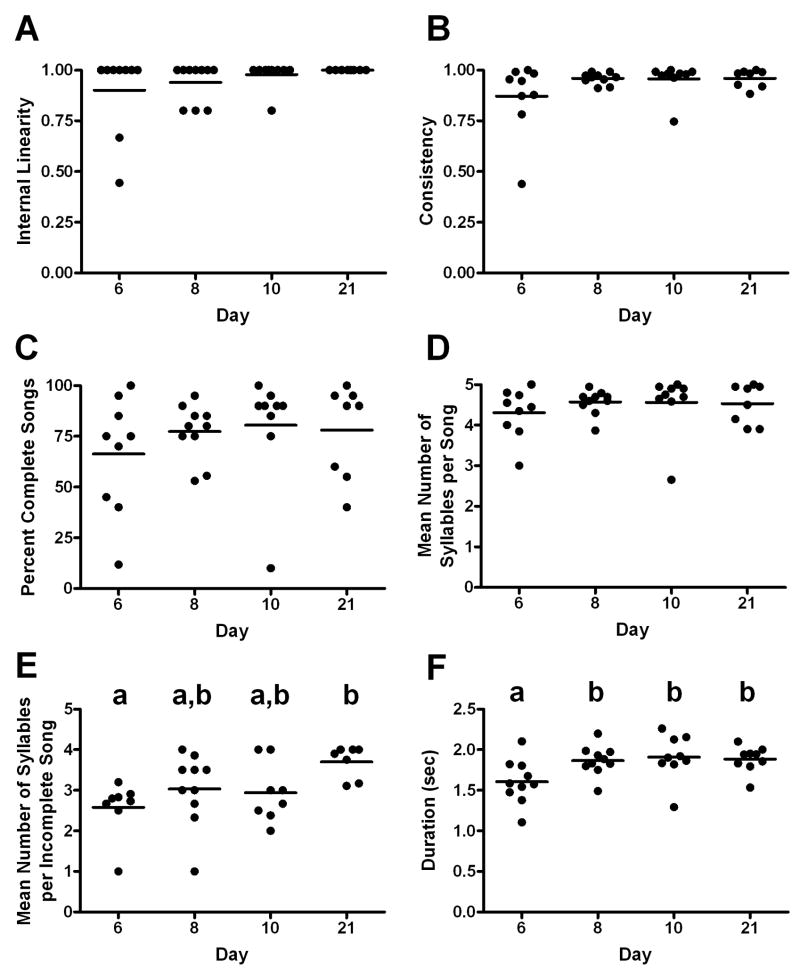
We assessed the stereotypy of song syntax on day 6, 8, 10 and 21. The stereotypy of song syntax changed minimally across days 6 and 21 (Fig. 6). To analyze song syntax, we first calculated internal linearity and found no significant differences across days (Fig. 6A; ANOVA: F3,9 = 1.52, P > 0.05). Similarly, consistency did not differ significantly across days (Fig. 6B; ANOVA: F3,9 = 1.54, P > 0.05). The percentage of a bird’s songs per day that were complete was also not significantly different across days (Fig. 6C; ANOVA: F3,9 = 0.59, P > 0.05). We also determined whether the number of syllables that the bird sang per song differed across days. To do this, we measured the mean number of syllables a bird sang per song, and found no significant differences across days (Fig. 6D, ANOVA: F3,9 = 0.60, P > 0.05). There was, however, a significant increase in the average number of syllables per incomplete song (Fig. 6E; ANOVA: F3,9 = 3.90, P = 0.024), with significantly more syllables sung per incomplete song on day 21 compared with day 6 (Fig. 6E; p=0.018). This result was reflected in mean song duration (Fig. 6F; ANOVA: F3,9 = 5.23, P = 0.006), where song on day 6 was significant shorter compared with day 8 (p=0.023), day 10 (p=0.014) and day 21 (p=0.021).
Figure 6.
Song syntax changes minimally between days 6 and 21 of LD photoperiod and systemic T treatment. Horizontal line indicates the mean. Different letters denote significant differences; groups with the same letter do not significantly differ (see Results for P values). Lack of letters indicates no significant differences. A) Internal linearity (a measure of the number of transitions between syllables) B) Consistency (a measure of the number of song variations) C) The percentage of complete songs out of the total number of songs sung. D) The mean number of syllables produced per song, including both complete and incomplete song. E) The mean number of syllables sung per incomplete song significantly increases from day 6 compared with day 21. F) Song duration significantly increases from day six compared with day eight.
Syntax stereotypy after the transition to nonbreeding condition could not be assessed
We transitioned five of the ten birds to nonbreeding conditions beginning on day 21. Given that these birds sang rarely if at all after the transition to nonbreeding conditions (see above), we were unable to assess stereotypy.
DISCUSSION
We found that systemic T and LD photoperiod typical of the breeding season changed song rate, phonological stereotypy, and the mean values of some spectral and temporal attributes of song, but at different times after the transition to breeding conditions. Song rate increased significantly by day 2, and reached maximal around day 6. Overall song phonological stereotypy increased significantly by day 8, and was maximal by day 10, with some individual syllables requiring more time to reach maximal stereotypy. Buzz duration increased significantly by day 21, with other changes in the spectral properties of syllables following a similar time course. We found few differences in song syntax between day 6 and 21. When birds were transferred from breeding to non-breeding conditions, song rate rapidly declined. These data suggest that different aspects of song behavior change on different timescales in response to changes in breeding condition.
Changes in song stereotypy generally parallel previously investigated growth of the motor pathway of the song control system. Overall song phonological stereotypy reached its maximum between days 8 and 10. This occurs after HVC volume and neuron number are increased significantly compared to non-breeding conditions (93% and 99% of maximal on day 7, respectively; Tramontin et al., 2000). Other morphological and electrophysiological properties of the song system have changed significantly before day 10. RA soma area is 93% of maximal by day 7 (Tramontin et al., 2000) and reaches its maximum between day 7 and day 11 (Tramontin et al., 2000; Meitzen et al., 2007a). Mean RA spontaneous firing rate is increased significantly from day 4 on day 11 (Meitzen et al., 2007a). nXIIts soma area is 94% of its maximum by day 7 (Tramontin et al., 2000). Finally, syrinx mass is 94% of maximal by day 7 (Tramontin et al., 2000). Thus, both overall song and individual syllables reach maximal stereotypy after HVC reaches its maximal breeding-condition state and after or concurrent with much of the growth of other song control system nuclei, suggesting that changes in the morphological and electrophysiological properties of the song control system are necessary for changes in song stereotypy. While these data are helpful for interpreting studies of seasonal changes of the neural substrate, further experiments testing the causal relationship between specific changes in the song control system and song behavior are necessary.
The speed at which song becomes stereotyped under seasonal-like conditions in the laboratory raises the hypothesis that a similar time course could occur in the field. Given the correct breeding cues, changes in song stereotypy and the song control system could occur quickly. While the speed at which song stereotypy increases has not been investigated in the field in Gambel’s white-crowned sparrows, circulating levels of T increase significantly from basal to breeding levels in a matter of weeks while males are migrating from wintering grounds to breeding territories in Alaska (Wingfield and Farner, 1978). In addition, studies of a closely related species, the song sparrow (Melospiza melodia), found that the song control system grows within a three-week period in late winter (Smith et al., 1997; Tramontin and Brenowitz, 1999; Tramontin et al., 2001; Meitzen et al., 2007b). These results predict that the increase in song stereotypy and the growth of song control system nuclei in the field could occur quite quickly.
Buzz duration takes longer to reach its maximum than song stereotypy or rate, following the growth of all measured neural properties of the song control system. Increases in buzz duration most closely match the time course of increases in RA neuron density, spontaneous firing rate, and in the volume of Area X, RA and nXIIts. Area X and RA volume are 80% and 82% of maximal, respectively; RA neuron density is intermediate on day 7, and reaches its maximum between day 7 and day 21 (Tramontin et al., 2000). The volume of nXIIts reaches maximum size between day 21 and days 43–45 (Smith et al., 1997b; Tramontin et al., 2000). RA spontaneous firing rate increased significantly from day 4 on day 11, and reaches its maximum by day 14 (Meitzen et al., 2007a). Studies of Gambel’s white-crowned sparrow song recorded in the field also found that buzz durations tended to be longer during the breeding season, although DeWolfe et al. (1974) did not statistically analyze their data and Smith et al. (1995) measured only the first buzz. A previous laboratory study of Gambel’s white-crowned sparrows also measured only the duration of the first buzz, but found no significant difference in buzz duration (Tramontin et al., 2000). The relatively long time course required for buzz duration may involve physiological processes other than those discussed above. One possibility is change in the muscle properties of the syrinx, the avian vocal organ. In the sexually dimorphic plainfin midshipman fish, for example, type I males have remarkably different sonic muscle properties (including myofibril Z-band and neuromuscular junction dimensions) than females or type II males, whose vocalizations are much shorter (Bass and Zakon, 2005). Similarly, male zebra finch syringes are increased in mass and muscle fiber diameter compared to females, who rarely sing (Nottebohm and Arnold, 1976; Wade and Buhlman, 2000). This speculation is tempered somewhat in that syringeal mass in Gambel’s white-crowned sparrows reaches ~94% of maximal mass within 7 days of exposure to LD photoperiod and systemic T (Tramontin et al., 2000). Mass, however, may not necessarily reflect other muscle properties such as aerobic capacity or maximum contraction rate.
We found no significant difference in the duration of the terminal buzz, which was highly variable (Table 1). The terminal buzz differs across geographically distinct Gambel’s white-crowned sparrow populations (Peyton and DeWolfe, 1968; Nelson, 1998; Chilton et al., 2002) and may communicate dialect identity in white-crowned sparrows in general, including the Gambel’s subspecies (Baptista, 1977; Chilton et al., 2002; Nelson and Soha, 2004; Nelson and Poesel, 2007). Seasonal changes in terminal buzz duration may thus not be advantageous, although it is unclear whether white-crowned sparrows communicate dialect identity during the non-breeding season.
The variability of song rate precludes a definitive comparison of its time course to that of song control system growth, although the data presented here suggest that, on average, birds begin singing and perhaps reach maximal song rate before morphological and electrophysiological characteristics of the song control system change fully (Tramontin et al., 2000; Sartor et al., 2005; Meitzen et al., 2007a). Systemic T and LD photoperiod might induce increases in song rate and song stereotypy via different neural mechanisms (Ball, 2004). When song control system growth is blocked by infusing androgen and estrogen receptor antagonists near HVC in white-crowned sparrows exposed to LD photoperiod and systemic T, song stereotypy differs significantly between treatment groups, but song rate does not (Meitzen et al., 2007a). Deafened male white-crowned sparrows greatly reduce song production (Konishi, 1965; Brenowitz et al., 2007), yet their song control systems grow and song becomes more stereotyped in response to LD photoperiod and systemic T (Brenowitz et al., 2007). Lesions of at least one area outside the song control system, the medial preoptic nucleus (POM), decrease song rate under some circumstances in starlings (Riters and Ball, 1999; Alger and Riters, 2006). In starlings, the POM is increased in volume in the spring breeding season (Riters et al., 2000). Thus, increases in song rate and song stereotypy may be mediated by different neural mechanisms, and an increase in song rate may not be sufficient or necessary for structural and electrophysiological changes in the song control system of Gambel’s white-crowned sparrows, or vice versa (additional evidence reviewed in Brenowitz, 2004; for an alternative view, see Ball et al., 2004; Sartor and Ball, 2005).
The time course of song control system growth is difficult to compare to that of the stereotypy of song syntax because syntax changed minimally between days 6 and 21, with only one significant exception: an increase in the number of syllables in incomplete songs by day 21. These results are consistent with those found for wild Gambel’s white-crowned sparrow song in the field; adult males in winter sometimes omit syllables (DeWolfe et al., 1974), with few other differences in overall song syntax (DeWolfe et al., 1974; Austen and Handford, 1991; Smith et al., 1995). Similarly, deafened white-crowned sparrows maintain normal syntax for prolonged periods (Konishi, 1965; Brenowitz et al., 2007), unlike other species (Nordeen and Nordeen, 1992; Woolley, 2004). The overall lack of change between non-breeding and breeding conditions in our study could be interpreted in at least two ways: first, that the stereotypy of song syntax does not change significantly between conditions, or second, that changes in syntax occur early (within the first 6 days of the seasonal-like manipulation used here). While the preponderance of evidence favors the first interpretation, formally we could not differentiate between these alternatives due to the low and extremely variable song rate during the first six days, which precluded analyzing song syntax (Fig. 1A).
Adults in other model systems with similar behaviors require varying amounts of time of exposure to systemic androgens in order to remodel neural structure and electrophysiology. Female aquatic clawed frogs exposed to systemic T, for instance, require eight to twelve weeks to reach their maximum masculinized “click” calling rate (Potter et al., 2004). Canaries require similar amounts of time for newly-learned syllables to become stereotyped (Nottebohm et al., 1986). Five to six weeks are needed for systemic T implants to lower the fundamental frequency of syllables in adult zebra finch song (Cynx et al., 2005). Responses to systemic doses of androgens in other model systems happen more quickly, closer to the ten days necessary for increases in white-crowned sparrow song stereotypy. In weakly electric fish, for example, systemic exposure to androgens in female and juvenile fish masculinizes electric organ discharges between one and two weeks (Bass and Zakon, 2005). On an even faster timescale (i.e., minutes or less) are rapid effects of hormones, which differ from the preceding examples in that they do not seem to require re-structuring of the neural substrate (Remage-Healey and Bass, 2006; Remage-Healey et al., 2008).
We conclude that different aspects of song behavior change on different time scales in response to changes in breeding condition. While song rate increased quickly, phonological stereotypy increased more gradually, and changes in buzz duration and some spectral attributes required the longest amount of time. These multiple time scales suggest that different neural mechanisms mediate different aspects of song behavior, and the time scale of changes in the song control system support this idea. Finally, this study indicates that measuring either song quantity or quality alone does not provide a complete picture of how song behavior changes during transitions in breeding physiology.
Acknowledgments
We thank Karin Lent and Kristen Richards for technical support and animal care, Stephanie Plamondon for assistance with using Sound Analysis Pro to analyze white-crowned sparrow song, and the members of the Perkel and Brenowitz laboratories for critical discussion and support. Grant Sponsor: NIH: MH068530 (D.J.P.), MH53032 (E.A.B), 5 T32 GM07108 (J.M. and C.K.T.); NSF: Graduate Research Fellowship (J.M.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alger SJ, Riters LV. Lesions to the medial preoptic nucleus differentially affect singing and nest box-directed behaviors within and outside of the breeding season in European starlings (Sturnus vulgaris) Behav Neurosci. 2006;120:1326–1336. doi: 10.1037/0735-7044.120.6.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austen MJW, Handford PT. Variation in the songs of breeding gambel’s white-crowned sparrows near Churchill, Manitoba. Condor. 1991;93:147–152. [Google Scholar]
- Ball GF. Pathways for hormonal influence of birdsong. In: Marler P, Slabbekoorn H, editors. Nature’s Music, The Science of Birdsong. San Diego: Elsevier; 2004. [Google Scholar]
- Ball GF, Auger CJ, Bernard DJ, Charlier TD, Sartor JJ, Riters LV, Balthazart J. Seasonal plasticity in the song control system: multiple brain sites of steroid hormone action and the importance of variation in song behavior. Ann N Y Acad Sci. 2004;1016:586–610. doi: 10.1196/annals.1298.043. [DOI] [PubMed] [Google Scholar]
- Baptista LE. Geographic variation in song and dialects of the Puget Sound White-crowned Sparrow. Condor. 1977;79:356–370. [Google Scholar]
- Bass AH, Zakon HH. Sonic and electric fish: at the crossroads of neuroethology and behavioral neuroendocrinology. Horm Behav. 2005;48:360–72. doi: 10.1016/j.yhbeh.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA. Plasticity of the adult avian song control system. Ann N Y Acad Sci. 2004;1016:560–585. doi: 10.1196/annals.1298.006. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA, Lent K, Rubel EW. Auditory feedback and song production do not regulate seasonal growth of song control circuits in adult white-crowned sparrows. J Neurosci. 2007;27:6810–6814. doi: 10.1523/JNEUROSCI.1248-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton G, Wiebe MO, Handford P. Large-scale geographic variation in songs of gambel’s white-crowned sparrows. Condor. 2002;104:378–386. [Google Scholar]
- Cynx J, Bean NJ, Rossman I. Testosterone implants alter the frequency range of zebra finch songs. Horm Behav. 2005;47:446–451. doi: 10.1016/j.yhbeh.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Del Negro C, Lehongre K, Edeline JM. Selectivity of canary HVC neurons for the bird’s own song: modulation by photoperiodic conditions. J Neurosci. 2005;25:4952–4963. doi: 10.1523/JNEUROSCI.4847-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWolfe BB, Kaska DD, Peyton LJ. Prominent variations in the songs of Gambel’s white-crowned sparrows. Bird Banding. 1974;45:224–252. [Google Scholar]
- Foster EF, Bottjer SW. Lesions of a telencephalic nucleus in male zebra finches: Influences on vocal behavior in juveniles and adults. J Neurobiol. 2001;46:142–165. doi: 10.1002/1097-4695(20010205)46:2<142::aid-neu60>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Konishi M. The role of auditory feedback in the control of vocalization in the white-crowned sparrow. Z Tierpsychol. 1965;22:770–783. [PubMed] [Google Scholar]
- Lucas JR, Freeberg TM, Krishnan A, Long GR. A comparative study of avian auditory brainstem responses: correlations with phylogeny and vocal complexity, and seasonal effects. J Comp Physiol A. 2002;188:981–992. doi: 10.1007/s00359-002-0359-x. [DOI] [PubMed] [Google Scholar]
- Lucas JR, Freeberg TM, Long GR, Krishnan A. Seasonal variation in avian auditory evoked responses to tones: a comparative analysis of Carolina chickadees, tufted titmice, and white-breasted nuthatches. J Comp Physiol A. 2007;193:201–215. doi: 10.1007/s00359-006-0180-z. [DOI] [PubMed] [Google Scholar]
- Maney DL, Goode CT, Lange HS, Sanford SE, Solomon BL. Estradiol modulates neural responses to song in a seasonal songbird. J Comp Neurol. 2008;511:173–186. doi: 10.1002/cne.21830. [DOI] [PubMed] [Google Scholar]
- Marler P, Tamura M. Culturally transmitted patterns of vocal behavior in sparrows. Science. 1964;146:1483–1486. doi: 10.1126/science.146.3650.1483. [DOI] [PubMed] [Google Scholar]
- Meitzen J, Moore IT, Lent K, Brenowitz EA, Perkel DJ. Steroid hormones act transsynaptically within the forebrain to regulate neuronal phenotype and song stereotypy. J Neurosci. 2007a;27:12045–57. doi: 10.1523/JNEUROSCI.3289-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzen J, Perkel DJ, Brenowitz EA. Seasonal changes in intrinsic electrophysiological activity of song control neurons in wild song sparrows. J Comp Physiol A. 2007b;193:677–683. doi: 10.1007/s00359-007-0222-1. [DOI] [PubMed] [Google Scholar]
- Meitzen J, Thompson CK. Seasonal-like growth and regression of the avian song control system: Neural and behavioral plasticity in adult male Gambel’s white-crowned sparrows. Gen Comp Endocrinol. 2008;157:259–265. doi: 10.1016/j.ygcen.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DA. Geographic variation in song of Gambel’s white-crowned sparrow. Behaviour. 1998;135:321–342. [Google Scholar]
- Nelson DA, Poesel A. Segregation of information in a complex acoustic signal: individual and dialect identity in white-crowned sparrow song. Animal Behaviour. 2007;74:1073–1084. [Google Scholar]
- Nelson DA, Soha JA. Perception of geographical variation in song by male Puget Sound white-crowned sparrows, Zonotrichia leucophrys pugetensis. Animal Behaviour. 2004;68:395–405. [Google Scholar]
- Nordeen KW, Nordeen EJ. Auditory feedback is necessary for the maintenance of stereotyped song in adult zebra finches. Behav Neural Biol. 1992;57:58–66. doi: 10.1016/0163-1047(92)90757-u. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. A brain for all seasons: cyclical anatomical changes in song control nuclei of the canary brain. Science. 1981;214:1368–1370. doi: 10.1126/science.7313697. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Arnold AP. Sexual dimorphism in vocal control areas of the songbird brain. Science. 1976;194:211–213. doi: 10.1126/science.959852. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Nottebohm ME, Crane L. Developmental and seasonal changes in canary song and their relation to changes in the anatomy of song-control nuclei. Behav Neural Biol. 1986;46:445–471. doi: 10.1016/s0163-1047(86)90485-1. [DOI] [PubMed] [Google Scholar]
- Park KH, Meitzen J, Moore IT, Brenowitz EA, Perkel DJ. Seasonal-like plasticity of spontaneous firing rate in a songbird pre-motor nucleus. J Neurobiol. 2005;64:181–191. doi: 10.1002/neu.20145. [DOI] [PubMed] [Google Scholar]
- Peyton LJ, DeWolfe BB. A distinctive song pattern in gambel’s white-crowned sparrow. Condor. 1968;70:385–386. [Google Scholar]
- Potter KA, Bose T, Yamaguchi A. Androgen-induced vocal transformation in adult female African clawed frogs. J Neurophysiol. 2005;94:415–428. doi: 10.1152/jn.01279.2004. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH. A rapid neuromodulatory role for steroid hormones in the control of reproductive behavior. Brain Res. 2006;1126:27–35. doi: 10.1016/j.brainres.2006.06.049. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 2008 Sep 28; doi: 10.1038/nn.2200. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters LV, Ball GF. Lesions to the medial preoptic area affect singing in the male European starling (Sturnus vulgaris) Horm Behav. 1999;36:276–286. doi: 10.1006/hbeh.1999.1549. [DOI] [PubMed] [Google Scholar]
- Riters LV, Eens M, Pinxten R, Duffy DL, Balthazart J, Ball GF. Seasonal changes in courtship song and the medial preoptic area in male European starlings (Sturnus vulgaris) Horm Behav. 2000;38:250–61. doi: 10.1006/hbeh.2000.1623. [DOI] [PubMed] [Google Scholar]
- Sartor JJ, Ball GF. Social suppression of song is associated with a reduction in volume of a song-control nucleus in European starlings (Sturnus vulgaris) Behav Neurosci. 2005;119:233–244. doi: 10.1037/0735-7044.119.1.233. [DOI] [PubMed] [Google Scholar]
- Sartor JJ, Balthazart J, Ball GF. Coordinated and dissociated effects of testosterone on singing behavior and song control nuclei in canaries (Serinus canaria) Horm Behav. 2005;47:467–476. doi: 10.1016/j.yhbeh.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Scharff C, Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implications for vocal learning. J Neurosci. 1991;11:2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinger BA, London SE. Neurosteroids and the songbird model system. J Exp Zoolog A Comp Exp Biol. 2006;305:743–748. doi: 10.1002/jez.a.303. [DOI] [PubMed] [Google Scholar]
- Smith GT, Brenowitz EA, Beecher MD, Wingfield JC. Seasonal changes in testosterone, neural attributes of song control nuclei, and song structure in wild songbirds. J Neurosci. 1997a;17:6001–6010. doi: 10.1523/JNEUROSCI.17-15-06001.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GT, Brenowitz EA, Wingfield JC. Roles of photoperiod and testosterone in seasonal plasticity of the avian song control system. J Neurobiol. 1997b;32:426–42. doi: 10.1002/(sici)1097-4695(199704)32:4<426::aid-neu6>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Smith GT, Brenowitz EA, Wingfield JC, Baptista LF. Seasonal changes in song nuclei and song behavior in Gambel’s white-crowned sparrows. J Neurobiol. 1995;28:114–125. doi: 10.1002/neu.480280110. [DOI] [PubMed] [Google Scholar]
- Tchernichovski O, Nottebohm F, Ho CE, Pesaran B, Mitra PP. A procedure for an automated measurement of song similarity. Anim Behav. 2000;59:1167–1176. doi: 10.1006/anbe.1999.1416. [DOI] [PubMed] [Google Scholar]
- Thompson CK, Bentley GE, Brenowitz EA. Rapid seasonal-like regression of the adult avian song control system. Proc Natl Acad Sci USA. 2007;104:15520–15525. doi: 10.1073/pnas.0707239104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramontin AD, Brenowitz EA. A field study of seasonal neuronal incorporation into the song control system of a songbird that lacks adult song learning. J Neurobiol. 1999;40:316–326. doi: 10.1002/(sici)1097-4695(19990905)40:3<316::aid-neu4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Tramontin AD, Brenowitz EA. Seasonal plasticity in the adult brain. Trends Neurosci. 2000;23:251–258. doi: 10.1016/s0166-2236(00)01558-7. [DOI] [PubMed] [Google Scholar]
- Tramontin AD, Hartman VN, Brenowitz EA. Breeding conditions induce rapid and sequential growth in adult avian song circuits: a model of seasonal plasticity in the brain. J Neurosci. 2000;20:854–861. doi: 10.1523/JNEUROSCI.20-02-00854.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramontin AD, Perfito N, Wingfield JC, Brenowitz EA. Seasonal growth of song control nuclei precedes seasonal reproductive development in wild adult song sparrows. Gen Comp Endocrinol. 2001;122:1–9. doi: 10.1006/gcen.2000.7597. [DOI] [PubMed] [Google Scholar]
- Tramontin AD, Wingfield JC, Brenowitz EA. Contributions of social cues and photoperiod to seasonal plasticity in the adult avian song control system. J Neurosci. 1999;19:476–483. doi: 10.1523/JNEUROSCI.19-01-00476.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramontin AD, Wingfield JC, Brenowitz EA. Androgens and estrogens induce seasonal-like growth of song nuclei in the adult songbird brain. J Neurobiol. 2003;57:130–140. doi: 10.1002/neu.10263. [DOI] [PubMed] [Google Scholar]
- Voigt C, Leitner S. Seasonality in song behaviour revisited: Seasonal and annual variants and invariants in the song of the domesticated canary (Serinus canaria) Horm Behav. 2008;54:383–378. doi: 10.1016/j.yhbeh.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Wade J, Buhlman L. Lateralization and effects of adult androgen in a sexually dimorphic neuromuscular system controlling song in zebra finches. J Comp Neurol. 2000;426:154–164. [PubMed] [Google Scholar]
- Wingfield JC, Farner D. The annual cycle of plasma irLH and steroid hormones in feral populations of the white-crowned sparrow, Zonotrichia leucophrys gambelii. Biol Reprod. 1978;19:1046–1056. doi: 10.1095/biolreprod19.5.1046. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Moore MC. Hormonal, social, and environmental factors in the reproductive biology of free-living male birds. In: Crews D, editor. Psychobiology of Reproductive Behavior: An Evolutionary Perspective. Englewood Cliffs, NJ: Prentice-Hall; 1987. [Google Scholar]
- Woolley SM. Auditory experience and adult song plasticity. Ann NY Acad Sci. 2004;1016:208–221. doi: 10.1196/annals.1298.017. [DOI] [PubMed] [Google Scholar]
- Wu W, Thompson JA, Bertram R, Johnson F. A statistical method for quantifying songbird phonology and syntax. J Neurosci Methods. 2008;174:147–154. doi: 10.1016/j.jneumeth.2008.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]