Abstract
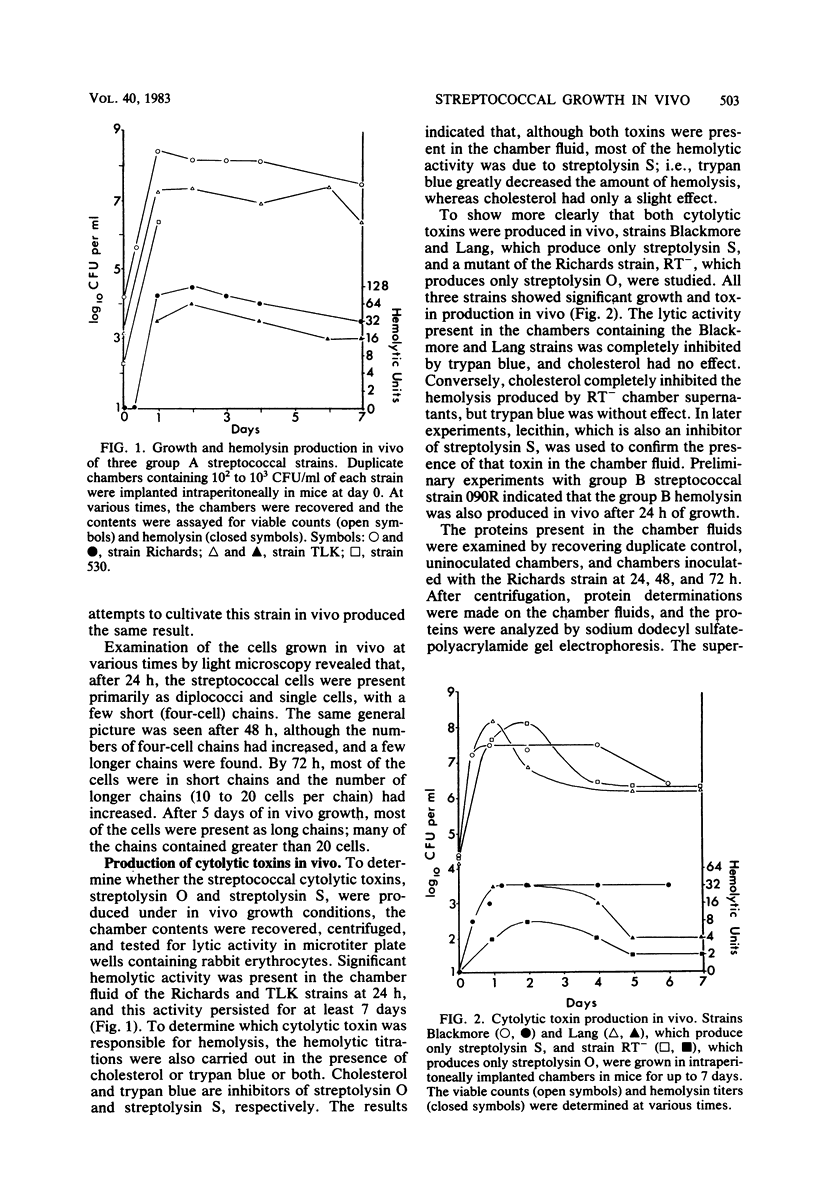
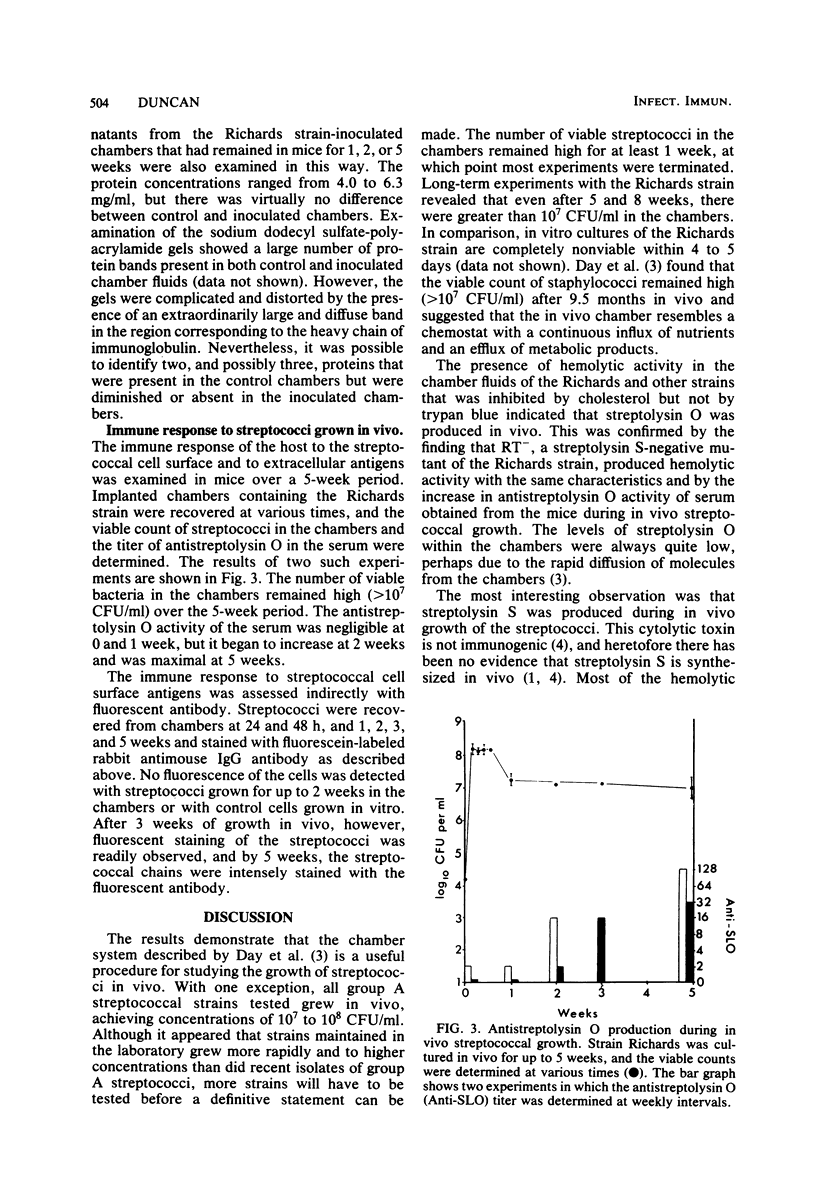
Streptococcal growth in vivo was studied with inoculated micropore filter chambers which were implanted into the peritoneal cavities of mice. Eight of nine group A strains and one group B strain grew in vivo, achieving concentrations of 10(7) to 10(9) CFU/ml in the chambers. Experiments with the Richards strain showed that the number of viable organisms remained high at 5 and 8 weeks after infection. The use of specific inhibitors and appropriate toxin-negative strains demonstrated that both cytolytic toxins produced by group A streptococci, streptolysin S and streptolysin O, were present in the culture fluids harvested from the chambers. This finding represents the first evidence that streptolysin S is produced in vivo. The host response to streptococci growing in vivo was examined by following the increase in serum antistreptolysin O levels. The response was first detected 2 weeks after chamber implantation and appeared to be maximal after 5 weeks. In addition, the production of antibody to streptococcal cell surface antigens was demonstrated indirectly with fluorescein-labeled anti-mouse immunoglobulin G.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alouf J. E. Streptococcal toxins (streptolysin O, streptolysin S, erythrogenic toxin). Pharmacol Ther. 1980;11(3):661–717. doi: 10.1016/0163-7258(80)90045-5. [DOI] [PubMed] [Google Scholar]
- Arko R. J. Neisseria gonorrhoeae: experimental infection of laboratory animals. Science. 1972 Sep 29;177(4055):1200–1201. doi: 10.1126/science.177.4055.1200. [DOI] [PubMed] [Google Scholar]
- Day S. E., Vasli K. K., Russell R. J., Arbuthnott J. P. A simple method for the study in vivo of bacterial growth and accompanying host response. J Infect. 1980 Mar;2(1):39–51. doi: 10.1016/s0163-4453(80)91773-9. [DOI] [PubMed] [Google Scholar]
- Finn T. M., Arbuthnott J. P., Dougan G. Properties of Escherichia coli grown in vivo using a chamber implant system. J Gen Microbiol. 1982 Dec;128(12):3083–3091. doi: 10.1099/00221287-128-12-3083. [DOI] [PubMed] [Google Scholar]
- GLADSTONE G. P., GLENCROSS E. J. Growth and toxin production of staphylococci in cellophane sacs in vivo. Br J Exp Pathol. 1960 Jun;41:313–333. [PMC free article] [PubMed] [Google Scholar]
- HOUSER E. D., BERRY L. J. The pathogenesis of staphylococcus infections. I. The use of diffusion chambers in establishing the role of staphylococcus toxins. J Infect Dis. 1961 Jul-Aug;109:24–30. doi: 10.1093/infdis/109.1.24. [DOI] [PubMed] [Google Scholar]
- KELLY D. K., WINN J. F. Renal lesions produced by group A, type 12, streptococci. Science. 1958 Jun 6;127(3310):1337–1337. doi: 10.1126/science.127.3310.1337. [DOI] [PubMed] [Google Scholar]
- Knöll H., Holm S. E., Gerlach D., Köhler W. Tissue cages for study of experimental streptococcal infection in rabbits. I. Production of erythrogenic toxins in vivo. Immunobiology. 1982;162(2):128–140. doi: 10.1016/S0171-2985(11)80024-0. [DOI] [PubMed] [Google Scholar]
- LAM G. T., SWEENEY F. J., Jr, WITMER C. M., WISE R. I. ABSCESS-FORMING FACTOR(S) PRODUCED BY STAPHYLOCOCCUS AUREUS. I. COLLODION BAG IMPLANTATION TECHNIQUE. J Bacteriol. 1963 Oct;86:611–615. doi: 10.1128/jb.86.4.611-615.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- TAN E. M., HACKEL D. B., KAPLAN M. H. Renal tubular lesions in mice produced by group A streptococci grown in intraperitoneal diffusion chambers. J Infect Dis. 1961 Jan-Feb;108:107–112. doi: 10.1093/infdis/108.1.107. [DOI] [PubMed] [Google Scholar]
- Veale D. R., Smith H., Witt K. A., Marshall R. B. Differential ability of colonial types of Neisseria gonorrhoeae to produce infection cutaneous perforated plastic chambers in guinea-pigs and rabbits. J Med Microbiol. 1975 May;8(2):325–335. doi: 10.1099/00222615-8-2-325. [DOI] [PubMed] [Google Scholar]
- Wagner M., Holm S. E., Wagner B. Growth of group B streptococci and development of surface antigens in tissue cages implanted in rabbits. Zentralbl Bakteriol Mikrobiol Hyg A. 1982 Jul;252(3):287–298. [PubMed] [Google Scholar]


