Abstract
The active form of vitamin D3, 1,25 dihydroxyvitamin D3 [1,25(OH)2D3], modulates proliferation and induces differentiation of many cancer cells. A new class of analogs of vitamin D3 has been synthesized, having 2 side-chains attached to carbon-20 (Gemini) and deuterium substituted on one side-chain. We have examined six of these analogs for their ability to inhibit growth of myeloid leukemia (HL-60), prostate (LNCaP, PC-3, DU145), lung (H520), colon (HT-29), and breast (MCF-7) cancer cell lines. Dose-response clonogenic studies showed that all 6 analogs had greater antiproliferative activities against cancer cells than 1,25(OH)2D3. Although they had similar potency, the most active of these analogs was BXL-01-0120. BXL-01-0120 was 529-fold more potent than 1,25(OH)2D3 in causing 50% clonal growth inhibition (ED50) of HL-60 cells. Pulse-exposure studies demonstrated that exposure to BXL-01-120 (10−9 M, 48 hours) resulted in 85% clonal inhibition of HL-60 growth. BXL-01-0120 (10−11 M, 4 days) induced the differentiation marker, CD11b. Also, morphologically differentiation was more prominent compared to 1,25(OH)2D3. Annexin V assay showed that BXL-01-0120 (10−10 M, 4 days) induced significantly (p<0.05) more apoptosis than 1,25(OH)2D3. In summary, these analogs have a unique structure resulting in extremely potent inhibition of clonal proliferation of various types of cancer cells, especially HL-60 cells. (201 words)
Keywords: Vitamin D, deuterated Gemini Vitamin D3, Antitumor
Introduction
Most cancer chemotherapy is usually toxic to normal cells; in contrast, vitamin D3 compounds cause few side-effects. The physiologically active form of vitamin D3, 1,25 dihydroxyvitamin D3, is a member of the seco-steroid hormone family, and controls calcium homeostasis and bone metabolism. 1,25(OH)2D3 can induce differentiation and inhibit the growth of a number of malignant cell types, including myeloid leukemia, as well as breast, prostate, colon, skin, and brain cancers. Several studies suggested that 1,25(OH)2D3 induces a significant G0/G1 arrest both by modulating the cyclin dependent kinase inhibitors (CDKIs) p21waf1 and p27kip1, and decreasing the levels of cyclin/cyclin-dependent kinases, c-myc and other growth-related proteins [1–4]. In an early clinical study, oral administration of 1,25(OH)2D3 to preleukemic patients was only partially effective [5]; therapeutic application of 1,25(OH)2D3 has been impeded by development of hypercalcemia [5, 6]. Therefore, vitamin D3 analogs have been synthesized that have enhanced potency to both inhibit clonal proliferation and induce differentiation of cancer cells, with reduced induction of hypercalcemia as compared to 1,25(OH)2D3.
In this study, a class of newly synthesized vitamin D3 analogs, deuterated Gemini, was examined. These compounds have a C-20 methyl group, a deuterium substituted side-chain, and a second side-chain that has a double or triple bond and a fluorine [7, 8]. Recently, several of these new deuterated Gemini were shown to have anticancer activity [9]. We have studied 6 novel deuterated analogs and found that each was markedly more potent than 1,25(OH)2D3 in inhibiting the clonal proliferation of a wide range of cancer cell types, even after a short pulse exposure. The analogs also caused differentiation, morphologic changes and apoptosis of HL-60 myeloid leukemia cells.
Materials and methods
2.1. Cell culture
Cancer cell lines used in this study included myeloid leukemia cell line (HL-60), prostate (PC-3, DU145 and LNCaP), lung (H520), colon (HT-29), and breast (MCF-7) cancer lines. Each was obtained from American Type Culture Collection (Rockville, MD), and they were maintained according to their recommendations. DU145 and MCF-7 cells were grown in Dulbecco’s modified Eagle’s Medium (DMEM) (Life Technologies Inc.) supplemented with 10% fetal calf serum (FCS) (Gemini Bio-Products, Calabasas, CA) and 10 units/ml penicillin-streptomycin (Life Technologies). HL-60, LNCaP, PC-3, H520 and HT-29 cells were cultured in RPMI-1640 with 10% FCS and antibiotics.
2.2. Vitamin D3 compounds
The vitamin D3 compounds were dissolved in absolute ethanol at 10−3 M, stored at −20°C, and protected from light. All analogs used in this study were synthesized by BioXell, Inc. The simplified code names and structures of the 1,25(OH)2D3 analogs are shown in Fig. 1. The starting concentrations of the analogs were determined via UV absorbance using their molar extinction coefficient at 264 nmol/l. For in vitro use, dilutions were made in the same tissue culture medium as those for cell culture. The maximal concentration of ethanol (diluent control) used in this study had no effect on cell growth.
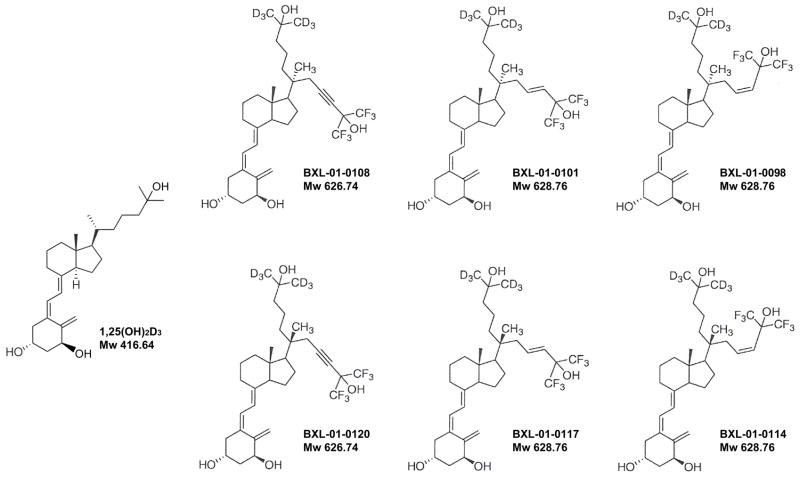
Figure 1.
The simplified code names and chemical structures of 1,25(OH)2D3 and deuterium modified vitamin D3 Gemini analogs. These analogs have a C-20 methyl group, a deuterium substituted on one side-chain, and a second side-chain that has a double or triple bond and a fluorine. Mw; Molecular weights.
2.3. Clonogenic growth
Single-cell suspensions of cells were enumerated and plated into 24-well flat-bottomed plates using a two-layer soft agar system with a total of 3 × 103 cells/well in a volume of 400 μl /well, as described previously [10]. After 10–14 days of culture, colonies were counted. Results were expressed as a mean percent of control plates containing no vitamin D3 compound. All experiments were done at least three times using triplicate wells per experimental point.
For pulse-exposure studies, HL-60 cells were incubated in liquid culture with either 1,25(OH)2D3 or BXL-01-0120, at 10−9 M for 24 and 48 hours. After incubation, these cells were carefully washed twice, counted, and plated in soft-agar in the absence of vitamin D compounds; and colonies were counted as described above. All experiments were done at least three times using triplicate wells per experimental point.
2.4. Differentiation
For measurement of cell surface marker, HL-60 cells were treated with vitamin D3 compounds for 96 hours and then examined for CD11b expression by FACScan (Becton Dickinson) using a FITC-labeled anti-CD11b antibody (DAKO, Carpinteria, CA).
2.5. Morphologic analysis
For Wright-Giemsa staining, HL-60 cells were treated with either diluent control or vitamin D3 analogs for 6 days. Cytospins of HL-60 cells were prepared and stained with Diff-Quick (Dade Behring, Dudingen, Switzerland).
2.6. Apoptosis analysis
For measurement of apoptosis, annexin V assay (Annexin V-FITC Apoptosis Detection Kit; Pharmingen, San Diego, CA) was performed according to the manufacturer’s instructions. Briefly, HL-60 cells were harvested after exposure to vitamin D3 compounds for 96 hours, washed twice with PBS, incubated with FITC-conjugated annexin V and PI for 15 minutes, and analyzed by FACScan (Becton Dickinson).
Results
3.1. Clonal inhibition of growth by vitamin D3 compounds
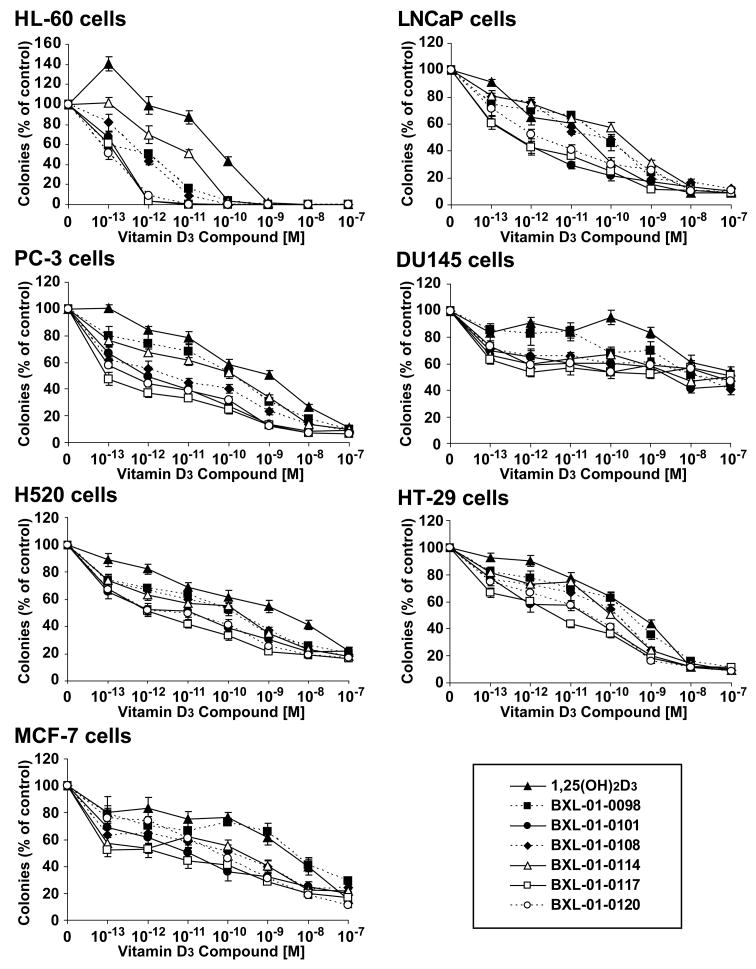
Deuterium-substituted Gemini compounds were examined for their ability to inhibit the clonogenic growth of human cancer cell lines established from prostate, lung, colon and breast cancers, as well as myeloid leukemia. Cells were grown in increasing concentrations of the vitamin D3 compounds. The mean ± SD number of colonies was graphed (Fig. 2). Remarkably, even concentration as low as 10−13 M had some inhibitory activity. The effective dose that inhibited 50% colony formation (ED50) was determined (Table 1). All of the analogs were nearly equal in potency, but BXL-01-0120 was slightly more potent. BXL-01-0120 was 529-, 18-, 1951-, 284-, 6-, and 78-fold more potent than 1,25(OH)2D3 in mediating 50% inhibition of clonal growth of HL-60, LNCaP, PC-3, H520, HT-29, and MCF-7 cells, respectively. All additional experiments focused on this compound.
Figure 2.
Dose-response effects of vitamin D3 compounds on clonal proliferation of several cancer cell lines. Clonal proliferation of HL-60 (leukemia), LNCaP, DU145, PC-3 (prostate), H520 (lung), HT-29 (colon) and MCF-7 (breast) cancer cell lines. Legend: (▴) 1,25(OH) 2D3; (▪) BXL-01-0098; (●) BXL -01-0101; (◆) BXL -01-0108;(Δ) BXL-01-0114; (□) BXL -01-0117; (^) BXL -01-0120. Results are expressed as a mean percent ± S.D. of control plates containing no vitamin D3 compounds. Results are the mean of three independent experiments with triplicate dishes.
Table 1.
Inhibition of clonal proliferation of tumor cell lines by vitamin D3 analogs
| Cell lines | 1,25(OH)2D3 | Deuterated Gemini Vitamin D3 (BXL-01-) |
|||||
|---|---|---|---|---|---|---|---|
| 0098 | 0114 | 0101 | 0117 | 0108 | 0120 | ||
| HL-60 | 7.4 × 10−11 | 1.0 × 10−12 | 9.1 × 10−12 | 1.6 × 10−13 | 1.4 × 10−13 | 5.1 × 10−13 | 1.4 × 10−13 |
| LNCaP | 1.9 × 10−11 | 6.4 × 10−11 | 2.5 × 10−10 | 4.9 × 10−13 | 3.2 × 10−13 | 5.8 × 10−11 | 1.5 × 10−12 |
| PC-3 | 8.0 × 10−10 | 1.1 × 10−10 | 1.4 × 10−10 | 8.8 × 10−13 | <1.0 × 10−13 | 3.4 × 10−12 | 4.1 × 10−13 |
| DU145 | N. R. | 1.8 × 10−8 | 3.8 × 10−9 | 2.9 × 10−9 | N.R. | 1.5 × 10−8 | 4.6 × 10−8 |
| H520 | 2.1 × 10−9 | 1.3 × 10−10 | 1.8 × 10−10 | 9.8 × 10−12 | 1.4 × 10−12 | 1.8 × 10−10 | 7.4 × 10−12 |
| HT-29 | 5.8 × 10−10 | 2.8 × 10−10 | 1.1 × 10−10 | 1.9 × 10−11 | 3.8 × 10−12 | 1.3 × 10−10 | 9.4 × 10−11 |
| MCF-7 | 3.1 × 10−9 | 4.9 × 10−9 | 2.1 × 10−10 | 1.0 × 10−11 | 2.4 × 10−12 | 1.2 × 10−10 | 4.0 × 10−11 |
Dose-response clonogenic assays in soft agar were performed; the data were plotted on semilogarithm graphs, and the curves were used to calculate the concentration of the analogs achieving a 50% inhibition (ED50) of clonal growth. N.R.: ED50 was not reached at 10−7 M analog. The analogs are abbereviated by their last digits which are shown on Fig. 1.
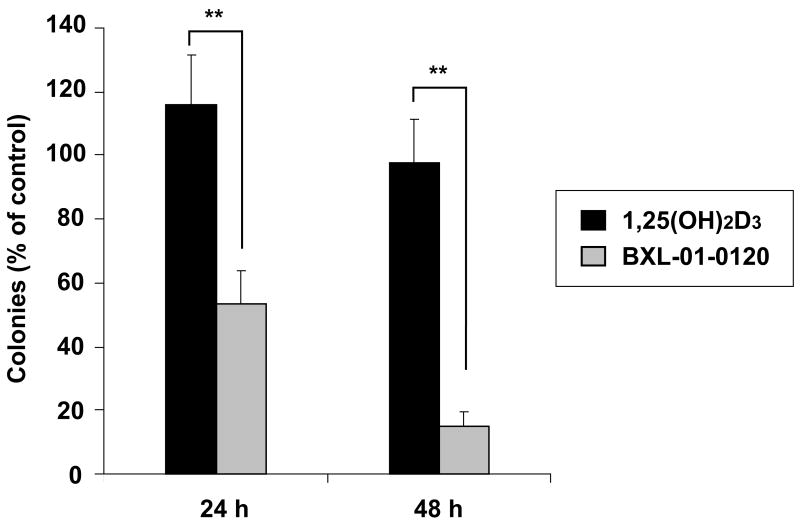
To examine whether the inhibition of clonogenic growth of cancer cells by vitamin D3 analogs was irreversible, HL-60 cells were cultured in liquid medium with 10−9 M of either 1,25(OH)2D3 or BXL-01-0120 for 24 and 48 hours, washed, counted and clonogenic assays performed in the absence of vitamin D3 compound (Fig. 3). Treatment with BXL-01-0120 caused 85% inhibition of colony formation after a 48 hours exposure; in contrast under the same conditions, treatment with 1,25(OH)2D3 caused < 5% decreased clonal growth.
Figure 3.
Effect of pulse-exposure of vitamin D3 compounds on clonal proliferation of HL-60 cells. HL-60 cells were incubated in liquid culture for either 24 or 48 hours with diluent control or 10−9 M of either 1,25(OH)2D3 or BXL-01-0120. After pulse-exposure to the compounds, cells were extensively washed, counted and clonogenic assay was performed. Results represent the number of colonies expressed as a percent of diluent control (Result: mean ± S.D. of triplicate plates). **; p<0.01
3.2. Differentiation of HL-60 cells by vitamin D3 compounds
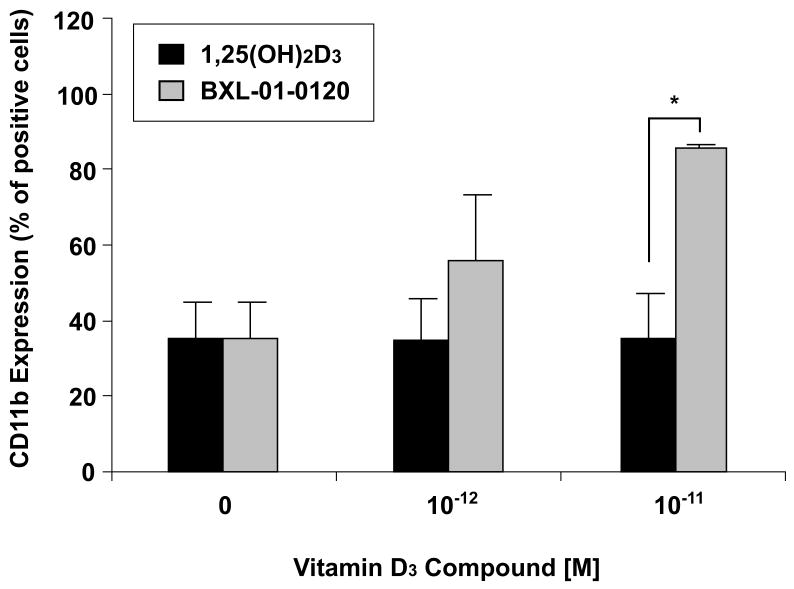
1,25(OH)2D3 and related compounds have been shown to induce monocytic differentiation of HL-60 cells. The expression of the cell surface protein CD11b is associated with the induction of differentiation. Therefore, this property was used to evaluate the potency of the new Gemini D3 analog using flow cytometry (Fig. 4). Exposure of HL-60 cells to either 1,25(OH)2D3 or BXL-01-0120 for 96 hours induced expression of CD11b in a dose-dependent manner. BXL-01-0120 (10−11 M) strongly stimulated expression of CD11b on 86% of HL-60 cells; in contrast, the same concentration of 1,25(OH)2D3 stimulated 35% of the cells to express CD11b.
Figure 4.
Dose-response effects of vitamin D3 compounds on differentiation of HL-60 cells. HL-60 cells were cultured with either 1,25(OH)2D3 or BXL-01-0120 at different concentrations for 96 hours and CD11b expression was analyzed by FACS analysis. *; p<0.05
3.3. Morphologic differentiation of HL-60 cells induced by vitamin D3 compounds
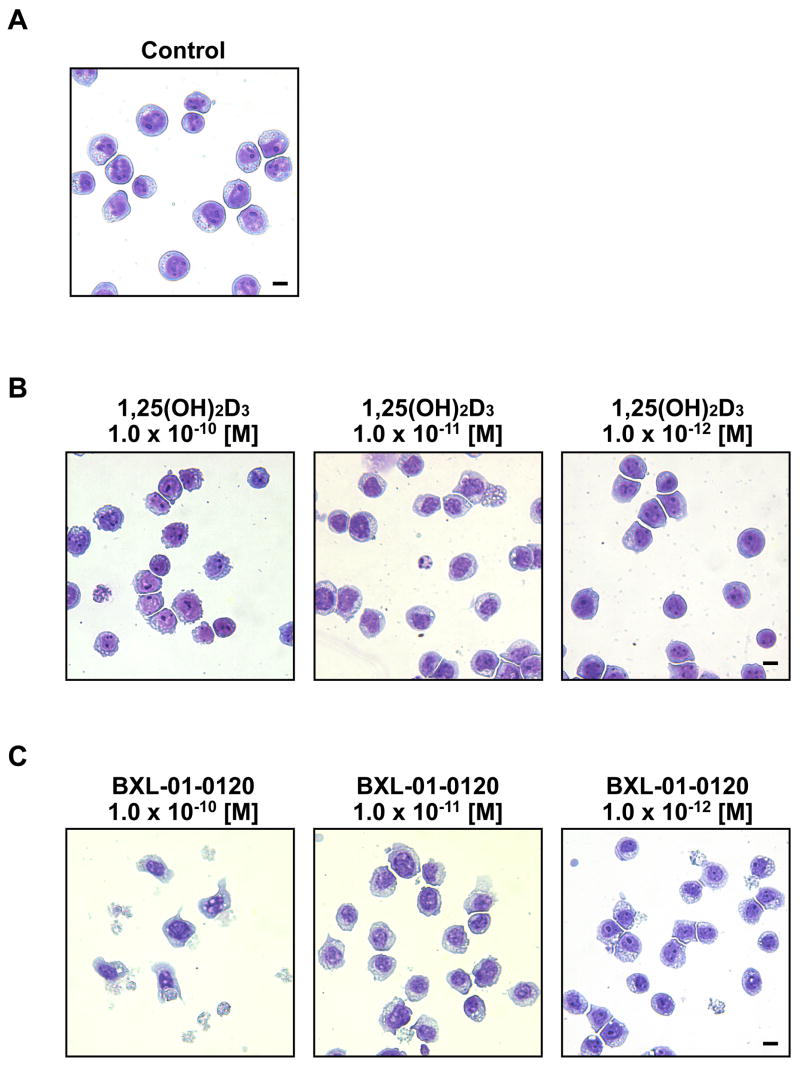
1,25(OH)2D3 (10−7, 10−8 M) and related compounds induce monocytic differentiation of HL-60 cells [3]. HL-60 cells treated with very low concentrations of BXL-01-0120 (10−11 to 10−12 M, 6 days) in liquid culture developed irregular and abundant vacuolated cytoplasm and irregular cell membrane. At 10−10 M of BXL-01-0120, visible cell death was prominent (Fig. 5). These changes were either not so visible (10−10 M) or much less pronounced (10−11M) in the cultures containing 1,25(OH)2D3. In contrast, HL-60 cultured in 1,25(OH)2D3 (10−12 M) looked similar to diluent control.
Figure 5.
Morphologic analysis of HL-60 cells. Cells were cultured with diluent control or vitamin D3 compounds for 6 days, fixed and stained with Wright-Giemsa. A. diluent control (Control); B. 10−10 to 10−12 M of 1,25(OH)2D3; C. 10−10 to 10−12 M of BXL-01-0120. (Scale bar = 10 μm.)
3.4. Induction of apoptosis
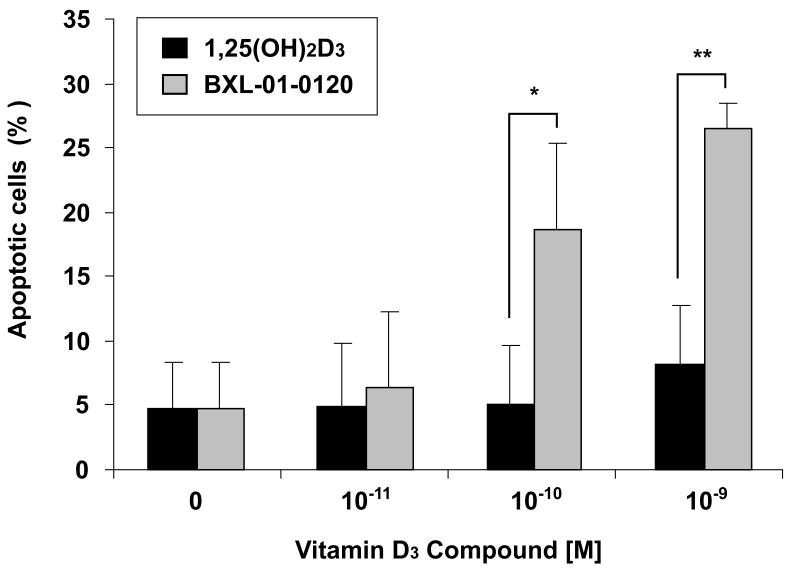
1,25(OH)2D3 and related compounds have been shown to cause modest apoptosis of several cancer cell types. HL-60 cells treated with BXL-01-0120 for 96 hours induced apoptosis (10−10 M, 19%; 10−9 M, 27%); in contrast, HL-60 cells cultured with 1,25(OH)2D3 had a similar percent of apoptotic cells as the diluent-treated control cells (10−10 M, 5%; 10−9 M, 8%) (Fig. 6).
Figure 6.
Ability of Vitamin D3 compounds to induce apoptosis. HL-60 cells were treated for 96 hours with either 1,25(OH)2D3 or BXL-01-0120 (10−11 to 10−9 M), followed by FACS analysis for apoptosis. Annexin V-FITC(+) and PI(±) represent cells in various stage of apoptosis. *; p<0.05, **; p<0.01
Discussion
Recently, several studies showed that vitamin D3 compounds with 2 side-chains on C-20 (Gemini) had greater antiproliferative activity than 1,25(OH)2D3 against a variety of cancer cell lines [9–14]. One of us (Milain Uskokovic) has synthesized a series of novel Gemini compounds, which have a deuterium substituted basic side-chain. We evaluated their biologic effects on cancer cell lines in this study. These analogs demonstrated remarkable abilities to inhibit the clonal proliferation of myeloid leukemia, prostate, lung, colon and breast cancer cells. Each had similar potency; for example, 10−13 to 10−12 M of each of the compounds inhibited 50 % clonal growth of HL-60 cells. For each analog, a cis- and a trans- form of the side-arms were studied (98/114; 101/117; 108/120) (Fig.1); and generally, they had similar activity. BXL-01-0120 was slightly more potent than the other analogs. In particular, it was 529-fold more potent than 1,25(OH)2D3 against the HL-60 cells.
The most resistant line was DU145 prostate cancer cells which are androgen-independent, and contain mutations of p16 and p53 [15, 16]. The 1,25(OH)2D3 was unable to inhibit 50% clonal growth of these cells even at 10−7 M; in contrast, five of 6 of the analogs achieved an ED50 ranging from 2.9 × 10−9 M to 4.6 × 10−8 M. Nevertheless, the dose-response curves showed a blunted response as compared to the antiproliferative activities of the analogs against other cancer cell lines. Interestingly, the other androgen-independent prostate cancer cell line (PC-3) was very sensitive to clonogenic growth inhibition by each of the vitamin D3 analogs (ED50 ranged between 1.1 × 10−10 M to <1.0 × 10−13 M). In fact, PC-3 was as sensitive to growth inhibition by the analogs as the androgen-sensitive LNCaP prostate cancer cells.
Induction of HL-60 differentiation is a commonly used marker to assess the potency of vitamin D compounds. Exposure of HL-60 myeloid leukemia cells to either 1,25(OH)2D3 or BXL-01-0120 induced the expression of the cell surface differentiation marker, CD11b (Fig.4). BXL-01-0120 (10−11 M) stimulated expression of CD11b on about 86% of cells, whereas the same concentration of 1,25(OH)2D3 induced expression on only 35% of cells. The analog also induced partial monocyte-like morphologic differentiation at very low concentrations (10−12, 10−11 M); in contrast, 1,25(OH)2D3 at these levels had little effect on morphology of the cells (Fig.5).
BXL-01-0120 (10−10 M; 96 hours) caused modest apoptosis of HL-60 cells (19%); in contrast, 1,25(OH)2D3 (10−10 M) stimulated only 5% apoptosis of these cells (Fig. 6). Other studies have also shown that vitamin D3 compounds can moderately promote apoptosis of a variety of tumor cell lines [17, 18]; but the mechanism of apoptosis is largely unknown. Previous studies showed that 1,25(OH)2D3 could downregulate the expression of not only the antiapoptotic protein Bcl-2, but it also could decrease several other antiapoptotic proteins in the Bcl-2 family, as well as the IAP-family of proteins [4]. Another study showed that vitamin D3 compounds induced apoptosis via a novel caspase-and p53-independent pathway, and apoptosis was inhibited by forced expression of Bcl-2 [19]. These findings suggested that vitamin D3 compounds might use several pathways to induce the modest apoptosis.
How these analogs achieve their remarkable antiproliferative activity is not totally clear. Gemini compounds bind to the VDR with similar avidity as 1,25(OH)2D3 [20]. Several of the structural changes probably slow metabolism of the active compound. For example, the double or triple bond at C-23, -24 prevents hydroxylation at these sites slowing inactivation [21]. Moreover, the fluorine or deuterium on the side-chain also may decrease metabolism of 1,25(OH)2D3 [22, 23]. Also, the presence of a second side-chain probably interfere with the orderly metabolic process [20].
We found that pulse-exposure to BXL-01-0120 (10−9 M) for 48 hours in liquid culture followed by removal of the analog still resulted in 85% inhibiton of clonal growth of HL-60 cells (Fig. 3). Of interest, a recent trial of pulse calcitriol in patients with refractory malignancies showed that weekly dosing of oral calcitriol permitted substantial dose-escalation with minimal toxicity [24]. High-dose intermittent calcitriol appears to be safe, feasible, and may have antitumor activity without significant hypercalcemia [25]. These data raise the possibility that pulsing of high doses of BXL-01-0120 may achieve serum levels sufficient for a biologic anticancer effect without significant hypercalcemia.
These deuterated Gemini compounds are some of the most potent vitamin D compounds tested to date. Another potent compound is 1,25(OH)2-20-epi-22-oxa-24,26,27-trishomo-D3 (KH1060) with an ED50 of 10–12 M as measured by the inhibition of clonogenic growth of HL-60 cells [26]. However, KH1060 also induced hypercalcemia at low concentrations (0.0125 μg/mouse every other day interperitoneally) [26]. Another deuterated Gemini analog (BXL-01-0072) induced effectively inhibited growth of a murine colon cancer cell line both in vitro and in vivo [9]. Deuterated Gemini analog administered to mice (4.8 pmol every other day for 2 weeks) did not cause hypercalcemia. In contrast, 1,25(OH)2D3 given in a similar schedule at a dose of 48 pmol, did cause hypercalcemia. More complete testing will be required to determine the relative calcemic affects of these analogs compared to 1,25(OH)2D3.
In summary, deuterated Gemini compounds strongly inhibited clonal proliferation of various types of cancer cells, especially HL-60 acute myeloid leukemia cells. Short pulse-exposure (48 hours) was adequate for the analog BXL-01-0120 to inhibit clonal growth of HL-60. Clinical trials of these analogs as maintenance therapy for acute myeloid leukemia and/or adjuvant therapy for prostate, colon, lung and breast cancer are warranted.
Acknowledgments
We thank members of our laboratory for helpful discussions. This work was supported by NIH grants, SWLF, Inger Foundation, the Tom Collier Memorial Regatta Foundation, as well as, Parker Hughes Fund. H. P. K. is the holder of the Mark Goodson endowed Chair in Oncology Research and is a member of the Jonsson Cancer Center and the Molecular Biology Institute, UCLA. The study is dedicated to David Golde, who was a mentor and friend.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schwaller J, Koeffler HP, Niklaus G, Loetscher P, Nagel S, Fey MF, Tobler A. Posttranscriptional stabilization underlies p53-independent induction of p21WAF1/CIP1/SDI1 in differentiating human leukemic cells. J Clin Invest. 1995;95:973–979. doi: 10.1172/JCI117806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang QM, Jones JB, Studzinski GP. Cyclin-dependent kinase inhibitor p27 as a mediator of the G1-S phase block induced by 1,25-dihydroxyvitamin D3 in HL60 cells. Cancer Res. 1996;56:264–267. [PubMed] [Google Scholar]
- 3.Elstner E, Linker-Israeli M, Umiel T, Le J, Grillier I, Said J, Shintaku IP, Krajewski S, Reed JC, Binderup L, Koeffler HP. Combination of a potent 20-epi-vitamin D3 analog (KH 1060) with 9-cis-retinoic acid irreversibly inhibits clonal growth, decreases bcl-2 expression, and induces apoptosis in HL-60 leukemic cells. Cancer Res. 1996;56:3570–3576. [PubMed] [Google Scholar]
- 4.Grillier I, Umiel T, Elstner E, Collins SJ, Koeffler HP. Alterations of differentiation, clonal proliferation, cell cycle progression and bcl-2 expression in RAR alpha-altered sublines of HL-60. Leukemia. 1997;11:393–400. doi: 10.1038/sj.leu.2400575. [DOI] [PubMed] [Google Scholar]
- 5.Koeffler HP, Hirji K, Itri L. 1,25-Dihydroxyvitamin D3: in vivo and in vitro effects on human preleukemic and leukemic cells. Cancer Treat Rep. 1985;69:1399–1407. [PubMed] [Google Scholar]
- 6.Koeffler HP, Amatruda T, Ikekawa N, Kobayashi Y, DeLuca HF. Induction of macrophage differentiation of human normal and leukemic myeloid stem cells by 1.25-dihydroxyvitamin D3 and its fluorinated analogues. Cancer Res. 1984;44:5624–5628. [PubMed] [Google Scholar]
- 7.Spina C, Tangpricha V, Yao M, Zhou W, Wolfe MM, Maehr H, Uskokovic M, Adorini L, Holick MF. Colon cancer and solar ultraviolet B radiation and prevention and treatment of colon cancer in mice with vitamin D and its Gemini analogs. J Steroid Biochem Mol Bio. 2005;97:111–120. doi: 10.1016/j.jsbmb.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Norman AW, Zhou JY, Henry HL, Uskokovic MR, Koeffler HP. Structure-function studies on analogues of 1 alpha,25-dihydroxyvitamin D3: differential effects on leukemic cell growth, differentiation, and intestinal calcium absorption. Cancer Res. 1990;50:6857–6864. [PubMed] [Google Scholar]
- 9.Spina CS, Ton L, Yao M, Maehr H, Wolfe MM, Uskokovic M, Adorini L, Holick MF. Selective vitamin D receptor modulators and their effects on colorectal tumor growth. J Steroid Biochem Mol Bio. 2007;103:757–762. doi: 10.1016/j.jsbmb.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 10.Munker R, Kobayashi T, Elstner E, Norman AW, Uskokovic M, Zhang W, Andreeff M, Koeffler HP. A new series of vitamin D analogs is highly active for clonal inhibition, differentiation, and induction of WAF1 in myeloid leukemia. Blood. 1996;88:2201–2209. [PubMed] [Google Scholar]
- 11.Spina CS, Tangpricha V, Uskokovic M, Adorinic L, Maehr H, Holick MF. Vitamin D and cancer. Anticancer Res. 2006;26:2515–2524. [PubMed] [Google Scholar]
- 12.O’Kelly J, Uskokovic M, Lemp N, Vadgama J, Koeffler HP. Novel Gemini-vitamin D3 analog inhibits tumor cell growth and modulates the Akt/mTOR signaling pathway. J Steroid Biochem Mol Biol. 2006;100:107–116. doi: 10.1016/j.jsbmb.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Lee HJ, Liu H, Goodman C, Ji Y, Maehr H, Uskokovic M, Notterman D, Reiss M, Suh N. Gene expression profiling changes induced by a novel Gemini Vitamin D derivative during the progression of breast cancer. Biochem Pharmacol. 2006;72:332–343. doi: 10.1016/j.bcp.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 14.Hisatake J, O’Kelly J, Uskokovic MR, Tomoyasu S, Koeffler HP. Novel vitamin D(3) analog, 21-(3-methyl-3-hydroxy-butyl)-19-nor D(3), that modulates cell growth, differentiation, apoptosis, cell cycle, and induction of PTEN in leukemic cells. Blood. 2001;97:2427–2433. doi: 10.1182/blood.v97.8.2427. [DOI] [PubMed] [Google Scholar]
- 15.Gaddipati JP, McLeod DG, Sesterhenn IA, Hussussian CJ, Tong YA, Seth P, Dracopoli NC, Moul JW, Srivastava S. Mutations of the p16 gene product are rare in prostate cancer. Prostate. 1997;30:188–194. doi: 10.1002/(sici)1097-0045(19970215)30:3<188::aid-pros7>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 16.Isaacs WB, Carter BS, Ewing CM. Wild-type p53 suppresses growth of human prostate cancer cells containing mutant p53 alleles. Cancer Res. 1991;51:4716–4720. [PubMed] [Google Scholar]
- 17.Welsh J. Induction of apoptosis in breast cancer cells in response to vitamin D and antiestrogens. Biochem Cell Biol. 1994;72:537–545. doi: 10.1139/o94-072. [DOI] [PubMed] [Google Scholar]
- 18.Vandewalle B, Wattez N, Lefebvre J. Effects of vitamin D3 derivatives on growth, differentiation and apoptosis in tumoral colonic HT 29 cells: possible implication of intracellular calcium. Cancer Lett. 1995;97:99–106. doi: 10.1016/0304-3835(95)03958-y. [DOI] [PubMed] [Google Scholar]
- 19.Mathiasen IS, Lademann U, Jaattela M. Apoptosis induced by vitamin D compounds in breast cancer cells is inhibited by Bcl-2 but does not involve known caspases or p53. Cancer Res. 1999;59:4848–4856. [PubMed] [Google Scholar]
- 20.Norman AW, Manchand PS, Uskokovic MR, Okamura WH, Takeuchi JA, Bishop JE, Hisatake JI, Koeffler HP, Peleg S. Characterization of a novel analogue of 1alpha,25(OH)(2)-vitamin D(3) with two side chains: interaction with its nuclear receptor and cellular actions. J Med Chem. 2000;43:2719–2730. doi: 10.1021/jm0000160. [DOI] [PubMed] [Google Scholar]
- 21.Uskokovic MR, Norman AW, Manchand PS, Studzinski GP, Campbell MJ, Koeffler HP, Takeuchi A, Siu-Caldera ML, Rao DS, Reddy GS. Highly active analogs of 1alpha,25-dihydroxyvitamin D(3) that resist metabolism through C-24 oxidation and C-3 epimerization pathways. Steroids. 2001;66:463–471. doi: 10.1016/s0039-128x(00)00226-9. [DOI] [PubMed] [Google Scholar]
- 22.Inaba M, Okuno S, Nishizawa Y, Imanishi Y, Katsumata T, Sugata I, Morii H. Effect of substituting fluorine for hydrogen at C-26 and C-27 on the side chain of 1,25-dihydroxyvitamin D3. Biochem Pharmacol. 1993;45:2331–2336. doi: 10.1016/0006-2952(93)90207-d. [DOI] [PubMed] [Google Scholar]
- 23.Halloran BP, Bikle DD, Castro ME, Gee E. Isotopic labeling affects 1,25-dihydroxyvitamin D metabolism. Biochemistry. 1989;28:1278–1281. doi: 10.1021/bi00429a049. [DOI] [PubMed] [Google Scholar]
- 24.Beer TM, Munar M, Henner WD. A Phase I trial of pulse calcitriol in patients with refractory malignancies. Cancer. 2001;91:2431–2439. [PubMed] [Google Scholar]
- 25.Trump DL, Hershberger PA, Bernardi RJ, Ahmed S, Muindi J, Fakih M, Yu WD, Johnson CS. Anti-tumor activity of calcitriol: pre-clinical and clinical studies. J Steroid Biochem Mol Biol. 2004;89–90:519–526. doi: 10.1016/j.jsbmb.2004.03.068. [DOI] [PubMed] [Google Scholar]
- 26.Luong QT, Koeffler HP. Vitamin D compounds in leukemia. J Steroid Biochem Mol Biol. 2005;97:195–202. doi: 10.1016/j.jsbmb.2005.06.017. [DOI] [PubMed] [Google Scholar]