Abstract
Besides their traditional role in maintaining central nervous system (CNS) homeostasis, astrocytes also participate in innate immune responses. Indeed, we have previously demonstrated that astrocytes are capable of recognizing bacterial pathogens such as S. aureus, a common etiologic agent of CNS infections, and respond with the robust production of numerous proinflammatory mediators. Suppression of Poly (ADP-ribose) polymerase-1 (PARP-1), a DNA repair enzyme, has been shown to attenuate inflammatory responses in several cell types including mixed glial cultures. However, a role for PARP-1 in regulating innate immune responses in purified astrocytes and the potential for multiple PARP family members to cooperatively regulate astrocyte activation has not yet been examined. The synthetic PARP-1 inhibitor PJ-34 attenuated the production of several proinflammatory mediators by astrocytes in response to S. aureus stimulation including nitric oxide, interleukin-1 beta (IL-1β), tumor necrosis factor-alpha (TNF-α), and CCL2. The release of all four mediators was partially reduced in PARP-1 knockout (KO) astrocytes compared to wild type cells. The residual inflammatory mediator expression detected in PARP-1 KO astrocytes was further blocked with PJ-34, suggesting either non-specific effects of the drug or actions on alternative PARP isoforms. Reduction of PARP-2 or PARP-3 expression by siRNA knock down revealed that these isoforms also contributed to inflammatory mediator regulation in response to S. aureus. Interestingly, the combined targeting of either PARP-1/PARP-2 or PARP-2/PARP-3 attenuated astrocyte inflammatory responses more effectively compared to knock down of either PARP alone, suggesting cooperativity between PARP isoforms. Collectively, these findings suggest that PARPs influence the extent of S. aureus-induced astrocyte activation.
Keywords: astrocytes, PARP, PJ-34, S. aureus, proinflammatory cytokines, chemokines, siRNA
INTRODUCTION
Brain abscesses result from the parenchymal colonization of pyogenic bacteria, such as Staphylococcus aureus (S. aureus), and represent a serious central nervous system (CNS) infection despite advances made in detection and therapy (Mathisen and Johnson 1997; Townsend and Scheld 1998; Lu et al. 2006). We and others have demonstrated using a mouse model of S. aureus-induced brain abscess that astrocyte activation is a hallmark feature of infection (Baldwin and Kielian 2004; Stenzel et al. 2004; Kielian et al. 2007). Astrocytes recognize S. aureus via Toll-like receptor 2 (TLR2), which triggers the production of numerous proinflammatory mediators including nitric oxide (NO), tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β), CXCL2 (also known as MIP-2; macrophage inflammatory protein-2), and CCL2 (also known as macrophage chemoattractant protein-1, MCP-1) (Esen et al. 2004; Phulwani et al. 2006; Farina et al. 2007). In this way, astrocytes participate in the early innate response to S. aureus by releasing mediators which amplify the host inflammatory reaction during brain abscess evolution (Kielian 2004). However, recent evidence suggests that this antibacterial response can extend beyond the infectious period (Baldwin and Kielian 2004; Kielian et al. 2007), which likely contributes, in part, to the excessive parenchymal damage that typifies brain abscesses (Townsend and Scheld 1998; Lu et al. 2002). Therefore, strategies devised to suppress chronic proinflammatory mediator production subsequent to pathogen removal may prove effective in attenuating brain abscess severity.
Poly (ADP-ribose) polymerases (PARPs) are enzymes that catalyze the transfer of ADP-ribose from NAD+ to various target proteins and play important roles in maintaining genomic stability, cell cycle regulation, and gene expression (Jagtap and Szabo 2005; Hassa and Hottiger 2008). Currently, 17 PARP family members have been identified, with PARP-1 representing the most extensively investigated isoform. PARP-1 mediates DNA repair by recruiting the appropriate proteins at DNA break points and also alters DNA structure, making the damaged site more accessible for repair proteins (D’Amours et al. 1999; Ame et al. 2004; Nguewa et al. 2005; Hassa et al. 2006). PARP-1 also influences NF-κB-dependent gene expression as well as the phosphorylation status of several alternative transcription factors including CREB, ATF-2, AP-1, and SP-1 (Oliver et al. 1999; Hassa et al. 2001; Ha et al. 2002; Hassa et al. 2003; Ha 2004; Nakajima et al. 2004; Hassa et al. 2005). Recent studies have demonstrated that PARP-1 contributes to dysregulated inflammation since PARP-1 inhibition attenuated the severity of several inflammatory diseases including experimental autoimmune encephalomyelitis (EAE), Parkinson’s disease, colitis, and asthma (Eliasson et al. 1997; Burkart et al. 1999; Jijon et al. 2000; Chiarugi 2002; Boulares et al. 2003; Iwashita et al. 2004; Hassa and Hottiger 2008). In addition, prolonged PARP-1 activation has been associated with apoptotic cell death in astrocytes (Alano et al. 2004; Ying et al. 2005; Zhu et al. 2005), which may be relevant to brain abscesses since we have proposed that continued astrocyte activation may impact, in part, the extent of brain parenchyma destruction during infection (Kielian 2004).
PARP-2 and PARP-3 interact with proteins that are also partners for PARP-1 and similarly, participate in DNA repair (Johansson 1999; Burkle 2001; de la Lastra et al. 2007; Rouleau et al. 2007). PARP-2 possesses the strongest sequence homology with PARP-1 (69%) than any other PARP family member (Ame et al. 2004; Nguewa et al. 2005). Recent studies have suggested a role for PARP-2 in inflammatory disease, where PARP-2 loss was protective in animal models of brain ischemia and colitis (Popoff et al. 2002; Kofler et al. 2006). Embryonic lethality of PARP-1/PARP-2 double KO mice also indicates strong compensatory effects mediated via PARP-2 (Menissier de Murcia et al. 2003). A relatively new member of the PARP family is PARP-3, which is capable of interacting with both PARP-1 and PARP-2 to participate in DNA repair processes (Rouleau et al. 2007).
Despite its described roles in inflammation, the importance of PARP-1 and other PARP family members in regulating astrocyte activation in response to S. aureus has not yet been examined. Although earlier reports have demonstrated that PARP-1 influences TNF-α and NO production in response to the gram-negative stimulus lipopolysaccharide (LPS), these studies were performed with mixed glial cultures containing astrocytes, microglia, and oligodendrocytes (Ha et al. 2002; Ha 2004; Nakajima et al. 2004). Therefore, a direct assessment of PARP effects on astrocytes cannot be implied. In addition, significant differences exist with regard to the nature of bacterial stimuli utilized. Specifically, we investigated responses to intact bacteria that trigger a plethora of cytokine as well as phagocytic pathways, whereas LPS selectively triggers the CD14/TLR4 complex (Akira 2006; O’Neill and Bowie 2007). To evaluate the importance of PARPs in controlling the innate immune properties of astrocytes, pharmacological or genetic approaches using the PARP inhibitor 2-(dimethylamino)-N-(5,6-dihydro-6-oxophenanthridin-2yl)acetamide (PJ-34) or primary astrocytes isolated from PARP-1 KO mice and siRNA-mediated knock down of various PARP isoforms, respectively, were employed. The results demonstrated the novel finding that PARPs 1-3 collaborate to regulate the extent of proinflammatory mediator release from activated astrocytes in response to bacterial stimuli. A better understanding of the complex relationships between various PARP isoforms and the inflammatory stage(s) they impact may lead to the exploitation of these enzymes as potential therapeutic targets for the future treatment of human inflammatory diseases.
MATERIALS AND METHODS
Mouse strains
PARP-1 KO and WT mice (both on a B6/129 background)(Wang et al. 1995) were obtained from Jackson Laboratories (Bar Harbor, ME). C57BL6 mice were purchased from Harlan Labs (Indianapolis, IN). The animal use protocol was approved by the University of Arkansas for Medical Sciences Animal Care and Use Committee and is in accord with NIH guidelines for the use of rodents.
Primary astrocyte cell culture and reagents
Primary astrocytes were derived from PARP-1 WT and KO mice or C57BL6 animals (1–4 days of age) as previously described (Esen et al. 2004; Phulwani et al. 2006). Briefly, mouse pups were euthanized using an overdose of inhaled isoflurane to obtain mixed glial cultures. Cerebri were collected under aseptic conditions and the meninges removed. Tissues were minced, resuspended in trypsin-EDTA (Mediatech Inc., Herndon, VA), and incubated at 37°C for 20 min. Subsequently, cells were resuspended in complete DMEM (4.5 g/L glucose, Mediatech Inc.) containing 10% FBS (Hyclone, Logan, UT), 200 mM L-glutamine, 100 U/ml penicillin, 0.1 mg/ml streptomycin and 0.25 μg/ml fungizone (all from Mediatech Inc.), OPI supplement (oxalacetic acid, pyruvate, insulin; Sigma, St. Louis, MO), and 0.5 ng/ml recombinant mouse GM-CSF (Biosource, Camarillo, CA). The cell suspension was further triturated and filtered through a 70 μm cell strainer. Subsequently, cells were centrifuged, resuspended in complete medium, and seeded into 150 cm2 flasks maintained in a humidified atmosphere of 95 % air/5 % CO2.
When cultures reached confluency (7–10 days), flasks were shaken overnight at 50 × g at 37°C to recover microglia. This was repeated for a minimum of three occurrences, which progressively diminished the number of contaminating microglia over time, whereupon flasks were trypsinized and cells resuspended in astrocyte medium (identical to the formulary for microglia described above with the omission of GM-CSF) supplemented with 0.1 mM of L-leucine methyl ester (L-LME; Sigma-Aldrich, St. Louis, MO), a microglial cytotoxic agent that has been extensively used as a method for microglial depletion (Esen et al. 2004; Hamby et al. 2006; Phulwani et al. 2006; Saura 2007). Astrocytes were cultured in L-LME-containing medium for at least 2 weeks prior to use in experiments and were not utilized until the third passage in culture (approximately day 35–42 in vitro). Continued passage of astrocytes also facilitated the elimination of residual microglia that remained firmly attached to the tissue culture flask surface following trypsinization to recover astrocytes. The purity of astrocyte cultures was evaluated by immunohistochemical staining using antibodies against CD11b (BD Pharmingen) and glial fibrillary acidic protein (GFAP; Invitrogen) to identify microglia and astrocytes, respectively. The purity of astrocyte cultures prepared in this manner was routinely ≥ 95%. A final observation to confirm the relative purity of astrocyte cultures prepared in this manner is provided by our extensive experience working with S. aureus and its cell wall product PGN in glia. Our studies have reproducibly demonstrated that these gram-positive microbial stimuli are poor inducers of iNOS expression and subsequent NO production in primary microglia, whereas they are potent activators of iNOS and NO in astrocytes (Esen et al. 2004; Esen and Kielian 2006; Kielian 2008).
Reagents
Heat-inactivated S. aureus (strain RN6390) was prepared as previously described (Kielian et al. 2002). PGN derived from S. aureus was obtained from InvivoGen (San Diego, CA). The synthetic PARP-1 inhibitor PJ-34 was purchased from Inotek Pharmaceuticals (Beverly, MA) and 3,4-Dihydro-5-[4-(1-piperidinyl)butoxy]-1(2H)-isoquinolinone (DPQ) and human recombinant PARP-1 were obtained from Alexis Biochemicals (San Diego, CA). Endotoxin levels in all cell culture reagents were verified < 0.03 EU/ml as determined by a Limulus amebocyte lysate assay (Associates of Cape Cod, Falmouth, MA).
Nitrite assay
Nitrite, a stable end product resulting from the reaction of NO with molecular oxygen, was used to quantitate NO levels in astrocyte-conditioned supernatants as previously described (Phulwani et al. 2006).
Cell viability assays
The effects of PARP pharmacological inhibitors on astrocyte viability were evaluated using a standard MTT assay as previously described (Esen et al. 2004).
Enzyme-linked immunosorbent assay (ELISA)
Quantitation of cytokine and chemokine levels in astrocyte-conditioned medium was performed using standard sandwich ELISA kits according to the manufacturer’s instructions (OptEIA mouse IL-1β and TNF-α, BD Pharmingen; DuoSet mouse CXCL2 and CCL2, R & D Systems, Minneapolis, MN).
Protein extraction and Western blot
Astrocytic nuclear extracts were prepared using a nuclear extraction kit (Panomics, Redwood City, CA). Calculation of total protein in astrocyte extracts was determined using a standard protein assay (bicinchoninic acid protein assay reagent, BCA; Bio-Rad, Hercules, CA). PARP-1 expression was evaluated by Western blot using a mouse anti-PARP-1 antibody (BD Biosciences, San Diego, CA) as previously described (Kielian et al. 2005).
RNA isolation and quantitative real-time RT-PCR (qRT-PCR)
Total RNA was isolated from primary astrocytes using the TriZol reagent and treated with DNAse1 (both from Invitrogen, Grand Island, NY) prior to use in qRT-PCR studies. The experimental procedure was performed as previously described (Phulwani et al. 2006). ABI Assays on Demand kits (Foster City, CA) were used to examine PARP-2, PARP-3, and CCL2 expression, whereas GAPDH and IL-1β primers and TAMRA TaqMan probes were synthesized by ABI based on previously published sequences (Esen et al. 2004; Tanga et al. 2005).
Short interfering RNA (siRNA)
siRNA oligonucleotides specific for PARP-2 and PARP-3 along with a scrambled oligonucleotide (negative control 1) were purchased from Ambion (Austin, TX). OPTI-MEM medium, Lipofectamine 2000, and Block IT FITC-conjugated oligonucleotide were purchased from Invitrogen. Initially, siRNA conditions were optimized using a siRNA starter kit (Ambion) for GAPDH knock down. To assess transfection efficiency, FITC-conjugated oligonucleotide (25 nM) was co-transfected in each treatment group along with siRNA oligonucleotide (100 nM) using Lipofectamine 2000. Average transfection efficiencies ranged from 70–80% as evident by the nuclear accumulation of FITC-conjugated oligonucleotide (Supplemental Fig. 1). Total RNA was collected at both 24 and 48 h post-transfection, whereupon qRT-PCR was performed to assess the extent and specificity of target gene knock down by siRNA oligonucleotides. A separate set of siRNA transfected astrocytes were stimulated with heat-inactivated S. aureus at 24 h post-transfection, whereupon cell-conditioned supernatants were collected 24 h following bacterial exposure to quantify the effects of PARP isoform knock down on proinflammatory mediator expression.
We did not evaluate the extent of PARP-2 or PARP-3 knock down by siRNA at the protein level due to the relative paucity of antibodies specific for these PARP isoforms for use in Western blotting. However, in our preliminary optimization experiments with GAPDH siRNA knock down, we were successful in obtaining an approximate 60–80% reduction in protein expression as determined by Western blotting. Although we cannot directly extend these findings to PARP isoform knock down at the protein level, qRT-PCR analysis demonstrated an approximate 50–70% decrease in PARP isoform mRNA levels in transfected astrocytes.
Statistics
Significant differences between PJ-34 and PARP-1 KO and WT treatment groups were determined using the Student’s t test at the 95% confidence interval, whereas differences between experimental groups following siRNA knock down of the various PARP isoforms was determined by a Student-Newman-Keuls method for multiple pair-wise comparisons. Both statistical analyses were performed using Sigma Stat (Chicago, IL).
RESULTS
Primary mouse astrocytes express PARP-1
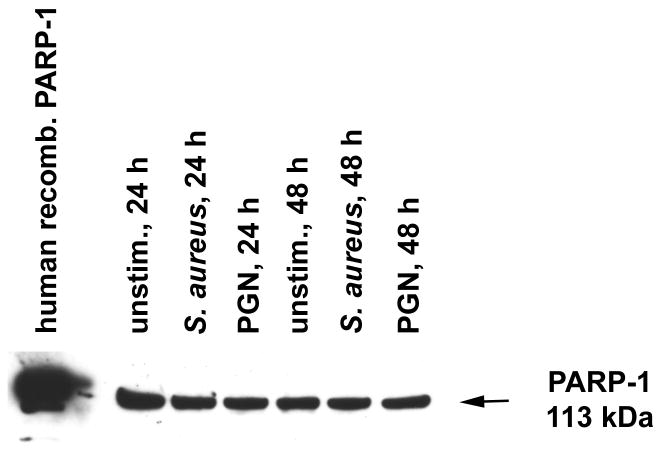
Recent studies have demonstrated that reactive astrocytes express PARP-1 in vivo, whereas other reports have suggested PARP-1 expression in astrocytes based on indirect evidence, most notably Poly(ADP-ribose) (PAR) formation (Chung et al. 2004; Kauppinen et al. 2006). PARP-1 protein expression has also been demonstrated in mixed glial cultures containing several cell types (Ha et al. 2002; Ha 2004; Nakajima et al. 2004). Here we show that purified astrocytes express PARP-1 constitutively in the nucleus with levels remaining unchanged even upon stimulation with the gram-positive stimuli S. aureus or PGN (Fig. 1). The expression of PARP-1 in astrocytes suggested that these cells may be responsive to PARP inhibitors.
Figure 1. Primary mouse astrocytes express PARP-1.
Astrocytes were seeded in 6-well plates at 2 × 106 cells per well. After 24 h, astrocytes were treated with heat-inactivated S. aureus (107 cfu/well) or PGN (10 μg/ml). At 24 or 48 h following bacterial stimulation, nuclear extracts were prepared and PARP-1 expression was evaluated by Western blot (40 μg of nuclear protein per sample). Human recombinant PARP-1 (far left lane) was used as a positive control. Results are representative of three independent experiments.
The PARP inhibitor PJ-34 attenuates proinflammatory mediator release from astrocytes
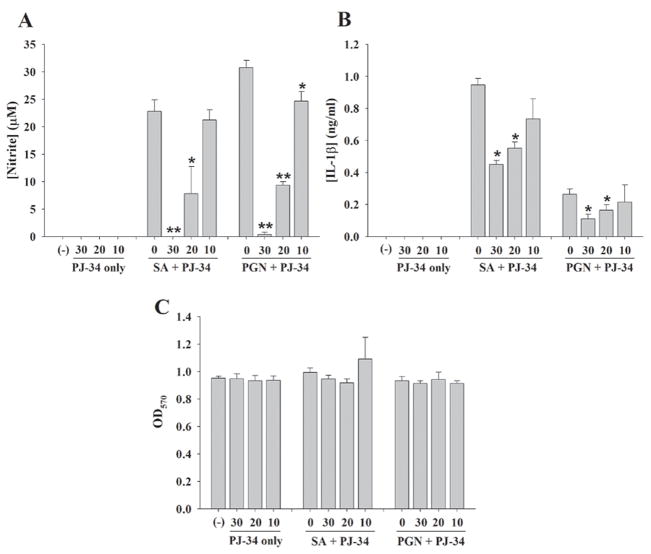
Bacterial stimuli such as intact S. aureus or its cell wall product PGN, induce robust astrocyte activation typified by the production of numerous proinflammatory mediators (Esen et al. 2004; Phulwani et al. 2006; Farina et al. 2007). We have previously demonstrated that both of these gram-positive bacterial stimuli are recognized via TLR2 in astrocytes (Esen et al. 2004). To evaluate whether PARPs impact astrocyte activation in response to these bacterial motifs, we investigated the effects of the PARP inhibitor PJ-34 on proinflammatory mediator release from stimulated astrocytes. PJ-34 led to a dose-dependent reduction in the expression of NO, IL-1β, CCL2, TNF-α, and CXCL2 in response to both S. aureus and PGN stimulation (Fig. 2 and data not shown). Cell viability assays revealed that PJ-34 was not cytotoxic at any of the concentrations examined, indicating that the anti-inflammatory effects observed in response to PJ-34 treatment were not due to cell death (Fig. 2C and 3C). In summary, these findings suggest that PARP activation delivers a proinflammatory signal(s) to astrocytes that can be partially blocked with PJ-34.
Figure 2. The PARP-1 inhibitor PJ-34 attenuated proinflammatory mediator release from S. aureus- or PGN-stimulated astrocytes.
Primary astrocytes were seeded in 96-well plates at 1 × 105 cells per well and incubated overnight. The following day, cells were pre-treated for 1 h with various concentrations of PJ-34 (10–30 μM) followed by stimulation with 107 cfu/well of heat-inactivated S. aureus (SA) or 10 μg/ml of PGN for 24 h. Subsequently, cell-free supernatants were collected and analyzed for NO (A) and IL-1β (B) using the Griess reagent and ELISA, respectively. Astrocyte viability was assessed using a standard MTT assay and the raw OD570 absorbance values are provided (C). Results are reported as the mean ± SD of three independent wells for each experimental treatment. Significant differences between astrocytes pretreated with S. aureus or PGN only versus cells exposed to the various concentrations of PJ-34 + S. aureus or PJ-34 + PGN are denoted with asterisks (*p < 0.05, **p < 0.001). Results are representative of six independent experiments.
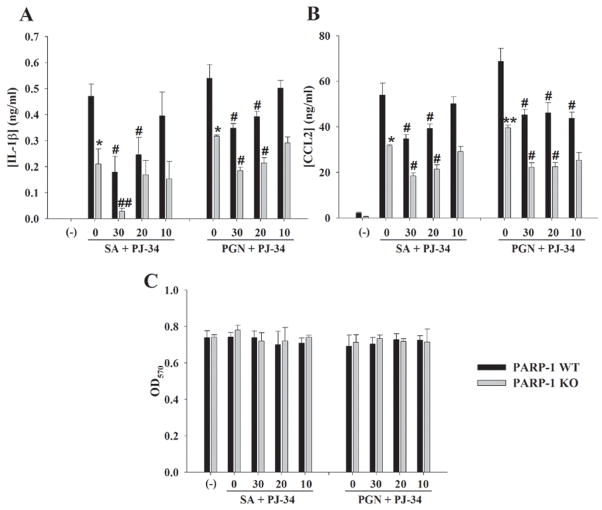
Figure 3. PARP-1 influences inflammatory mediator production in S. aureus or PGN-stimulated astrocytes.
PARP-1 WT and KO astrocytes were seeded in 96-well plates at 1 × 105 cells per well and incubated overnight. The following day, cells were pre-treated for 1 h with various concentrations of PJ-34 (10–30 μM) followed by stimulation with 107 cfu/well of heat-inactivated S. aureus (SA) or 10 μg/ml of PGN for 24 h. Subsequently, cell-free supernatants were collected and analyzed for IL-1β (A) and CCL2 (B) by ELISA. Astrocyte viability was assessed using a standard MTT assay and the raw OD570 absorbance values are provided (C). Results are reported as the mean ± SD of three independent wells for each experimental treatment. Significant differences between PARP-1 WT and KO astrocytes stimulated with S. aureus or PGN alone are denoted with asterisks (*p < 0.05). In addition, significant changes between PARP-1 WT or KO astrocytes treated with S. aureus or PGN only versus cells exposed to the various concentrations of PJ-34 + S. aureus or PJ-34 + PGN are denoted with a pound sign (#p < 0.05, ##p < 0.001). Results are representative of three independent experiments.
PARP-1 KO astrocytes demonstrate defects in proinflammatory mediator production in response to bacterial stimuli
Recent studies have demonstrated that PARP-1 KO glia have defective inflammatory signaling responses to the gram-negative antigen LPS (Ha et al. 2002; Ha 2004; Nakajima et al. 2004). However, these studies were performed with mixed glial cultures (containing microglia, astrocytes, and oligodendrocytes) confounding an assessment of PARP-1 effects on an individual cell type. In addition, the functional importance of PARP-1 in mediating astrocyte activation in response to the gram-positive pathogen S. aureus, a prevalent CNS pathogen, or its cell wall product PGN has not yet been examined. These microbial stimuli are important from a therapeutic standpoint since S. aureus can colonize the CNS and the prevalence of methicillin-resistant S. aureus (MRSA) in CNS infections such as brain abscess continues to rise (Roche et al. 2003; Jones et al. 2004; Sifri et al. 2007). In contrast to PARP inhibition with PJ-34, only a subset of inflammatory mediators was affected in PARP-1 KO astrocytes. Specifically, PARP-1 KO cells demonstrated a partial suppression of NO, IL-1β, TNF-α, and CCL2 production compared to WT astrocytes in response to S. aureus and PGN (Figs. 3 and 6), whereas no effect on CXCL2 expression was observed (data not shown). Treatment of PARP-1 KO astrocytes with PJ-34 further attenuated NO, IL-1β, TNF-α, and CCL2 production in response to microbial stimuli (Fig. 3 and data not shown). This finding was unexpected since PJ-34 has been reported as a specific PARP-1 inhibitor (Jagtap and Szabo 2005). Collectively, these findings suggest that PARP-1 impacts specific aspects of the astrocytic proinflammatory signaling pathway in response to gram-positive microbial stimuli. The ability of PJ-34 to further attenuate astrocyte activation is suggestive of either compensatory effects exerted via other PARP family members or alternatively, non-specific effects of PJ-34 on molecules unrelated to the PARP family.
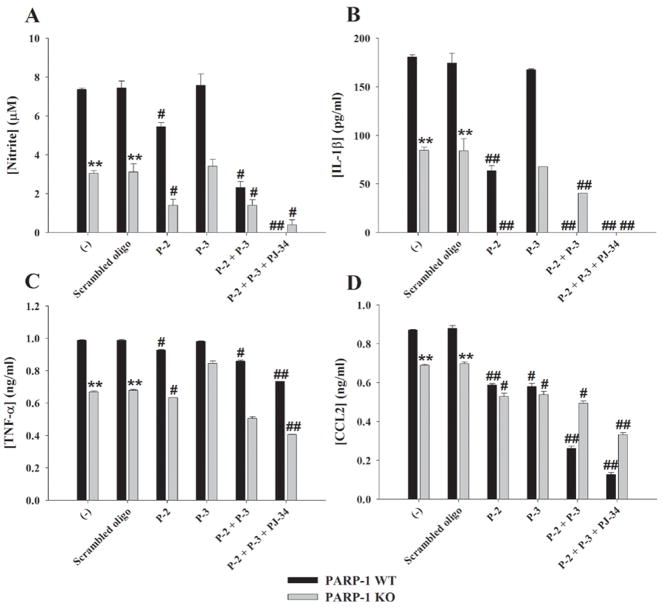
Figure 6. PARP-1, PARP-2, and PARP-3 interact to regulate proinflammatory mediator production in astrocytes.
PARP-1 WT and KO astrocytes were seeded in 6-well plates at 1 × 106 cells per well. After 24 h, cells received transfection reagent alone (−) or were transfected with either a scrambled oligonucleotide (negative control) or siRNA oligonucleotides directed against PARP-2 and/or PARP-3 (100 nM). At 23 h following transfection, cells in selected wells were pre-treated with PJ-34 (10–30 μM) for 1 h. Subsequently, all wells were stimulated with 108 cfu of heat-inactivated S. aureus. Cell-free supernatants were collected at 24 h following bacterial exposure and analyzed for NO release using the Griess reagent (A) and IL-1β (B), TNF-α (C), and CCL2 (D) production by ELISA. Results are reported as the mean ± SD of two independent wells for each experimental treatment. Significant differences between PARP-1 WT and KO astrocytes are denoted with asterisks (*p < 0.05, **p < 0.001). Significant changes between astrocytes transfected with scrambled control oligonucleotide versus cells transfected with siRNAs for i) PARP-2; ii) PARP-3; iii) PARP-2 + PARP-3; or iv) PJ-34 + PARP-2 + PARP-3 are denoted with a pound sign (#p < 0.05, ##p < 0.001). Results are representative of three independent experiments.
Primary astrocytes express PARP-2 and PARP-3
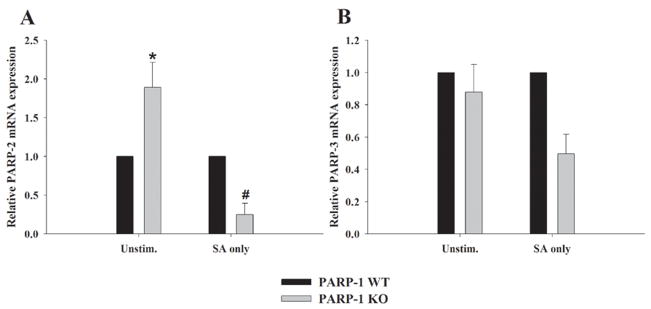
When considering the additional potential targets of PJ-34 in PARP-1 KO astrocytes, likely candidates included PARP-2 and/or PARP-3, both of which interact with proteins that are also partners for PARP-1 and participate in DNA repair (Rouleau et al. 2007). However, to our knowledge, the demonstration of these alternative PARP isoforms in astrocytes has not yet been reported. To determine if astrocytes express PARP-2 and/or PARP-3, and whether the expression of one or both of these isoforms is altered in PARP-1 KO cells, qRT-PCR analysis was performed (Fig. 4). PARP-2 levels were constitutively higher in resting PARP-1 KO astrocytes, suggesting that this isoform may play a compensatory role in the context of PARP-1 loss (Fig. 4A). Under resting conditions, PARP-1 WT and KO astrocytes expressed PARP-3 at roughly equivalent levels (Fig. 4B). Both PARP-2 and PARP-3 expression were reduced in response to astrocyte activation with S. aureus (Fig. 4).
Figure 4. Primary astrocytes express PARP-2 and PARP-3.
PARP-1 WT and KO astrocytes were seeded in 6-well plates at 1 × 106 cells per well. After 24 h, cells were treated with 107 cfu/well of heat-inactivated S. aureus (SA). Total RNA was collected from cells at 6 h following S. aureus stimulation and PARP-2 and PARP-3 mRNA expression was quantified by qRT-PCR. Gene expression levels were calculated after normalizing PARP-2 or PARP-3 signals against the housekeeping gene GAPDH and are presented as relative mRNA expression units (mean ± SEM of two independent experiments). Significant differences in PARP-2 expression between unstimulated PARP-1 WT versus KO astrocytes are denoted by asterisks (*, p < 0.05), whereas differences in PARP-2 levels between S. aureus-stimulated PARP-1 WT versus KO cells are noted by a pound sign (#, p < 0.05).
Effects of PARP-2 and PARP-3 on astrocyte activation
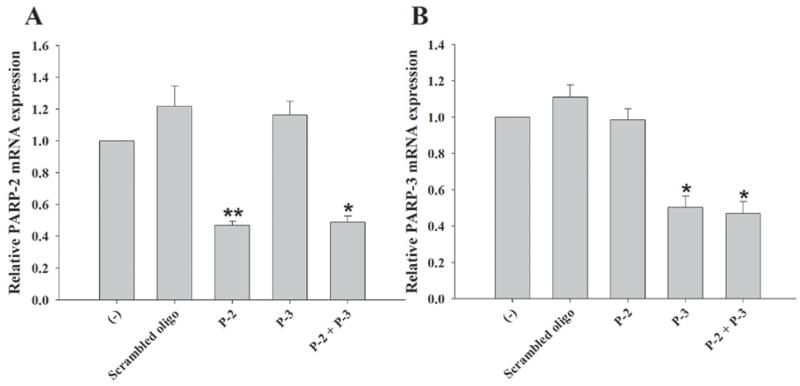
PARP-1/PARP-2 double KO mice are not viable beyond the embryonic period (Menissier de Murcia et al. 2003) and PARP-3 KO animals have not yet been generated, which prevented their use as tools to investigate potential redundancies of these PARP isoforms in regulating astrocytic responses to microbial stimuli. To assess the potential roles of PARP-2 and PARP-3 on astrocyte activation, both PARP isoforms were attenuated by siRNA knock down. We examined PARP-2 and PARP-3 siRNA knock down in PARP-1-deficient astrocytes to determine whether the various PARP family members cooperate to regulate proinflammatory mediator release. The efficiency of our lipid-based transfection approach reached approximately 80% based on the nuclear accumulation of FITC-conjugated oligonucleotide that was co-transfected along with siRNA oligonucleotides (Supplemental Fig. 1). In preliminary experiments designed to identify optimal siRNA conditions, an approximate 80% knock down of GAPDH mRNA and protein levels was achieved (data not shown). Upon transfection with siRNAs directed against PARP-2 or PARP-3, qRT-PCR analysis revealed that mRNA expression of both isoforms was reduced approximately 50–70% at 24 h following transfection (data not shown). This level of PARP isoform knock down was maintained out to 48 h post-transfection, the latest time point evaluated (Fig. 5). Importantly, no significant alternations in PARP-2 or PARP-3 expression were observed when astrocytes were transfected with an equivalent amount of negative scrambled oligonucleotide (Fig. 5), indicating that the observed reductions in PARP-2 and PARP-3 following siRNA treatment were not the result of non-specific effects. To confirm the specificity of siRNA oligonucleotides for targeting select PARP isoforms, PARP-3 mRNA expression was examined in cells knocked down for PARP-2 and vice-versa. Neither PARP-2 nor PARP-3 siRNAs significantly affected the expression of the other, confirming the specificity of siRNA transcript targeting (Fig. 5). Additionally, co-transfection with both PARP-2 and PARP-3 siRNAs was capable of attenuating both isoforms simultaneously. The combined knock down of PARP-2 and PARP-3 expression by siRNA in PARP-1 KO astrocytes provided the most comprehensive reduction in PARPs examined in this study. In fact, to our knowledge this report represents the first investigation into the combined actions of PARPs 1-3 in regulating inflammatory responses in any cell type.
Figure 5. Targeted knock down of PARP-2 and PARP-3 expression in primary astrocytes by siRNA.
PARP-1 WT astrocytes were seeded in 6-well plates at 1 × 106 cells per well. After 24 h, cells were transfected with either a scrambled oligonucleotide (negative control) or siRNA oligonucleotides directed against PARP-2 and/or PARP-3 (100 nM). Total RNA was collected at 48 h following transfection for evaluating the degree of PARP-2 (A) or PARP-3 (B) knock down by qRT-PCR. Gene expression levels were calculated after normalizing PARP-2 or PARP-3 signals against the housekeeping gene GAPDH and are presented as relative mRNA expression units relative to astrocytes exposed to transfection reagent alone (−) (mean ± SD of two separate wells of transfected cells). Specificity of oligonucleotide targeting was confirmed by analyzing PARP-2 mRNA expression in astrocytes transfected with PARP-3 siRNA and vice-versa. Significant differences between control transfected and siRNA treated astrocytes are denoted with asterisks (*p < 0.05, **p < 0.001). Results are representative of three independent experiments.
Upon confirmation of siRNA knock down of PARP-2 and PARP-3, subsequent experiments examined the consequences of multiple PARP inhibition on astrocyte proinflammatory mediator production in response to S. aureus. In WT cells, PARP-2 knock down led to a significant attenuation in the expression of NO, IL-1β, TNF-α, and CCL2, suggesting that PARP-2 also plays a critical role in dictating the extent of proinflammatory mediator release in response to bacterial stimuli (Fig. 6). Interestingly, although PARP-3 siRNA had little/no impact on astrocyte activation in WT cells, the simultaneous knock down of both PARP-2 and PARP-3 led to an additive inhibitory effect on the release of NO, IL-1β, TNF-α, and CCL2 in response to S. aureus compared to each PARP siRNA individually (Fig. 6). These findings indicate that PARP-2 and PARP-3 can cooperate to influence astrocyte responses to bacterial stimuli.
With regard to PARP-1 KO astrocytes, NO, IL-1β, TNF-α, and CCL2 production was significantly inhibited in response to bacterial stimulation compared to WT cells (Fig. 6). Treatment of PARP-1 KO astrocytes with PARP-2 siRNA further attenuated the production of these mediators, whereas PARP-3 knock down had little/no effect, similar to what was observed in WT cells (Fig. 6). Interestingly, the simultaneous knock down of both PARP-2 and PARP-3 in KO astrocytes (representing a global reduction in all three PARP isoforms) did not cause a further reduction in proinflammatory mediator release compared to the simultaneous inactivation of both PARP-1 and PARP-2. This finding suggests that the majority of PARP modulatory effects on astrocyte inflammatory molecule production can be attributed to the simultaneous actions of at least two PARP isoforms, whereas the involvement additional PARP family members (at least the ones examined in this study) do not further influence astrocyte activation. Transfection of PARP-1 WT and KO astrocytes with negative scrambled oligonucleotide did not affect proinflammatory mediator expression, ruling out any non-specific effects of siRNA (Fig. 6). Interestingly, even with the simultaneous knock down of multiple PARP isoforms, PJ-34 still exerted anti-inflammatory effects (Fig. 6). This could be explained by the action of PJ-34 on residual PARP-2 and/or PARP-3 expression, as siRNA only afforded a 50–70% knock down in expression levels. An alternative possibility is that PJ-34 exerts effects on molecule(s) other than PARPs 1-3.
DISCUSSION
Recent evidence suggests that the host immune response directed towards pathogen eradication also contributes to the excessive damage of surrounding non-infected tissue during brain abscess development (Baldwin and Kielian 2004; Kielian 2004). Since astrocyte activation is a hallmark feature of this disease and these cells produce numerous proinflammatory mediators in response to bacterial stimuli (Baldwin and Kielian 2004; Esen et al. 2004; Stenzel et al. 2004; Kielian et al. 2007), it is possible that astrocytes contribute to the pathological immune response that continues beyond effective bacterial neutralization from the brain. This possibility prompted us to investigate approaches that could modulate astrocyte activation in vitro that could potentially be used in future in vivo studies for improving brain abscess outcome. One such approach was PARP inhibition, based on earlier reports demonstrating that PARP-1 is pivotal for regulating immune cell activation in response to LPS (Olszanecki et al. 2006; Aldinucci et al. 2007). We employed a comprehensive strategy to investigate the functional importance of PARPs in regulating astrocyte responses to S. aureus including the use of PARP pharmacological inhibitors, PARP-1 KO astrocytes, and siRNA for knocking down PARP-2 and PARP-3. The summation of our findings demonstrated that PARPs influence the extent of proinflammatory mediator release by astrocytes in a cooperative manner.
PJ-34, a water-soluble phenanthridinone derivative and reported PARP-1 inhibitor, attenuated astrocyte activation by downregulating the production of numerous proinflammatory mediators including NO, IL-1β, TNF-α, and CCL2. However, the immune modulatory effects of PJ-34 on astrocyte activation were observed at concentrations far exceeding its IC50 for inhibiting PARP enzymatic activity (10–30 μM versus 20 nM, respectively). The concentrations of PJ-34 used in the current study were similar to other reports demonstrating immune modulatory effects in disparate cell types (Hasko et al. 2002). Our findings with PJ-34 raised the question as to whether the compound exerted non-specific anti-inflammatory effects via molecules unrelated to the PARP family. Since PJ-34 acts as a competitive inhibitor of PARP enzymatic activity by blocking the NAD+ binding site within the catalytic domain, it could be predicted that PJ-34 may affect other enzymes which depend upon efficient interaction with NAD+ to mediate their functions (Jagtap and Szabo 2005). In addition, PJ-34 is a water-soluble molecule which likely restricts its accumulation intracellularly, suggesting that PJ-34 may elicit localized effects at the cell membrane. One potential candidate is CD38, a plasma membrane-associated ecto-enzyme that possesses ADP-ribosyl cyclase activity owing to the presence of a NAD+ binding domain (Young et al. 2006). Interestingly, CD38 has been shown to regulate both innate and adaptive immune responses and could serve as another target to explain the anti-inflammatory effects of PJ-34 (Lund 2006; Partida-Sanchez et al. 2007). Indeed, CD38 expression has been demonstrated in astrocytes in further support of this possibility (Verderio et al. 2001; Bruzzone et al. 2004). PJ-34 also modulates the activity of transient receptor potential cation channel, subfamily M, member 2 (TRPM2)-mediated calcium influx into HEK293 cells (Fonfria et al. 2004). Since calcium transients play an important role in regulating immune responses, such an effect of PJ-34 on calcium levels may have influenced proinflammatory mediator production. However, these potential PARP-1-independent targets of PJ-34 in astrocytes remain highly speculative and could be a topic for future studies examining mechanistic effects of this compound. Alternatively, there may simply be a disconnect between the effective concentrations of PJ-34 required for inhibiting PARP enzymatic activity versus inflammatory responses.
To directly demonstrate whether PARP-1 mediates astrocyte activation, PARP-1 KO and WT astrocytes were exposed to bacterial stimuli and assayed for proinflammatory mediator production. The results indicated that PARP-1 selectively influences S. aureus-induced astrocyte activation as PARP-1 loss attenuated the expression of specific inflammatory mediators including IL-1β, TNF-α, NO, and CCL2, whereas other molecules were not affected. Selectivity in the repertoire of proinflammatory mediators regulated by PARP-1 has been previously described using mixed glial cultures stimulated with LPS (Ha et al. 2002; Ha 2004; Nakajima et al. 2004).
To determine whether alternative PARP family members compensated for the loss of PARP-1 in KO astrocytes, we performed siRNA knock down for select PARP family members possessing a close resemblance to PARP-1 in terms of structure, function, and subcellular localization, namely PARP-2 and PARP-3. Via siRNA, we achieved approximately a 50–70% knock down of PARP-2 and/or PARP-3 expression in primary astrocytes. The combined knock down of these alternative PARP family members in PARP-1 KO astrocytes produced, to our knowledge, the most comprehensive analysis of PARP involvement in inflammatory responses examined to date. Interestingly, the simultaneous reduction of PARP-1 and PARP-2 (achieved by siRNA for PARP-2 in PARP-1 KO astrocytes) demonstrated more significant inhibition compared to PARP-1 + PARP-3 knock down, suggesting that PARP-2 predominates over PARP-3. In addition, the combined knock down of PARP-2 and PARP-3 in WT astrocytes exerted higher immunosuppressive effects compared to the individual targeting of PARP-2 or PARP-3. Collectively, these findings could be explained by the fact that PARP-1, -2 and -3 can interact to form heterodimers in various proportions within the nucleus. It is possible that the partial loss of one PARP may not have perturbed the balance of heterodimer formation for influencing astrocytic immune responses. Indeed, our data suggests that inflammatory responses in astrocytes are compromised when a minimum of two PARPs are knocked down simultaneously, such that binding partners for the remaining abundantly expressed PARP are not readily available to warrant effector responses (i.e. modulating cell activation) and no additional defects are observed when more than two isoforms are reduced. However, this possibility remains highly speculative at this time and requires further investigation to be either validated or refuted.
Surprisingly, PJ-34 still attenuated the residual proinflammatory mediator production observed in S. aureus-stimulated PARP-1 KO astrocytes simultaneously knocked down for both PARP-2 and PARP-3. This finding raises the possibility that other PARP family members apart from PARPs 1-3 influence astrocytic responses to S. aureus, with the caveat that PJ-34 represents a PARP-specific inhibitor. One likely candidate is short PARP-1 (sPARP-1), an alternative splice product of the PARP-1 gene (Sallmann et al. 2000). sPARP-1 has sequence homology with the PARP-1 catalytic domain and has been shown to be activated via DNA damage-independent mechanisms in mouse embryonic fibroblasts (Sallmann et al. 2000). Another possibility is that our siRNA approach only enabled a 50–70% reduction in PARP-2 and PARP-3 expression. Therefore, residual PARP-2 and/or PARP-3 levels might have been subject to the inhibitory actions of PJ-34 resulting in further anti-inflammatory effects. It is also plausible that PJ-34 modulated astrocyte activation via pathways independent of PARP enzymatic activity since another potent PARP enzymatic inhibitor, DPQ, failed to alter S. aureus-induced astrocyte activation (data not shown). This finding raises the possibility that PARPs regulate astrocyte activation via a scaffolding action through interactions with other cell signaling molecules and not via enzymatic activity. Indeed, several recent studies have shown that PARP-1 acts as a co-activator for NF-κB to regulate glial activation via physical interactions that are independent of PARP enzymatic activity (Hassa et al. 2001; Nakajima et al. 2004; Jagtap and Szabo 2005). A separate study has demonstrated that PARP-1 regulates the expression of the NF-κB co-activator p300/CBP (Hassa et al. 2003; Hassa et al. 2005), suggesting that PARP enzymatic activity may be dispensable for regulating astrocyte activation in response to inflammatory stimuli.
Finally, the relationship between PARP-2 and PARP-3 mRNA levels in PARP-1 KO astrocytes suggested a complicated pattern of expression regulation. Specifically, PARP-2 mRNA levels were elevated in PARP-1 KO astrocytes under resting conditions but were significantly attenuated following S. aureus exposure. In contrast, no alterations in constitutive PARP-3 mRNA expression were observed between PARP-1 KO and WT astrocytes, whereas S. aureus treatment did reduce PARP-3 levels, although this difference did not reach statistical significance. We originally examined the expression of alternative PARP isoforms based on the finding that PJ-34 was still capable of attenuating proinflammatory mediator release in PARP-1 KO astrocytes. Clearly, the differential expression of these other PARP family members in astrocytes both under resting and activation conditions suggests a complex regulatory pathway, although it is difficult to speculate extensively on potential mechanisms since little information is currently available regarding the factors controlling the coordinate expression of PARP isoforms. Indeed, our findings demonstrating the involvement of two PARP family members in regulating maximal S. aureus-dependent astrocyte activation reinforces this level of complexity. The fact that PARP-1/PARP-2 double KO mice are not viable but individual PARP KOs are (Menissier de Murcia et al. 2003; Huber et al. 2004), suggests that PARP-1 and PARP-2 exhibit overlapping but non-redundant functions. Since PARP-1 is the major poly(ADP-ribos)ylating enzyme of eukaryotic cells (Jagtap and Szabo 2005), PARP-1 loss may have contributed to the compensatory increase of PARP-2 in astrocytes. In addition, it is also likely that PARP-1 negatively regulates PARP-2 and PARP-3 expression. This speculation is based on studies where PARP-1 has been shown to regulate the expression of several proteins in both positive and negative manners (Carrillo et al. 2004). However, to best of our knowledge, no one has yet reported a direct negative role of PARP-1 on PARP-2 and PARP-3 transcriptional levels. Interestingly, we observed attenuation of PARP-2 and PARP-3 expression in PARP-1 KO cells only upon exposure to S. aureus. This finding might be the result of non-specific effects of inflammation on PARP-2 and PARP-3 expression. Absence of such effects in the presence of PARP-1 suggests that it serves as the primary PARP family member involved in regulating astrocytic immune responses to S. aureus. However, we failed to see attenuation of PARP-1 expression in wild type astrocytes following S. aureus stimulation, which might be due to the high abundance of PARP-1 in eukaryotic cells (1 × 106 − 2 × 106 copies/cell) (Jagtap and Szabo 2005; Hassa et al. 2006).
Our findings demonstrating a role for PARP-1 in regulating astrocytic proinflammatory mediator release in response to intact S. aureus are in partial agreement with earlier reports using mixed glial cells (Ha et al. 2002; Ha 2004; Nakajima et al. 2004). For example, all studies concur that TNF-α and NO are regulated by PARP-1, in part, via a NF-κB-dependent pathway (Ha et al. 2002; Ha 2004; Nakajima et al. 2004). However, important differences between the current study and previous reports were also observed. In particular, there are some disparities regarding the targets of PARP-1 inhibition. For example, we observed that PARP-1 loss attenuated CCL2 production, whereas this chemokine was not affected in studies by Ha et al. (Ha et al. 2002). In addition, there are discrepancies regarding the effectiveness of PARP-1 inhibitors (i.e. DPQ and PJ-34) on glial production of proinflammatory mediators, which may reflect variations in the efficacy of PARP inactivation and/or PARP-1 enzymatic activity-independent mechanism(s). Overall, these differences could be explained by several factors including the purity of cell cultures, dose of PARP inhibitors employed, efficacy of PARP inactivation (i.e. use of cells from gene-deficient mice versus siRNA), and/or the type of stimulus used (i.e. soluble activators such as LPS versus particulate intact bacteria). Indeed, our study utilized relatively pure astrocyte cultures, whereas others have examined PARP-1 function in mixed glial cultures that contain a significant number of microglia (Ha et al. 2002; Ha 2004; Nakajima et al. 2004).
Variable effects of PARP-1 inhibition on gene expression may reflect influences of multiple regulatory pathways. Therefore, although several proinflammatory genes are regulated, in part, by NF-κB, many are also controlled by other transcription factors that may not be influenced by PARP activity. Indeed, p38 MAPK-dependent signaling is impacted by PARP-1’s ability to regulate the phosphorylation status of CREB and ATF-2 (Ha 2004), two transcription factors that influence the expression of TNF-α and IL-1β. With regard to the current study, it is interesting to consider two aspects of potential PARP-1 involvement in regulating proinflammatory mediator production in astrocytes. First, it is known that astrocytes require TLR2 to recognize intact S. aureus and signal proinflammatory mediator release (Esen et al. 2004). TLR2 engagement leads to the activation of NF-κB and p38 MAPK pathways (Akira and Takeda 2004; O’Neill and Bowie 2007), both of which are impacted by PARP-1 (Hassa et al. 2001; Ha et al. 2002; Hassa et al. 2003; Ha 2004; Nakajima et al. 2004). Second, TNF-α and IL-1β are produced by S. aureus-activated astrocytes and can act in an autocrine/paracrine manner to augment cell activation (Esen et al. 2004; Phulwani et al. 2006). Since both receptors utilize p38 MAPK signaling cascades this represents another level whereby PARP-1 can influence this positive feedback loop to regulate astrocyte activation.
Several novel findings have clarified the contribution of PARP family members in regulating astrocyte activation. First, PARP-1, -2 and -3 demonstrate cooperative roles in regulating the production of astrocytic proinflammatory mediators in response to bacterial stimuli. Second, astrocytes express PARP-2 and PARP-3 and this report, to our knowledge, is the first to document a role for the latter in regulating inflammation in any cell type. Overall, these studies suggest that targeting multiple PARP family members may effectively attenuate astrocyte activation and may be a useful therapeutic strategy in experimental paradigms where inappropriate immune responses contribute to the pathogenic destruction of normal brain parenchyma, typical of brain abscess and other neuroinflammatory diseases. This possibility is beyond the scope of the current report; however, it remains a plausible avenue of investigation in future studies.
Supplementary Material
Primary astrocytes were seeded in 6-well plates at 1 × 106 cells per well. After 24 h, cells were transfected with a scrambled (negative) or siRNA oligonucleotides directed against PARP-2 and/or PARP-3 (100 nM) in conjunction with a FITC-conjugated oligonucleotide (25 nM) to determine transfection efficiency. Subsequently, nuclear uptake of FITC-conjugated oligonucleotide was visualized by florescence microscopy (10X). Results are representative of eight independent experiments.
Acknowledgments
The authors would like to thank Gail Wagoner for excellent technical assistance. This work was supported by the NIH National Institute of Mental Health (RO1 NS40730) to T.K. and the NINDS supported Core Facility at UAMS (P30 NS047546).
References
- Akira S. TLR signaling. Curr Top Microbiol Immunol. 2006;311:1–16. doi: 10.1007/3-540-32636-7_1. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Alano CC, Ying W, Swanson RA. Poly(ADP-ribose) polymerase-1-mediated cell death in astrocytes requires NAD+ depletion and mitochondrial permeability transition. J Biol Chem. 2004;279:18895–18902. doi: 10.1074/jbc.M313329200. [DOI] [PubMed] [Google Scholar]
- Aldinucci A, Gerlini G, Fossati S, Cipriani G, Ballerini C, Biagioli T, Pimpinelli N, Borgognoni L, Massacesi L, Moroni F, Chiarugi A. A key role for poly(ADP-ribose) polymerase-1 activity during human dendritic cell maturation. J Immunol. 2007;179:305–312. doi: 10.4049/jimmunol.179.1.305. [DOI] [PubMed] [Google Scholar]
- Ame JC, Spenlehauer C, de Murcia G. The PARP superfamily. Bioessays. 2004;26:882–893. doi: 10.1002/bies.20085. [DOI] [PubMed] [Google Scholar]
- Baldwin AC, Kielian T. Persistent immune activation associated with a mouse model of Staphylococcus aureus-induced experimental brain abscess. J Neuroimmunol. 2004;151:24–32. doi: 10.1016/j.jneuroim.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Boulares AH, Zoltoski AJ, Sherif ZA, Jolly P, Massaro D, Smulson ME. Gene knockout or pharmacological inhibition of poly(ADP-ribose) polymerase-1 prevents lung inflammation in a murine model of asthma. Am J Respir Cell Mol Biol. 2003;28:322–329. doi: 10.1165/rcmb.2001-0015OC. [DOI] [PubMed] [Google Scholar]
- Bruzzone S, Verderio C, Schenk U, Fedele E, Zocchi E, Matteoli M, De Flora A. Glutamate-mediated overexpression of CD38 in astrocytes cultured with neurones. J Neurochem. 2004;89:264–272. doi: 10.1111/j.1471-4159.2003.02326.x. [DOI] [PubMed] [Google Scholar]
- Burkart V, Wang ZQ, Radons J, Heller B, Herceg Z, Stingl L, Wagner EF, Kolb H. Mice lacking the poly(ADP-ribose) polymerase gene are resistant to pancreatic beta-cell destruction and diabetes development induced by streptozocin. Nat Med. 1999;5:314–319. doi: 10.1038/6535. [DOI] [PubMed] [Google Scholar]
- Burkle A. Physiology and pathophysiology of poly(ADP-ribosyl)ation. Bioessays. 2001;23:795–806. doi: 10.1002/bies.1115. [DOI] [PubMed] [Google Scholar]
- Carrillo A, Monreal Y, Ramirez P, Marin L, Parrilla P, Oliver FJ, Yelamos J. Transcription regulation of TNF-alpha-early response genes by poly(ADP-ribose) polymerase-1 in murine heart endothelial cells. Nucleic Acids Res. 2004;32:757–766. doi: 10.1093/nar/gkh239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarugi A. Inhibitors of poly(ADP-ribose) polymerase-1 suppress transcriptional activation in lymphocytes and ameliorate autoimmune encephalomyelitis in rats. Br J Pharmacol. 2002;137:761–770. doi: 10.1038/sj.bjp.0704934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YH, Joo KM, Lee YJ, Shin DH, Cha CI. Reactive astrocytes express PARP in the central nervous system of SOD(G93A) transgenic mice. Brain Res. 2004;1003:199–204. doi: 10.1016/j.brainres.2004.01.010. [DOI] [PubMed] [Google Scholar]
- D’Amours D, Desnoyers S, D’Silva I, Poirier GG. Poly(ADP-ribosyl)ation reactions in the regulation of nuclear functions. Biochem J. 1999;342(Pt 2):249–268. [PMC free article] [PubMed] [Google Scholar]
- de la Lastra CA, Villegas I, Sanchez-Fidalgo S. Poly(ADP-ribose) polymerase inhibitors: new pharmacological functions and potential clinical implications. Curr Pharm Des. 2007;13:933–962. doi: 10.2174/138161207780414241. [DOI] [PubMed] [Google Scholar]
- Eliasson MJ, Sampei K, Mandir AS, Hurn PD, Traystman RJ, Bao J, Pieper A, Wang ZQ, Dawson TM, Snyder SH, Dawson VL. Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat Med. 1997;3:1089–1095. doi: 10.1038/nm1097-1089. [DOI] [PubMed] [Google Scholar]
- Esen N, Kielian T. Central role for MyD88 in the responses of microglia to pathogen-associated molecular patterns. J Immunol. 2006;176:6802–6811. doi: 10.4049/jimmunol.176.11.6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esen N, Tanga FY, DeLeo JA, Kielian T. Toll-like receptor 2 (TLR2) mediates astrocyte activation in response to the Gram-positive bacterium Staphylococcus aureus. J Neurochem. 2004;88:746–758. doi: 10.1046/j.1471-4159.2003.02202.x. [DOI] [PubMed] [Google Scholar]
- Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007 doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Fonfria E, Marshall IC, Benham CD, Boyfield I, Brown JD, Hill K, Hughes JP, Skaper SD, McNulty S. TRPM2 channel opening in response to oxidative stress is dependent on activation of poly(ADP-ribose) polymerase. Br J Pharmacol. 2004;143:186–192. doi: 10.1038/sj.bjp.0705914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha HC. Defective transcription factor activation for proinflammatory gene expression in poly(ADP-ribose) polymerase 1-deficient glia. Proc Natl Acad Sci U S A. 2004;101:5087–5092. doi: 10.1073/pnas.0306895101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha HC, Hester LD, Snyder SH. Poly(ADP-ribose) polymerase-1 dependence of stress-induced transcription factors and associated gene expression in glia. Proc Natl Acad Sci U S A. 2002;99:3270–3275. doi: 10.1073/pnas.052712399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamby ME, Uliasz TF, Hewett SJ, Hewett JA. Characterization of an improved procedure for the removal of microglia from confluent monolayers of primary astrocytes. J Neurosci Methods. 2006;150:128–137. doi: 10.1016/j.jneumeth.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Hasko G, Mabley JG, Nemeth ZH, Pacher P, Deitch EA, Szabo C. Poly(ADP-ribose) polymerase is a regulator of chemokine production: relevance for the pathogenesis of shock and inflammation. Mol Med. 2002;8:283–289. [PMC free article] [PubMed] [Google Scholar]
- Hassa PO, Hottiger MO. The diverse biological roles of mammalian PARPS, a small but powerful family of poly-ADP-ribose polymerases. Front Biosci. 2008;13:3046–3082. doi: 10.2741/2909. [DOI] [PubMed] [Google Scholar]
- Hassa PO, Haenni SS, Elser M, Hottiger MO. Nuclear ADP-ribosylation reactions in mammalian cells: where are we today and where are we going? Microbiol Mol Biol Rev. 2006;70:789–829. doi: 10.1128/MMBR.00040-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassa PO, Covic M, Hasan S, Imhof R, Hottiger MO. The enzymatic and DNA binding activity of PARP-1 are not required for NF-kappa B coactivator function. J Biol Chem. 2001;276:45588–45597. doi: 10.1074/jbc.M106528200. [DOI] [PubMed] [Google Scholar]
- Hassa PO, Buerki C, Lombardi C, Imhof R, Hottiger MO. Transcriptional coactivation of nuclear factor-kappaB-dependent gene expression by p300 is regulated by poly(ADP)-ribose polymerase-1. J Biol Chem. 2003;278:45145–45153. doi: 10.1074/jbc.M307957200. [DOI] [PubMed] [Google Scholar]
- Hassa PO, Haenni SS, Buerki C, Meier NI, Lane WS, Owen H, Gersbach M, Imhof R, Hottiger MO. Acetylation of poly(ADP-ribose) polymerase-1 by p300/CREB-binding protein regulates coactivation of NF-kappaB-dependent transcription. J Biol Chem. 2005;280:40450–40464. doi: 10.1074/jbc.M507553200. [DOI] [PubMed] [Google Scholar]
- Huber A, Bai P, de Murcia JM, de Murcia G. PARP-1, PARP-2 and ATM in the DNA damage response: functional synergy in mouse development. DNA Repair (Amst) 2004;3:1103–1108. doi: 10.1016/j.dnarep.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Iwashita A, Tojo N, Matsuura S, Yamazaki S, Kamijo K, Ishida J, Yamamoto H, Hattori K, Matsuoka N, Mutoh S. A novel and potent poly(ADP-ribose) polymerase-1 inhibitor, FR247304 (5-chloro-2-[3-(4-phenyl-3,6-dihydro-1(2H)-pyridinyl)propyl]-4(3H)-quinazo linone), attenuates neuronal damage in in vitro and in vivo models of cerebral ischemia. J Pharmacol Exp Ther. 2004;310:425–436. doi: 10.1124/jpet.104.066944. [DOI] [PubMed] [Google Scholar]
- Jagtap P, Szabo C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov. 2005;4:421–440. doi: 10.1038/nrd1718. [DOI] [PubMed] [Google Scholar]
- Jijon HB, Churchill T, Malfair D, Wessler A, Jewell LD, Parsons HG, Madsen KL. Inhibition of poly(ADP-ribose) polymerase attenuates inflammation in a model of chronic colitis. Am J Physiol Gastrointest Liver Physiol. 2000;279:G641–651. doi: 10.1152/ajpgi.2000.279.3.G641. [DOI] [PubMed] [Google Scholar]
- Johansson M. A human poly(ADP-ribose) polymerase gene family (ADPRTL): cDNA cloning of two novel poly(ADP-ribose) polymerase homologues. Genomics. 1999;57:442–445. doi: 10.1006/geno.1999.5799. [DOI] [PubMed] [Google Scholar]
- Jones ME, Draghi DC, Karlowsky JA, Sahm DF, Bradley JS. Prevalence of antimicrobial resistance in bacteria isolated from central nervous system specimens as reported by U.S. hospital laboratories from 2000 to 2002. Ann Clin Microbiol Antimicrob. 2004;3:3. doi: 10.1186/1476-0711-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauppinen TM, Chan WY, Suh SW, Wiggins AK, Huang EJ, Swanson RA. Direct phosphorylation and regulation of poly(ADP-ribose) polymerase-1 by extracellular signal-regulated kinases 1/2. Proc Natl Acad Sci U S A. 2006;103:7136–7141. doi: 10.1073/pnas.0508606103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian T. Immunopathogenesis of brain abscess. J Neuroinflammation. 2004;1:16. doi: 10.1186/1742-2094-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian T, Mayes P, Kielian M. Characterization of microglial responses to Staphylococcus aureus: effects on cytokine, costimulatory molecule, and Toll-like receptor expression. J Neuroimmunol. 2002;130:86–99. doi: 10.1016/s0165-5728(02)00216-3. [DOI] [PubMed] [Google Scholar]
- Kielian T, Esen N, Bearden ED. Toll-like receptor 2 (TLR2) is pivotal for recognition of S. aureus peptidoglycan but not intact bacteria by microglia. Glia. 2005;49:567–576. doi: 10.1002/glia.20144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian T, Esen N, Liu S, Phulwani NK, Syed MM, Phillips N, Nishina K, Cheung AL, Schwartzman JD, Ruhe JJ. Minocycline Modulates Neuroinflammation Independently of Its Antimicrobial Activity in Staphylococcus aureus-Induced Brain Abscess. Am J Pathol. 2007;171:1199–1214. doi: 10.2353/ajpath.2007.070231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian T, Syed MMd, Liu S, Phillips N, Wagoner G, Drew PD, Esen N. The synthetic PPAR-γ agonist ciglitazone attenuates neuroinflammation and accelerates encapsulation in bacterial brain abscesses. Journal of Immunology. 2008;180:5004–5016. doi: 10.4049/jimmunol.180.7.5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler J, Otsuka T, Zhang Z, Noppens R, Grafe MR, Koh DW, Dawson VL, de Murcia JM, Hurn PD, Traystman RJ. Differential effect of PARP-2 deletion on brain injury after focal and global cerebral ischemia. J Cereb Blood Flow Metab. 2006;26:135–141. doi: 10.1038/sj.jcbfm.9600173. [DOI] [PubMed] [Google Scholar]
- Lu CH, Chang WN, Lui CC. Strategies for the management of bacterial brain abscess. J Clin Neurosci. 2006;13:979–985. doi: 10.1016/j.jocn.2006.01.048. [DOI] [PubMed] [Google Scholar]
- Lu CH, Chang WN, Lin YC, Tsai NW, Liliang PC, Su TM, Rau CS, Tsai YD, Liang CL, Chang CJ, Lee PY, Chang HW, Wu JJ. Bacterial brain abscess: microbiological features, epidemiological trends and therapeutic outcomes. Qjm. 2002;95:501–509. doi: 10.1093/qjmed/95.8.501. [DOI] [PubMed] [Google Scholar]
- Lund FE. Signaling properties of CD38 in the mouse immune system: enzyme-dependent and -independent roles in immunity. Mol Med. 2006;12:328–333. doi: 10.2119/2006-00099.Lund. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathisen GE, Johnson JP. Brain abscess. Clin Infect Dis. 1997;25:763–779. doi: 10.1086/515541. quiz 780–761. [DOI] [PubMed] [Google Scholar]
- Menissier de Murcia J, Ricoul M, Tartier L, Niedergang C, Huber A, Dantzer F, Schreiber V, Ame JC, Dierich A, LeMeur M, Sabatier L, Chambon P, de Murcia G. Functional interaction between PARP-1 and PARP-2 in chromosome stability and embryonic development in mouse. Embo J. 2003;22:2255–2263. doi: 10.1093/emboj/cdg206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima H, Nagaso H, Kakui N, Ishikawa M, Hiranuma T, Hoshiko S. Critical role of the automodification of poly(ADP-ribose) polymerase-1 in nuclear factor-kappaB-dependent gene expression in primary cultured mouse glial cells. J Biol Chem. 2004;279:42774–42786. doi: 10.1074/jbc.M407923200. [DOI] [PubMed] [Google Scholar]
- Nguewa PA, Fuertes MA, Valladares B, Alonso C, Perez JM. Poly(ADP-ribose) polymerases: homology, structural domains and functions. Novel therapeutical applications. Prog Biophys Mol Biol. 2005;88:143–172. doi: 10.1016/j.pbiomolbio.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Oliver FJ, Menissier-de Murcia J, de Murcia G. Poly(ADP-ribose) polymerase in the cellular response to DNA damage, apoptosis, and disease. Am J Hum Genet. 1999;64:1282–1288. doi: 10.1086/302389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszanecki R, Gebska A, Jawien J, Jakubowski A, Korbut R. Inhibition of NOS-2 induction in LPS-stimulated J774.2 cells by 1, 5-isoquinolinediol, an inhibitor of PARP. J Physiol Pharmacol. 2006;57:109–117. [PubMed] [Google Scholar]
- O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- Partida-Sanchez S, Rivero-Nava L, Shi G, Lund FE. CD38: an ecto-enzyme at the crossroads of innate and adaptive immune responses. Adv Exp Med Biol. 2007;590:171–183. doi: 10.1007/978-0-387-34814-8_12. [DOI] [PubMed] [Google Scholar]
- Phulwani NK, Feinstein DL, Gavrilyuk V, Akar C, Kielian T. 15-deoxy-Delta12, 14-prostaglandin J2 (15d-PGJ2) and ciglitazone modulate Staphylococcus aureus-dependent astrocyte activation primarily through a PPAR-gamma-independent pathway. J Neurochem. 2006;99:1389–1402. doi: 10.1111/j.1471-4159.2006.04183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoff I, Jijon H, Monia B, Tavernini M, Ma M, McKay R, Madsen K. Antisense oligonucleotides to poly(ADP-ribose) polymerase-2 ameliorate colitis in interleukin-10-deficient mice. J Pharmacol Exp Ther. 2002;303:1145–1154. doi: 10.1124/jpet.102.039768. [DOI] [PubMed] [Google Scholar]
- Roche M, Humphreys H, Smyth E, Phillips J, Cunney R, McNamara E, O’Brien D, McArdle O. A twelve-year review of central nervous system bacterial abscesses; presentation and aetiology. Clin Microbiol Infect. 2003;9:803–809. doi: 10.1046/j.1469-0691.2003.00651.x. [DOI] [PubMed] [Google Scholar]
- Rouleau M, McDonald D, Gagne P, Ouellet ME, Droit A, Hunter JM, Dutertre S, Prigent C, Hendzel MJ, Poirier GG. PARP-3 associates with polycomb group bodies and with components of the DNA damage repair machinery. J Cell Biochem. 2007;100:385–401. doi: 10.1002/jcb.21051. [DOI] [PubMed] [Google Scholar]
- Sallmann FR, Vodenicharov MD, Wang ZQ, Poirier GG. Characterization of sPARP-1. An alternative product of PARP-1 gene with poly(ADP-ribose) polymerase activity independent of DNA strand breaks. J Biol Chem. 2000;275:15504–15511. doi: 10.1074/jbc.275.20.15504. [DOI] [PubMed] [Google Scholar]
- Saura J. Microglial cells in astroglial cultures: a cautionary note. J Neuroinflammation. 2007;4:26. doi: 10.1186/1742-2094-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifri CD, Park J, Helm GA, Stemper ME, Shukla SK. Fatal brain abscess due to community-associated methicillin-resistant Staphylococcus aureus strain USA300. Clin Infect Dis. 2007;45:e113–117. doi: 10.1086/522171. [DOI] [PubMed] [Google Scholar]
- Stenzel W, Soltek S, Schluter D, Deckert M. The intermediate filament GFAP is important for the control of experimental murine Staphylococcus aureus-induced brain abscess and Toxoplasma encephalitis. J Neuropathol Exp Neurol. 2004;63:631–640. doi: 10.1093/jnen/63.6.631. [DOI] [PubMed] [Google Scholar]
- Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci U S A. 2005;102:5856–5861. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend GC, Scheld WM. Infections of the central nervous system. Adv Intern Med. 1998;43:403–447. [PubMed] [Google Scholar]
- Verderio C, Bruzzone S, Zocchi E, Fedele E, Schenk U, De Flora A, Matteoli M. Evidence of a role for cyclic ADP-ribose in calcium signalling and neurotransmitter release in cultured astrocytes. J Neurochem. 2001;78:646–657. doi: 10.1046/j.1471-4159.2001.00455.x. [DOI] [PubMed] [Google Scholar]
- Wang ZQ, Auer B, Stingl L, Berghammer H, Haidacher D, Schweiger M, Wagner EF. Mice lacking ADPRT and poly(ADP-ribosyl)ation develop normally but are susceptible to skin disease. Genes Dev. 1995;9:509–520. doi: 10.1101/gad.9.5.509. [DOI] [PubMed] [Google Scholar]
- Ying W, Alano CC, Garnier P, Swanson RA. NAD+ as a metabolic link between DNA damage and cell death. J Neurosci Res. 2005;79:216–223. doi: 10.1002/jnr.20289. [DOI] [PubMed] [Google Scholar]
- Young GS, Choleris E, Lund FE, Kirkland JB. Decreased cADPR and increased NAD+ in the Cd38−/− mouse. Biochem Biophys Res Commun. 2006;346:188–192. doi: 10.1016/j.bbrc.2006.05.100. [DOI] [PubMed] [Google Scholar]
- Zhu K, Swanson RA, Ying W. NADH can enter into astrocytes and block poly(ADP-ribose) polymerase-1-mediated astrocyte death. Neuroreport. 2005;16:1209–1212. doi: 10.1097/00001756-200508010-00015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primary astrocytes were seeded in 6-well plates at 1 × 106 cells per well. After 24 h, cells were transfected with a scrambled (negative) or siRNA oligonucleotides directed against PARP-2 and/or PARP-3 (100 nM) in conjunction with a FITC-conjugated oligonucleotide (25 nM) to determine transfection efficiency. Subsequently, nuclear uptake of FITC-conjugated oligonucleotide was visualized by florescence microscopy (10X). Results are representative of eight independent experiments.