Abstract
Borrelia burgdorferi invasion of mammalian joints results in genesis of Lyme arthritis. Other than spirochete lipids, existence of protein antigens, which are abundant in joints and participate in B. burgdorferi-induced host inflammatory response, is unknown. Here, we report that major products of the B. burgdorferi basic membrane protein (bmp) A/B operon that are induced in murine and human joints, possess inflammatory properties. Compared to the wild type B. burgdorferi, an isogenic bmpA/B mutant induced significantly lower levels of pro-inflammatory cytokines TNF-α and IL-1β in cultured human synovial cells, which could be restored using bmpA/B-complemented mutants, and more directly, upon addition of recombinant BmpA, but not BmpB or control spirochete proteins. Non-lipidated and lipidated versions of BmpA induced similar levels of cytokines, and remained unaffected by treatment with lipopolysaccharide inhibitor, polymyxin B. The bmpA/B mutant was also impaired in the induction of NF-κB and p38 MAP kinase signaling pathways in synovial cells, which were activated by non-lipidated BmpA. These results show that a protein moiety of BmpA can induce cytokine responses in synovial cells via activation of the NF-κB and p38 MAP kinase pathways and thus, could potentially contribute to the genesis of Lyme arthritis.
Keywords: Borrelia burgdorferi, Lyme disease, pro-inflammatory cytokines, BmpA
1. Introduction
Lyme borreliosis is a prevalent tick-borne human disease caused by Borrelia burgdorferi or related spirochetes [1]. Ixodes ticks, while feeding on a mammal, deposit spirochete on the dermis. The bacteria soon spread to a variety of host organs, most often in joints where a robust host inflammatory responses result in complications of Lyme arthritis. Without antibiotic therapy, approximately 60% of the infected patients develop Lyme arthritis [1]. The physical presence of spirochetes in the joint tissue is indispensible for the development of Lyme arthritis [2], however, the identity of the precise molecular components of spirochetes that trigger inflammation in the host remains largely unclear [1].
B. burgdorferi-induced joint inflammation is primarily orchestrated by inflammatory cytokines and chemokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1α, IL-1β, IL-6, IL-8, IL-12p70, and IL-18, IL-23/IL-17 and interferon (IFN)-γ [1, 3, 4]. As spirochetes lack lipopolysaccharide (LPS), lipoproteins are thought to be the primary mediator of B. burgdorferi-induced inflammatory responses [5], which are mediated by specific toll-like receptors (TLRs), such as TLR1 and TLR2, in conjunction with CD14 [6–8]. However, the fact that TLR2-deficient mice, more importantly MyD88-knockout mice, the shared adaptor molecule for most TLRs-induced signaling, also develop joint inflammation, suggesting that TLR-independent receptors are also important in the pathogenesis of Lyme arthritis [9]. Multiple subtypes of integrins and Fcγ receptors are examples of non-TLR receptors that recognize specific spirochete ligands, such as p66 [10], BBB07 [11] or unidentified B. burgdorferi antigens [12], and are likely to be associated with host inflammatory responses. In addition to cell surface events that initiate B. burgdorferi-cell activation, details of intracellular signaling pathways that control the expression of immunomodulators have also been studied. B. burgdorferi activates distinct signaling pathways, for example, NF-κB, p38 MAPK, JNK and JAK/STAT that contribute to the expression of a variety of target cytokines, chemokines, matrix metalloproteinases (MMP) [13–16], thereby contributing to the genesis of Lyme arthritis [1]. These studies established the paradigm that Lyme arthritis results from a multifactorial inflammatory response that is triggered by diverse host-pathogen interactions and the activation of signaling cascades that induces immnomodulatory molecules contributing to the genesis of inflammatory disease.
The B. burgdorferi basic membrane protein (bmp) A/B operon encodes two paralogous lipoproteins, BmpA and BmpB [17]. BmpA, however, is the major product of the operon, which is expressed at levels 4-fold higher than BmpB [18]. BmpA/B proteins are exposed on the microbial surface [19], highly antigenic [20], and induced in infected murine and human joints [19]. A recent study indicated that bmpA/B mutants, although infectious in mice, were unable to persist in joints, and thus, induced less severe arthritis [19]. We therefore sought to determine whether the products of the bmpA/B operon could directly induce inflammatory cytokine responses in joint derived cells. Elucidation of the inflammation cascades mediated by specific B. burgdorferi antigens that are induced locally in joints might shed light on the mechanisms of the complex arthritic disorder caused by the pathogen.
2. Materials and methods
2.1. Borrelia burgdorferi isolates and the human synovial cell line
An infectious isolate of B. burgdorferi B31 and isogenic mutants were used throughout the study [19]. The human synovial cell line SW 982 (ATCC, Manassas, VA) was grown in L-15 medium (Invitrogen, Carlsbad, CA) supplemented with 2% glutamine and 10% fetal calf serum (Invitrogen) at 37°C in a humidified incubator without CO2. The cells were grown to 80% confluence in 12-well (2×105 cells/well) plates before use.
2.2. Production of lipidated and non-lipidated recombinant Borrelia burgdorferi proteins and antibodies
Recombinant non-lipidated BmpA and BmpB proteins [19], BB0365 [21] and lipidated OspA [22] were produced in Escherichia coli as detailed. Lipidated BmpA was produced in E. coli using the bacterial expression vector, pGEX-6P1 (GE Healthcare, Piscataway, NJ) and the following primers with EcoRI and XhoI restriction sites (5′-3′): ACG AAT TCA TGA ATA AAA TAT TGT TGT TGA, AGC TCG AGT TAA ATA AAT TCT TTA AGA AA). Expression, purification and enzymatic cleavage of the glutathione transferase (GST) fusion protein were carried out as described previously [19]. Generation of rabbit antisera against non-lipidated BmpA has been described previously [19].
2.3. Stimulation of synovial cells with B. burgdorferi and recombinant proteins
Synovial cells were exposed to B. burgdorferi with a multiplicity of infection (MOI) of 1:50 [23], different concentrations of recombinant proteins (30 nanogram - 1μg/ml), lipopolysaccharide (LPS, 5μg/ml, Sigma Chemical Co. St. Louis, MO) and LPS inhibitor, polymyxin B (10μg/ml, Sigma) as described [5].
2.4. Quantitative RT-PCR
Quantitative RT-PCR (qRT-PCR) analyses were performed to measure quantities of cytokine transcripts as described [19, 21]. Briefly, at indicated time points following stimulation, synovial cells were harvested and total RNA was purified with the TRIzol reagent (Invitrogen). Total RNA was digested with RNase-free DNaseI (NEB, Ipswich, MA) followed by purification on RNeasy mini spin columns (Invitrogen) and transcribed to first-strand cDNA using a commercial kit (Stratagene, La Jolla, CA). Following forward and reverse primers were used for qRT-PCR analysis: TNF-α, 5′-TCT CCT TCC TGA TCG TGG C-3′ and 5′-TGA AGA GGA CCT GGG AGT AGA-3′; IL-1β, 5′-CAA AGG ATA TGG AAA CAA AAG-3′ and 5′-GGA ACG TGC TGT CAG AGG TC-3′; β-actin, 5′-GAC GAC ATG GAG AAA ATC TG-3′ and 5′-AGG TCT CAA ACA TGA TCT GG-3′. Quantities of cytokine mRNA in each sample were normalized based on the respective quantities of β-actin as described [19, 21].
2.5. ELISA
Following stimulation, culture supernatants were centrifuged at 2500g for 5 min to obtain cell-free samples. TNF-α and IL-1β protein levels were measured using commercially available cytokine capture-ELISA kits (BD Bioscience, Franklin Lakes, NJ), according to the manufacturer’s instructions.
2.6. Western blot analysis
Western blot analysis was performed as described [14] using antibodies that recognize total and phosphorylated forms of IκB-α (Cell Signaling Inc. Danvers, MA) and p38 MAP kinase (Cell Signaling Inc) proteins. The immunoblot signals were revealed by horseradish peroxidase-conjugated secondary antibodies (Sigma Chemical Co) and chemiluminence substrate (GE Healthcare). Western blots were also imaged in a Chemidoc XRS (Bio-rad laboratories, Hercules, CA) for densitometric analyses of relative protein levels using Quantity One software (Bio-rad).
2.7. Electrophoretic mobility shift assays (EMSA)
Nuclear extracts of synovial cells were separated using a commercially available kit (PIERCE Biotech, Rockford, IL) following incubation of 0, 5, 10, 15, 30 and 60 minutes in the absence or presence of BmpA (0.1μg/ml) or LPS (5μg/ml). Binding reactions were performed using 2 μg of nuclear protein in the presence of specific 32P-end-labeled double stranded oligonucleotide as described [14] with or without unlabeled oligonucleotide probe corresponded to the consensus NF-kB binding site (AGT TGA GGG GAC TTT CCC AGG C). A oligonucleotide probe corresponding to AP-1 binding site was used as a control [24]. Super-shift and competition assays were performed using antibodies against p50 and RelA (Santa Cruz Biotechnology, Santa Cruz, CA).
2.8. Reporter gene assays
The activation of the NF-κB or p38 MAP kinase pathways in transfected synovial cells was assessed as described before [25] using signal transduction pathway activation assay (PathDetect reporting systems, Stratagene). Briefly, cells were co-transfected using effectene transfection reagent (Qiagen, Valencia, CA) with either 400ng of pNF-κB-Luc or 400ng and 40 ng of pFR-Luc and pFA-CHOP plasmids, respectively. Cells were also transfected with 50ng of phRL-TK plasmid as an internal control. Twenty-four hours following transfection, cells were exposed to recombinant proteins in the presence or absence of polymyxin B as described earlier. BmpA antibodies (1: 100 dilution) were added in some wells. In addition, selected wells also received either TLR2 agonist Pam3CSK4 (5 nanogram/ml, InvivoGen, San Diego, CA) or LPS (5μg/ml) and TLR2 or TLR4 neutralization antibodies (10μg/ml, InvivoGen). After 18 hours of incubation, the cells were lysed in lysis buffer (Promega, Madison, WI) and Firefly and Renilla luciferase activities were determined by the Dual Luciferase reporter system (Promega).
2.9. Statistical analysis
Results are expressed as the mean ± standard error (SEM) derived from at least three independent experiments done in duplicate. The significance of the differences between the mean values of the groups was evaluated by Student’s t test. P < 0.05 was considered significant.
3. Results
3.1. BmpA/B-deficient B. burgdorferi fails to induce pro-inflammatory cytokines in cultured human synovial cells
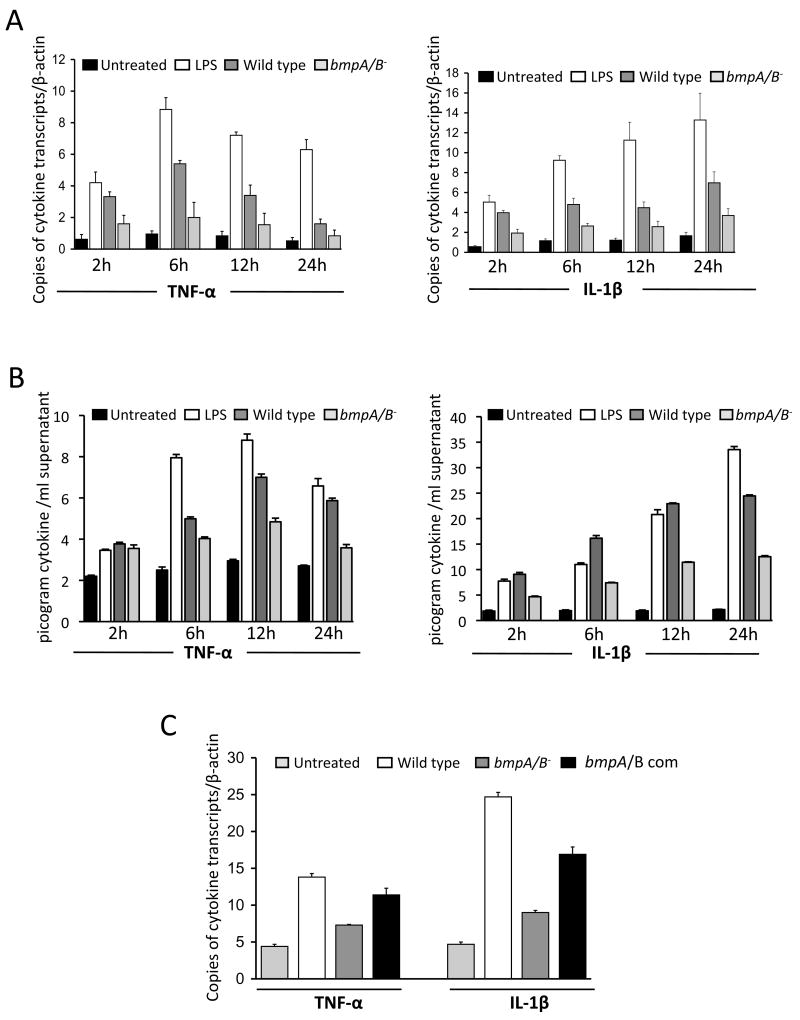
It has been reported that B. burgdorferi bmpA/B genes are preferentially induced in infected murine and human joint tissues, and that infection with bmpA/B mutants leads to less severe Lyme arthritis [19]. To assess if bmpA/B mutant B. burgdorferi are defective in activating cellular inflammatory responses, human synovial cells (ATCC cell line SW982) were exposed to B. burgdorferi at a multiplicity of infection (MOI) of 1:50 and harvested at 2, 6, 12 and 24 hours following spirochete treatment. As controls, parallel wells were left untreated or exposed to lipopolysaccharide (LPS). Transcript and secreted protein levels of two pro-inflammatory cytokines, TNF-α and IL-1β, which are implicated in the pathogenesis of Lyme arthritis [1, 3, 4] were analyzed by quantitative RT-PCR (qRT-PCR) and ELISA, respectively. Compared to wild type spirochetes, bmpA/B-deficient B. burgdorferi induced significantly lower levels of both cytokine transcripts at all indicated time points (Fig. 1A, P < 0.001). Consistent with the mRNA level, the amounts of TNF-α and IL-1β proteins were also significantly reduced in the supernatants of cultured synovial cells exposed to bmpA/B mutants (Fig 1B, P < 0.0002). These results suggest that deletion of bmpA/B genes impairs the ability of the spirochete to induce TNF-α and IL-1β production in synovial cells. A bmpA/B-complemented isolate that restored the production of both BmpA and BmpB proteins [19] was able to induce significantly higher levels of TNF-α and IL-1β transcripts than bmpA/B mutants (P< 0.05), indicating that the phenotypic defects of bmpA/B mutants for the induction of TNF-α and IL-1β can be attributed to the loss of the bmpA/B gene products (Fig. 1C). These experiments indicate that B. burgdorferi bmpA/B gene products can directly induce selected pro-inflammatory cytokines in human synovial cells.
Fig. 1.
Deficiency of B. burgdorferi bmpA/B operon impairs production of TNF-α and IL-1β. (A) A B. burgdorferi isolate deficient in BmpA/B proteins (BmpA/B−) is significantly impaired in its ability to induce TNF-α (left panel) and IL-1β (right panel) expression in cultured human synovial cell line SW 982. Cells were exposed to wild type B. burgdorferi (dark gray bar) and isogenic bmpA/B mutants (bmpA/B−, light gray bar) for 2, 6, 12 and 24 hours. Total RNA was isolated and TNF-α, IL-1β and β-actin transcript levels were measured by quantitative RT-PCR analysis, and the levels of cytokines were further normalized to β-actin levels. Expression of cytokines in untreated cells (black bar) and cells exposed to lipopolysaccharide (LPS, white bar) at indicated time points were used as negative and positive controls, respectively. Bars represent the mean ± standard error (SEM) from three independent experiments. bmpA/B mutant-exposed transcript levels were significantly lower than those treated with wild type spirochetes at all time points (P < 0.001). (B) B. burgdorferi bmpA/B mutant is impaired to induce secretion of TNF-α and IL-1β proteins. Synovial cells (SW 982) were exposed to wild type B. burgdorferi (dark gray bar) and isogenic bmpA/B mutants (bmpA/B−, light gray bar) for 2, 6, 12 and 24 hours. The cells without treatment, or exposed to lipopolysaccharide (LPS) were used as controls. Supernatants were evaluated for TNF-α (left panel) and IL-1β (right panel) by enzyme-linked immunosorbent assay. Bars represent the mean ± standard error (SEM) from three independent experiments. bmpA/B mutant-induced cytokine levels were significantly lower than those of wild type spirochetes at all time points (P < 0.0002), except for TNF-α levels at 2 hour time point (P > 0.05). (C) bmpA/B-complemented B. burgdorferi is able to induce higher levels of cytokines than bmpA/B mutants. Synovial cells were exposed to wild type or genetically manipulated B. burgdorferi isolates for 12 hours and transcript levels of TNF-α and IL-1β were detected by quantitative RT-PCR analysis and normalized to the level of β-actin. The cells without treatment were used as a negative control. Bars represent the mean ± standard error from three independent experiments. Difference between bmpA/B-complemented and bmpA/B− B. burgdorferi is significant (P< 0.05).
3.2. BmpA activates pro-inflammatory cytokine production via non-lipidated portion of the lipoprotein
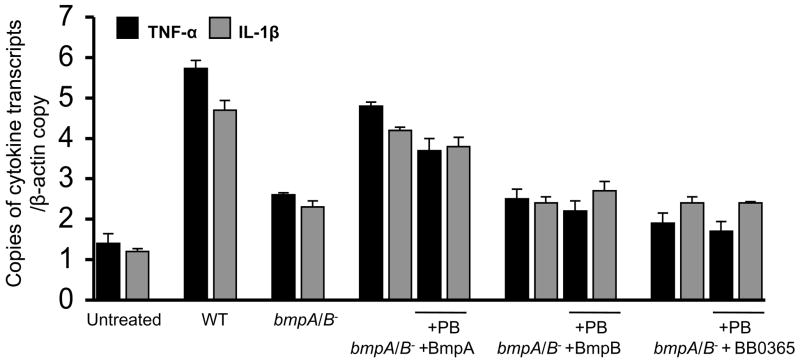
As B. burgdorferi possesses numerous membrane lipoproteins with common lipid modifications [17], we next studied whether the defect of bmpA/B mutants to induce cytokine response was due to the loss of the non-lipidated portion of lipoproteins. Synovial cells were treated with bmpA/B mutants (MOI of 1:50) in the presence or absence of either non-lipidated BmpA or BmpB proteins (300 nanogram/ml). As control, equal amounts of non-lipidated BB0365 were added in parallel wells to assess the effect of an unrelated B. burgdorferi protein. BmpA, BmpB and BB0365 were produced using the same expression vector and E. coli isolate. Additional wells were also treated with polymyxin B (10μg/ml) to exclude the effects of contaminating endotoxins. Results showed that non-lipidated BmpA was the most potent inducer of cytokine expression, and significantly reconstituted the capability of bmpA/B mutant B. burgdorferi to induce the expression of TNF-α and IL-1β (P< 0.01), whereas BmpB or BB0365 proteins were unable to rescue the mutant ability to induce the cytokine response (Fig. 2). Polymyxin B did not influence BmpA-induced cytokine response, indicating that contaminated bacterial endotoxins are not responsible for the effect (Fig. 2). As BmpA alone was sufficient to restore the ability of the bmpA/B mutant to induce an inflammatory response, subsequent studies were focused on BmpA.
Fig. 2.
Recombinant BmpA selectively reconstitutes the ability of bmpA/B mutant B. burgdorferi to induce cytokine expression. Synovial cells were exposed to B. burgdorferi bmpA/B mutants (bmpA/B−) for 12 hours in the absence or presence of recombinant B. burgdorferi proteins (300 nanogram/ml), BmpA, BmpB and BB0365 (control). Parallel wells were treated with 10 ug/ml of polymyxin B (PB) before addition of B. burgdorferi. Expression of TNF-α (black bar) and IL-1β (gray bar) were measured by quantitative RT-PCR analysis and normalized to the level of β-actin. Levels of cytokine transcripts in the untreated cells and in cells exposed to either wild type spirochetes or bmpA/B mutants were shown. Bars represent the mean ± standard error from three independent experiments. Unlike corresponding concentration of BmpB and BB0365, only BmpA (300 nanogram/ml) was able to reconstitute the ability of bmpA/B− to induce the cytokine response (P< 0.05).
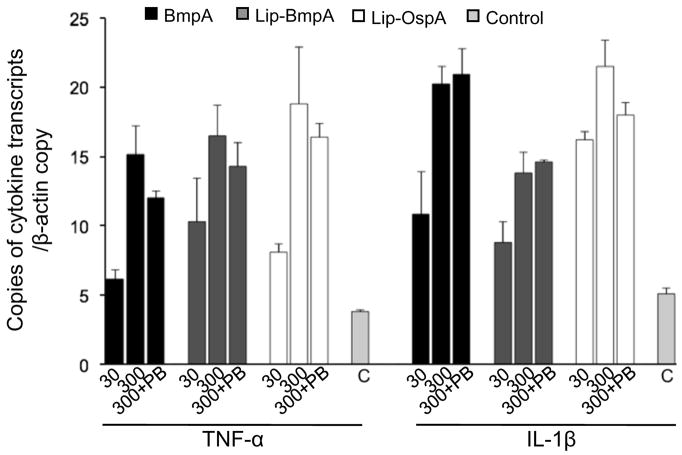
We then investigated whether the non-lipidated BmpA protein, in the absence of B. burgdorferi, could directly induce cytokine production in the synovial cells. Cells (2×105 cells/ml) were treated with non-lipidated BmpA or the control protein, BB0365, at two concentrations (30 and 300 nanogram/ml), and transcript levels of TNF-α and IL-1β were measured by qRT-PCR at 12 hours after treatment. To assess if the lipid modification of BmpA could influence the cytokine response, parallel wells were also treated with equal concentrations of lipidated BmpA. Lipidated OspA, a known inducer of TNF-α and IL-1β [26], was used as a control. As an additional control, parallel wells were also treated with polymyxin B. Results showed that, compared to untreated control, non-lipidated BmpA was able to induce both TNF-α and IL-1β expression, which was not statistically different from that of lipidated BmpA (Fig. 3), indicating that the lipid modification of the BmpA protein is not necessary to induce pro-inflammatory cytokine production in synovial cells. As expected, lipidated OspA induced TNF-α and IL-1β transcripts. In contrast, BB0365 failed to induce a significant cytokine response, and polymyxin B did not influence the cellular cytokine expression, suggesting that the ability of BmpA to induce cytokine expression was likely specific and independent of endotoxin contamination. Together, these data indicate that the non-lipidated portion of the B. burgdorferi BmpA lipoprotein is capable of inducing the production of pro-inflammatory cytokines by synovial cells.
Fig. 3.
Non-lipidated and lipidated BmpA directly induce comparable levels of TNF-α and IL-1β transcripts. Synovial cells were treated with either 30 or 300 nanogram/ml of recombinant non-lipidated (black bar) or lipidated (gray bar) BmpA proteins in the absence or presence of 10ug/ml polymyxin B (PB) for 12 hours. Amounts of TNF-α and IL-1β mRNA were measured by quantitative RT-PCR analysis and normalized to the level of β-actin. Levels of cytokine transcripts in the untreated cells (control) and in cells exposed to lipidated B. burgdorferi OspA (Lip-OspA, white bar) were shown. Bars represent the mean ± standard error from three independent experiments. Difference in the levels of cytokine transcripts between non-lipidated and lipidated BmpA exposed cells are non-significant (P>0.05), but higher than untreated cells (P< 0.001).
3.3. bmpA/B mutant B. burgdorferi fails to activate NF-κB and p38 MAPK signaling events
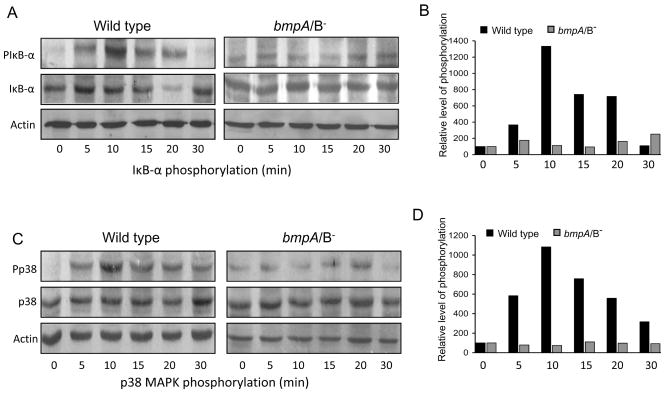
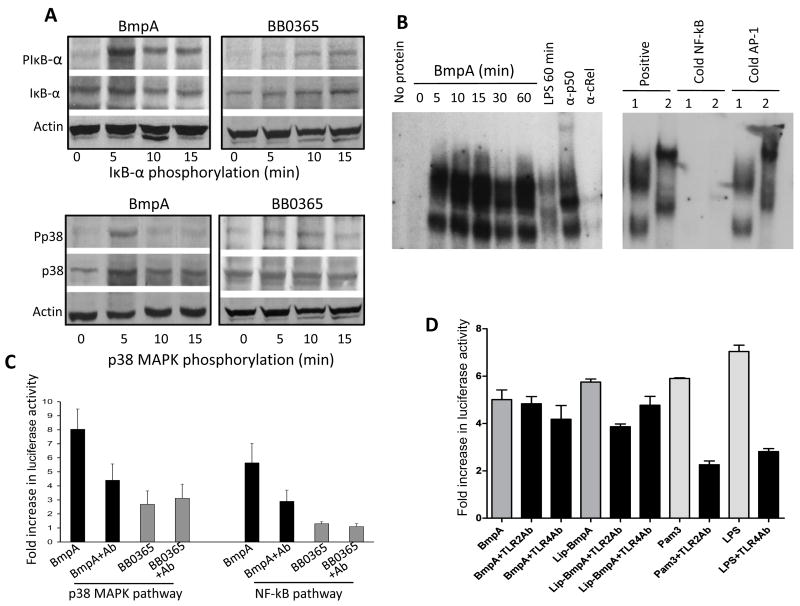
We then explored the signaling events in synovial cells that might influence the ability of bmpA/B mutant B. burgdorferi to induce pro-inflammatory cytokine expression. We chose to assess two key signaling pathways associated with genesis of Lyme arthritis, NF-κB [15] and p38 MAP kinase [14] pathways, which are also known to regulate expression of inflammatory cytokines [1]. To compare the activation of these two pathways, synovial cells were exposed to equal numbers of wild type or bmpA/B mutant B. burgdorferi at a MOI of 1:50 and the phosphorylation status of cellular IκB-α (as a surrogate marker of NF-κB activation) and p38 MAP kinase were assessed after 0, 5, 10, 15, 20 and 30 minutes of the spirochete treatment. Western blot analyses showed that phosphorylation status of IκB-α and p38 MAPK were rapidly enhanced with the exposure of wild type spirochetes, and an approximate 10-fold increase of phosphorylation was noted within the first few minutes of spirochete treatment (Fig. 4). In contrast, cells exposed to bmpA/B mutant B. burgdorferi failed to display a significant increase in the phosphorylation status of either IκB-α or p38 MAP kinase (Fig. 4). These results suggest that the reduced TNF-α and IL-1β expression in synovial cells exposed to bmpA/B mutants (Fig. 1) could be linked to the impaired ability of mutants to activate NF-κB and p38 MAP kinase signaling pathways.
Fig. 4.
bmpA/B mutant B. burgdorferi has impaired ability to induce phosphorylation of IκB-α and p38. (A) Phosphorylation of IκB-α. Cells were exposed to wild type and bmpA/B mutant spirochetes and harvested at 0, 5, 10, 15, 20 and 30 min following B. burgdorferi exposure. Western blot analysis was performed on whole cell lysates using antibodies that specifically recognize phosphorylated (PIκB-α) and total (IκB-α) forms of IκB-α proteins. Cellular actin level was used as a loading control. A representative of three experiments is presented. (B) Densitometric analysis of IκB-α phosphorylation. Relative densities of phosphorylated and total IκB-α levels in synovial cells exposed to wild type (black bar) and bmpA/B mutant B. burgdorferi (gray bar) were determined by densitometric scan. Data represented as the relative ratio of phosphorylated and total IκB-α levels, and the values of 0 minute time points, were considered as 100%. (C) and (D) Phosphorylation of p38 MAPK. The cell lysates were assessed by Western blotting using antibodies that specifically recognize phosphorylated (Pp38) and total (p38) forms of p38 MAPK proteins, followed by densitometric analysis with similar parameters as described for IκB-α in (A) and (B) above.
3.4. Non-lipidated BmpA directly activates NF-κB and p38 MAPK pathway in synovial cells
As BmpA directly induced the expression of TNF-α and IL-1β in synovial cells (Fig. 3), we next assessed whether the recombinant protein could directly induce the activation of NF-κB and p38 MAP kinase in synovial cells. Similar to wild type B. burgdorferi-induced phosphorylation of synovial cells (Fig. 4), phosphorylation of IκB-α and p38 MAP kinase was readily evident within 5 minutes of recombinant BmpA treatment (Fig. 5A), while the control protein, BB0365, did not show obvious phosphorylation of either protein (Fig. 5A). Similarly, electrophoretic mobility shift assay (EMSA) showed that BmpA induced the formation of complexes with the consensus binding sequence that was similar to those obtained with LPS (Fig. 5B). Super-shift assays further confirmed the specificity of the EMSA assay and showed that the heterodimeric NF-kB induced by BmpA contains p50 and RelA (Fig. 5B). Finally, we assessed whether BmpA can induce the NF-κB and p38 MAP kinase reporter genes expression in the transfected synovial cells. To accomplish this, cells were transfected with NF-κB and p38 MAP kinase reporter plasmids or control plasmids, as detailed in the Materials and Methods section. Twenty-four hours after transfection, cells were exposed to equal concentration (300 nanogram/ml) of either non-lipidated BmpA or the control B. burgdorferi protein, BB0365, for 18 hours. As additional controls, parallel wells were also treated with polymyxin B and BmpA antibodies (1: 100 dilution) to assess the specificity of BmpA-induced reporter gene expression. BmpA increased the activity of NF-κB and p38 MAP kinase reporting systems to increase by 8- and 5-fold, respectively, which was significantly higher (P< 0.01) than that induced by the control BB0365 protein (Fig. 5C). Polymyxin B did not influence the ability of BmpA to induce the reporter gene expression (data not shown). BmpA antibodies significantly blocked the effects of BmpA-mediated reporter gene expression (P< 0.05), but had no effect on BB0365-induced wells, further suggesting that the activation of NF-κB and p38 MAP kinase can be solely attributed to BmpA (Fig. 5C). Of note, neutralizing antibodies against either TLR2 or TLR4 were unable to inhibit non-lipidated BmpA-mediated activation of NF-κB pathways in transfected synovial cells; TLR2 antibodies, however, significantly blocked similar activation of NF-κB pathway mediated by lipidated BmpA (P<0.004, Fig 5D).
Fig. 5.
Non-lipidated BmpA activates NF-κB or p38 MAPK signaling pathways in synovial cells. (A) Phosphorylation of IκB-α and p38 MAPK. Cells were harvested at indicated times after treatment with non-lipidated BmpA or BB0365 (300 nanogram/ml), and phosphorylation of IκB-α was assessed by Western blotting using antibodies that specifically recognize phosphorylated (PIκB-α) and total (IκB-α) forms of IκB-α proteins (upper panels). Lower panels indicate phosphorylation of p38 MAPK assessed using antibodies that specifically recognize phosphorylated (Pp38) and total (p38) forms of p38 MAPK proteins. (B) BmpA-mediated induction NF-κB was assessed by electromobility shift assays (EMSA). The left panel shows a time course of nuclear extracts binding to an oligonucleotide probe containing consensus NF-κB binding sites. Synovial cells were stimulated for the indicated times with BmpA or LPS (control). The panel also shows a representative super shift assay using antibodies specific for p50 and c-Rel. The reactions using BmpA- (1) or LPS- (2) induced nuclear extracts were performed in the absence or presence of excess (100X) unlabeled oligonucleotides (cold NF-κB, right panel). The competition reactions were also carried out with cold AP-1 consensus binding sequences (negative control). (C) Activation of NF-κB or p38 MAPK signal transduction pathways in transfected synovial cells. Cells were co-transfected with phRL-TK plasmid (internal control) and pNF-κB-Luc (NF-κB reporter) or pFR-Luc and pFA-CHOP (p38 reporter). Transfected cells were exposed to 300 nanogram/ml of either non-lipidated recombinant proteins, BmpA (black bar) or control B. burgdorferi protein, BB0365 (gray bar) in the presence or absence of BmpA antibodies (1: 100 dilution). After 18 hours of incubation, luciferase activities were determined in each sample, and differential transfection efficiencies were normalized using Renilla luciferase activities. Specific luciferase activities of each group were presented as fold increase compared to the corresponding zero time values. Bars represent the mean ± standard error from three independent experiments. Luciferase reporter gene expression in BmpA exposed cells is significantly higher than that of BB0365 treated cells (P< 0.001), which is abrogated by the addition of BmpA antibody (P< 0.01). (D) TLR antibody-mediated inhibition of BmpA-induced activation of NF-κB signal transduction pathways in transfected cells. Synovial cells were co-transfected with phRL-TK plasmid (internal control) and pNF-κB-Luc (NF-κB reporter) plasmids, exposed to either 300 nanogram/ml of recombinant BmpA proteins (dark gray bars) or known TLR2 or TLR4 agonists, Pam3CSK4 or LPS, respectively (light gray bars). In addition, selected cells were also incubated with either TLR2 or TLR4 antibodies (black bars). After 18 hr of incubation, luciferase activities were determined and presented as fold increase relative to the corresponding zero time values. Bars represent the mean ± standard error from three independent experiments. TLR2 antibody significantly reduced the luciferase expression in cells exposed to either lipidated BmpA or Pam3CSK4 (P<0.004), but did not alter the reporter gene expression in cells exposed to non-lipidated BmpA (P>0.05). TLR4 antibody, while reduced the LPS-mediated luciferase expression (P<0.005), did not influence either lipidated or non-lipidated BmpA-mediated reporter gene expression (P>0.05).
4 Discussion
Lyme arthritis is a multifactorial host inflammatory response triggered by the B. burgdorferi invasion of the joint tissue [1]. Here, we show that the major product of the B. burgdorferi bmpA/B operon, BmpA, which is selectively induced in infected murine and human joints [19], triggers expression of two pro-inflammatory cytokines, TNF-α and IL-1β, in cultured human synovial cells. While spirochete lipids are regarded as the primary mediator of B. burgdorferi-induced host inflammatory response [5, 15, 26], BmpA induced the cytokine expression through the non-lipidated portion of the lipoprotein, and also activated NF-κB and p38 MAP kinase pathways. Exploration of specific contributions of joint-inducible B. burgdorferi gene products, such as BmpA, in the development of inflammatory responses might provide new insights into the pathogenesis of Lyme arthritis.
B. burgdorferi infection in joints induces expression of selective inflammatory cytokines that are triggered by distinct cell surface events [1, 7, 10–12]. Contrary to infiltrating immune cells, such as macrophages or monocytes, where phagocytosis of B. burgdorferi is required for activation of cytokine response [27], resident joint cells, such as synovial cells, are likely to be non-phagocytic, thus, might rely on the interaction with the spirochete or its derived components via cell surface receptors. While TLR2 is known to recognize spirochete lipids [7, 8], integrins [10, 11] and Fcγ receptors [12] have also been shown to interact with specific B. burgdorferi ligands, resulting in cellular inflammatory responses. Additionally, a recent study implicated TLR5, a known receptor for bacterial flagellin, as a possible participant in B. burgdorferi-induced cellular cytokine activation [13]. Although we have determined that synovial cells used in the current study produce detectable levels of TLR2, TLR4 and TLR5 mRNA and protein (data not shown), and are also known to express multiple subtypes of integrins [28], their involvement in BmpA-mediated cytokine production remains unclear. While lipidated BmpA-induced activation of synovial cells could be mediated by TLR2 (Fig. 5D), our studies suggest that BmpA could trigger similar inflammatory responses that are independent of its lipid modification, and likely involve a protein moiety of the lipoprotein. Non-lipidated BmpA-mediated activation of synovial cells is specific to the concerned antigen, rather endotoxin contamination as polymyxin B had minimal inhibitory effects. This is further supported by the observations that although each non-lipidated recombinant protein used in our study used the same expression vector and E. coli isolates, only BmpA, unlike BmpB (Fig. 2) and BB0365 (Fig. 2 and Fig 5), selectively activated the synovial cells. BmpA is paralogous to 3 additional members of B. burgdorferi bmp gene family [17], and structurally most similar to BmpB (49% amino acid identity). However, BmpB failed to induce significant cytokine responses, when compared to BmpA, suggesting that unique epitope/s selectively present in BmpA are responsible for the pro-inflammatory cytokine response in synovial cells.
B. burgdorferi burden in infected joints is correlated with the severity of Lyme arthritis [1], which, at least in part, depends on the localized production of inflammatory cytokines [1]. TNF-α and IL-1β are two key pro-inflammatory cytokines that are readily induced by B. burgdorferi when exposed to human or murine cells in vitro [26, 27] and in infected mice or Lyme disease patients [4, 29]. B. burgdorferi-mediated TNF-α and IL-1β induction in joint tissues is also shown to contribute to the subsequent production of additional immnomodulatory molecules, such as IL-8 [3], which is a potent chemoattractant for polymorphonuclear cells. Lyme arthritis could be characterized by a massive influx of immune cells from the blood vessels, including polymorphonuclear cells, which significantly contributes to the etiology of arthritis [30]. Since BmpA undergoes a spatial and temporal upregulation in infected murine joints that coincides with development of inflammation [19], our results strongly suggest that BmpA may participate in the B. burgdorferi-induced activation of pro-inflammatory cytokine in synovial cells via NF-κB and p38 MAP kinase signaling pathways, thereby contributing to the outcome of joint inflammation.
In conclusion, we show that a joint-inducible, antigenic and surface-exposed lipoprotein of B. burgdorferi, BmpA, is directly capable of inducing pro-inflammatory cytokine responses via a protein moiety. Identification of cell surface events, including participation of putative host receptor/s that mediate BmpA-induced inflammatory responses, will provide new insights into the pathogenic mechanisms of the multifactorial joint disorders caused by the Lyme disease pathogen.
Acknowledgments
This work was supported by grants from Arthritis Foundation (Maryland Chapter) and National Institutes of Health AR055323 (UP), AI49200 (EF) and a Faculty Research Grant (JA). U.P is the recipient of a Scientist Development Grant from American Heart Association. E.F. is an Investigator of the Howard Hughes Medical Institute. We sincerely thank Deborah Shroder and Caitlin Hester for excellent technical help.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steere AC, Glickstein L. Elucidation of Lyme arthritis. Nat Rev Immunol. 2004;4:143–152. doi: 10.1038/nri1267. [DOI] [PubMed] [Google Scholar]
- 2.Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med. 1994;330:229–234. doi: 10.1056/NEJM199401273300401. [DOI] [PubMed] [Google Scholar]
- 3.Straubinger RK, Straubinger AF, Summers BA, Erb HN, Harter L, Appel MJ. Borrelia burgdorferi induces the production and release of proinflammatory cytokines in canine synovial explant cultures. Infect Immun. 1998;66:247–258. doi: 10.1128/iai.66.1.247-258.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shin JJ, Glickstein LJ, Steere AC. High levels of inflammatory chemokines and cytokines in joint fluid and synovial tissue throughout the course of antibiotic-refractory lyme arthritis. Arthritis Rheum. 2007;56:1325–1335. doi: 10.1002/art.22441. [DOI] [PubMed] [Google Scholar]
- 5.Radolf JD, Norgard MV, Brandt ME, Isaacs RD, Thompson PA, Beutler B. Lipoproteins of Borrelia burgdorferi and Treponema pallidum activate cachectin/tumor necrosis factor synthesis. Analysis using a CAT reporter construct. J Immunol. 1991;147:1968–1974. [PubMed] [Google Scholar]
- 6.Sellati TJ, Bouis DA, Caimano MJ, Feulner JA, Ayers C, Lien E, Radolf JD. Activation of human monocytic cells by Borrelia burgdorferi and Treponema pallidum is facilitated by CD14 and correlates with surface exposure of spirochetal lipoproteins. J Immunol. 1999;163:2049–2056. [PubMed] [Google Scholar]
- 7.Hirschfeld M, Kirschning CJ, Schwandner R, Wesche H, Weis JH, Wooten RM, Weis JJ. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J Immunol. 1999;163:2382–2386. [PubMed] [Google Scholar]
- 8.Alexopoulou L, Thomas V, Schnare M, Lobet Y, Anguita J, Schoen RT, Medzhitov R, Fikrig E, Flavell RA. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat Med. 2002;8:878–884. doi: 10.1038/nm732. [DOI] [PubMed] [Google Scholar]
- 9.Bolz DD, Sundsbak RS, Ma Y, Akira S, Kirschning CJ, Zachary JF, Weis JH, Weis JJ. MyD88 plays a unique role in host defense but not arthritis development in Lyme disease. J Immunol. 2004;173:2003–2010. doi: 10.4049/jimmunol.173.3.2003. [DOI] [PubMed] [Google Scholar]
- 10.Coburn J, Cugini C. Targeted mutation of the outer membrane protein P66 disrupts attachment of the Lyme disease agent, Borrelia burgdorferi, to integrin alphavbeta3. Proc Natl Acad Sci U S A. 2003;100:7301–7306. doi: 10.1073/pnas.1131117100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behera AK, Durand E, Cugini C, Antonara S, Bourassa L, Hildebrand E, Hu LT, Coburn J. Borrelia burgdorferi BBB07 interaction with integrin alpha3beta1 stimulates production of pro-inflammatory mediators in primary human chondrocytes. Cell Microbiol. 2008;10:320–331. doi: 10.1111/j.1462-5822.2007.01043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Talkington J, Nickell SP. Role of Fc gamma receptors in triggering host cell activation and cytokine release by Borrelia burgdorferi. Infect Immun. 2001;69:413–419. doi: 10.1128/IAI.69.1.413-419.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin OS, Isberg RR, Akira S, Uematsu S, Behera AK, Hu LT. Distinct roles for MyD88 and Toll-like receptors 2, 5, and 9 in phagocytosis of Borrelia burgdorferi and cytokine induction. Infect Immun. 2008;76:2341–2351. doi: 10.1128/IAI.01600-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olson CM, Hedrick MN, Izadi H, Bates TC, Olivera ER, Anguita J. p38 mitogen-activated protein kinase controls NF-kappaB transcriptional activation and tumor necrosis factor alpha production through RelA phosphorylation mediated by mitogen- and stress-activated protein kinase 1 in response to Borrelia burgdorferi antigens. Infect Immun. 2007;75:270–277. doi: 10.1128/IAI.01412-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wooten RM, Modur VR, McIntyre TM, Weis JJ. Borrelia burgdorferi outer membrane protein A induces nuclear translocation of nuclear factor-kappa B and inflammatory activation in human endothelial cells. J Immunol. 1996;157:4584–4590. [PubMed] [Google Scholar]
- 16.Behera AK, Hildebrand E, Uematsu S, Akira S, Coburn J, Hu LT. Identification of a TLR-independent pathway for Borrelia burgdorferi-induced expression of matrix metalloproteinases and inflammatory mediators through binding to integrin alpha 3 beta 1. J Immunol. 2006;177:657–664. doi: 10.4049/jimmunol.177.1.657. [DOI] [PubMed] [Google Scholar]
- 17.Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, White O, Ketchum KA, Dodson R, Hickey EK, Gwinn M, Dougherty B, Tomb JF, Fleischmann RD, Richardson D, Peterson J, Kerlavage AR, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams MD, Gocayne J, Weidman J, Utterback T, Watthey L, McDonald L, Artiach P, Bowman C, Garland S, Fuji C, Cotton MD, Horst K, Roberts K, Hatch B, Smith HO, Venter JC. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 18.Dobrikova EY, Bugrysheva J, Cabello FC. Two independent transcriptional units control the complex and simultaneous expression of the bmp paralogous chromosomal gene family in Borrelia burgdorferi. Mol Microbiol. 2001;39:370–378. doi: 10.1046/j.1365-2958.2001.02220.x. [DOI] [PubMed] [Google Scholar]
- 19.Pal U, Wang P, Bao F, Yang X, Samanta S, Schoen R, Wormser GP, Schwartz I, Fikrig E. Borrelia burgdorferi basic membrane proteins A and B participate in the genesis of Lyme arthritis. J Exp Med. 2008;205:133–141. doi: 10.1084/jem.20070962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bryksin AV, Godfrey HP, Carbonaro CA, Wormser GP, Aguero-Rosenfeld ME, Cabello FC. Borrelia burgdorferi BmpA, BmpB, and BmpD proteins are expressed in human infection and contribute to P39 immunoblot reactivity in patients with Lyme disease. Clin Diagn Lab Immunol. 2005;12:935–940. doi: 10.1128/CDLI.12.8.935-940.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pal U, Dai J, Li X, Neelakanta G, Luo P, Kumar M, Wang P, Yang X, Anderson JF, Fikrig E. A differential role for BB0365 in the persistence of Borrelia burgdorferi in mice and ticks. J Infect Dis. 2008;197:148–155. doi: 10.1086/523764. [DOI] [PubMed] [Google Scholar]
- 22.Pal U, de Silva AM, Montgomery RR, Fish D, Anguita J, Anderson JF, Lobet Y, Fikrig E. Attachment of Borrelia burgdorferi within Ixodes scapularis mediated by outer surface protein A. J Clin Invest. 2000;106:561–569. doi: 10.1172/JCI9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore MW, Cruz AR, LaVake CJ, Marzo AL, Eggers CH, Salazar JC, Radolf JD. Phagocytosis of Borrelia burgdorferi and Treponema pallidum potentiates innate immune activation and induces gamma interferon production. Infect Immun. 2007;75:2046–2062. doi: 10.1128/IAI.01666-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Izadi H, Motameni AT, Bates TC, Olivera ER, Villar-Suarez V, Joshi I, Garg R, Osborne BA, Davis RJ, Rincon M, Anguita J. c-Jun N-terminal kinase 1 is required for Toll-like receptor 1 gene expression in macrophages. Infect Immun. 2007;75:5027–5034. doi: 10.1128/IAI.00492-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiss-Toth E, Bagstaff SM, Sung HY, Jozsa V, Dempsey C, Caunt JC, Oxley KM, Wyllie DH, Polgar T, Harte M, O’Neill A, Qwarnstrom LEE, Dower SK. Human tribbles, a protein family controlling mitogen-activated protein kinase cascades. J Biol Chem. 2004;279:42703–42708. doi: 10.1074/jbc.M407732200. [DOI] [PubMed] [Google Scholar]
- 26.Radolf JD, Arndt LL, Akins DR, Curetty LL, Levi ME, Shen Y, Davis LS, Norgard MV. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytes/macrophages. J Immunol. 1995;154:2866–2877. [PubMed] [Google Scholar]
- 27.Cruz AR, Moore MW, La Vake CJ, Eggers CH, Salazar JC, Radolf JD. Phagocytosis of Borrelia burgdorferi, the Lyme disease spirochete, potentiates innate immune activation and induces apoptosis in human monocytes. Infect Immun. 2008;76:56–70. doi: 10.1128/IAI.01039-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spessotto P, Cervi M, Mucignat MT, Mungiguerra G, Sartoretto I, Doliana R, Colombatti A. beta 1 Integrin-dependent cell adhesion to EMILIN-1 is mediated by the gC1q domain. J Biol Chem. 2003;278:6160–6167. doi: 10.1074/jbc.M208322200. [DOI] [PubMed] [Google Scholar]
- 29.Defosse DL, Johnson RC. In vitro and in vivo induction of tumor necrosis factor alpha by Borrelia burgdorferi. Infect Immun. 1992;60:1109–1113. doi: 10.1128/iai.60.3.1109-1113.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown CR, Blaho VA, Loiacono CM. Susceptibility to experimental Lyme arthritis correlates with KC and monocyte chemoattractant protein-1 production in joints and requires neutrophil recruitment via CXCR2. J Immunol. 2003;171:893–901. doi: 10.4049/jimmunol.171.2.893. [DOI] [PubMed] [Google Scholar]