Abstract
Objective
To evaluate the clinical impact and cost-effectiveness of HLA-B*5701 testing to guide selection of first-line HIV regimens in the United States.
Design
Cost-effectiveness analysis using a simulation model of HIV disease. The prevalence of HLA-B*5701 and the probabilities of confirmed and unconfirmed severe systemic hypersensitivity reaction (HSR) among patients taking abacavir testing HLA-B*5701 positive and negative were from the PREDICT-1 trial. The monthly costs of abacavir and tenofovir-based regimens were $1,135 and $1,139; similar virologic efficacy was assumed and this assumption was varied in sensitivity analysis.
Subjects
Simulated cohort of patients initiating HIV therapy.
Interventions
1) first-line abacavir, lamivudine, and efavirenz without pre-treatment HLA-B*5701 testing; 2) the same regimen with HLA-B*5701 testing; 3) first-line tenofovir, emtricitabine, and efavirenz.
Main Outcome Measures
Quality-adjusted life expectancy (QALYs) and lifetime medical costs discounted at 3% p.a., cost-effectiveness ratios ($/QALY).
Results
Abacavir-based treatment without HLA-B*5701 testing resulted in a projected 30.93 years life expectancy, 16.23 discounted QALYs, and $472,200 discounted lifetime cost per person. HLA-B*5701 testing added 0.04 quality-adjusted months at an incremental cost of $110, resulting in a cost-effectiveness ratio of $36,700/QALY compared to no testing. Initiating treatment with a tenofovir-based regimen increased costs without improving QALYs. HLA-B*5701 testing remained the preferred strategy only if abacavir-based treatment had equal efficacy and cost less per month than tenofovir-based treatment. Results were also sensitive to the cost of HLA-B*5701 testing and the prevalence of HLA*B5701.
Conclusions
Pharmacogenetic testing for HLA-B*5701 is cost-effective only if abacavir-based treatment is as effective and costs less than tenofovir-based treatment.
Keywords: abacavir, antiretroviral therapy, cost-effectiveness, genetic testing, HIV
BACKGROUND
Abacavir is a nucleoside reverse transcriptase inhibitor (NRTI) used as part of combination antiretroviral therapy (ART) for HIV. It has proven efficacy in first-line ART regimens, with few drug long-term toxicities observed [1]. In a small proportion of patients however, treatment with abacavir may be associated with systemic hypersensitivity reaction (HSR), a multi-organ system process that can be severe enough to cause hospitalization or death [2]. Both retrospective and prospective studies have demonstrated a strong association between the presence of the HLA-B*5701 allele and the risk of HSR in patients taking abacavir [3, 4]. Furthermore, in a randomized, prospective clinical trial, patients screened for HLA-B*5701 before abacavir therapy experienced dramatically reduced rates of both clinically-diagnosed and immunologically confirmed HSR [5].
HIV treatment guidelines issued by the United States Department of Health and Human Services in February 2008 recommend abacavir as a preferred component of initial ART only for patients who test negative for HLA-B*5701 [6]. Given the safety and efficacy of abacavir-based treatment in the absence of HSR, our goal was to evaluate the long-term clinical impact and cost-effectiveness of pre-treatment HLA-B*5701 testing to guide initial HIV therapy.
METHODS
Analytic overview
We used the Cost-Effectiveness of Preventing AIDS Complications (CEPAC) model, a widely published simulation state transition model of HIV disease [7, 8], along with data reported in the literature, to evaluate the clinical impact and cost-effectiveness of HLA-B*5701 testing to guide selection of first-line HIV treatment regimens in patients in the United States.
Upon entering the model, simulated patients initiated a first-line ART regimen consisting of a fixed-dose combination of abacavir and lamivudine with efavirenz or a fixed-dose combination of tenofovir, emtricitabine, and efavirenz. For patients initiating treatment that included abacavir, we compared “universal testing” versus “no testing.” In the universal testing strategy, all patients were tested for HLA-B*5701 prior to ART initiation. Abacavir-based treatment was selected for patients testing HLA-B*5701 negative and tenofovir-based treatment was selected for patients testing HLA-B*5701 positive. In the no testing strategy, all patients were initiated on abacavir-based treatment. In either strategy, patients taking abacavir who developed a suspected HSR were treated in office-based or inpatient settings according to the severity of their symptoms, and were then switched to tenofovir-based treatment. Patients switched to tenofovir-based treatment who subsequently developed treatment-limiting tenofovir-associated nephrotoxicity were switched to a fixed-dose combination of zidovudine and lamivudine with efavirenz.
In the comparison strategy, all patients in the model initiated tenofovir-based treatment without initial HLA-B*5701 testing. For patients who developed treatment-limiting tenofovir-associated nephrotoxicity, we considered three alternatives to guide drug substitution: 1) “substitution HLA-B*5701 testing,” where those testing HLA-B*5701 negative were switched to abacavir-based treatment and those testing HLA-B*5701 positive were switched to zidovudine-based treatment, 2) substituting abacavir-based treatment without testing, and 3) substituting zidovudine-based treatment without testing.
Model
We used the CEPAC model to project the long-term outcomes of each possible combination of initial drug regimens, toxicities (mild, severe, or fatal suspected HSR; treatment-limiting tenofovir-associated nephrotoxicity; or no toxicity), and drug substitutions. Details regarding the CEPAC model structure, data, and assumptions have been previously described (see Appendix). We then calculated the results for each strategy as the weighted average of these projected outcomes using probabilities derived from published studies as the basis for weighting. These probabilities included the incidence of immunologically confirmed HSR in patients taking abacavir with and without HLA-B*5701 testing, and the reduction in suspected HSR diagnoses observed with HLA-B*5701 testing. Tables 1 and 2 report baseline model inputs and ranges used in sensitivity analyses.
Table 1.
Baseline inputs for model variables
| Variable | Baseline value | Range evaluated | Reference |
|---|---|---|---|
| Cohort characteristics | |||
| Age, mean years ± SD | 36.0±9.8 | --- | [8, 31] |
| Percentage of male subjects | 74 | --- | [31] |
| CD4 cell count, mean cells/μl ± SD | 276±201 | --- | [31] |
| Distribution of initial HIV RNA (copies/ml) | [32] | ||
| >100,000 | 0.13 | --- | |
| 30,001–100,000 | 0.13 | --- | |
| 10,001–30,000 | 0.25 | --- | |
| 3,001–10,000 | 0.25 | --- | |
| 501–3000 | 0.16 | --- | |
| 0–500 | 0.08 | --- | |
| Abacavir HSR probabilities | |||
| Prevalence of HLA-B*5701 | 0.057a | 0.00 – 0.20 | [5, 20] |
| Probability of immunologically confirmed HSR on abacavir | |||
| If test HLA-B*5701 positive | 0.479 | 0.479 – 1.00 | [4, 5] |
| If test HLA-B*5701 negative | 0.000 | 0.00 – 0.03 | [5, 33] |
| Severity of immunologically confirmed HSR on abacavir | |||
| Mild | 0.585 | 0.500 – 0.654 | [9] |
| Severe, non-fatal | 0.408 | 0.344 – 0.492 | [9] |
| Fatal | 0.007 | 0.006 – 0.008 | [2, 9] |
| Probability of unconfirmed HSR on abacavir | |||
| With HLA-B*5701 testing | 0.034 | 0.00 – 0.10 | [5, 34] |
| Without HLA-B*5701 testing | 0.051 | 0.05 – 0.10 | [5, 34] |
| Probability of treatment limiting nephrotoxicity on tenofovir | 0.021 | 0.00 – 1.00 | [13] |
| Efficacy of antiretroviral therapy (% HIV RNA suppressed to <400 copies/ml at 48 weeks) | |||
| 1st line abacavir/lamivudine + efavirenz | 81 | --- | assumption |
| 1st line tenofovir/emtricitabine + efavirenz | 81 | --- | [10, 35] |
| 1st line Zidovudine/lamivudine (substitution) + efavirenz | 70 | --- | [10, 35] |
HSR = systemic hypersensitivity reaction; SD = standard deviation
Among the control group population that could be evaluated for immunologically confirmed HSR.
Table 2.
Baseline costs
| Variable | Baseline value | Range | Reference |
|---|---|---|---|
| HLA-B*5701 testing cost | |||
| Cost per test ($)a | 68 | 68 – 341 | [15, 16] |
| Cost of antiretroviral therapy ($/month) | |||
| 1st line: abacavir/lamivudine + efavirenz | 1,135 | 1,129 – 1,139 | [17] |
| 1st line: tenofovir/emtricitabine/efavirenz | 1,139 | --- | [17] |
| 1st line: zidovudine/lamivudine + efavirenz | 1,081 | --- | [17] |
| Toxicity costs ($) | |||
| Abacavir hypersensitivity reaction | |||
| Mild HSR | 105 | 53 – 210 | [16, 18] |
| Severe HSR | 3,566 | 1,783 – 7,132 | [8] |
| Fatal HSR | 31,999 | 15,999 – 63,998 | [8] |
| Tenofovir-associated treatment-limiting nephrotoxicity | $194 | 97 – 388 | [8, 16] |
| Abacavir hypersensitivity reaction quality of life decrements | |||
| Mild HSR | 0.01b | 0.01 – 0.05 | [11] |
| Severe HSR | 0.04c | 0.04 – 0.20 | [12] |
| Fatal HSR | 0.18d | 0.18 – 0.90 | [7] |
HSR = systemic hypersensitivity reaction
Primary analysis assumes cost is equivalent to Medicare reimbursement using CPT codes 83890; 83893 (x3); 83896 (x3); 83898; 83912
A quality-of-life decrement of 0.08 was assessed for 3 days out of 1 month.
A quality-of-life decrement of 0.15 was assessed for 7 days out of 1 month.
Represents the quality-of-life decrement occurring in the last month of life compared to chronic HIV
Results are reported as quality-adjusted life expectancies and lifetime direct medical costs in 2006 U.S. dollars, both discounted to present value at an annual rate of 3. All cost-effectiveness ratios are calculated on an incremental basis by ranking strategies from least to most expensive and comparing each strategy to the next most expensive strategy. Cost-effectiveness ratios are reported as cost per quality-adjusted life year ($/QALYs).
Abacavir HSR data
Based on the results from the control group of the PREDICT-1 trial, a randomized multicenter trial conducted in Europe and Australia to evaluate the efficacy of HLA-B*5701 screening for HSR in patients taking abacavir, the probability of testing HLA-B*5701 positive is 5.7% and the probability of immunologically confirmed HSR for HLA-B*5701 positive patients taking abacavir is 2.7%. The rate of immunologically-confirmed HSR is, therefore, 47.9% in HLA-B*5701-positive patients. In PREDICT-1, none of the HLA-B*5701-negative patients taking abacavir developed immunologically confirmed HSR [5]. Other studies indicate that 58.5% of all immunologically-confirmed HSR cases are mild (grade 1 or 2) and 41.5% are severe (grade 3 or 4) [9], and that 1.7% of the severe cases are fatal [2]. Based on the results of the PREDICT-1 trial, suspected HSR that would not be immunologically confirmed is diagnosed in 5.1% of patients taking abacavir when HLA-B*5701 testing is unavailable and 3.4% when testing is available [5]. In sensitivity analyses, we examined different assumptions for a population with a race/ethnicity composition relevant to U.S. clinical settings.
Efficacy of antiretroviral regimens
In the primary analysis, the efficacy of first-line regimens was derived from a clinical trial in which 81% of patients treated with tenofovir and emtricitabine with efavirenz achieved HIV RNA <400 copies/ml at 48 weeks and the mean CD4 cell count increase was 190 cells/uL [10]. We assumed equivalent efficacy for abacavir and lamivudine with efavirenz, and varied this assumption in sensitivity analyses. Based on the same clinical trial results, patients in the simulation who switched to zidovudine and lamivudine with efavirenz achieved a viral suppression rate of 70% with HIV RNA <400 copies/ml at 48 weeks and a mean CD4 cell count increase of 158 cells/uL [10]. We used published clinical trial data to estimate the virologic efficacy of each of 5 subsequent line of ART (see Appendix).
Toxicities
The incidence of NRTI toxicities and quality-of-life decrements (on a scale from 0 to 1.00, where 0 is death or worst possible health and 1.00 is perfect health) associated with these toxicities in the simulation were derived from the literature. Quality-of-life decrements were 0.08 for 3 days for mild HSR [11], 0.15 for 7 days for severe HSR [12], and 0.36 for 15 days for fatal HSR [7]. The probability of nephrotoxicity severe enough to require tenofovir discontinuation was derived from a clinical trial that reported an incidence of 1.1% in patients on tenofovir for 1 year [13]. Consistent with the low risk of tenofovir discontinuation in those initially tolerating the drug [14], the incidence of nephrotoxicity was halved in each subsequent year. The average time patients spent on tenofovir-containing first-line regimens in the model was 7.9 years, resulting in an estimated cumulative incidence of discontinuations due to renal events of 2.1% during first-line treatment. Nephrotoxicity requiring tenofovir discontinuation was assumed to cause a quality-of-life decrement equivalent to mild HSR. Additional chronic toxicities considered in the model included lipoatrophy and neuropathy (see Appendix).
Costs
In the primary analysis, the cost of HLA-B*5701 testing was $68, derived from the Medicare fee schedule and the Medicare reimbursement codes used by one national commercial laboratory (LabCorp, Burlington, NC) [15, 16]. The monthly costs of abacavir and lamivudine fixed-dose combination with efavirenz ($1,135), tenofovir, emtricitabine, and efavirenz fixed-dose combination ($1,139), and zidovudine and lamivudine fixed-dose combination with efavirenz ($1,081) are average wholesale prices, adjusted to reflect discounts to Medicaid programs and a $4 per month retail pharmacy dispensing fee for each efavirenz or fixed-dose combination prescription [8, 17]. The monthly costs of subsequent regimens ranged from $1,549 to $3,338 (see Appendix).
The cost of mild HSR treated in an outpatient setting was $105, based on costs of a follow-up clinic visit, a complete blood count, a blood culture, and chemistries that included tests for renal function and liver enzymes, all from Medicare fee schedules [16, 18]. For severe HSR, costs for non-fatal ($3,566) and fatal ($31,999) cases were equivalent to the costs of treating similar acute events in the CEPAC model [8]. The cost of treatment-limiting nephrotoxicity was $194, based on costs of a follow-up clinic visit, a complete blood count, a urinalysis, and a chemistry panel that includes a test for renal function and phosphorous (which would be repeated monthly for 6 months) [16, 18].
Assumptions
We assumed that the probability of an unconfirmed HSR diagnosis is not affected by the prevalence of the HLA-B*5701 allele in the population, and that patients with unconfirmed HSR incur the same cost and quality-of-life effects as patients with immunologically confirmed mild HSR. Because immunologic confirmatory testing (i.e. skin patch testing) is not recommended as a clinical tool [6], we also assumed that skin patch testing would not occur and that either tenofovir and emtricitabine or zidovudine and lamivudine would be substituted for abacavir and lamivudine in these patients, depending on the strategy being evaluated.
Patients who failed on an abacavir-based treatment were assumed to have developed abacavir resistance and could not re-use this drug on subsequent regimens. Patients who failed on a tenofovir and emtricitabine-containing regimen could re-use this drug combination in order to retain the beneficial effects of resistance mutations that typically emerge with these drugs [19], unless they developed treatment-limiting tenofovir-associated nephrotoxicity. Patients who could use neither abacavir nor tenofovir in subsequent regimens used zidovudine-based treatment and, in the event of discontinuation of zidovudine due to toxicity, were treated with lamivudine along with other active agents.
Sensitivity analyses
Because the prevalence of HLA-B*5701 and the incidence of unconfirmed HSR vary by race [5, 20], we examined the impact of simulating a population whose race/ethnicity composition is relevant in U.S. clinical settings, instead of the European and Australian subjects enrolled in the PREDICT-1 trial. In this analysis, the cohort had a racial and ethnic composition equivalent to patients initiating HIV treatment at HIV Research Network sites in the U.S. (49.6% black, 27.2% white, 20.6% Hispanic, and 2.6% other race/ethnicity) [21], with a probability of testing HLA-B*5701 positive of 2.1% in blacks and 6.2% in whites, Hispanics, and those of other race/ethnicity [22] resulting in an average prevalence of 4.1%. This analysis also took into account the observation that whites are twice as likely to receive an unconfirmed HSR diagnosis compared to non-whites [5], but the proportion of patients with a positive or negative test result for HLA-B*5701 who develop confirmed HSR is unaffected by race/ethnicity [23]. We also considered a population restricted to patients with HIV RNA <100,000 copies/ml at initiation of first-line ART due to recent data finding a lower efficacy of abacavir and lamivudine in patients with initial HIV RNA >100,000 copies/ml [24 2008, 2008]. To evaluate the impact of other data uncertainties, we conducted sensitivity analyses using wide ranges of plausible input data (Tables 1 and 2).
RESULTS
Primary analysis
Initiating abacavir-based treatment in all patients without testing for HLA-B*5701 was the least expensive first-line strategy among all alternatives considered. With this strategy, 2.7% of patients developed confirmed HSR (1.6% of patients developed mild HSR, 1.1% developed severe HSR, and 0.02% developed fatal HSR). An additional 5.1% had an unconfirmed HSR diagnosis and were switched from abacavir to tenofovir-based treatment. Patients initiating this strategy had a life expectancy of 371.17 months (30.93 years), a discounted, quality-adjusted life expectancy of 194.75 months (16.23 QALYs), and an average discounted lifetime cost of $472,200 (Table 3).
Table 3.
Cost-Effectiveness Results for HLA-B*5701 Strategies to Guide Selection of First-Line Antiretroviral Regimens.
| Strategy | Confirmed HSR (%) | Total HSR (%) | Lifetime Cost per Person ($) | Quality-Adjusted Life-Months per Person | Cost-Effectiveness Ratio ($/QALY) |
|---|---|---|---|---|---|
| No testing, initiate with abacavir-based therapy | 2.73 | 7.83 | 472,210 | 194.75 | --- |
| No testing, initiate with tenofovir-based therapy, substitute zidovudine and lamivudine a | 0 | 0 | 472,290 | 194.71 | Dominated b |
| Universal HLA-B*5701 testing | 0 | 3.36 | 472,320 | 194.79 | 36,700 |
| Initiate with tenofovir based therapy, substitution HLA-B*5701 testing a | 0 | 0.07 | 472,550 | 194.79 | Dominated b |
| No testing, initiate with tenofovir-base therapy, substitute abacavir and lamivudine a | 0.06 | 0.16 | 472,550 | 194.79 | Dominated b |
QALY: Quality-Adjusted Life Year; all costs and quality-adjusted life months or years discounted at 3% p.a.
HSR: systemic hypersensitivity reaction
if nephrotoxicity requiring tenofovir discontinuation occurs
A dominated strategy has a higher cost and an equal or lower quality-adjusted life expectancy compared to the previous strategy.
With the universal testing strategy, no patients developed mild, severe, or fatal HSR, and 3.4% had an unconfirmed HSR diagnosis. Additionally, 5.7% were initially assigned to the tenofovir-based regimen due to a positive HLA-B*5701 test result. Universal testing strategy patients had an incremental gain of 0.04 discounted, quality-adjusted life months with an incremental discounted lifetime cost of $110. The cost-effectiveness ratio of universal testing, compared to no testing and initiating all patients on abacavir-based treatment, was $36,700/QALY.
Initiating patients on tenofovir-based treatment and substituting abacavir and lamivudine if treatment-limiting nephrotoxicity occurs, with or without HLA-B*5701 testing prior to making the substitution, resulted in a similar quality-adjusted life expectancy of 194.79 QALYs but was $230 more expensive than universal testing. Substituting the more toxic, less effective zidovudine-based regimen if treatment-limiting nephrotoxicity occurs resulted in 0.07 fewer quality-adjusted life months compared to universal testing. Hence, at current drug costs and assuming equal efficacy between abacavir-based and tenofovir-based treatment, universal testing is preferred to initiating patients on tenofovir-based treatment.
Sensitivity analyses varying efficacy of treatment
Universal testing remained preferred to strategies that involve starting patients on tenofovir-based treatment in terms of cost-effectiveness only if both treatments were assumed to be equally effective. If the proportion of patients with HIV RNA <400 copies/ml at 48 weeks was 1% lower on abacavir-based treatment than on tenofovir-based treatment, universal testing resulted in a lower discounted quality-adjusted life expectancy (16.22 QALYs) than starting patients on tenofovir-based treatment. It also resulted in a higher lifetime cost, because the lower cost of the abacavir-based regimen was offset by the need to switch sooner to a more expensive therapy.
Sensitivity analyses varying the cost of the HLA-B*5701 test
At half the primary analysis test cost ($34), the cost-effectiveness ratio for universal testing compared to no testing decreased from $36,700/QALY to $25,300/QALY. At double the primary analysis test cost ($136), roughly equivalent to the lowest cost reported by hospital-based laboratories, the cost-effectiveness ratio for universal testing compared to no testing increased to $59,700/QALY, and universal testing had a cost-effectiveness ratio less than $100,000/QALY as long as the monthly cost of abacavir-based therapy was at least $3 less than tenofovir-based therapy (Table 4). At five times the primary analysis test cost ($340), roughly equivalent to the highest cost reported by a hospital-based laboratory, initiating tenofovir-based treatment, and substituting abacavir without HLA-B*5701 testing if treatment-limiting nephrotoxicity occurs, was the most cost-effective strategy compared to no testing. The incremental cost-effectiveness of universal testing compared to initiating tenofovir-based treatment was $480,000/QALY. When the monthly cost of abacavir-based therapy was $6 less than tenofovir-based therapy (instead of $4 per month less in the primary analysis), universal testing became preferred to initiating with tenofovir-based treatment and had a cost-effectiveness ratio of $128,300/QALY.
Table 4.
Sensitivity Analysis: Cost-effectiveness Ratios for Universal HLA-B*5701 Testing ($/QALY)
| Strategy | Half Primary analysis Test Cost ($34)a | Primary analysis Test Cost ($68) | Twice Primary analysis Test Costa ($136) |
|---|---|---|---|
| Abacavir-based treatment less effective than tenofovir-based treatment | Dominatedb | Dominatedb | Dominatedb |
| Abacavir-based therapy monthly cost advantage versus tenofovir based therapy: | |||
| $4 (primary analysis) | 25,300 | 36,700 | 59,700 |
| $2 | 24,700 | 36,000 | 120,000b |
| $0 | 432,000 b | 840,000b | 1,656,000 b |
| US ART initiation cohort (HLA-B*5701 prevalence ~4.1%) | 29,500 | 45,200 | 77,100 |
| HIV RNA <100,000 copies/ml cohort | 24,300 | 35,400 | 57,700 |
| Universal testing eliminates all unconfirmed HSR diagnoses | 22,700 | 33,500 | 55,300 |
| Universal testing provides no reduction in unconfirmed HSR diagnoses | 26,700 | 38,700 | 62,100 |
QALY: Quality-Adjusted Life Year
Note: Cost-effectiveness ratios are for universal testing compared to no testing unless otherwise indicated.
Equivalent to the lowest cost reported by hospital-based laboratories.
Compared to tenofovir-based first-line therapy; “Dominated” indicates that universal HLA-B*5701 testing has lower quality–adjusted life expectancy and a higher cost compared to tenofovir-based first-line therapy.
Sensitivity analyses varying population characteristics
Results were similar for patients with racial/ethnic characteristics comparable to patients initiating HIV treatment in the U.S. The rank ordering of results did not change, but the cost-effectiveness ratio for universal testing compared to no testing increased to $45,200/QALY, reflecting the higher proportion of non-whites tested and the lower prevalence of HLA-B*5701 in this population (4.1% versus 5.7% in the primary analysis). Results were also similar if the population was restricted to patients with HIV RNA <100,000 copies/ml at initiation of first-line ART (Table 4).
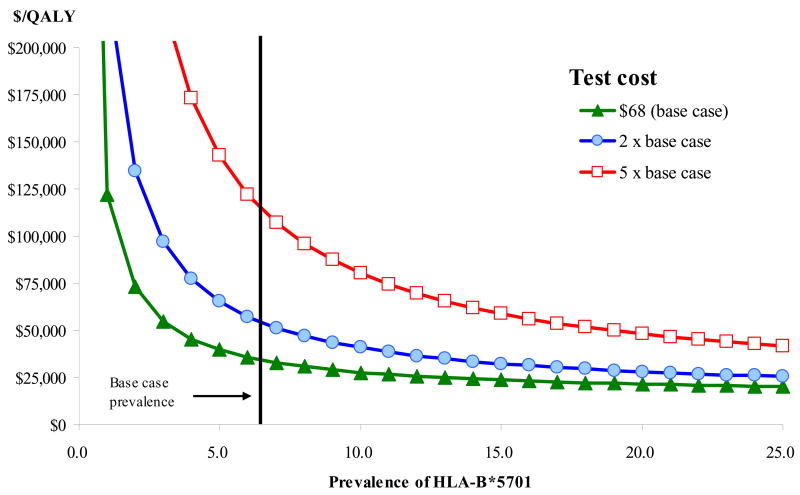
Results varied according to the prevalence of HLA-B*5701 in the population (Figure 1). With the primary analysis test cost of $68, the cost-effectiveness ratio of universal testing remained below $100,000/QALY as long as the prevalence of HLA-B*5701 was greater than 1.4% and remained below $50,000/QALY as long as the prevalence of HLA-B*5701 was greater than 3.6%, compared to 5.7% in the primary analysis. At twice the primary analysis test cost, these thresholds became 2.9% and 7.4%.
Figure 1.
Cost-effectiveness Ratio for HLA-B*5701 Testing versus No Testing by Population Prevalence of HLA-B*5701
Other sensitivity analyses
If HLA-B*5701 testing eliminated all unconfirmed HSR diagnoses, universal testing became more attractive, with a cost-effectiveness ratio of $33,500/QALY assuming the primary analysis test cost. Conversely, when testing provided no benefit in reducing the probability of unconfirmed HSR diagnoses, the cost-effectiveness ratio increased to $38,700/QALY (Table 4). Results were sensitive to the probability of confirmed HSR among HLA-B*5701 positive patients when test costs were higher: at twice the primary analysis test cost, the probability of confirmed HSR among HLA-B*5701 positive patients must be below 59.1% (versus 47.9% in the primary analysis) to be cost-effective at the $50,000/QALY threshold.
As long as the monthly cost of abacavir-based treatment was at least $2 lower than tenofovir based treatment, the universal testing strategy had a lower discounted lifetime cost compared to tenofovir-based treatment. Varying the proportions of confirmed HSR that were mild, severe, and fatal within published ranges did not have a meaningful impact on cost-effectiveness findings, nor did varying the cost or quality-of-life decrements associated with HSR or tenofovir-related nephrotoxicity. If patients unable to use abacavir or tenofovir substituted new regimens without any risk of additional toxicities, the cost-effectiveness of universal testing became $35,000/QALY, compared to $36,700/QALY in the primary analysis.
DISCUSSION
For some medications, pre-treatment genetic screening can significantly improve drug safety [25]. In HIV therapy, important new discoveries are emerging that describe genetic associations with severe adverse drug events [26]. Although genetic screening can reduce the incidence of toxicity, additional expenditures are required to conduct the genetic test. Cost-effectiveness analysis can assist decision makers in evaluating the value of conducting pharmacogenetic tests.
We conducted a cost-effectiveness analysis of pharmacogenetic testing in HIV-infected patients in the U.S., taking into account the implications for initial regimen selection and subsequent treatment options. We found that HLA-B*5701 testing to guide selection of a first-line ART regimen is cost-effective, with a cost-effectiveness ratio below the commonly-accepted thresholds in the U.S. of $50,000-$100,000/QALY. The results were critically dependent on the comparable efficacy and lower cost of abacavir-based treatment compared to tenofovir-based treatment.
We found that the economic value of testing also depends on the cost of the test and the prevalence of HLA-B*5701, although a higher test cost could be offset by a reduction in the price of abacavir-based treatment. In non-US settings, treatment and testing costs will vary depending on the site and prevalence will vary depending on race/ethnicity characteristics of the population. The findings reported here are consistent with an analysis conducted in the United Kingdom before the PREDICT-1 results became available, which found that the incremental cost of testing per abacavir hypersensitivity reaction avoided was sensitive to prevalence and medication costs (when test costs were not varied) [27]. In the U.S., cost considerations may be particularly important for public programs such as the AIDS Drug Assistance Programs funded by the Ryan White CARE Act. These state-administered programs are major payers for HIV drugs, but do not generally pay for laboratory tests, and in some states tight budget limits have already led to cost-containment strategies.
This analysis has several limitations, and follow-up cost-effectiveness analyses will be valuable as uncertainties regarding therapeutic options and future genetic tests are resolved. We project long-term survival based on sequential lines of ART of varying efficacy based on data available from clinical trials; the actual regimens to be chosen in the future remain unknown, especially in light of recently approved agents with novel mechanisms of action. We did not take into account the possibility of increased risks of cardiovascular events associated with abacavir reported recently [29], and we did not consider alternatives to HLA-B*5701 testing that may be less expensive but have similar test performance characteristics in predicting abacavir hypersensitivity reactions. A less expensive test may result in a more attractive cost-effectiveness ratio, depending on the test characteristics.
The assumption about the reduction in the rate of HSR diagnoses after testing may be conservative, because it was derived from the PREDICT-1 trial in which clinicians were blinded to whether patients experiencing symptoms had tested HLA-B*5701 negative or had been assigned to the control arm. Although we found that the cost-effectiveness results were only moderately sensitive to this assumption, our model does not fully reflect the benefits to providers and patients of avoiding such a diagnosis, including fewer physician-patient contacts and fewer worries about a potentially life-threatening adverse event.
Most importantly, we found that HLA-B*5701 testing was only cost-effective under the assumption of equal efficacy between abacavir-based therapy and tenofovir-based therapy. In one randomized, prospective study of first-line ART, abacavir/lamivudine was found to be non-inferior to tenofovir/emtricitabine when both were used in combination with ritonavir-boosted lopinavir [30]. Our findings were unchanged when we restricted the analysis to patients with HIV RNA <100,000 copies/ml, based on interim results from a second blinded study that is still underway [24 2008, 2008]. However, if this study’ results ultimately demonstrate a lower efficacy overall of abacavir/lamivudine regardless of baseline HIV RNA, then starting with a tenofovir-based treatment is more cost effective, regardless of whether HLA-B*5701 testing is performed.
Based on currently available data, use of genetic testing for HLA-B*5701 to guide selection of initial treatment for HIV in the United States is effective and is cost-effective using a threshold of $50,000/QALY. However, the cost-effectiveness ratio is highly dependent on the comparable efficacy of abacavir-based treatment to tenofovir-based treatment, on the relative costs of the drugs, and on the cost of the HLA-B*5701 test itself. With the many highly effective options for initial HIV treatment now available in the U.S., pharmaceutical manufacturers and test providers need to ensure that the cost of this innovation is commensurate with the value that it provides.
Acknowledgments
The authors gratefully acknowledge Caroline E. Sloan, A.B., Sarah Chung, B.S., A. David Paltiel, Ph.D, and Bingxia Wang, Ph.D for their assistance.
Financial Support: National Institute of Allergy and Infectious Diseases (K24 AI062476, K25 AI50436 and R37 AI42006), National Institute on Drug Abuse (K01 DA017179).
Footnotes
AUTHOR CONTRIBUTIONS
All co-authors participated in the study and contributed to the analysis and interpretation of the data and to the development and critical revision of the manuscript. Bruce R. Schackman conceived and designed the study. Paul E. Sax provided clinical expertise, and Elena Losina performed statistical analyses and provided epidemiological experience. Rochelle P. Walensky and Kenneth A. Freedberg provided modelling expertise. Callie A. Scott provided analytic, technical, administrative and logistical support for the study. Bruce R. Schackman drafted the manuscript, to which all authors contributed. All authors have seen and approved the final version of the manuscript.
Potential Conflicts of Interest: Dr. Sax has been a consultant for Abbott, Bristol-Myers Squibb, Gilead, and GlaxoSmithKline. He has received honoraria for teaching from Abbott, Bristol-Myers Squibb, Gilead, GlaxoSmithKline, Merck, Tibotec, and Virco, and grant support from Pfizer, Merck, and GlaxoSmithKline.
References
- 1.Castillo SA, Hernandez JE, Brothers CH. Long-term safety and tolerability of the lamivudine/abacavir combination as components of highly active antiretroviral therapy. Drug Saf. 2006;29:811–826. doi: 10.2165/00002018-200629090-00005. [DOI] [PubMed] [Google Scholar]
- 2.Hetherington S, McGuirk S, Powell G, Cutrell A, Naderer O, Spreen B, et al. Hypersensitivity reactions during therapy with the nucleoside reverse transcriptase inhibitor abacavir. Clin Ther. 2001;23:1603–1614. doi: 10.1016/s0149-2918(01)80132-6. [DOI] [PubMed] [Google Scholar]
- 3.Hetherington S, Hughes AR, Mosteller M, Shortino D, Baker KL, Spreen W, et al. Genetic variations in HLA-B region and hypersensitivity reactions to abacavir. Lancet. 2002;359:1121–1122. doi: 10.1016/S0140-6736(02)08158-8. [DOI] [PubMed] [Google Scholar]
- 4.Rauch A, Nolan D, Martin A, McKinnon E, Almeida C, Mallal S. Prospective genetic screening decreases the incidence of abacavir hypersensitivity reactions in the Western Australian HIV cohort study. Clin Infect Dis. 2006;43:99–102. doi: 10.1086/504874. [DOI] [PubMed] [Google Scholar]
- 5.Mallal S, Phillips E, Carosi G, Molina JM, Workman C, Tomazic J, et al. HLA-B*5701 Screening for Hypersensitivity to Abacavir. N Engl J Med. 2008;358:568–579. doi: 10.1056/NEJMoa0706135. [DOI] [PubMed] [Google Scholar]
- 6.DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents -- A Working Group of the Office of AIDS Research Advisory Council (OARAC) [(Accessed March 5 2008)];Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2008 http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf.
- 7.Freedberg KA, Losina E, Weinstein MC, Paltiel AD, Cohen CJ, Seage GR, et al. The cost effectiveness of combination antiretroviral therapy for HIV disease. N Engl J Med. 2001;344:824–831. doi: 10.1056/NEJM200103153441108. [DOI] [PubMed] [Google Scholar]
- 8.Schackman BR, Gebo KA, Walensky RP, Losina E, Muccio T, Sax PE, et al. The lifetime cost of current human immunodeficiency virus care in the United States. Med Care. 2006;44:990–997. doi: 10.1097/01.mlr.0000228021.89490.2a. [DOI] [PubMed] [Google Scholar]
- 9.Yeni P, Sutherland-Phillips D, Wannamaker P, Hernandez J, Brothers C, Yau L, et al. Reported incidence and severity of suspected abacavir hypersensitivity reactions (HSR) through at least 6 weeks in a large, controlled clinical trial using a once-daily (OAD) abacavir 600mg/lamivudine 300mg tablet (ABC/3TC FDC) dual nucleoside backbone with a boosted protease inhibitor: The KLEAN study [abstract TuPe2.4C08]. 3rd IAS Conference on HIV Pathogenesis, Treatment and Prevention; Rio de Janeiro, Brazil. July 24–27 2005. [Google Scholar]
- 10.Gallant JE, DeJesus E, Arribas JR, Pozniak AL, Gazzard B, Campo RE, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med. 2006;354:251–260. doi: 10.1056/NEJMoa051871. [DOI] [PubMed] [Google Scholar]
- 11.Dodek P, Phillips P. Questionable history of immediate-type hypersensitivity to penicillin in Staphylococcal endocarditis: treatment based on skin-test results versus empirical alternative treatment--A decision analysis. Clin Infect Dis. 1999;29:1251–1256. doi: 10.1086/313435. [DOI] [PubMed] [Google Scholar]
- 12.Pepper PV, Owens DK. Cost-effectiveness of the pneumococcal vaccine in healthy younger adults. Med Decis Making. 2002;22:S45–57. doi: 10.1177/027298902237705. [DOI] [PubMed] [Google Scholar]
- 13.Johnson MA, Gathe JC, Jr, Podzamczer D, Molina JM, Naylor CT, Chiu YL, et al. A once-daily lopinavir/ritonavir-based regimen provides noninferior antiviral activity compared with a twice-daily regimen. J Acquir Immune Defic Syndr. 2006;43:153–160. doi: 10.1097/01.qai.0000242449.67155.1a. [DOI] [PubMed] [Google Scholar]
- 14.Arribas J, Pozniak AL, Gallant JE, DeJesus E, Campo RE, Chen SS, et al. Three-year safety and efficacy of emtricitabine (FTC)/tenofovir DF (TDF) and efavirenz (EFV) compared to fixed dose zidovudine/lamivudine (AZT/3TC) and EFV in antiretroviral treatment-naïve patients [abstract WEPEB029]. 4th IAS Conference on HIV Pathogenesis, Treatment and Prevention; Sydney, Australia. July 22–25 2007. [Google Scholar]
- 15.Laboratory Corporation of America. [(Accessed March 5 2008)];HLA-B5701 Test. 2007 http://www.labcorp.com/datasets/labcorp/html/chapter/mono/hl002200.htm.
- 16.Centers for Medicare and Medicaid Services. Clinical Laboratory Fee Schedule. http://www.cms.hhs.gov/ClinicalLabFeeSched/01_overview.asp (Accessed 5 March 2008); 2006.
- 17.Red Book. Montvale, NJ: Thomson PDR; 2006. [Google Scholar]
- 18.Centers for Medicare and Medicaid Services. [(Accessed March 5 2008)];Medicare Physician Fee Schedule. 2006 http://www.cms.hhs.gov/PhysicianFeeSched/01_Overview.asp#TopOfPage.
- 19.Cong ME, Heneine W, Garcia-Lerma JG. The fitness cost of mutations associated with human immunodeficiency virus type 1 drug resistance is modulated by mutational interactions. J Virol. 2007;81:3037–3041. doi: 10.1128/JVI.02712-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips EJ. Genetic screening to prevent abacavir hypersensitivity reaction: are we there yet? Clin Infect Dis. 2006;43:103–105. doi: 10.1086/504878. [DOI] [PubMed] [Google Scholar]
- 21.Losina E, Schackman BR, Sadownik S, Gebo KA, Walensky RP, Weinstein MC, et al. Disparities in survival attributable to suboptimal HIV care in the US: Influence of gender and race/ethnicity [abstract 142]. 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles, CA. February 25–28 2007. [Google Scholar]
- 22. [(Accessed March 5 2008)];Allele frequencies in worldwide populations. www.allelefrequencies.net.
- 23.Saag M, Balu R, Brachman P, Brothers C, Stancil B, Mosteller M, et al. High sensitivity of HLA-B*5701 in whites and blacks in immunologically-confirmed cases of abacavir hypersensitivity (ABC HSR) [abstract WEAB305]. 4th IAS Conference on HIV Pathogenesis, Treatment and Prevention; Sydney, Australia. July 22–25 2007. [Google Scholar]
- 24.NIAID, NIH. [Febraury 28, 2008];NIAID Modifies HIV Antiretroviral Treatment Study: Combination Therapy that Includes ABC/3TC Found Less Effective in Subgroup of Antiretroviral-Naïve Individuals. http://www3.niaid.nih.gov/news/newsreleases/2008/actg5202bulletin.htm.
- 25.Phillips KA, Veenstra DL, Oren E, Lee JK, Sadee W. Potential role of pharmacogenomics in reducing adverse drug reactions: a systematic review. JAMA. 2001;286:2270–2279. doi: 10.1001/jama.286.18.2270. [DOI] [PubMed] [Google Scholar]
- 26.Ma Q, Brazeau D, Forrest A, Morse GD. Advances in pharmacogenomics of antiretrovirals: an update. Pharmacogenomics. 2007;8:1169–1178. doi: 10.2217/14622416.8.9.1169. [DOI] [PubMed] [Google Scholar]
- 27.Hughes DA, Vilar FJ, Ward CC, Alfirevic A, Park BK, Pirmohamed M. Cost-effectiveness analysis of HLA B*5701 genotyping in preventing abacavir hypersensitivity. Pharmacogenetics. 2004;14:335–342. doi: 10.1097/00008571-200406000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Josephson F, Albert J, Flamholc L, Gisslen M, Karlstrom O, Lindgren SR, et al. Antiretroviral treatment of HIV infection: Swedish recommendations 2007. Scand J Infect Dis. 2007;39:486–507. doi: 10.1080/00365540701383154. [DOI] [PubMed] [Google Scholar]
- 29.Sabin CA, Worm SW, Weber R, Reiss P, El-Sadr W, Dabis F, et al. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration. Lancet. 2008;371:1417–1426. doi: 10.1016/S0140-6736(08)60423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith K, Fine D, Patel P, Bellos N, Sloan L, Lackey P, et al. Efficacy and safety of abacavir/lamivudine compared to tenofovir/emtricitabine in combination with once-daily lopinavir/ritonavir through 48 weeks in the HEAT study [abstract 774]. 15th Conference on Retroviruses and Opportunistic Infections; Boston, Massachusetts. February 3–6 2008. [Google Scholar]
- 31.Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, Miller MD, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292:191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 32.Samet JH, Freedberg KA, Savetsky JB, Sullivan LM, Stein MD. Understanding delay to medical care for HIV infection: the long-term non-presenter. AIDS. 2001;15:77–85. doi: 10.1097/00002030-200101050-00012. [DOI] [PubMed] [Google Scholar]
- 33.Mallal S, Nolan D, Witt C, Masel G, Martin AM, Moore C, et al. Association between presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. Lancet. 2002;359:727–732. doi: 10.1016/s0140-6736(02)07873-x. [DOI] [PubMed] [Google Scholar]
- 34.Zucman D, Truchis P, Majerholc C, Stegman S, Caillat-Zucman S. Prospective screening for human leukocyte antigen-B*5701 avoids abacavir hypersensitivity reaction in the ethnically mixed French HIV population. J Acquir Immune Defic Syndr. 2007;45:1–3. doi: 10.1097/QAI.0b013e318046ea31. [DOI] [PubMed] [Google Scholar]
- 35.Pozniak AL, Gallant JE, DeJesus E, Arribas JR, Gazzard B, Campo RE, et al. Tenofovir disoproxil fumarate, emtricitabine, and efavirenz versus fixed-dose zidovudine/lamivudine and efavirenz in antiretroviral-naive patients: virologic, immunologic, and morphologic changes--a 96-week analysis. J Acquir Immune Defic Syndr. 2006;43:535–540. doi: 10.1097/01.qai.0000245886.51262.67. [DOI] [PubMed] [Google Scholar]