Abstract
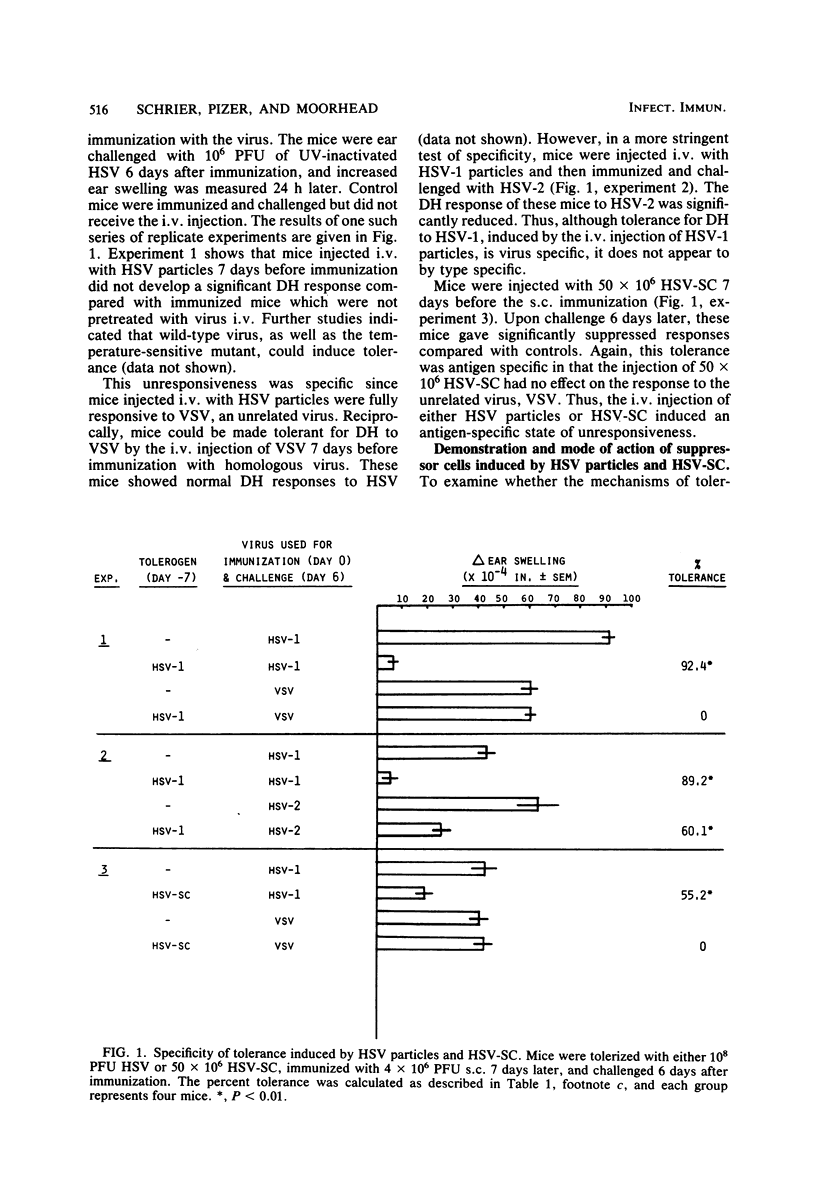
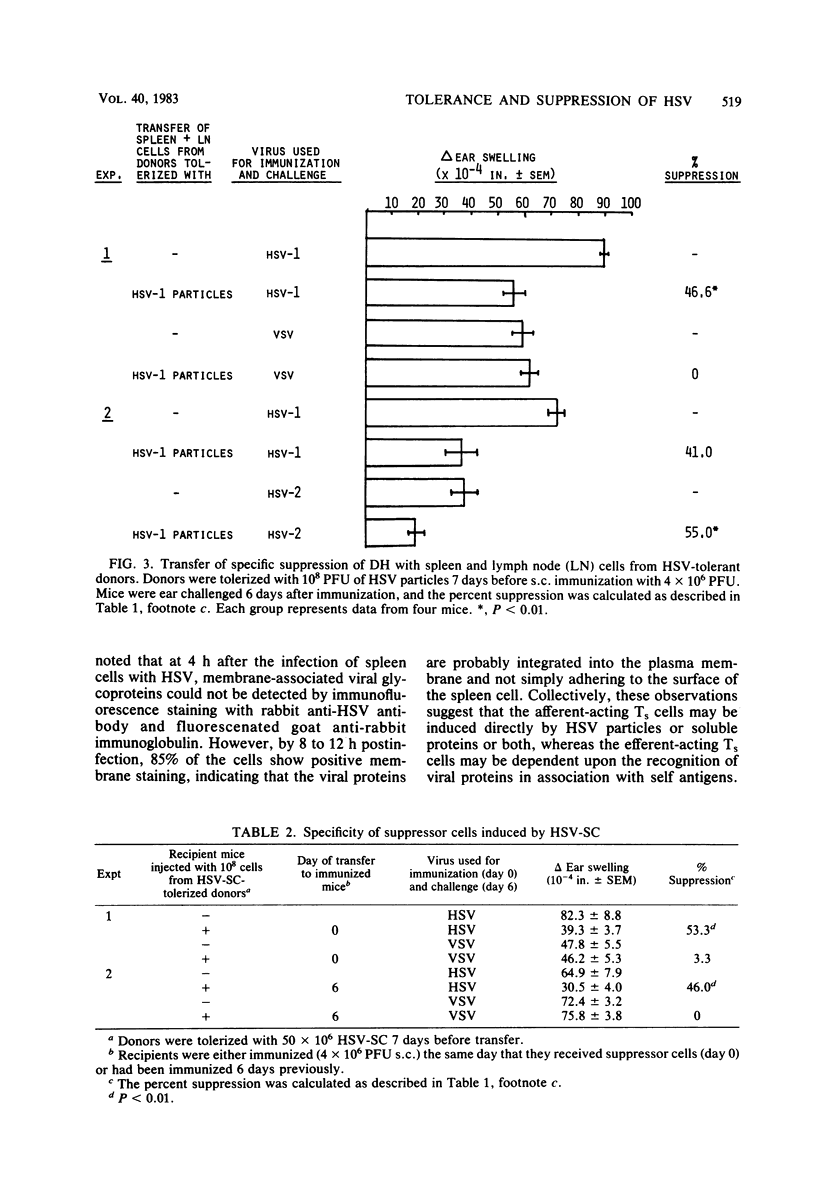
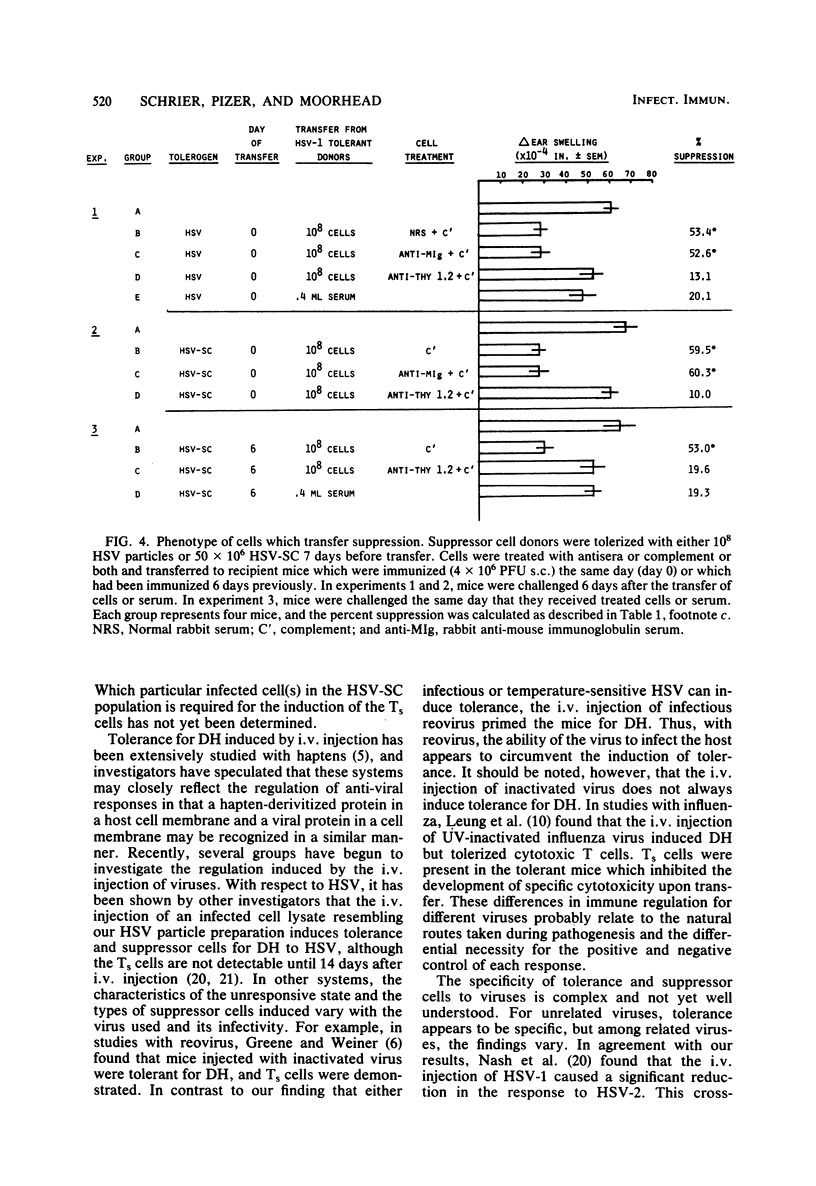
In this report, we examine tolerance (hyporesponsiveness) and suppression of delayed hypersensitivity (DH) to herpes simplex virus (HSV) in mice, using two different forms of tolerogen: HSV particles and HSV-infected spleen cells. The intravenous injection of mice with either HSV particles or spleen cells 7 days before subcutaneous immunization with virus induced a profound state of unresponsiveness. This unresponsive state was mediated, at least in part, by suppressor T cells (Ts), which were demonstrated by passive transfer to naive recipients. However, different types of Ts were induced depending on the form of the tolerogen. The injection of HSV particles induced Ts which suppressed the induction but not the expression of DH. On the other hand, the injection of HSV spleen cells induced two types of Ts: one which inhibited the induction of the DH response and one which inhibited the expression of DH to HSV. Both tolerance and Ts are virus specific (i.e., the DH response to an unrelated virus was not inhibited) but not type specific for HSV type 1 and HSV type 2. Since both virus particles and virus-infected cells may be present in the blood during HSV infection, the induction of this type of immune regulation may influence the outcome of both acute and latent HSV infections.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D., Young L. S., Meyer R. D., Blevins A. H. Infectious complications of neoplastic disease. Med Clin North Am. 1971 May;55(3):729–745. doi: 10.1016/s0025-7125(16)32514-7. [DOI] [PubMed] [Google Scholar]
- BAKER W. H. J., LAWTON A. M., MCCARTHY K. Primary generalized infection caused by herpes simplex virus. Br Med J. 1952 Dec 20;2(4798):1334–1336. doi: 10.1136/bmj.2.4798.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach B. A., Sherman L., Benacerraf B., Greene M. I. Mechanisms of regulation of cell-mediated immunity. II. Induction and suppression of delayed-type hypersensitivity to azobenzenearsonate-coupled syngeneic cells. J Immunol. 1978 Oct;121(4):1460–1468. [PubMed] [Google Scholar]
- Burns W., Billups L. C., Notkins A. L. Thymus dependence of viral antigens. Nature. 1975 Aug 21;256(5519):654–656. doi: 10.1038/256654a0. [DOI] [PubMed] [Google Scholar]
- Greene M. I., Weiner H. L. Delayed hypersensitivity in mice infected with reovirus. II. Induction of tolerance and suppressor T cells to viral specific gene products. J Immunol. 1980 Jul;125(1):283–287. [PubMed] [Google Scholar]
- Howes E. L., Taylor W., Mitchison N. A., Simpson E. MHC matching shows that at least two T-cell subsets determine resistance to HSV. Nature. 1979 Jan 4;277(5691):66–68. doi: 10.1038/277067a0. [DOI] [PubMed] [Google Scholar]
- Johnson R. T., Mims C. A. Pathogenesis of viral infections of the nervous system. N Engl J Med. 1968 Jan 4;278(1):23–contd. doi: 10.1056/NEJM196801042780106. [DOI] [PubMed] [Google Scholar]
- Lawman M. J., Rouse B. T., Courtney R. J., Walker R. D. Cell-mediated immunity against herpes simplex induction of cytotoxic T lymphocytes. Infect Immun. 1980 Jan;27(1):133–139. doi: 10.1128/iai.27.1.133-139.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K., Ashman R. B., Ertl H. C., Ada G. L. Selective suppression of the cytotoxic T cell response to influenza virus in mice. Eur J Immunol. 1980 Nov;10(11):803–810. doi: 10.1002/eji.1830101102. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Russell S. M. Delayed-type hypersensitivity to influenza virus. Induction of antigen-specific suppressor T cells for delayed-type hypersensitivity to hemagglutinin during influenza virus infection in mice. J Exp Med. 1980 Apr 1;151(4):799–814. doi: 10.1084/jem.151.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf J. F., Hamilton D. S., Reichert R. W. Herpetic keratitis in athymic (nude) mice. Infect Immun. 1979 Dec;26(3):1164–1171. doi: 10.1128/iai.26.3.1164-1171.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. D., Sy M. S., Claman H. N. Suppressor T cell mechanisms in contact sensitivity. I. Efferent blockade by syninduced suppressor T cells. J Immunol. 1978 Jul;121(1):265–273. [PubMed] [Google Scholar]
- Moorhead J. W. Tolerance and contact sensitivity to DNFA in mice. VIII. Identification of distinct T cell subpopulations that mediate in vivo and in vitro manifestations of delayed hypersensitivity. J Immunol. 1978 Jan;120(1):137–144. [PubMed] [Google Scholar]
- Moorhead J. W. Tolerance and contact sensitivity to DNFB in mice. VI. Inhibition of afferent sensitivity by suppressor T cells in adoptive tolerance. J Immunol. 1976 Sep;117(3):802–806. [PubMed] [Google Scholar]
- Moorhead J. W., Walters C. S., Claman H. N. Immunologic reactions to haptens on autologous carriers. I. Participation of both thymus-derived and bone marrow-derived cells in the secondary in vitro response. J Exp Med. 1973 Feb 1;137(2):411–423. doi: 10.1084/jem.137.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller S. A., Herrmann E. C., Jr, Winkelmann R. K. Herpes simplex infections in hematologic malignancies. Am J Med. 1972 Jan;52(1):102–114. doi: 10.1016/0002-9343(72)90012-5. [DOI] [PubMed] [Google Scholar]
- Nagafuchi S., Oda H., Mori R., Taniguchi T. Mechanism of acquired resistance to herpes simplex virus infection as studied in nude mice. J Gen Virol. 1979 Sep;44(3):715–723. doi: 10.1099/0022-1317-44-3-715. [DOI] [PubMed] [Google Scholar]
- Nahmias A. J., Alford C. A., Korones S. B. Infection of the newborn with herpesvirus hominis. Adv Pediatr. 1970;17:185–226. [PubMed] [Google Scholar]
- Nash A. A., Gell P. G., Wildy P. Tolerance and immunity in mice infected with herpes simplex virus: simultaneous induction of protective immunity and tolerance to delayed-type hypersensitivity. Immunology. 1981 May;43(1):153–159. [PMC free article] [PubMed] [Google Scholar]
- Nash A. A., Phelan J., Gell P. G., Wildy P. Tolerance and immunity in mice infected with herpes simplex virus: studies on the mechanism of tolerance to delayed-type hypersensitivity. Immunology. 1981 Jun;43(2):363–369. [PMC free article] [PubMed] [Google Scholar]
- Nash A. A., Phelan J., Wildy P. Cell-mediated immunity in herpes simplex virus-infected mice: H-2 mapping of the delayed-type hypersensitivity response and the antiviral T cell response. J Immunol. 1981 Apr;126(4):1260–1262. [PubMed] [Google Scholar]
- Nash A. A., Quartey-Papafio R., Wildy P. Cell-mediated immunity in herpes simplex virus-infected mice: functional analysis of lymph node cells during periods of acute and latent infection, with reference to cytotoxic and memory cells. J Gen Virol. 1980 Aug;49(2):309–317. doi: 10.1099/0022-1317-49-2-309. [DOI] [PubMed] [Google Scholar]
- Oakes J. E. Role for cell-mediated immunity in the resistance of mice to subcutaneous herpes simplex virus infection. Infect Immun. 1975 Jul;12(1):166–172. doi: 10.1128/iai.12.1.166-172.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfizenmaier K., Jung H., Starzinski-Powitz A., Röllinghoff M., Wagner H. The role of T cells in anti-herpes simplex virus immunity. I. Induction of antigen-specific cytotoxic T lymphocytes. J Immunol. 1977 Sep;119(3):939–944. [PubMed] [Google Scholar]
- Rager-Zisman B., Allison A. C. Mechanism of immunologic resistance to herpes simplex virus 1 (HSV-1) infection. J Immunol. 1976 Jan;116(1):35–40. [PubMed] [Google Scholar]
- Rand K. H., Rasmussen L. E., Pollard R. B., Arvin A., Merigan T. C. Cellular immunity and herpesvirus infections in cardiac-transplant patients. N Engl J Med. 1977 Jun 16;296(24):1372–1377. doi: 10.1056/NEJM197706162962402. [DOI] [PubMed] [Google Scholar]
- Russell A. S. Cell-mediated immunity to herpes simplex virus in man. J Infect Dis. 1974 Feb;129(2):142–146. doi: 10.1093/infdis/129.2.142. [DOI] [PubMed] [Google Scholar]
- Schrier R. D., Pizer L. I., Moorhead J. W. Delayed hypersensitivity to herpes simplex virus: murine model. Infect Immun. 1982 Feb;35(2):566–571. doi: 10.1128/iai.35.2.566-571.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer E. S., Andersen H. K. Clinically evident, non-terminal infections with herpesviruses and the wart virus in immunosuppressed renal allograft recipients. Br Med J. 1970 Aug 1;3(5717):251–254. doi: 10.1136/bmj.3.5717.251. [DOI] [PMC free article] [PubMed] [Google Scholar]


