Abstract
Background & Aims
Smooth muscle is essential for maintaining homeostasis for many body functions and provides adaptive responses to stresses imposed by pathological disorders. Identified cell signaling networks have defined many potential mechanisms for initiating smooth muscle contraction with or without myosin regulatory light chain (RLC) phosphorylation by myosin light chain kinase (MLCK). We generate tamoxifen-inducible and smooth muscle-specific MLCK knockout (KO) mice and provide direct loss-of-function evidence that shows the primary importance of MLCK in phasic smooth muscle contractions.
Methods
We used the Cre-loxP system to establish Mlck floxed mice in which exons 23, 24, and 25 were flanked by two loxP sites. Smooth muscle-specific MLCK KO mice were generated by crossing Mlck floxed mice with SM-CreERT2 (ki) mice followed by tamoxifen treatment. The phenotype was assessed by histological, biochemical, molecular, cell biological and physiological analyses.
Results
Targeted deletion of MLCK in adult mouse smooth muscle resulted in severe gut dysmotility characterized by weak peristalsis, dilation of the digestive tract and reduction of feces excretion and food intake. There was also abnormal urinary bladder function and lower blood pressure. Isolated muscles showed a loss of RLC phosphorylation and force development induced by K+-depolarization. The kinase knockout also markedly reduced RLC phosphorylation and force development with acetylcholine (ACh) which activates Ca2+-sensitizing signaling pathways.
Conclusions
MLCK and its phosphorylation of RLC are required physiologically for smooth muscle contraction and essential for normal gastrointestinal motility.
Smooth muscles are responsible for contraction of the hollow organs in the body such as the gastrointestinal tract, urinary bladder, blood vessels and the uterus. Normal contractility is essential for maintaining homeostasis and adaptive responses to stresses imposed by pathological disorders. Smooth muscle contractility is regulated by a network of signaling pathways centered on the molecular motor myosin as well as membrane properties associated with calcium handling and cell adhesion.1–5 Depolarization of the cell membrane activates voltage-gated Ca2+-channels resulting in Ca2+ influx and activation of myosin cross-bridge cycling on actin filaments by RLC phosphorylation catalyzed by Ca2+/calmodulin-dependent MLCK. However, agonist stimulation of G-protein coupled receptors on smooth muscle cell surfaces may recruit other regulatory elements. One scheme for gastrointestinal smooth muscle is that the initial increase in [Ca2+]i is rapidly dissipated, resulting in MLCK inactivation.3 Maintenance of RLC phosphorylation and muscle force are then regulated by several signaling pathways involving Ca2+-independent kinase(s) and inhibition of myosin phosphatase. There is also evidence that force maintenance switches to thin-filament regulation independent of RLC phosphorylation after the initial increase in [Ca2+]i where unphosphorylated RLC does not completely inhibit myosin ATPase activity.6,7
These conflicting proposals depend largely on results obtained by correlating changes in measured responses such as [Ca2+]i, extents of protein phosphorylations and force development with interventions by chemical inhibitors in isolated muscles or cells in culture. However, elucidation of MLCK function in vivo is crucial for understanding the complex regulatory processes involved in smooth muscle contraction. Smooth muscle MLCK containing a kinase catalytic core with substrate binding sites and several structural motifs is ubiquitously expressed in different cells in the body.8–11 Conventional deletion of MLCK expression leads to embryonic or perinatal lethality12 making it impossible to determine the functional importance of MLCK in contraction of mature smooth muscle. Thus, we crossed mice containing floxed MLCK alleles with SM-CreERT2 (ki) mice expressing a tamoxifen-activated Cre recombinase driven by the SM-22 promoter to delete MLCK expression specifically in smooth muscles of adult mice.
Materials and Methods
Generation of floxed Mlck mice and tissue-specific knockout mice (MLCKSMKO)
BAC (bacterial artificial chromosome)-retrieval methods were used for constructing the targeting vector.13,14 In brief, the Mlck locus encoding a portion of the kinase domain was retrieved from a 129/sv BAC clone bMQ 366n04 (provided by Sanger Institute) by a retrieval vector containing two homologous arms. Exons 23 to 25 which encode the ATP-binding site of the kinase were flanked by two loxP sites and an frt-Neo-frt cassette as a positive selection marker. Additionally, this deletion causes an out-of-frame reading shift and thereby generates a premature stop codon and a loss-of-function allele (Figure 1). Embryonic stem (ES) W4 cells were electroporated with the linearized targeting vector, selected, then expanded for Southern blot analysis. Chimeric mice were generated by injecting ES cells into C57BL/6 blastocysts followed by transfer to pseudopregnant mice. To generate MLCKSMKO mice, chimeric mice were crossed with SM-CreERT2 (ki) mice expressing a tamoxifen-inducible Cre recombinase under control of the SM22 promoter. Subsequent breeding yielded genotypes for experiments. Tamoxifen was injected i.p. for five consecutive days at a dose of 1mg per day as described.15 The tamoxifen (100 mg, Sigma, T5648) was dissolved in 0.5 ml ethanol followed by 9.5 ml sunflower oil at a concentration of 10 mg/ml and stored at −20°C for up to one month. All mice used in this study were of a mixed 129/B6 background.
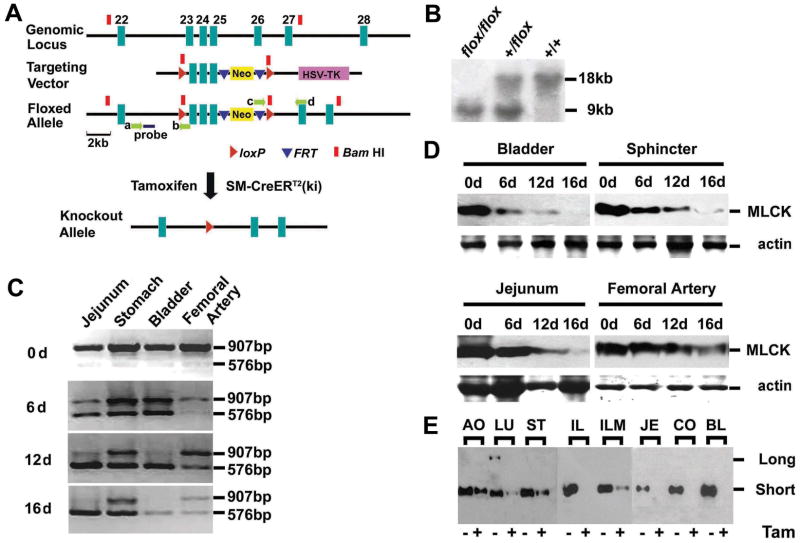
Figure 1.
Targeted disruption of Mlck gene in smooth muscle. (A) Schematic representation of Mlck smooth muscle-specific knockout strategy. The 8.9-kb genomic DNA fragment containing Mlck exons 23–25 was subcloned from 129/sv BAC using gap repair. The floxed Neo cassette was targeted upstream of exon 23 and excision of the floxed Neo cassette left behind a single loxP site (arrowheads in red) at the targeted locus. The single PGK-Neo cassette flanked by FRT sites (arrowheads in blue) and a downstream loxP site, was then introduced downstream of exon 25. The Neo cassette contains a BamHI site (blocks in red) which is favorable for Southern blot analysis. The floxed allele (Mlckflox) was formed after homologous recombination in ES cells. Mice containing the floxed allele were crossed with SM-CreERT2 (ki) mice that express a tamoxifen-activated Cre recombinase to generate Mlck+/flox; SM-CreERT2 and Mlckflox/flox; SM-CreERT2 mice. The ablation of exons 23–25 was induced by tamoxifen injection. The probe used for Southern blot analysis is shown as a solid blue bar, and the locations of the PCR primers a–d are indicated by green arrows. (B) Tail DNA isolated from homozygous (flox/flox) floxed, heterozygous (+/flox) and wild-type (+/+) mice was digested with BamHI and analyzed by Southern blot. The wild-type and floxed allele yield 18kb and 9kb fragments respectively. (C) RT-PCR assay for MLCK mRNA. Various smooth muscle tissues were collected from MLCKSMKO mice induced with tamoxifen at different time points. The mRNA containing exons 23–25 was amplified by RT-PCR. The products in size of 907bp and 576bp reflect wild-type and mutated MLCK respectively. (D) Western blots of MLCK in tamoxifen-treated tissues collected at indicated days (d). Total actin stained with Coomassie Brilliant Blue G-250 was used as protein loading control. Tissues included the urinary bladder, internal anal sphincter, jejunum and femoral artery. (E) Western blots of MLCK protein from floxed mice treated with tamoxifen (Tam+) or its vehicle (Tam-) for 16 days. The amount of loaded protein was normalized by total actin control. AO, aorta; LU, lung; ST, stomach; IL, ileum; ILM, ileum mucosa; JE, jejunum; CO, colon; BL, bladder.
All experiments were conducted in accordance with Animal Care and Use Committee of Model Animal Research Center.
Western blot analysis
Western blot analyses were performed for measurement of MLCK and other protein expressions.16 Briefly, tissue samples were collected and frozen quickly in 10% trichloroacetic acid and 10 mM dithiothreitol in acetone precooled to a slush at −80°C. After homogenizing thoroughly, the sample pellet was washed three times with ether for 5 minutes each and dried to remove residual ether. The protein was dissolved completely in 8 M urea solution. Protein concentration was measured with bicinchoinic acid (BCA) protein assay reagent. Equal amounts of protein were loaded for 6% SDS-PAGE followed by protein transfer to a nitrocellulose membrane. The membrane was then probed with a monoclonal antibody to MLCK (Sigma K36) and secondary antibody sequentially. The membrane was incubated in Super Signal West Dura substrate (PIERCE) before exposure to film. Antibodies for other proteins were to ILK (Sigma), ROCK-1 (Santa Cruz), MYPT1 (Upstate), phospho-MYPT1[Thr-696] (Upstate), phospho-MYPT1[Thr-850] (Upstate), RLC,16 smooth muscle heavy chain 2 (SM-MHC, Abcam), telokin (Santa Cruz), SM22 (Sigma) and Cre (Novagen).
Measurement of myosin regulatory light chain phosphorylation
Urea/glycerol-PAGE electrophoresis was used for measurement of RLC phosphorylation where the nonphosphorylated RLC is separated from the monophosphorylated RLC.16 The phosphorylated RLC bands were verified by anti-phospho-myosin light chain [pSer19] antibody (data not shown, Sigma). The percent phosphorylated RLC was determined by quantification with Jieda 801 Image Analysis system 3.3.2: Jiangsu JEDA Science-Technology Development Co., Ltd, Nanjing, China.
Analysis of smooth muscle contractility
Mice of either sex were sacrificed by cervical dislocation. Segments (6 mm long) from jejunum and ileum were mounted in a longitudinal orientation in an organ bath. Tension was recorded isometrically at 37°C by a force transducer (MLT0202, ADInstruments) connected to a PowerLab (ADInstruments) recording device. Resting tension was set to 0.1–0.3 g. Force measurements were performed as described17 and detailed in Supplementary Methods. Force per cross-sectional area was calculated from histological measurements of the longitudinal muscle layer.
Measurements of smooth muscle functions in vivo
The charcoal transit test was performed with minor modification.18 Mice deprived of food overnight were administrated 100 μl of charcoal test meal (5% (w/v) charcoal in 0.9% NaCl) by orogastric gavage. The mice were sacrificed 90 minutes after receiving the test meal, and the distance traveled by the black test meal was measured. Intestinal motility was expressed by the ratio of the distance traveled by the test meal to the total length of the small intestine (pylorus-cecum). Freely moving mice were individually placed on alkaline phenol-red immersed filter papers and short term voluntary urination was analyzed.19 After one hr, the number of small (<0.2 cm2) and large (>0.2 cm2) spots and the total area of urine spots were measured. Blood pressure was also measured as described.20
Adenovirus infection for MLCK expression
Jejunum smooth muscle strips (6 mm) with epithelium removed were infected with MLCK-expressing adenovirus (Adv-MLCK) and cultured up to 60 h. Adv-MLCK was prepared by releasing MLCK fragments (2456bp and 3278bp) with HindIII/BamHI digestion from pEGFP-MLCK21016 and introducing into pShuttle-IRES-GFP-1 vector followed by virus preparation according to the guide of an AdEasy-1 kit (Strategene).
Statistical analysis
Data are presented as the mean±SEM. Differences between groups were determined by Student’s t-test with significance at p<0.05.
Results
Generation and characterization of MLCKSMKO mice
We established a mouse strain with loxP sites flanking exons 23–25 of the coding region for the kinase ATP-binding site (Figure 1 and Supplementary Figure 1). Mice containing the floxed Mlck (Mlckflox allele) gene were crossed with SM-CreERT2 (ki) mice expressing a tamoxifen-activated Cre recombinase15 to ablate Mlck expression in adult smooth muscle. Immunohistochemistry showed tissue-specific expression of CreERT2 in the smooth muscle layers in the ileum and nuclear translocation of Cre ERT2 after tamoxifen induction (Supplementary Figure 1D). However, different tissues had different amounts of CreERT2 where arterial expression of Cre was lower than expression in intestinal or bladder smooth muscles (Supplementary Figure 1B). The activated CreERT2 induced by tamoxifen caused deletion of exons 23–25 in various smooth muscle-containing organs (Supplementary Figure 1C). To determine whether deletion of targeted exons altered Mlck gene expression after tamoxifen injection, we measured MLCK mRNA and protein contents in different tissues. RT-PCR analysis showed that the Mlck gene in jejunum and bladder was converted 12 days after the start of tamoxifen injections (Figure 1C). Western blots showed that the gene conversion caused a loss of MLCK protein in a time-dependent manner (Figure 1D). Immunohistochemistry analysis showed no MLCK protein in the smooth muscle layer of MLCK-inactivated ileum (Supplementary Figure 1E). The loss of kinase protein reflects the turnover of MLCK, which showed an estimated half-life of 5 days in bladder, jejunum and the internal anal sphincter muscles. MLCK expression was not completely abolished in arterial tissues by 16 days (Figure 1D), consistent with the low Cre expression (Supplementary Figure 1B). MLCK protein decreased about 50% in the aorta and femoral artery in contrast to the apparent depletion in ileum, jejunum and bladder (Figure 1D and E). Interestingly, stomach tissue also showed only partial deletion of MLCK protein where RT-PCR analysis was more similar to the femoral artery, as compared to jejunum and bladder. The differential expression of SM-CreERT2 (ki) in mice is also observed with LacZ analysis, indicating some selectivity of SM22 promoter activation and greater recombination efficiency in phasic smooth muscle cells.15 MLCK expression in intestinal mucosa was also decreased (Figure 1E), which may be accounted for by effects on microvascular smooth muscle and/or an unhealthy condition of the mucosa in the knockout. Because the recombination efficiency appears to be 100% in phasic smooth muscles13, additional functional characterizations focused on these tissues.
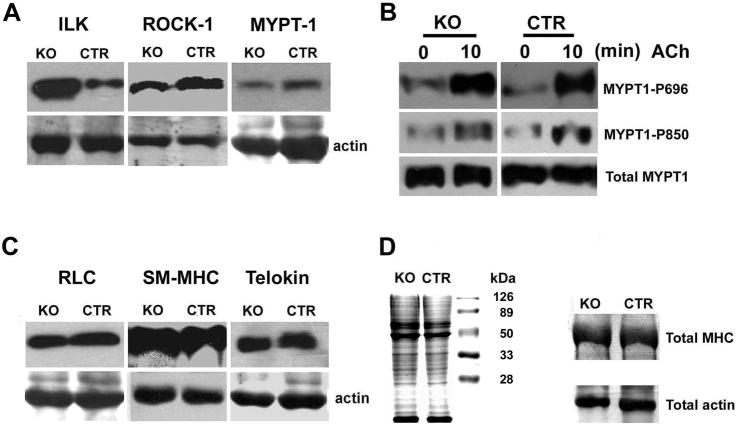
To assess potential compensatory expressions of contraction-related proteins in MLCK knockout mice, we quantified by Western blot the relative amounts of integrin-linked kinase (ILK), Rho-associated coiled-coil-forming protein kinase 1 (ROCK1), myosin phosphatase protein targeting subunit of the RLC phosphatase (MYPT1), RLC, smooth muscle myosin heavy chain and telokin. The results showed an increased expression of ILK (4.4±0.1 fold) but no significant changes in the other proteins (Figure 2A, C and D). Analysis by RT-PCR showed the relative amounts of Zip kinase mRNA in tissues from MLCK knockout and control mice were not changed (data not shown). Additionally, further analysis of ileal smooth muscle from MLCK knockout mice showed no extra MLCK protein fragments arising from mistranslation as detected by Western blotting with the K36 monoclonal antibody that binds within residues 2–42 of the kinase and with anti-telokin antibody that binds with the very C-terminal region of the kinase21 (data not shown).
Figure 2.
Expression of contractile and related regulatory proteins in jejunum from MLCKSMKO mice. Western blot of ILK, ROCK1, MYPT1, RLC, SM-MHC and telokin (A and C) from MLCKSMKO (KO) and CTR jejunum. The samples were resolved by separate SDS-PAGE and total actin stained with Coomassie brilliant blue was used as loading control. MYPT1 phosphorylations at residues 696 and 850 in response to ACh were measured by anti-MYPT1-P696 and anti-MYPT1-P850 antibody (B). Ileal total proteins (30 μg) in MLCKSMKO and CTR were resolved by SDS-PAGE and stained with Coomassie brilliant blue (left side of D). To visualize more clearly the total myosin heavy chain and actin, we performed electrophoresis with different amounts of protein loading (100 μg) for comparisons of KO and CTR samples (right side of D).
Birth of pups including Mlck floxed mice with (Mlckflox/flox; SM-CreERT2 and Mlck+/flox; SM-CreERT2) or without (Mlckflox/flox and Mlck+/flox) CreERT2 occurred in the expected Mendelian ratio. The floxed mice were fertile and reached adulthood without any obvious morphological or behavioral abnormalities. The amounts of MLCK in wild-type, Mlckflox/flox and Mlckflox/flox; SM-CreERT2 smooth muscles were similar (data not shown), indicating that insertion of recombination elements did not interfere with expression of MLCK. In subsequent experiments, we used Mlck+/flox:SM-CreERT2 mice with tamoxifen treatment as controls (CTR). MLCKSMKO represents Mlckflox/flox:SM-CreERT2 mice induced with tamoxifen (five consecutive daily injections with 1 mg tamoxifen per mouse).
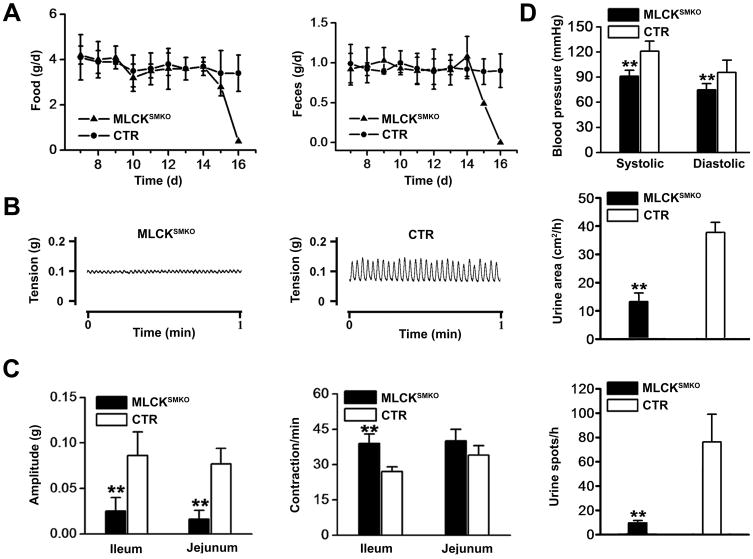
Macrophenotypic analysis showed abnormal gastrointestinal motility (vide infra) in addition to abnormal urinary bladder function and lower blood pressure (Figure 3D). Mice displayed uroschesis during the late period after tamoxifen treatment. Both the number and size of urine spots were decreased significantly. In addition, systolic and diastolic blood pressures significantly decreased from 121±11 to 96±15 and 91±7 to 75±7 mm Hg, respectively. These phenotypes are consistent with the decreased expression of MLCK in smooth muscle cells after tamoxifen treatment.
Figure 3.
Knockout of smooth muscle MLCK attenuates gastrointestinal motility. (A) Time course for food intake (left) and feces excretion (right) after tamoxifen treatment. Days were numbered with the start of tamoxifen injection. The value of each point represents the mean±SEM of MLCKSMKO and the CTR mice (n=3 each group). (B) Representative spontaneous contractions are shown for ileal segments from MLCKSMKO (left) and CTR (right) mice. (C) Quantification of the contraction amplitudes (left) and frequencies (right) are shown. (D) Tail blood pressure of MLCKSMKO and CTR mice was measured 15–16 days after beginning of tamoxifen injection (top). Micturition assay was performed at day 15. The total area of urine spots per hour (middle) and total number of urine spots per hour (bottom) were quantified. Bars represent means ± SEM, n=3–9, **p<0.01 (t-test).
Deletion of MLCK in adult mice caused a failure of gastrointestinal motility
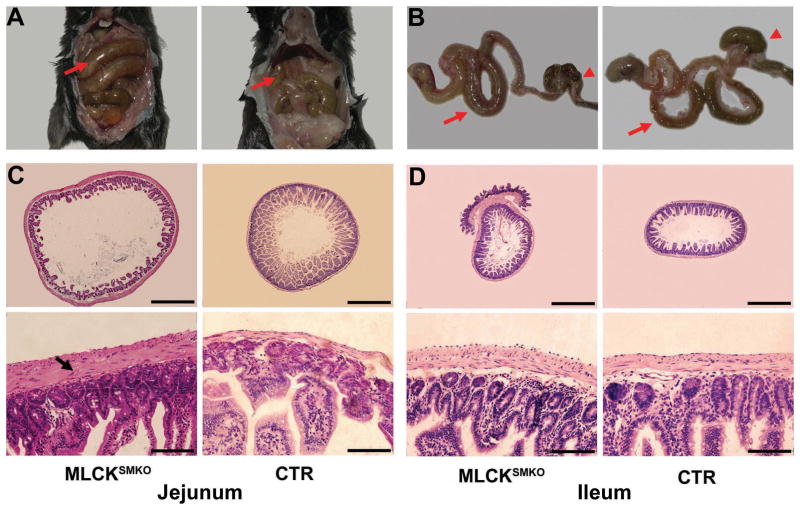
The most obvious phenotypic changes in MLCKSMKO mice were in the phasic smooth muscle systems. MLCKSMKO mice had reduced intestinal motility, an enlarged abdomen and severe spinal sinuosity at 14 to 16 days after starting tamoxifen induction. During this period, excretion of feces and intake of food were markedly reduced (Figure 3A). Using the charcoal transit test18, we found a failure of gastro-emptying in MLCKSMKO mice. All animals died with symptoms of a paralytic gut within 17 days after tamoxifen injection. Necropsy showed grossly extended jejunum and ileum containing abundant liquid (Figure 4A and B). In addition, dysmotility led to mucosal inflammation as reflected in the increased bacterial content, changes in hematological profile and expression of proinflammatory cytokines in jejunum from knockout animals (Supplementary Figure 2). The colon size appeared normal with solid but incompletely separated feces. Peristaltic contractile tension of ileum and jejunum tissues decreased significantly (Figure 3B and C). In contrast, the peristaltic frequency of ileal smooth muscle from MLCKSMKO mice increased 30%, probably due to compensatory effects from pacemaker cells (Figure 3C). Control mice receiving tamoxifen treatment in the same manner had a normal gastrointestinal motility, indicating no adverse effects due to the tamoxifen administration in vivo.
Figure 4.
Abnormal gastrointestinal tract of MLCKSMKO mice. Appearance of gastrointestinal tract of a MLCKSMKO and a CTR mouse in situ (A) and out of the body (B). Note the dilated jejunum (arrows) and compacted feces in caecum and colon (arrowheads) in the MLCKSMKO mouse. Histological examination of the small intestinal tract shows tissue changes with MLCK knockout in smooth muscle. Transverse sections of jejunum (C) and ileum (D) from MLCKSMKO and CTR mice were stained with hematoxylin and eosin. The lumen of the MLCKSMKO jejunum has a larger diameter with rare intestinal villus and a hypertrophic smooth muscle layer (indicated by an arrow). The morphology of ileum from MLCKSMKO mice appears normal except for the hypertrophic smooth muscle layer. Scale bars for C and D: upper row, 500 μm; lower row, 50 μm.
Histological analyses revealed dramatic changes in the intestinal tract (Figure 4C and D). At the late stage of tamoxifen induction, the small intestine was dilated with the average diameter increased significantly (1.3±0.2-fold) for jejunum (1659±108 vs 1265±125 μm, p<0.001). The average diameter of the ileum did not change significantly (1.1±0.2-fold; 1022±152 vs 956±88 μm, p=0.44). Interestingly, the cross-sectional thickness of jejunum muscle layer increased (2.8±0.8-fold for the longitudinal muscle layer and 2.6±0.9-fold for the circular muscle layer; p<0.0001) while the thickness of ileum muscle increased but less (1.3±0.3-fold for longitudinal muscle and 1.5±0.4-fold for circular muscle, (p<0.01). Inspection of the jejunum cross-section revealed noticeably enlarged muscle cells. Further analysis of the number of nuclei in an arc of the wall equal to one-tenth the circumference showed no differences between jejunum from MLCKSMKO and CTR mice (longitudinal muscle: 99±21 vs. 92±12, p=0.69; circular muscle: 91±5 vs. 87±8, p=0.77).22 Thus, the thicker muscle layers were attributed largely to smooth muscle cell hypertrophy. Dysmotility induces inflammation that may contribute to the thickening of the muscle layer.23 We extended our analysis of the composition of proteins in hypertrophic muscles. Jejunum smooth muscles from MLCKSMKO mice showed a similar general protein-staining pattern relative to wild-type mice after SDS-PAGE, and a comparable ratio of smooth muscle myosin and actin (Figure 2D). Thus, the MLCK deletion and resulting selective hypertrophy did not result in significant down-regulation of the primary contractile and associated regulatory proteins that could account for hypomotility associated with a change in the smooth muscle cell phenotype.
Contraction was impaired in MLCKSMKO smooth muscles
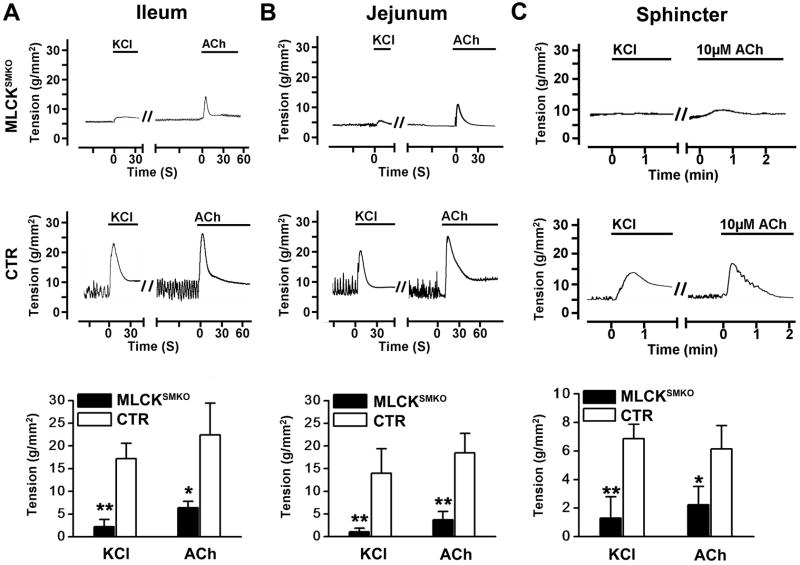
To determine the contractile properties of MLCK-deficient smooth muscle, we measured the tension developed in isolated strips in response to KCl or the muscarinic agonist, acetylcholine (ACh).17 The ileal strips from MLCKSMKO mice developed only 13% of the tension developed by control (CTR) tissues and did not show a transient peak during the initial phase with KCl stimulation (Figure 5A). Similar results were obtained with jejunal tissues (Figure 5B). The responses to KCl were similar with and without the muscarinic antagonist atropine (Figure 6A and B), indicating KCl did not act by stimulating ACh release from neurons in the phasic ileal smooth muscle tissue. To assess the function of MLCK in a smooth muscle that normally maintains high tone in vivo, the internal anal sphincter (IAS) was studied.24 The tension developed by IAS muscle from MLCKSMKO mice also decreased significantly in response to KCl (Figure 5C). These results collectively demonstrate an essential role for MLCK for robust, depolarization-induced smooth muscle contractions.
Figure 5.
Contractions in intestinal smooth muscle and internal anal sphincter (IAS). Representative recordings of ileum (A) and jejunum (B), and IAS (C) from MLCKSMKO (upper row) and CTR (middle row) mice treated with 87 mM KCl or 1μM ACh. Bars show duration of stimulation. Quantification of contraction responses to KCl and ACh (bottom row) for ileum (A), and jejunum (B) and IAS (C). Bars represent means±SEM, n=4–10, **p<0.01, *p<0.05 (t-test).
Figure 6.
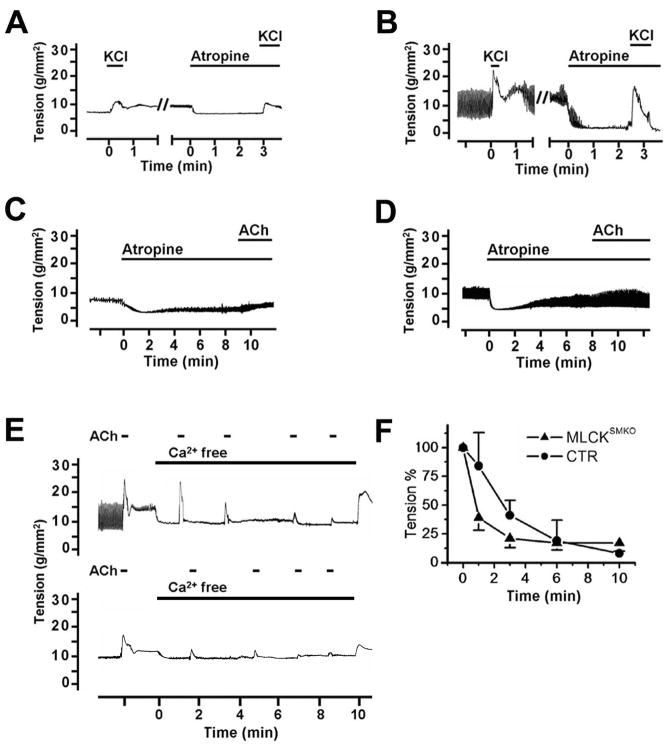
Effects of atropine and Ca2+ depletion on smooth muscle contractility. KCl-induced contraction was not blocked by atropine (1μM) in ileal smooth muscle from MLCKSMKO (A) or CTR (B) mice. ACh (1μM) induced contractions in ileum from MLCKSMKO (C) and control (D) mice were inhibited by atropine. Depletion of Ca2+ in ileal strips by 1 mM EGTA inhibited the contractile responses to repeated exposure to ACh (E). Quantitation of contractile responses of MLCKSMKO and CTR muscles to calcium depletion were normalized (F). Values are means±SEM (n=3–4).
The intestinal smooth muscles from MLCKSMKO mice displayed only 20–28% tension responses to ACh compared to CTR muscle (Figure 5). The contraction responses were decreased significantly for both jejunum and ileum. Treatment with atropine abolished the ACh-induced contraction, indicating a muscarinic-specific response (Figure 6C and D). The force developed by IAS muscle in response to ACh also decreased significantly (Figure 5C). These results show that MLCK plays a central but not exclusive role in agonist-mediated contractions.
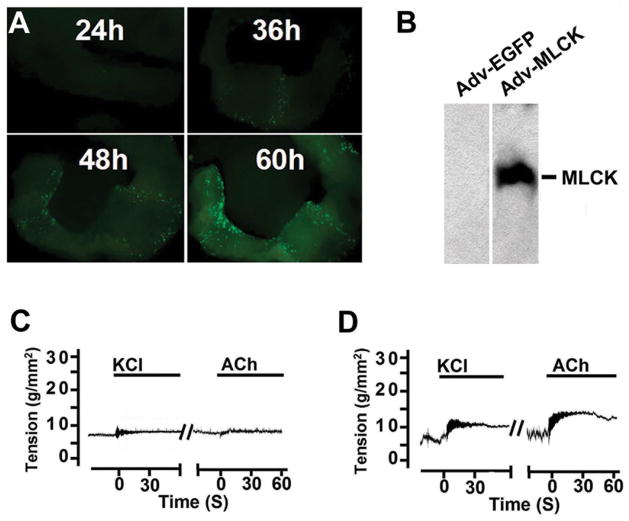
To confirm the functional role of MLCK in smooth muscle contraction, MLCK was expressed after the knockout in jejunum smooth muscle. Tissue strips without epithelium were infected with adenovirus in culture 60 hours for expression of MLCK and GFP. The smooth muscle tissues showed a time-dependent expression of GFP fluorescence as well as MLCK protein (Figure 7A and B) although not all of the smooth muscle cells were infected. The tension developed by the MLCK-deficient muscle partially recovered in response to KCl or ACh in contrast to the tissue not expressing MLCK (Figure 7C and D).
Figure 7.
Expression of exogenous MLCK partially restores contraction of MLCKSMKO smooth muscle. (A) A jejunum smooth muscle strip (6 mm) was infected with MLCK-expressing adenovirus (Adv-MLCK) and cultured up to 60 h. The GFP expression driven by IRES as a tag of MLCK expression in a smooth muscle strip was examined by a dissecting fluorescence microscopy. (B) Exogenous MLCK was detected by Western blot in strips collected at 60 h. The two panels shown were from the same gel, but different lanes. Typical force recordings in response to 87 mM KCl or 1 μM ACh are shown for MLCK-deficient muscle infected with (C) Adv-GFP control virus or (D) Adv-MLCK virus.
RLC phosphorylation responses were inhibited in MLCKSMKO smooth muscles
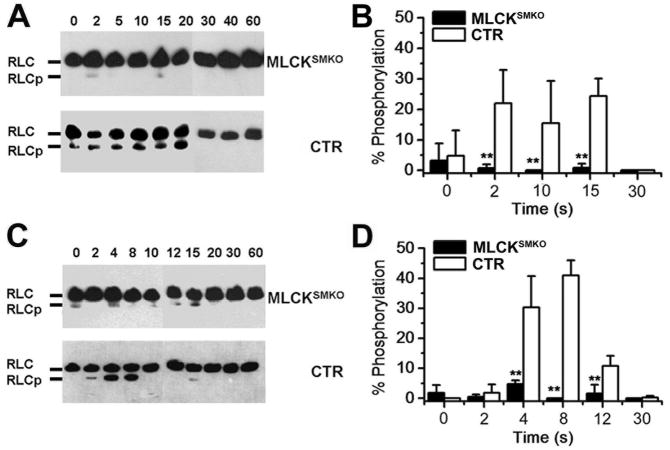
MLCK is a dedicated protein kinase that phosphorylates only myosin RLCs, therefore we measured RLC phosphorylation in response to KCl and ACh. In CTR ileal muscle, RLC phosphorylation increased to 24.3±5.8% by 15 sec after KCl stimulation and then declined (Figure 8A and B). Maximal RLC phosphorylation was significantly less in MLCKSMKO muscle after KCl stimulation (0.8±1.4 %). These results are consistent with the marked attenuation of tension development by isolated muscles from MLCKSMKO mice. Thus, MLCK appears to be the only significant kinase that phosphorylates RLC to initiate depolarization-mediated contraction. With ACh-induced contractions, RLC phosphorylation increased to 41±5.0 % at 8 sec in CTR ileal tissues (Figure 8C and D). In MLCKSMKO smooth muscle, however, RLC was phosphorylated only to 4.8±1.2 % at 4 sec after ACh stimulation with no significant RLC phosphorylation by 8 sec. Thus, MLCK-mediated RLC phosphorylation also plays a central role in agonist-mediated contraction.
Figure 8.
Time course of RLC phosphorylation in ileal smooth muscle from MLCKSMKO and CTR mice. RLC phosphorylation was measured in quick-frozen ileum smooth muscles from MLCKSMKO and control mice treated with 87 mM KCl (A) or 1μM ACh (C) as shown by representative Western blots of glycerol/urea PAGE gels. B and D show quantitation of RLC phosphorylation with KCl and ACh treatments, respectively. Bars represent means±SEM, n=4–10, **p<0.01 (t-test).
To determine the possible involvement of compensatory mechanisms in MLCK-deficient muscle, we measured MYPT1 phosphorylation mediated by a G protein-coupled receptor pathway that leads to inhibition of myosin phosphatase activity. MYPT1 phosphorylation for both Thr696 and Thr850 showed comparable responses to ACh in tissue from MLCKSMKO and CTR mice (Figure 2B). Thus, the reduction of RLC phosphorylation in MLCK-deficient muscle was not caused by a reduction of MYPT1 phosphorylation. Additionally, the similar amounts of expression of ROCK, MYPT1 and telokin protein in MLCK-deficient muscle relative to CTR tissues shows an apparent lack of a generalized, compensatory response from these regulatory proteins (Figure 2A and C). ILK expression increased but did not lead to a predicted increase in RLC phosphorylation.25
Depletion of Ca2+ inhibited the smaller contraction to ACh in MLCKSMKO smooth muscles
Because there were partial contractile and RLC phosphorylation responses with ACh, we determined if they were Ca2+-dependent. Depletion of Ca2+ with EGTA in ileum tissue strips inhibited the robust contractile response as well as the smaller contraction to ACh in CTR and MLCKSMKO tissues, respectively (Figure 6E and F). These results show that the small contraction induced by ACh in MLCKSMKO muscle (Figure 5A and B) is Ca2+-dependent.
Discussion
RLC phosphorylation plays an important role in the initial development of smooth muscle contraction.1–5 However, there are experimental conditions where RLC phosphorylation is dissociated from force development.6,7 Other protein kinases, such as ZIP kinase (zipper-interacting kinase), ILK and ROCK1, can phosphorylate RLC in a Ca2+-independent manner and thereby, could potentially regulate smooth muscle contraction.25–28 Thus, it is predicted that MLCK-deficient smooth muscle might still contract through a compensatory, non-RLC phosphorylation mechanism or through RLC phosphorylation by a Ca2+-independent kinase. However, targeted deletion of MLCK shows a significant loss of RLC phosphorylation and force development induced by K+-depolarization, suggesting an essential role for MLCK in the normal depolarization-mediated contractions of phasic smooth muscles in vivo. Ca2+-sensitization by ROCK1 has been reported to play a role in K+-induced contraction of some phasic smooth muscles.29,30 The current results indicate that, to the extent of its involvement in intestinal contractility, ROCK1 action is secondary to the initiation of force through MLCK-dependent RLC phosphorylation. The kinase knockout also markedly reduced RLC phosphorylation and force development in response to ACh, indicating that MLCK plays a central role in agonist-mediated contractions. Therefore, we conclude that MLCK is an essential kinase for RLC phosphorylation and contraction of phasic smooth muscle and that its role is not compensated by other cellular mechanisms.
After stimulating with ACh, the MLCKSMKO muscle generates a small contraction together with a small amount of RLC phosphorylation. This effect may not be attributable to a compensatory response via a Ca2+-independent mechanism, because the small contraction was inhibited effectively by Ca2+ depletion. These results also emphasize that Ca2+-independent kinases do not play a primary role in physiological contractile responses by direct phosphorylation of RLC, particularly in phasic smooth muscles,25 although we cannot directly rule out some involvement of overexpressed ILK in the MLCK knockout smooth muscle combined with RhoA-mediated inhibition of myosin phosphatase activity. It is also possible that the small amount of RLC phosphorylated in MLCKSMKO tissues in response to ACh is due to trace amounts of residual MLCK because only a small fraction (<20%) of the kinase is normally activated during a maximal contraction response.16
The physiological importance of MLCK is strongly supported by the robust gut dysmotility phenotype in MLCKSMKO mice. The depletion of MLCK in other types of smooth muscle (e.g. vascular) appears slower or incomplete due to expression of Cre via the SM22 promoter, thus other experimental approaches are needed for detailed analysis of these smooth muscle tissues. After induction with tamoxifen, MLCKSMKO mice showed primary symptoms of a paralytic gut that became progressively severe without any phenotypic reversion. Gastrointestinal motility depends on rhythmic contraction of intestinal smooth muscle, which is regulated by electro-mechanical coupling between smooth muscle cells and interstitial cells of Cajal.31,32 G protein-coupled receptor mediated contraction also plays an important role in gastrointestinal motility.32,33 However, mice lacking M2/M3-type muscarinic receptors did not have an abnormal phenotype, suggesting that G protein-coupled receptor pathway is not essential for gastrointestinal motility.34 The present study shows that loss of MLCK causes severe gut dysmotility, a phenotype similar to that found in the Cav1.2 L-type calcium channel knockout mice.35–37 Thus, Ca2+ influx through voltage-gated Cav1.2 L-type calcium channel with activation of Ca2+/calmodulin-dependent MLCK for RLC phosphorylation appears to be the central mechanism for intestinal motility. The components of the MLCK and RLC-regulatory signaling pathway may therefore be critical targets for diseases showing intestinal hypermotility. MLCKSMKO mice may also serve as a model for intestinal hypomotility diseases and visceral myopathies.
In summary, MLCK is essential for normal RLC phosphorylation and contraction in adult phasic smooth muscles, with its absence leading to prominent phenotypes in gastrointestinal responses and functions.
Supplementary Material
Acknowledgments
We thank N.Copeland (National Cancer Institute, USA) for providing materials of BAC-retrieval system. This work was supported by the MOST (2007CB947100), National Natural Science Funding of China (30570911), 973 program (2005CB522501) and grants from NIH (HL080536 and HL026043, USA) and the Moss Heart Fund.
Abbreviations in this paper
- ACh
acetylcholine
- IAS
internal anal sphincter
- ILK
integrin-linked kinase
- KO
knockout
- MLCK
myosin light chain kinase
- MYPT1
myosin phosphatase protein targeting subunit of the RLC phosphatase
- RLC
regulatory light chain
- ROCK1
Rho-associated coiled-coil-forming protein kinase 1
- ZIP kinase
zipper-interacting kinase
Footnotes
Conflicts of interest: There are no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kamm KE, Stull JT. Dedicated myosin light chain kinases with diverse cellular functions. J Biol Chem. 2001;276:4527–4530. doi: 10.1074/jbc.R000028200. [DOI] [PubMed] [Google Scholar]
- 2.Somlyo AP, Somlyo AV. Ca2+-sensitivity of smooth and non-muscle myosin II: modulation by G Proteins, kinases and myosin phosphatase. Physiological Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 3.Murthy KE. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol. 2006;68:11.1–11.30. doi: 10.1146/annurev.physiol.68.040504.094707. [DOI] [PubMed] [Google Scholar]
- 4.Ito M, Nakano T, Erdödi F, et al. Myosin phosphatase: structure, regulation and function. Mol Cell Biochem. 2004;259:197–209. doi: 10.1023/b:mcbi.0000021373.14288.00. [DOI] [PubMed] [Google Scholar]
- 5.Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- 6.Himpens B, Matthijs G, Somlyo AV, et al. Cytoplasmic free calcium, myosin light Chain phosphorylation, and force in Phasic and tonic smooth muscle. J Gen Physiol. 1988;92:713–729. doi: 10.1085/jgp.92.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan KG, Gangopadhyay SS. Invited review: cross-bridge regulation by thin filament-associated proteins. J Appl Physiol. 2001;91:953–962. doi: 10.1152/jappl.2001.91.2.953. [DOI] [PubMed] [Google Scholar]
- 8.Watterson DM, Schavocky JP, Guo L, et al. Analysis of the kinase-related protein gene found at human chromosome 3q21 in a multi-gene cluster: Organization, expression, alternative splicing, and polymorphic marker. J Cell Biochem. 1999;75:481–491. [PubMed] [Google Scholar]
- 9.Smith L, Su X, Lin P, et al. Identification of a novel actin binding motif in smooth muscle myosin light chain kinase. J Biol Chem. 1999;274:29433–29438. doi: 10.1074/jbc.274.41.29433. [DOI] [PubMed] [Google Scholar]
- 10.Herring BP, Dixon S, Gallagher PJ. Smooth muscle myosin light chain kinase expression in cardiac and skeletal muscle. Am J Physiol Cell Physiol. 2000;279:C1656–C1664. doi: 10.1152/ajpcell.2000.279.5.C1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang CX, Chen HQ, Chen C, et al. Microfilament-binding properties of N-terminal extension of the isoform of smooth muscle long myosin light chain kinase. Cell Res. 2006;16:367–376. doi: 10.1038/sj.cr.7310047. [DOI] [PubMed] [Google Scholar]
- 12.Somlyo AV, Wang H, Choudhury N, et al. Myosin light chain kinase knockout. J Muscle Res Cell Motil. 2004;25:241–242. doi: 10.1023/b:jure.0000038362.84697.c0. [DOI] [PubMed] [Google Scholar]
- 13.Lee EC, Yu D, Marinez de Valasco J, et al. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics. 2001;73:56–65. doi: 10.1006/geno.2000.6451. [DOI] [PubMed] [Google Scholar]
- 14.Liu PT, Jenkins NA, Copeland NG. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 2003;13:476–484. doi: 10.1101/gr.749203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuhbandner S, Brummer S, Metzger D, et al. Temporally controlled somatic mutagenesis in smooth muscle. Genesis. 2000;28:15–22. doi: 10.1002/1526-968x(200009)28:1<15::aid-gene20>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 16.Isotani E, Zhi G, Lau KS, et al. Real-time evaluation of myosin light chain kinase activation in smooth muscle tissues from a transgenic calmodulin-biosensor mouse. Proc Natl Acad Sci. 2004;101:6279–6284. doi: 10.1073/pnas.0308742101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson DP, Sutherland C, Walsh MP. Ca2+ activation of smooth muscle contraction: evidence for the involvement of calmodulin that is bound to the triton insoluble fraction even in the absence of Ca2+ J Biol Chem. 2002;277:2186–2192. doi: 10.1074/jbc.M110056200. [DOI] [PubMed] [Google Scholar]
- 18.Rossi J, Herzig KH, Voikar V, et al. Alimentary tract innervation deficits and dysfunction in mice lacking GDNF family receptor 2. J Clin Invest. 2003;112:707–716. doi: 10.1172/JCI17995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birder LA, Nakamura Y, Kiss S, et al. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV. Nat Neurosci. 2002;5:856–860. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- 20.DiPetrillo K, Tsaih SW, Sheehan S, et al. Genetic analysis of blood pressure in C3H/HeJ and SWR/J mice. Physiol Genomics. 2004;17:215–220. doi: 10.1152/physiolgenomics.00212.2003. [DOI] [PubMed] [Google Scholar]
- 21.Blue EK, Goeckeler ZM, Jin YJ, et al. 220- and 130-kDa MLCKs have distinct tissue distributions and intracellular localization patterns. Am J Physiol Cell Physiol. 2002;282:C451–C460. doi: 10.1152/ajpcell.00333.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niessen P, Rensen S, Van Deursen J, et al. Smoothelin-a is essential for functional intestinal smooth muscle contractility in mice. Gastroenterology. 2005;129:1592–1601. doi: 10.1053/j.gastro.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 23.Won KJ, Suzuki T, Hori M, et al. Motility disorder in experimentally obstructed intestine: relationship between muscularis inflammation and disruption of the ICC network. Neurogastroenterol Motil. 2006;18:53–61. doi: 10.1111/j.1365-2982.2005.00718.x. [DOI] [PubMed] [Google Scholar]
- 24.Patel CA, Rattan S. Spontaneously tonic smooth muscle has characteristically higher levels of RhoA/ROK compared with the phasic smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2006;291:G830–G837. doi: 10.1152/ajpgi.00130.2006. [DOI] [PubMed] [Google Scholar]
- 25.Ihara E, Moffat L, Ostrander J, et al. Characterization of protein kinase pathways responsible for Ca2+ sensitization in rat ileal longitudinal smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2007;293:G699–G710. doi: 10.1152/ajpgi.00214.2007. [DOI] [PubMed] [Google Scholar]
- 26.Niiro N, Ikebe M. Zipper-interacting protein kinase induces Ca2+-free smooth muscle contraction via myosin light chain phosphorylation. J Biol Chem. 2001;276:29567–29674. doi: 10.1074/jbc.M102753200. [DOI] [PubMed] [Google Scholar]
- 27.Deng JT, Van Lierop JE, Sutherland C, et al. Ca2+-independent smooth muscle contraction, a novel function for integrin-linked kinase. J Biol Chem. 2001;276:16365–16373. doi: 10.1074/jbc.M011634200. [DOI] [PubMed] [Google Scholar]
- 28.Hagerty L, Weitzel DH, Chambers J, et al. ROCK1 phosphorylates and activates zipper-interacting protein kinase. J Biol Chem. 2007;282:4884–4893. doi: 10.1074/jbc.M609990200. [DOI] [PubMed] [Google Scholar]
- 29.Shabir S, Borisova L, Wray S, et al. Rho-kinase inhibition and electromechanical coupling in rat and guinea-pig ureter smooth muscle: Ca2+-dependent and -independent mechanisms. J Physiol. 2004;560:839–55. doi: 10.1113/jphysiol.2004.070615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amobi NI, Chung IP, Smith IC. Attenuation of contractility in rat epididymal vas deferens by Rho kinase inhibitors. Auton Autacoid Pharmacol. 2006;26:169–81. doi: 10.1111/j.1474-8673.2006.00367.x. [DOI] [PubMed] [Google Scholar]
- 31.Horowitz B, Ward SM, Sanders KM. Cellular and molecular basis for electrical rhythmicity in gastrointestinal muscles. Annu Rev Physiol. 1999;61:19–43. doi: 10.1146/annurev.physiol.61.1.19. [DOI] [PubMed] [Google Scholar]
- 32.Lecci A, Santicioli P, Maggi CA. Pharmacology of transmission to gastrointestinal muscle. Curr Opin Pharmacol. 2002;2:630–641. doi: 10.1016/s1471-4892(02)00225-4. [DOI] [PubMed] [Google Scholar]
- 33.Hansen MB. Neurohumoral Control of Gastrointestinal Motility. Physiol Res. 2003;52:1–30. [PubMed] [Google Scholar]
- 34.Matsui M, Motomura D, Fujikawa T, et al. Mice lacking M2 and M3 muscarinic acetylcholine receptors are devoid of cholinergic smooth muscle contractions but still viable. J Neurosci. 2002;22:10627–10632. doi: 10.1523/JNEUROSCI.22-24-10627.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moosmang S, Schulla V, Welling A, et al. Dominant role of smooth muscle L-type calcium channel Cav1.2 for blood pressure regulation. EMBO J. 2003;22:6027–6034. doi: 10.1093/emboj/cdg583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wegener JW, Schulla V, Lee TS, et al. An essential role of Cav1.2 L-type calcium channel for urinary bladder function. FASEB J. 2004;18:1159–1161. doi: 10.1096/fj.04-1516fje. [DOI] [PubMed] [Google Scholar]
- 37.Wegener JW, Schulla V, Koller A, et al. Control of intestinal motility by the Cav1.2 L-type calcium channel in mice. FASEB J. 2006;20:1260–1262. doi: 10.1096/fj.05-5292fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.