Abstract
Hormonal levels fluctuate during the breeding season in many anurans, but the identity of the hormones that modulate breeding behavior and their effects remain unclear. We tested the influence of a combined treatment of progesterone and prostaglandin on phonotaxis, the key proceptive reproductive behavior of female anurans. First, we found that female gray treefrogs (Hyla versicolor) treated with progesterone and prostaglandin exhibited phonotaxis to synthetic male advertisement signals significantly more often than animals treated with ringers vehicle or uninjected controls. Responsive females had greater levels of plasma progesterone and estradiol compared to both control groups, suggesting that these steroids may be promoting phonotaxis. Second, we found that the selectivity of hormonally-induced phonotaxis in H. versicolor was similar to that observed in freshly captured breeding animals. Females made the same choices between acoustic signals after hormone treatments in tests of frequency, call rate and pulse rate, compared to their responses without treatment immediately after collection from the breeding chorus. The preference for a longer call was, however, significantly weaker after hormone induction of phonotaxis. Hormonally primed females were also less likely to respond in any test and took longer to respond than did freshly collected females. Consequently, our study shows how progesterone-prostaglandin induced phonotaxis in female treefrogs influences both the quality and quantity of phonotaxis, relative to that exhibited by naturally breeding females.
Keywords: Phonotaxis, Progesterone, Prostaglandin, Estradiol, Anuran, Mate choice, Hyla versicolor
INTRODUCTION
Reproductive hormones play a major role in initiating and modulating mating behavior in a wide variety of taxa (e.g. Crews and Moore 2005; Moore et al., 2005; Wingfield 2005). Hormones can change the morphology of ornaments used to attract mates, the production and probability of responding to sexual signals, and other courtship behaviors. Hormones may also alter the sensitivity of sensory systems or the way that signals are perceived (e.g. Aitken and Capranica 1984; Hultcrantz et al., 2006; Penna et al., 1992; Sisneros et al., 2004), which has important implications for variability in mate choice or changes in female choosiness (Jennions and Petrie 1997). Therefore, the hormonal regulation of mating behavior cannot be fully understood without knowledge about the influence of these hormones on mate choice.
Positive phonotaxis is the orientation and movement toward a sound source (a signaler) by a receiver. In most anurans this is the primary proceptive mating behavior observed in gravid females, which approach calling males to initiate mating. Because breeding females also show this behavior in response to playbacks of conspecific calls through loudspeakers, the acoustic signal rather than other sensory stimuli is sufficient to elicit phonotaxis. In one study of anurans the highest probability of phonotactic responses occurred when estradiol and progesterone levels were greatest (Lynch and Wilczynski 2005), suggesting that these steroids may be important modulators of anuran phonotaxis.
Progesterone and estradiol levels increase in female anurans during the period encompassing reproductive events, when phonotaxis occurs (Harvey et al., 1997; Itoh and Ishii 1990; Lynch and Wilczynski 2005; Medina et al., 2004). In the clawed frog, Xenopus laevis, receptive behaviors (thigh adduction, lack of a ticking vocalization) that would immediately follow phonotaxis in the wild were not induced by increases in estradiol or progesterone alone, but were induced by both together (Kelley 1982). Whether progesterone or estradiol are specifically involved in the changes in phonotaxis behavior that occur at this time is, however, unknown. Progesterone and prostaglandin injections were used together to induce phonotaxis in American toads (Bufo americanus; Schmidt 1985), but progesterone alone may not result in phonotaxis (Schmidt 1969, this is his assertion, data are unreported). However, Schmidt (1985) did not test uninjected females, making it difficult to determine whether phonotaxis would have also occurred in these animals without the injection of hormones.
Several lines of evidence suggest that prostaglandins are also involved in regulating phonotaxis. A combined human chorionic gonadotropin (HCG) and progesterone induction of phonotaxis can be halted with a prostaglandin inhibitor and reinstated with prostaglandin F2α injection (Schmidt 1984), but prostaglandin alone was generally ineffective in eliciting phonotaxis (Schmidt 1985). Thus prostaglandin may be necessary but not sufficient to induce phonotactic behavior. Prostaglandin F2α levels increase in ovulating frogs, possibly from GnRH stimulation of oviducts (Gobbetti and Zerani 1992), suggesting its involvement in the control of oviposition, a behavior that would naturally follow phonotaxis. Consequently, prostaglandin F2α is elevated near the time of phonotaxis and may be an important modulator of phonotactic behavior.
Phonotaxis is usually selective. Animals respond to a circumscribed set of sounds, with properties similar to those of long-range signals produced by conspecific individuals and sometimes including those of closely related species (Gerhardt and Huber 2002). In “choice” situations, however, breeding females show remarkable selectivity that not only excludes responses to the signals of other species in favor of conspecific signals but also shows discrimination among conspecific signals with subtle acoustic differences (review: Gerhardt and Huber 2002). If there is an interaction between a female’s hormonal state and her reception of auditory signals, then we would predict that hormonal state could influence her phonotactic behavior and selectivity. Hormonal profiles do change seasonally in anurans (reviewed in Rastogi et al., 2005), and there is some evidence that hormonal state does influence sensory perception. Seasonal changes in midbrain auditory neuron sensitivity occur in Hyla chrysoscelis, the sister species of H. versicolor, showing that females had lower auditory thresholds during the breeding season, compared to the thresholds of frogs tested outside the breeding period (Hillery 1984). Steroids (estradiol or testosterone) have been shown to increase the evoked responses of midbrain auditory neurons (Yovanof and Feng 1983) or the number of neurons that respond in some anurans (Aitken and Capranica 1984; Urano and Gorbman 1981), but not others (Penna et al., 1992). Furthermore, estradiol has been associated with increases in gene expression in the torus semicircularis (Lynch and Wilczynski 2008) a region of sensorimotor integration in anurans (Endepols and Walkowiak 2001). Thus, the probability of phonotaxis and its selectivity are likely to be influenced by a female’s hormonal state.
Prior studies investigating the hormonal control of phonotaxis have focused on inducement of phonotaxis to a single - typically invariant - species-specific call (e.g. Boyd 1994; Kelley 1982; Picker 1983; Schmidt 1984). Alternative acoustic stimuli were used in phonotaxis studies of the túngara frog, Physalaemus pustulosus; however, the alternative call varied in several acoustic parameters simultaneously (Lynch et al., 2005; Lynch and Wilczynski 2005; Lynch et al., 2006). A more complete understanding of the hormonal control of anuran phonotaxis requires learning how different hormones contribute to various aspects of female selection criteria. Our subjects were gray treefrogs, Hyla versicolor, for which an extensive body of knowledge about phonotactic selectivity exists (Gerhardt and Huber 2002). Here we explicitly test if modulation of phonotaxis by progesterone and prostaglandin influences either the quantity (probability and number) or quality (speed and selectivity) of responses in four tests of alternative acoustic signals in which there was a single difference in the value of an acoustic property of known behavioral significance (Gerhardt and Doherty 1988; Gerhardt et al., 2000; Gerhardt 2005a, 2005b; Klump and Gerhardt 1987).
METHODS
All procedures outlined in this study were approved by the University of Missouri Animal Care and Use Committee protocol #1910. Animals were collected under Missouri Department of Conservation Wildlife collector’s permits #12923 and #12343. Hyla versicolor females were initially collected in amplexus from a natural breeding chorus in the Thomas Baskett Wildlife Conservation Area near Ashland, MO, USA during the 2005 (April 5-June 22) and 2006 (April 13-June 6) breeding seasons.
Acoustic Testing Procedure
We evaluated female responsiveness and selectivity by means of playbacks of synthetic advertisement calls that were generated using custom designed software (by J. Schwartz) and modified using Cool Edit (Syntrillium Co, Phoenix, AZ, USA). Our standard call was 837 ms (18 pulses) long with a pulse rate of 20 pulses/s; the spectrum consisted of two components of 1.1 and 2.2 kHz, with the amplitude of the low-frequency component 6 dB less than that of the high-frequency component. The call period was 4 s. Our standard synthetic call is equivalent to a call from an “average” male from our population (descriptions and variability of natural calls can be found in Gerhardt et al., 1996). In two-speaker tests, there was no statistically significant difference in the proportion of females choosing the standard synthetic call and pre-recorded exemplars in two-alternative, forced-choice tests (Gerhardt 1978). We tested this standard call against one of four alternative, less attractive stimuli: 1) Call Duration test - a shorter alternative call (645 ms =14 pulses); 2) Pulse Rate test - with a faster pulse-rate alternative (30 pulses/s); 3) Call Rate test - with a slower alternative call rate (8 s call period); and 4) Spectral test - with a call of higher frequency (1.4 + 2.8 kHz peaks, with the amplitude of the low-frequency component 6 dB less than that of the high-frequency component). Previous experiments showed that field-collected, gravid (or “reproductively active”) females taken from amplexus preferred the standard call to these, or similar, alternatives (Gerhardt and Doherty 1988; Gerhardt et al., 2000; Gerhardt 2005a, 2005b; Klump and Gerhardt 1987). Every female was tested with all four of these acoustic tests, with at least 30 min separating successive tests. The order of tests was haphazard, and the speaker broadcasting the standard call was haphazardly alternated to minimize the risk of side biases, none of which were detected.
All tests were conducted in the semi-anechoic chamber described in Gerhardt (1995) at 20±1°C. For each test, females were placed in a small hardware cloth cage midway between two Analog-Digital-Systems 200 speakers that were separated by 2 m. The sound pressure level (SPL re 20 μbar, fast root-mean-square) of the stimuli was equalized at 85 dB SPL at this release point with a Larsen-Davis 800B sound level meter. Females were released by remotely removing the top of the cage after alternating stimuli from the acoustic tests described above were broadcast at least three times, with equal periods of silence between successive presentations of alternatives.
During testing, frogs were observed with a remote camera and infrared illumination. A response was tabulated when a female moved to within 10 cm of one of the speakers after showing phonotactic orientation movements, such as head and body scanning that occurred during or shortly after several calls (not necessarily each call) in a playback series (Rheinlaender et al., 1979). We also recorded the time to make a choice. A “no response” was recorded when the female failed to show phonotactic behavior within 10 min of release; some females remained in the release cage and others wandered around randomly in the chamber without showing phonotactic orientation movements.
Experiment 1: Hormonal inducement of phonotaxis
We tested female gray treefrogs (n=45) for phonotaxis, using the four acoustic choices outlined above, under three different treatments: 1) hormonal priming with progesterone and prostaglandin F2α; 2) control injections with amphibian ringers (vehicle) and 3) no injection. Our treatment of progesterone and prostaglandin (both from Sigma-Aldrich, St Louis, MO, USA) was a procedure modified from Schmidt (1985). Dosages were modified because in preliminary tests the recommended dosages for American toads (Bufo americanus) (Schmidt 1985) resulted in unacceptable levels of mortality and poor responses from surviving gray treefrogs. Based on Schmidt’s (1985) equation of: dose=((body mass/100 g)0.666)*K we adjusted dosages so that K=2 mg progesterone was used (note that this is a non-linear relationship between body mass and dose, which is approximately equivalent to 36 mg/kg body mass over the range tested). This resulted in near zero mortality. We adjusted prostaglandin dosages to improve responses such that K=1200 μg (approximately equal to 21.8 mg/kg body mass over the tested range). Progesterone was injected intraperitoneally into the right medial side, posterior to the liver. Intramuscular injections of prostaglandin were administered into the thighs, with dosages divided evenly between both legs, 19±1 hours after progesterone administration. Frogs that were tested under the ringers treatment were injected with equivalent volumes of amphibian ringers at the same time as hormonally treated frogs. All frogs were tested under all three treatments with at least three weeks between treatments. The order of treatments for each frog was randomized.
Frogs tested in experiment 1 were all long-term captives that had been housed individually for greater than eighteen months. All testing was completed between September and November of 2007. The person observing the phonotaxis tests was blind to which treatments the frogs received.
To assess how our hormonal treatment might be influencing hormonal levels, at the conclusion of testing each day a sample of blood (~100 μl) was collected from each frog via cardiac puncture. Blood samples were stored up to 24 hr at 2–8 °C and then centrifuged to separate and remove the plasma. Plasma was then stored at −20 °C until assayed. We also sampled frogs in the field approaching the breeding chorus to determine if our treatments were at physiologically relevant levels.
Hormonal analyses were done with commercial radioimmunoassay kits (Progesterone: Coat-a-Count TKPG-2, Siemens, Los Angeles, CA; Estradiol: ImmuChem 07-138102, MP Biomedicals, Orangeburg, NY). All samples were run in duplicate. Samples were diluted to 10 μl sample in 90 μl zero standard buffer prior to assay. Kits were validated using serial dilution of a pooled sample of Hyla versicolor plasma. Curves generated from these serial dilutions were parallel to the standard curves (data not shown). Mean intra-assay coefficients of variation for progesterone and estradiol were both 17.1% (both based on 6 standards run with each assay). Inter-assay coefficients of variation were 7.1% for four progesterone assays and 13.7% for five estradiol assays. The minimum detection limit for the progesterone assay was 0.05 ng/ml and for the estradiol assay was 10 pg/ml.
Experiment 2: Changes in selectivity in hormonally induced frogs
We tested individual female gray treefrogs both during the breeding season and after administration of hormones outside of the breeding season to compare natural versus progesterone-prostaglandin induced phonotactic. Females were initially collected from amplexus in breeding choruses (n=109) in 2005 and 2006. After collection, females were held in coolers on melting ice for up to 7 days, and then warmed to 20 °C in an incubator prior to testing. All females were tested with the four phonotaxis tests outlined in the acoustic testing procedure above. The number of days between collection and testing did not affect the choices females made (see Results).
Females that responded in all four phonotactic tests during the breeding season (n=66) were toe-clipped for identification and housed in captivity until subsequent testing following hormonal priming in September - November of the same year. Hormonally primed females were acclimated overnight at 20 °C in the same incubator used for breeding season tests, and the hormonal priming and phonotactic testing procedure was the same as described above. Not all females responded in every test when hormonally primed; therefore, some females were treated as many as three times in an attempt to obtain responses for all tests. At least two weeks separated successive hormonal treatments to minimize possible carryover effects. The person recording the responses of hormonally primed females did not have knowledge of their previous responses during the breeding season.
Statistical analysis
Multiple responses from the same female to different tests are non-independent; therefore, we used Cochran’s Q test to compare the proportions of females responding to different treatments in experiment 1 and to compare the responses of breeding season and hormonally primed frogs in experiment 2. To determine if the proportions of frogs responding to the standard call were different across years or across different dates within the same year we used chi-square analysis.
We wanted to know if hormone primed frogs took the same amount of time to make choices as the same frogs tested during the breeding season, therefore we used Wilcoxon sign-rank tests to compare matched-pair responses for differences in the time to make a choice between these groups. ANOVA was used to test if there was an influence of the number of days since capture on the time to make a choice. Effects of days since capture on the probability of a female responding were analyzed with a log-likelihood test.
The distribution of hormone levels was non-normal, so levels of progesterone and estradiol were log-transformed to achieve normality prior to statistical analysis. The values reported here are, however, the untransformed values. Because each female was tested with all treatments, but not all females responded, differences in hormonal levels between treatments were analyzed with a random-effects mixed-model for repeated-measures. All statistical analyses were performed using JMP software (SAS Institute, Cary, NC, USA), except for the Cochran’s tests, which were hand calculated. Values are presented as means ± standard errors.
RESULTS
Experiment 1: Hormonal inducement of phonotaxis
Positive phonotaxis was observed significantly more often in female treefrogs treated with progesterone and prostaglandin than in females receiving either amphibian ringers or no injections (Table 1). This result was robust, regardless of the acoustic stimulus tested (Table 1). Additionally, if we restrict our analysis to only those frogs that responded, hormone injected frogs were likely to respond in significantly more tests (2.8± 0.2 tests) compared to ringers-injected (1.4± 0.1 tests) or uninjected frogs (1.5± 0.2 tests) (n=101 frog-tests, X2=32.79, p<0.0001). Fewer frogs responded in the ringers- injected treatment compared to the uninjected controls (Table 1), suggesting that the injections themselves had a depressive effect on female responses. Date of testing did not influence the likelihood of a female responding (n=101 frog tests, X2=1.256, p=0.262).
Table 1.
The percent of frogs exhibiting phonotaxis in four tests of acoustic selectivity for hormonally injected (n=38), vehicle (ringers) injected (n=38), and untreated females (n=42). The percent shown reflects the total number of frogs responding, regardless of whether they chose the standard or alternative call.
| % of frogs responding to calls: | |||||
|---|---|---|---|---|---|
| Treatment: | Un-injected | Ringers | Progesterone/prostaglandin | Cochran’s Q | p |
| Pulse rate | 7 | 8 | 53 | 25.185 | <0.001 |
| Spectral | 12 | 3 | 45 | 18.995 | <0.001 |
| Call duration | 12 | 5 | 39 | 14.709 | <0.001 |
| Call rate | 10 | 3 | 45 | 20.667 | <0.001 |
|
| |||||
| All tests combined | 26 | 13 | 66 | 19.185 | <0.001 |
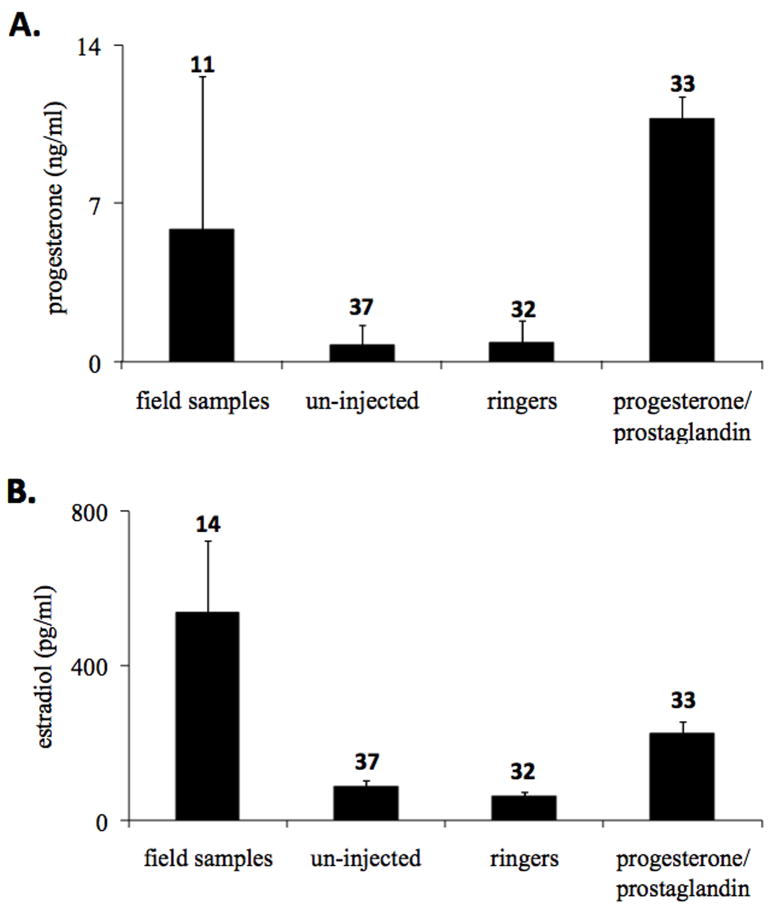
Hormonally primed frogs had greater progesterone levels than ringers-injected or uninjected frogs (F2, 92=20.88, p<0.0001) (Fig. 1-A). This indicates our injections of progesterone had the expected effect. Estradiol levels were elevated as well in hormonally primed frogs when compared to ringers-injected and uninjected frogs (F2, 96 =13.12, p<0.0001) (Fig. 1-B). Measured hormone levels of progesterone-prostaglandin treated frogs were not significantly different from wild females naturally approaching a breeding chorus (progesterone: F1,43=2.225, p=0.1431; estradiol F1,46=1.697, p=0.1991) (Fig. 1-A+B), so our treatments were within the natural physiological range.
Fig. 1.
Plasma progesterone (A) and estradiol (B) levels for frogs treated with progesterone and prostaglandin, amphibian ringers (vehicle), uninjected controls and wild-caught breeding females. Both plasma progesterone and estradiol levels were significantly greater in progesterone-prostaglandin treated frogs, relative to the same frogs under the ringers or uninjected control treatments (both p<0.0001). Hormone levels of progesterone-prostaglandin treated females were not significantly different from field-collected animals (progesterone: p=0.1431; estradiol: p=0.1991). Values are presented as mean ± SE with sample sizes above each bar.
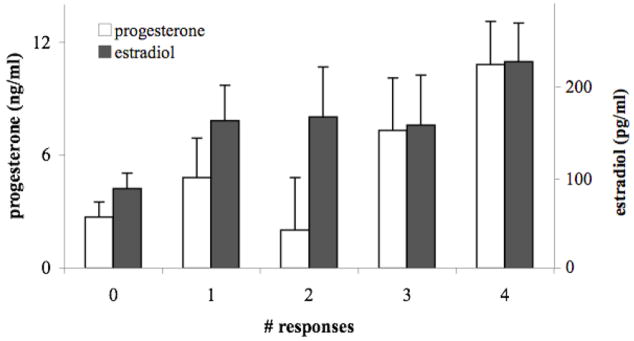
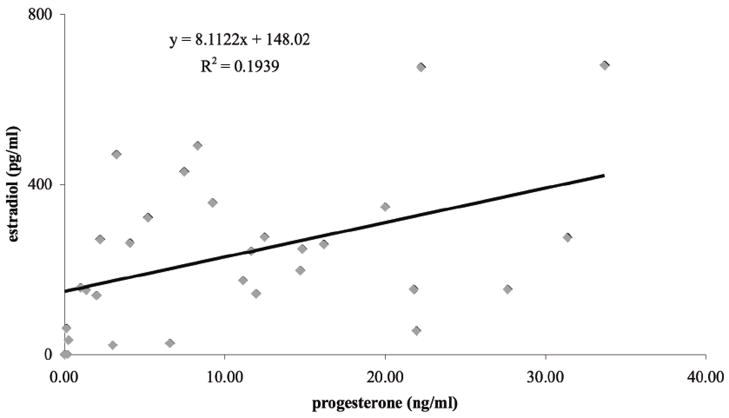
The number of times a frog responded was positively correlated with both progesterone (F1,91=7.378, p=0.0079) (Fig. 2) and estradiol levels (F1,95=4.875, p=0.0296) (Fig. 2). Progesterone levels were positively correlated with estradiol levels in hormonally treated frogs (Fig. 3). Neither progesterone (F1,95=0.557, p=0.447) nor estradiol levels (F1,95=0.410, p=0.514) were influenced by the date of treatment.
Fig. 2.
Mean (±SE) plasma progesterone and estradiol levels as a function of the number of tests in which a frog responded regardless of treatment. Females that responded to more tests had greater progesterone (p=0.0079) and estradiol (p=0.0296) levels.
Fig. 3.
Relationship between plasma progesterone and estradiol concentrations in female frogs injected with progesterone and prostaglandin.
Experiment 2: Changes in selectivity in hormonally induced frogs
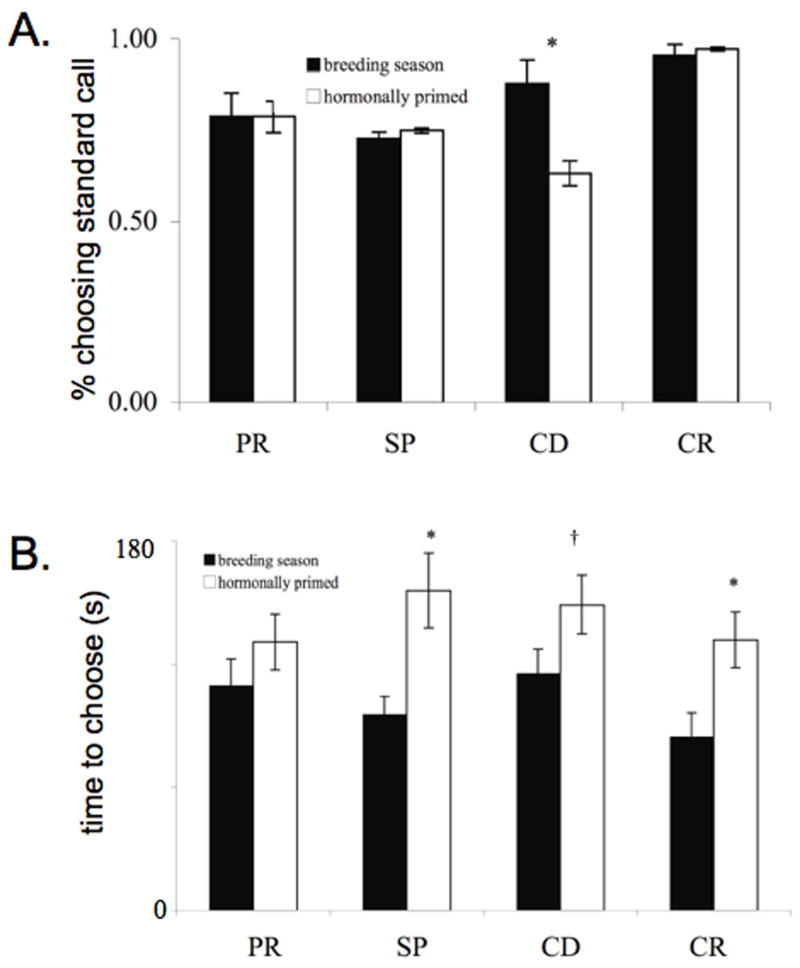
A majority of females chose the standard call in all tests during the breeding season and after hormonal priming (Fig. 4-A). There were no significant differences between the proportion of females choosing the standard call during the breeding season and after induction of phonotaxis in tests of differences in pulse rate, frequency or call rate (pulse rate: n=33, Q=0.143, p=0.721; spectral: n=36, Q=0.077, p=0.857; call rate: n=35, Q=1.00, p=0.343). The proportion of females choosing the call of longer duration was significantly lower when females were hormonally induced than when tested during the breeding season (n=38, Q=12.00, p<0.001), although a statistically significant majority (24 of 38 females, binomial test: p = 0.035) still preferred the longer call (Fig. 4-A).
Fig. 4.
(A) Comparison of the choices made during the breeding season by untreated females and by the same females following progesterone-prostaglandin treatment. (B) Comparison of the time required to make a choice by untreated females tested during the breeding season and the same females following progesterone-prostaglandin treatment. PR=pulse rate test, SP=spectral test, CD=call duration test, CR=call rate test, *=significant at p=0.001. †=marginally significant at p=0.059.
When comparing breeding season vs. non-breeding season choices, 28 of the 46 females that responded, changed their choice to the other alternative stimulus in at least one test. Nine of the 28 individuals that responded differently did so in more than one test. With the exception of the call-duration test, females that switched call preferences were as likely to switch from standard to alternative calls as they were to switch from alternative to standard calls. All 12 females that responded differently in the call-duration test switched from a preference for the standard call during the breeding season to the short-call alternative when hormonally primed.
Hormonally induced frogs took longer to respond than frogs tested during the breeding season. This difference was statistically significant for the spectral and call rate discrimination tests, and marginally significant for the test of call duration discrimination (Fig. 4-B) (pulse rate: n=33, Z=87.0, p=0.122; spectral: n=36, Z=200.0, p=0.0009; call duration: n=35, Z=129.5, p=0.0593; call rate: n=35, Z=199.0, p=0.0005).
Females were more likely to respond phonotactically during the breeding season than after hormone treatments (Table 2). Twenty seven percent (116 of 426) of all frog tests resulted in a “no response” during the breeding season, while 51% (149 of 291) of the tests of the hormone-primed frogs resulted in a “no response.” Tests of the difference in call rate resulted in fewer “no responses” when compared to the other three tests for both breeding-season and hormone-primed individuals (Table 2).
Table 2.
Percent of tests resulting in a “no response” (frogs that did not approach either stimuli within 10 min) for breeding season and the same females hormonally primed after the breeding season.
| Test | % of breeding season tests resulting in “no response” | % of hormone primed tests resulting in “no response” |
|---|---|---|
| Pulse rate | 32 | 58 |
| Spectral | 30 | 54 |
| Call duration | 33 | 47 |
| Call rate | 10 | 43 |
|
| ||
| All tests combined | 27 | 51 |
Of the 50 frogs tested after hormonal priming, twenty-four were administered hormones on multiple occasions (up to three administrations, each at least 14 days apart) in an attempt to get responses in all four tests. Of these 24 frogs, nine responded only during the first administration; four responded only after the second administration; ten responded on multiple occasions; and one never responded.
During the breeding season, 66 of 109 females responded in all four tests. The number of days between collection and testing influenced the likelihood of an individual’s responding (n=109, X2=28.41, p<0.0001) but did not influence the animal’s choice (i.e. whether she chose the standard or alternate call) (n=66, pulse rate: X2=0.36, p=0.548; spectral: X2=0.06, p=0.810; call duration: X2=1.00, p=0.317; call rate: X2=0.12, p=0.729). There was also no significant effect of the number of days since collection on the time required to make a choice (F4,57=0.758, p=0.557). Of those frogs that responded, the proportion of frogs responding to the standard call during the breeding season was not significantly different between 2005 and 2006 (n=66; pulse rate: X2=1.72, p=0.190; spectral: X2=1.23, p=0.267; call duration: X2=1.18, p=0.278; call rate: X2=1.27, p=0.260).
To confirm that the response of female frogs to hormone treatments was consistent between years (i.e. that hormonally primed frogs of experiment 1 were comparable to those primed in experiment 2), we tested for differences in the proportion of hormonally-primed females responding to any test. There was no effect of year on the proportion of females responding after progesterone-prostaglandin treatment (n=132, X2=2.40, p=0.301). There was also no effect of year (2005–2007) on the proportion of females choosing the standard call when hormonally primed (pulse rate: n=53, X2=3.10, p=0.213; spectral: n=53, X2=4.03, p=0.134; call duration: n=53, X2=0.50, p=0.780; call rate: n=52, X2=0.87, p=0.646)
DISCUSSION
Progesterone-prostaglandin treatment of female gray treefrogs induced phonotaxis toward playbacks of synthetic advertisement calls. Our study confirmed that this induction of phonotaxis by progesterone-prostaglandin is similar to that exhibited by naturally breeding females in terms of the selectivity of such responses to three of the four acoustic parameters tested. Females were, however, significantly less likely to choose calls of longer duration after progesterone-prostaglandin treatment, although a significant majority of treated females still chose the long-duration call. Furthermore, the probability of a phonotactic response after hormonal treatment was significantly reduced, compared to naturally breeding frogs. Because neither progesterone (Schmidt 1969, Kelley 1982) nor prostaglandin alone (Schmidt 1985) consistently resulted in phonotaxis in previous studies, our results support the hypothesis that the combined effect of the two hormones is sufficient for eliciting this behavior in frogs.
In our study, frogs that were treated with progesterone and prostaglandin had elevated levels of estradiol, a steroid that has also been implicated in the regulation of receptive behavior (Kelley 1982). However, elevated levels of estradiol (Diakow et al., 1978; Kelley 1982) or progesterone (Kelley 1982) alone have not been shown to promote receptive behaviors in other species of anurans. Prostaglandin has been shown to increase estradiol release in post reproductive Rana esculenta, resulting in increased ovarian mass, but prostaglandin did not induce ovulation when administered alone (Gobbetti et al., 1990). This observation suggests that prostaglandins could be responsible for the increased estradiol levels noted here. Progesterone alone may also contribute to the observed increase in estradiol through its metabolism into estradiol, or it could be promoting estradiol release or synthesis through another pathway. We noted an increase in estradiol after our hormonal administrations, raising the possibility that estradiol might mediate the influence of these hormones on the phonotaxis we observed. Because Kelley (1982) found that administration of progesterone and estradiol together was needed to induce the receptive behaviors that naturally follow phonotaxis, our hormonal treatment may be effectively equivalent to hers. Further work will be needed to confirm this hypothesis and to learn whether the progesterone, prostaglandin, or an interaction of the two is responsible for the estradiol increase. Additionally, while we can conclude that our progesterone-prostaglandin treatment induced the observed phonotaxis behavior, we can not determine whether it was the progesterone, prostaglandin, estradiol, or some combination thereof that ultimately caused this behavior.
Steroid levels in breeding frogs may increase in wild frogs via gonadotropins. HCG has been shown to increase plasma estradiol in a manner consistent with naturally phonotactic females (Lynch et al., 2006) and may be responsible for increases in progesterone as well (Morrill et al., 2006; Thornton 1972). Both Xenopus laevis (Picker 1983) and Physalaemus pustulosus (Lynch et al., 2006) respond to HCG injections with phonotactic behavior. However, since estradiol levels did not change across the stages of reproduction when phonotaxis would occur in R. esculenta (Gobbetti and Zerani 1992), changes in estradiol may not be necessary or sufficient for eliciting phonotaxis in all species of anurans. Of course, some minimum level of estradiol may still be needed to induce this behavior.
Females of H. versicolor usually made the same choices between acoustic signals when their phonotaxis was induced by injections of progesterone and prostaglandin as they did after being collected in a breeding chorus and tested within a few days. As expected from the results of previously published studies (Gerhardt 2005a, 2005b; Gerhardt and Doherty 1988; Klump and Gerhardt 1987), females preferred the standard call in both conditions. Hormonally induced females did show a significantly weaker preference in the call-duration test than when tested shortly after capture; however, a majority of females still preferred the longer call. The weakening of a preference for call duration is noteworthy. Gray treefrog females use call duration as a cue to evaluate male quality (Gerhardt et al., 1996). Choosing longer calling males has been shown to convey genetic benefits for traits that improve offspring survival (Welch et al., 1998); consequently, a weakening of this preference could have implications for reproductive fitness.
Hormonally primed females were also less likely to respond in any test and usually took longer to respond than did freshly collected females. Similar results were found in comparisons of phonotaxis in P. pustulosus captured during the breeding season (Lynch et al., 2005) with that of hormonally primed (with HCG) post-mating animals (Lynch et al., 2006): there was no obvious difference in the proportion of females responding to the species-typical call, though this was not explicitly tested.
Our combination of progesterone-prostaglandin (modified from Schmidt 1985; see Methods) was usually effective in inducing phonotaxis in post-reproductive females. Administration of HCG or arginine vasotocin (AVT) has also been shown to induce phonotaxis in female anurans (Boyd 1994; Kelley 1982; Lynch et al., 2006; Picker 1983; Schmidt 1985). While the relationships between the hormones that may be involved in female anuran reproduction are not yet well understood, there is some suggestion that the actions of both HCG and AVT may overlap or coincide with the actions of progesterone and/or prostaglandin. Gonadotropins increase prostaglandin production in anuran interrenal glands (Gobbetti and Zerani 1991), ovaries, and oviducts (Gobbetti and Zerani 1992) during the reproductive period. Gonadotropins also increase progesterone production in the follicles during the same period (Chang et al., 1997; Kwon et al., 1993). Furthermore, both AVT-induced (Diakow and Nemiroff 1981) and HCG-induced (Weintraub et al., 1985; Schmidt 1984) receptive behaviors can be inhibited by a prostaglandin inhibitor. Our results may thus prove to be similar to hormonal induction of phonotaxis using these other hormones, although this awaits further confirmation.
Mature ovarian follicles and oviposition were not required for the observed induction of phonotaxis in gray treefrogs. We have successfully used this protocol with three different species in the gray treefrog complex (Hyla versicolor, H. chrysoscelis and H. arenicolor) in every season and in almost every month of the year, and oviposition is only occasionally observed (Gordon and Gerhardt, unpublished). The reduction in phonotactic responses in other anurans that occurs when HCG is used to induce this behavior may be a result of insufficient numbers of well-developed follicles, as suggested by Picker (1983) to explain why only about 30% of the females of African clawed frogs, Xenopus laevis, responded after injections of HCG. The fact that all female Xenopus induced to phonotactic receptivity with HCG oviposited in a separate study (Weintraub et al., 1985) corroborates this assertion. Lynch et al., (2006) inferred that only females of P. pustulosus with mature follicles respond to HCG treatment, though this was not explicitly tested. P. pustulosus breeds repeatedly throughout much of the year (Ryan 1985), while the hylid treefrogs we studied have a narrower period of reproductive activity. Though it is possible that these North American Hyla maintain mature follicles throughout the year, our protocol does not appear to depend on females having eggs in the same state as in the breeding season.
Numerous studies have used hormones to elicit reproductive behavior such as vocalization, amplexus, and phonotaxis in frogs and toads (e.g. Boyd 1994; Gerhardt et al., 1994; Kelley 1982; Noble and Aronson 1942). These studies have either sought to discover causal relationships between hormones and mating behavior (e.g. Lynch et al. 2006; Weintraub et al., 1985) or used hormonal treatment as a practical means of assessing variation in mating behaviors among individuals, populations, or species under the same environmental conditions (e.g. Gerhardt 1994; Gerhardt et al., 1996). The latter application implicitly assumes that the selectivity of individuals under treatments that induce such behavior in the laboratory reflects natural conditions. Here we have shown that these behaviors are, in fact, similar.
Acknowledgments
We thank Vince Marshall for discussions of test selection; and Ray Semlitsch, Sunny Boyd, Mark Bee and two anonymous reviewers for helpful comments on the manuscript. Shannan Tucker, Calan McConkey, Shelli Hellman, Tyler Cook, Mitch Tucker, Samantha Bisges, Chris Tegtmeyer, Abby Evers, and Amberly Woods provided assistance catching and testing frogs. Carl Gerhardt was funded by grants from the National Science Foundation (IBN0091993) and National Institute of Health (NIH R01 DC05760). RLF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- Aitken PG, Capranica RR. Auditory input to a vocal nucleus in the frog Rana pipiens, hormonal and seasonal effects. Exp Brain Res. 1984;57:33–39. doi: 10.1007/BF00231129. [DOI] [PubMed] [Google Scholar]
- Boyd SK. Arginine vasotocin facilitation of advertisement calling and call phonotaxis in bullfrogs. Horm Behav. 1994;28:232–240. doi: 10.1006/hbeh.1994.1020. [DOI] [PubMed] [Google Scholar]
- Chang KJ, Kim JW, Im WB, Kang HM, Kwon HB. Differential effects of gonadotropin and orthovanadate on oocyte maturation, ovulation, and prostaglandin synthesis by Rana ovarian follicles in vitro. J Exp Zool. 1997;277:155–165. [PubMed] [Google Scholar]
- Crews D, Moore MC. Historical contributions of research on reptiles to behavioral Neuroendocrinology. Horm Behav. 2005;48:384–394. doi: 10.1016/j.yhbeh.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Diakow C, Nemiroff A. Vasotocin, prostaglandin and female reproductive behavior in the frog Rana pipiens. Horm Behav. 1981;15:86–93. doi: 10.1016/0018-506x(81)90037-4. [DOI] [PubMed] [Google Scholar]
- Diakow C, Wilcox JN, Woltmann R. Female frog reproductive behavior elicited in the absence of the ovaries. Horm Behav. 1978;11:183–189. doi: 10.1016/0018-506x(78)90047-8. [DOI] [PubMed] [Google Scholar]
- Endpols H, Walkowiak W. Integration of ascending and descending inputs in the auditory midbrain of anurans. J Comp Physiol, A. 2001;186:1119–1133. doi: 10.1007/s003590000159. [DOI] [PubMed] [Google Scholar]
- Gerhardt HC. Discrimination of intermediate sounds in a synthetic call continuum by female green treefrogs. Science. 1978;199:1089–1091. doi: 10.1126/science.628833. [DOI] [PubMed] [Google Scholar]
- Gerhardt HC. Reproductive character displacement of female mate choice in the grey treefrog Hyla chrysoscelis. Anim Behav. 1994;47:959–969. [Google Scholar]
- Gerhardt HC. Phonotaxis in female frogs and toads: execution and design of experiments. In: Klump GM, Dooling RR, Fay RR, Stebbins WC, editors. Animal Psychophysics: Design and Conduct of Sensory Experiments. Birkhäuser Verlag; Basel: 1995. pp. 209–220. [Google Scholar]
- Gerhardt HC. Acoustic spectral preferences in two cryptic species of gray treefrogs: implications for mate choice and sensory mechanisms. Anim Behav. 2005a;70:39–49. [Google Scholar]
- Gerhardt HC. Advertisement-call preferences in diploid-tetraploid treefrogs (Hyla chrysoscelis and Hyla versicolor): implications for mate choice and the evolution of communication systems. Evolution. 2005b;59:395–408. [PubMed] [Google Scholar]
- Gerhardt HC, Doherty JA. Acoustic communication in the gray treefrog, Hyla versicolor: evolutionary and neurobiological implications. J Comp Physiol, A. 1988;162:261–278. [Google Scholar]
- Gerhardt HC, Dyson ML, Tanner SD. Dynamic properties of the advertisement calls of gray treefrogs: patterns of variability and female choice. Behav Ecol. 1996;7:7–18. [Google Scholar]
- Gerhardt HC, Dyson ML, Tanner SD, Murphy CG. Female treefrogs do not avoid heterospecific calls during phonotactic approaches to conspecific calls: implications for mechanisms of mate choice. Anim Behav. 1994;47:1323–1332. [Google Scholar]
- Gerhardt HC, Huber F. Acoustic Communication in Insects and Anurans. University of Chicago Press; Chicago: 2002. [Google Scholar]
- Gerhardt HC, Tanner SD, Corrigan CM, Walton HC. Female preference functions based on call duration in the gray treefrog (Hyla versicolor) Behav Ecol. 2000;11:663–669. [Google Scholar]
- Gobbetti A, Zerani M, Carnevali O, Botte V. Prostaglandin F2-alpha in female water frog, Rana esculenta - plasma levels during the annual cycle and effects of exogenous PGF2-alpha on circulating sex hormones. Gen Comp Endocrinol. 1990;80:175–180. doi: 10.1016/0016-6480(90)90162-f. [DOI] [PubMed] [Google Scholar]
- Gobbetti A, Zerani M. Gonadotropin-releasing hormone stimulates biosynthesis of prostaglandin F2-alpha by the interrenal gland of the water frog, Rana esculenta, in vitro. Gen Comp Endocrinol. 1991;84:434–439. doi: 10.1016/0016-6480(91)90092-k. [DOI] [PubMed] [Google Scholar]
- Gobbetti A, Zerani M. A possible involvement of prostaglandin F2-alpha (PGF2-alpha) in Rana esculenta ovulation - effects of mammalian gonadotropin releasing hormone on invitro PGF2-alpha and 17-Beta-estradiol production from ovary and oviduct. Gen Comp Endocrinol. 1992;87:163–170. doi: 10.1016/0016-6480(92)90018-f. [DOI] [PubMed] [Google Scholar]
- Harvey LA, Propper CR, Woodley SK, Moore MC. Reproductive endocrinology of the explosively breeding desert spadefoot toad, Scaphiopus couchii. Gen Comp Endocrinol. 1997;105:102–113. doi: 10.1006/gcen.1996.6805. [DOI] [PubMed] [Google Scholar]
- Hillery CM. Seasonality of two midbrain auditory responses in the treefrog Hyla chrysoscelis. Copeia. 1984;1984:844–852. [Google Scholar]
- Hultcrantz M, Simonoska R, Stenberg AE. Estrogen and hearing: a summary of recent investigations. Acta Otolaryngol (Stockh) 2006;126:10–14. doi: 10.1080/00016480510038617. [DOI] [PubMed] [Google Scholar]
- Itoh M, Ishii S. Changes in plasma levels of gonadotropins and sex steroids in the toad Bufo japonicus in association with behavior during the breeding season. Gen Comp Endocrinol. 1990;80:451–464. doi: 10.1016/0016-6480(90)90194-q. [DOI] [PubMed] [Google Scholar]
- Jennions MD, Petrie M. Variation in mate choice and mating preferences: a review of causes and consequences. Biol Rev. 1997;72:283–327. doi: 10.1017/s0006323196005014. [DOI] [PubMed] [Google Scholar]
- Kelley DB. Female sex behaviors in the south African clawed frog, Xenopus laevis: gonadotropin-releasing, gonadotropic and steroid hormones. Horm Behav. 1982;16:158–174. doi: 10.1016/0018-506x(82)90016-2. [DOI] [PubMed] [Google Scholar]
- Klump GM, Gerhardt HC. Use of non-arbitrary acoustic criteria in mate choice by female gray tree frogs. Nature. 1987;326:286–288. [Google Scholar]
- Kwon HB, Ahn RS, Lee WK, Im WB, Lee CC, Kim K. Changes in the activities of steroidogenic enzymes during the development of ovarian follicles in Rana nigromaculata. Gen Comp Endocrinol. 1993;92:225–232. doi: 10.1006/gcen.1993.1158. [DOI] [PubMed] [Google Scholar]
- Lynch KS, Crews D, Ryan MJ, Wilczynski W. Hormonal state influences aspects of female mate choice in the túngara Frog (Physalaemus pustulosus) Horm Behav. 2006;49:450–457. doi: 10.1016/j.yhbeh.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch KS, Rand AS, Ryan MJ, Wilczynski W. Plasticity in female mate choice associated with changing reproductive states. Anim Behav. 2005;69:689–699. [Google Scholar]
- Lynch KS, Wilczynski W. Gonadal steroids vary with reproductive stage in a tropically breeding female anuran. Gen Comp Endocrinol. 2005;143:51–56. doi: 10.1016/j.ygcen.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Lynch KS, Wilczynski W. Reproductive hormones modify reception of species-typical communication signals in a female anuran. Brain Behav Evol. 2008;71:143–150. doi: 10.1159/000111460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina MF, Ramos I, Crespo CA, Gonzalez-Calvar S, Fernandez SN. Changes in serum sex steroid levels throughout the reproductive cycle of Bufo arenarum females. Gen Comp Endocrinol. 2004;136:143–151. doi: 10.1016/j.ygcen.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Moore FL, Boyd SK, Kelley DB. Historical perspective: hormonal regulation of behaviors in amphibians. Horm Behav. 2005;48:373–383. doi: 10.1016/j.yhbeh.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Morrill GA, Schatz F, Kostellow A, Bloch E. Gonadotropin stimulation of steroid synthesis and metabolism in the Rana pipiens ovarian follicle: sequential changes in endogenous steroids during ovulation, fertilization and cleavage stages. J Steroid Biochem Mol Biol. 2006;99:129–138. doi: 10.1016/j.jsbmb.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Noble GK, Aronson LR. The sexual behavior of anura - the normal mating pattern of Rana pipiens. Bull Am Mus Nat Hist. 1942;80:127–142. [Google Scholar]
- Penna M, Capranica RR, Somers J. Hormone-induced vocal behavior and midbrain auditory-sensitivity in the green treefrog, Hyla cinerea. J Comp Phys A. 1992;170:73–82. doi: 10.1007/BF00190402. [DOI] [PubMed] [Google Scholar]
- Picker MD. Hormonal induction of the aquatic phonotactic response of Xenopus. Behavior. 1983;84:74–90. [Google Scholar]
- Rastogi RK, Iela L, di Meglio M, Di Fiore MM, D’Aniello B, Pinelli C, Fiorentino M. Hormonal regulation of reproductive cycles in amphibians. In: Heatwole H, editor. Amphibian Biology Vol 6 Endocrinology. Surrey, Beatty and Sons; Chipping Norton, Australia: 2005. pp. 2045–2177. [Google Scholar]
- Rheinlaender J, Gerhardt HC, Yager D, Capranica RR. Accuracy of phonotaxis in the green treefrog (Hyla cinerea) J Comp Phys. 1979;133:247–255. [Google Scholar]
- Ryan MJ. The Túngara Frog: A Study in Sexual Selection and Communication. University of Chicago Press; Chicago: 1985. [Google Scholar]
- Schmidt RS. Preoptic activation of mating call orientation in female anurans. Behav. 1969;35:114–127. doi: 10.1163/156853970x00150. [DOI] [PubMed] [Google Scholar]
- Schmidt RS. Mating call phonotaxis in the female American toad: induction by hormones. Gen Comp Endocrinol. 1984;55:150–156. doi: 10.1016/0016-6480(84)90139-4. [DOI] [PubMed] [Google Scholar]
- Schmidt RS. Prostaglandin-induced mating call phonotaxis in female American toad: facilitation by progesterone and arginine vasotocin. J Comp Physiol, A. 1985;156:823–829. [Google Scholar]
- Sisneros JA, Forlano PM, Deitcher DL, Bass AH. Steroid dependent auditory plasticity leads to adaptive coupling of sender and receiver. Science. 2004;305:404–407. doi: 10.1126/science.1097218. [DOI] [PubMed] [Google Scholar]
- Thornton VF. A progesterone-like factor detected by bioassay in the blood of the toad (Bufo bufo) shortly before induced ovulation. Gen Comp Endocrinol. 1972;18:133–139. doi: 10.1016/0016-6480(72)90090-1. [DOI] [PubMed] [Google Scholar]
- Urano A, Gorbman A. Effects of pituitary hormonal treatment on responsiveness of anterior preoptic neurons in male leopard frogs, Rana pipiens. J Comp Physiol, A. 1981;141:163–171. [Google Scholar]
- Welch AM, Semlitsch RD, Gerhardt HC. Call duration as an indicator of genetic quality in male gray tree frogs. Science. 1998;280:1928–1930. doi: 10.1126/science.280.5371.1928. [DOI] [PubMed] [Google Scholar]
- Weintraub AS, Kelley DB, Bockman RS. Prostaglandin E2 induces receptive behaviors in female Xenopus laevis. Horm Behav. 1985;19:386–399. doi: 10.1016/0018-506x(85)90036-4. [DOI] [PubMed] [Google Scholar]
- Wingfield JC. Historical contributions of research on birds to behavioral neuroendocrinology. Horm Behav. 2005;48:395–402. doi: 10.1016/j.yhbeh.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Yovanof S, Feng AS. Effects of estradiol on auditory evoked responses from the frog’s auditory midbrain. Neurosci Lett. 1983;36:291–297. doi: 10.1016/0304-3940(83)90015-0. [DOI] [PubMed] [Google Scholar]