Abstract
Improgan, a cimetidine derivative which lacks activity at known histamine, opioid or cannabinoid receptors, acts by an unknown mechanism in the periaqueductal gray (PAG) and raphe magnus (RM) to stimulate descending, analgesic circuits. These circuits may utilize cannabinoid mechanisms. To characterize further the nature of these circuits, the effects of intracerebral (i.c.) microinjections of rimonabant (a CB1 receptor inverse agonist) were studied on antinociceptive responses following i.c. microinjections of improgan and the cannabinoid agonist WIN 55,212 (WIN) in rats. Separate intra-RM injections of improgan (30 μg) and WIN (8 μg) produced near-maximal antinociception on both the hot plate (HP) and tail flick (TF) nociceptive tests. Pretreatment with intra-RM rimonabant (20 μg) antagonized the antinociception produced by both intra-RM improgan and intra-RM WIN, but had no effects when given alone. Similar studies with improgan demonstrated rimonabant-sensitive sites within the dorsal and ventrolateral PAG. However, intra-RM pretreatment with rimonabant had no effect on antinociceptive responses following intra-PAG improgan. These studies show that improgan activates pain-relieving mechanisms in the PAG and the RM, both of which may utilize local cannabinoid mechanisms.
Keywords: pain, analgesia, cannabinoids, rimonabant, periaqueductal gray, improgan
1. Introduction
Improgan, a compound derived from the H2 antagonist cimetidine, produces strong antinociception in rodents following intracerebroventricular (icv) administration (Li et al., 1996). Extensive testing with tail-pinch, tail flick, hot plate, and neuropathic pain assays (Hough, 2004 and in preparation) shows a broad antinociceptive efficacy; a lack of activity by improgan on locomotor and rotorod tests suggest a true analgesic (vs. motor impairment) action (Li et al., 1997). Several congeners of improgan with considerably higher potency and/or brain-penetrating properties have been recently discovered (Hough et al., 2005; Hough et al., 2006; Hough et al., 2007).
More is known about the anatomical sites of improgan action than is known about its mechanism. CNS mapping studies have shown that improgan, like cannabinoids and opioids, acts in the dorsal PAG (DPAG), the ventrolateral PAG (VLPAG), and RM (Nalwalk et al., 2004). Unlike these other analgesics, however, improgan has no direct activity in the spinal cord (Nalwalk et al., 2004). Descending circuits connecting the PAG, RM, and spinal cord are thought to mediate improgan antinociception (Hough et al., 2001). The antinociceptive mechanism is not mediated by opioids (Hough et al., 2000), and improgan lacks activity on known receptors for histamine (Mobarakeh et al., 2003), opioids (Hough et al., 2000), cannabinoids (Hough et al., 2006), and over 100 other receptors (Hough, 2004). Thus, improgan produces non-opioid antinociception by actions in the PAG and RM, but the improgan receptor has not been found.
Evidence is accumulating that a cannabinoid mechanism may be relevant for improgan analgesia. Cannabinoid-blocking doses of rimonabant (SR141716A, the CB1 antagonist/inverse agonist) antagonize improgan antinociception in rats and mice (Hough et al., 2002). However, radioligand binding assays and GTP-γ-S functional assays have confirmed that improgan does not directly bind to, block or activate CB1 or CB2 receptors (Hough et al., 2002, 2006). These findings imply that improgan might indirectly activate CB1 receptors (e.g. by releasing endocannabinoids). Improgan studies with germ-line CB1 null mice gave equivocal results which neither support nor refute the relevance of CB1 receptors (Hough et al., 2002). In a recently-reported study, acute improgan antinociception was reduced by chronic cannabinoid pretreatment, also in support of some type of improgan-cannabinoid interaction (Nalwalk et al., 2006). Improgan was also found to produce mild hypothermia in rats, a response which was attenuated by rimonabant (Salussolia et al., 2007). Very recently, Gehani et al. (2007) described the existence of rimonabant congeners with very low CB1 affinities which retained improgan-blocking properties, suggesting the possible relevance of non-CB1, non-CB2 cannabinoid receptors linked to improgan antinociception. Because the rimonabant-induced antagonism of improgan is an important clue to improgan’s mechanism of action, the present study investigated the rimonabant sensitivity of improgan antinociception following microinjections into three brain stem areas relevant to analgesic circuits.
2. Results
Various combinations of vehicle, improgan, WIN, and rimonabant were administered by i.c. injections into the RM, VLPAG, or DPAG (see Fig. 1 for placements). Microinjections of improgan into the RM and DPAG had no observable untoward effects. Injections of improgan into the VLPAG occasionally produced a motor syndrome previously described as “explosive motor behavior” (EMB), consisting of uncontrolled jumping, stereotyped circling, excessive running, sometimes accompanied by vocalization (Nalwalk et al., 2004). Improgan-induced EMB is further discussed below.
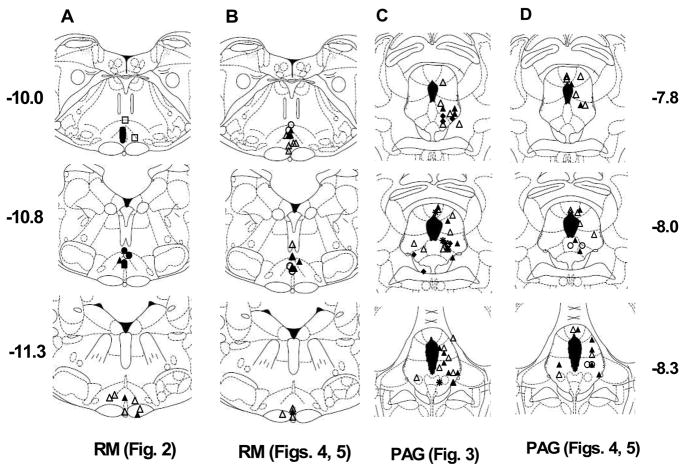
Figure 1.
Placements for all successful brain microinjections. A, B: Raphe magnus (RM) injections for experiments shown in Fig. 2 (A) and Figs. 4–5 (B) are depicted in drawings of three coronal sections (−10.0, −10.8 and −11.3 mm from bregma, labeled far left in A). Actual AP placements ranged from −9.68 to −10.04, −10.3 to −11.0, and −11.3 to −11.6, respectively. C, D: Periaqueductal grey (PAG) injection sites for experiments shown in Fig. 3 (C) and Figs. 4–5 (D) are depicted in drawings of −7.8, −8.0 and −8.3 mm from bregma (labeled far right in D). Actual AP placements ranged from −7.3 to −7.8 (for −7.8 plate) and −8.3 to −9.16 (for −8.3 plate). Placements shown in the left PAG were from improgan-treated subjects displaying mild motor changes (EMB, n=5 in C, n=2 in D, see Results); those drawn in the right PAG showed no such effects. Symbols represent each subject’s experimental group, designated here as in the legends of subsequent figures. Open circle: Veh/Veh; open triangle: Veh/Imp; filled circle: SR/Veh; filled triangle: SR/Imp [Figs. 2, 4, same as SR20/Imp in Fig. 3]; open square: Veh/Win; filled square: SR/Win; asterisk: SR5/Imp; filled diamond: SR40/Imp. Section drawings are from (Paxinos and Watson, 1986).
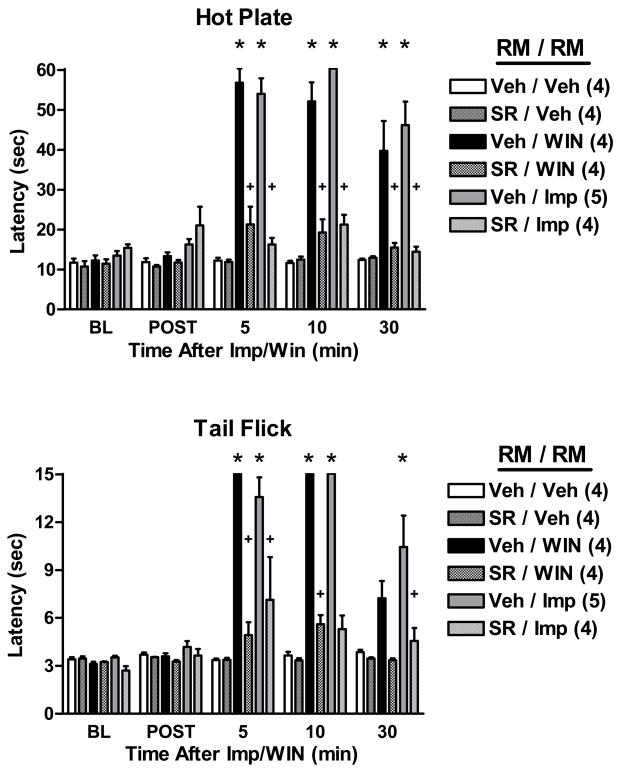
The effects of i.c. rimonabant or vehicle (administered as a pretreatment into the RM) were studied on nociceptive responses following intra-RM administration of improgan, WIN, or vehicle (Fig. 2). In vehicle-pretreated subjects, both improgan (30 μg) and WIN (8 μg) elicited strong reductions in HP and TF nociceptive responding when administered into the RM. Rimonabant pretreatment (20 μg) produced complete or nearly-complete antagonism of both improgan and WIN antinociception (Fig. 2). ANOVA of the HP data of Fig. 2 (between groups #1: rimonabant pretreatment; between groups #2: antinociceptive treatments; within groups [repeated measures]: time) found highly significant (P <0.001) main effects of rimonabant pretreatment, antinociceptive treatments, and time, with a significant (P < 0.001) pretreatment by treatment by time interaction. Identical results were obtained when a comparable ANOVA was performed on the TF data of Fig. 2. Rimonabant given alone did not change nociceptive thresholds (Fig. 2).
Figure 2.
Effects of rimonabant on the antinociception induced by WIN 55,212-2 or improgan in the raphe magnus (RM). Rats with single cannula placements aimed at the RM (Fig. 1) were baseline tested (BL) with both the HP (top) and TF (bottom) assays, then received a microinjection of either vehicle (Veh, 100% DMSO) or rimonabant (SR, 20 μg) and were retested 4.5 min later (POST). Animals then received a second microinjection of Veh (60% DMSO), WIN 55,212-2 (WIN, 8 μg) or improgan (Imp, 30 μg) into the same location. Antinociceptive latencies (s, ordinate, mean ± SEM, number of subjects in parentheses) were recorded 5, 10 and 30 min after the end of the second injection. Times shown on the abscissa (min) reflect the time after the second microinjection. *P < 0.005 vs. Veh/Veh control at the same time point; +P < 0.005 vs. same analgesic treatment and time in the absence of rimonabant.
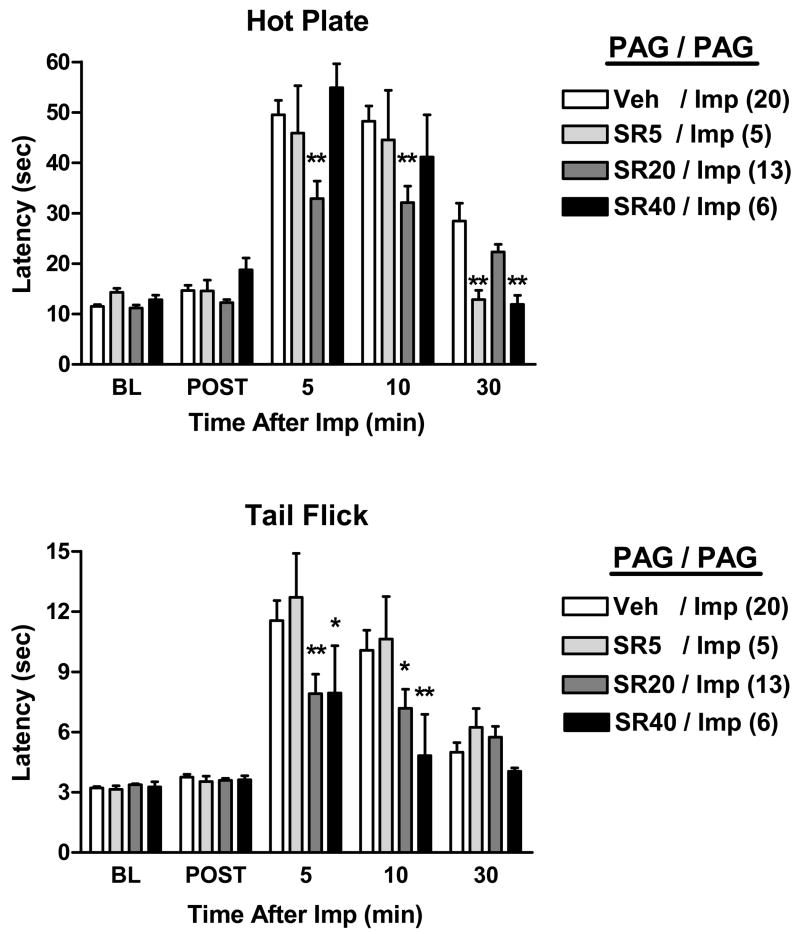
Additional single-cannula experiments were performed with drug injections made into two areas of the PAG (Fig. 3). In these experiments, however, three doses of rimonabant (5, 20 and 40 μg) were studied. ANOVA of the HP data of Fig. 3 (between groups #1: cannula placement [DPAG vs. VLPAG]; between groups #2: rimonabant pretreatment; within groups [repeated measures]: time) found significant main effects of rimonabant (P<0.001) and time (P<0.001), with a significant (P< 0.001) rimonabant by time interaction. The same results were obtained from the comparable ANOVA of the TF data of Fig. 3 (for main effect of rimonabant, P= 0.017). Because there were no significant main effects or interaction terms involving PAG region in either the HP or TF data sets (indicating no differences between the two areas), results from these two regions were pooled (Fig. 3). Following vehicle pretreatments, intra-PAG improgan produced large increases in both HP and TF nociceptive latencies. Intra-PAG pretreatment with the lowest dose of rimonabant (5 μg) was largely without effect (Fig. 3). Pretreatment with the larger dose (20 μg) reduced improgan antinociception by about 50% on both tests at 5 and 10 min after administration (Fig. 3). The largest dose of rimonabant (40 μg) tended to further reduce the antinociception on the TF, but not on the HP test (Fig. 3). A dose-related antagonism of improgan was most evident on the TF test at the 10 min point (Fig. 3).
Figure 3.
Effect of three doses of rimonabant on improgan antinociception in the PAG. Two consecutive drug injections were made through a single cannula located in either the dorsal or ventrolateral PAG (see Fig. 1). Experiments were identical with those described in Fig. 2, except for cannula locations, and all subjects received improgan. Pre-treatment consisted of either vehicle (Veh) or rimonabant (5, 20, or 40 μg, SR5, SR20, SR40, respectively). Data from DPAG and VLPAG experiments showed no significant differences, and were pooled. Results are shown in the format of Fig. 2. *,**P < 0.05, 0.01 vs. Veh/Imp at the same time.
Presently, a total of 50 intra-PAG injections of improgan (DPAG=11, VLPAG=39) produced no EMB in the DPAG, and severe EMB in 5 of the VLPAG injections; data from the latter subjects were not collected. Six other VLPAG subjects showed mild motor changes that did not prevent testing. One of these six had a placement outside of the VLPAG target area and was not used. Data from the other five were included in Fig. 3, (total n= 44); location of injections in these subjects are shown in the left PAG in Fig. 1. Rimonabant pretreatment did not affect the incidence of EMB at either dose level (data not shown).
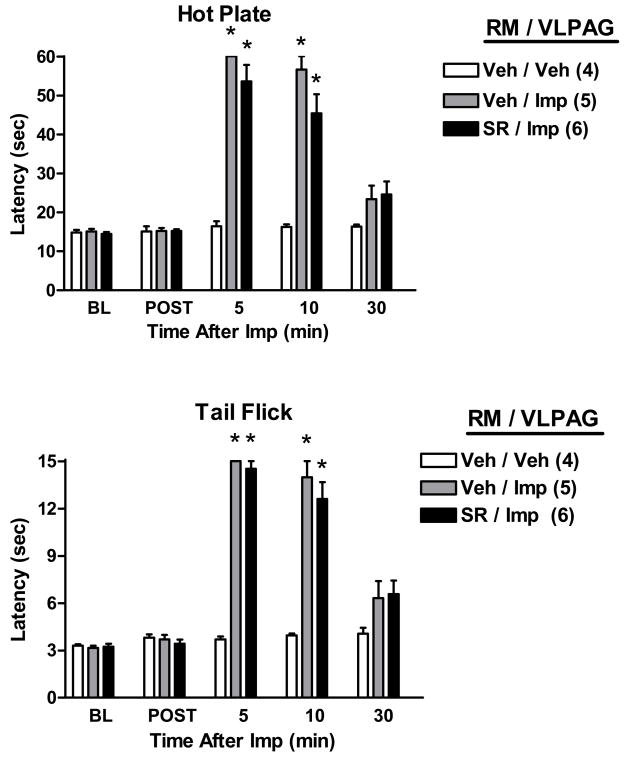
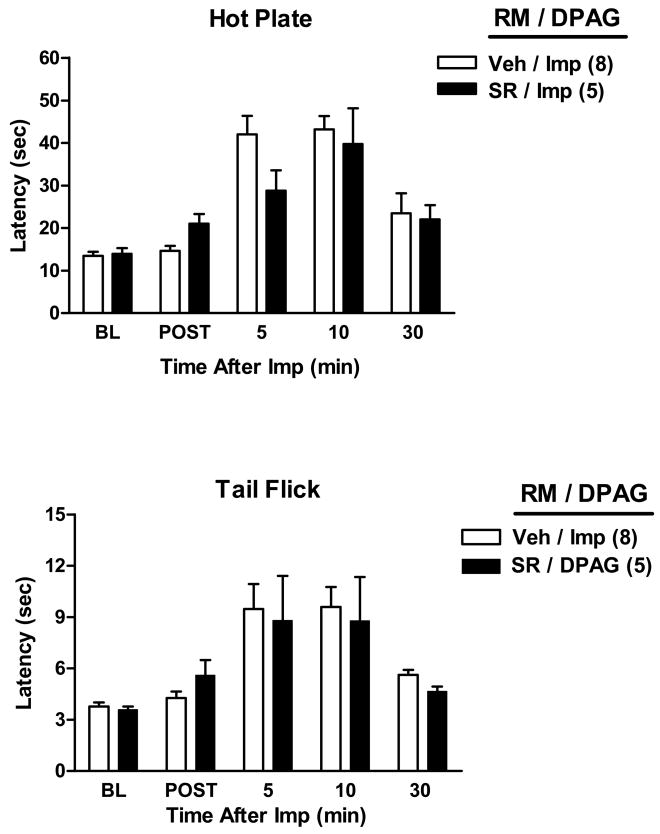
Because of the known significance of PAG-RM circuitry in analgesic mechanisms, the possibility that improgan antinociception elicited from the PAG might have a cannabinoid component in the RM was investigated in double-cannulated subjects (Figs. 4–5). Improgan microinjections into either the VLPAG (Fig. 4) or DPAG (Fig. 5) increased both HP and TF nociceptive latencies. ANOVA of HP data (Fig. 4) from vehicle-RM treated subjects (between groups: VLPAG improgan vs. VLPAG vehicle; within groups [repeated measures]: time) found highly significant (P<0.001) main effects of improgan, time and a significant (P<0.01) improgan by time interaction term. Identical results were obtained with TF data of Fig. 4. However, pretreatment with rimonabant into the RM had no effect on the antinociception elicited by intra-VLPAG (Fig. 4) or DPAG (Fig. 5) improgan injections. For both sets of data (Figs. 4–5), this was substantiated by separate ANOVAs of data from improgan-treated subjects (between groups: pretreatment with rimonabant vs. vehicle; within groups [repeated measures]: time), which found no significant effects of rimonabant (P > 0.05) in either the HP or TF data. Interestingly, in subjects receiving any intra-RM pretreatment, severe EMB was noted following intra-VLPAG improgan (Fig. 4) in 10/21 subjects (48%), data from which were not collected. Two others from the remaining 11 in this group showed mild changes (data from which were included). Locations of the injections in these subjects are shown in the left PAG in Fig. 1D. No EMB was produced in RM-cannulated subjects receiving intra-DPAG vehicle, intra-DPAG improgan, or intra-VLPAG vehicle.
Figure 4.
Effect of rimonabant microinjection into the RM on antinociception induced by improgan microinjection into the ventrolateral PAG (VLPAG). Single drug microinjections were made into two different stereotaxically placed cannulas. Rats were baseline (BL) tested as described above, then received an i.c. injection of either vehicle (Veh, 100% DMSO) or rimonabant (SR, 20 μg) into the RM, and were re-tested 4.5 min later (POST). Animals then received a second microinjection of saline (Veh) or improgan (Imp, 30 μg) into the VLPAG. Results are shown in the format of Figs. 2 and 3. *P < 0.001 vs. Veh RM/Veh VLPAG at the same time point.
Figure 5.
Effect of rimonabant microinjection into the RM on antinociception induced by improgan microinjection into the dorsal PAG (DPAG). Rats were baseline (BL) tested as above, then received an i.c. injection of either vehicle (Veh, 100% DMSO) or rimonabant (SR, 20 μg) into the RM., and were re-tested 4.5 min later (POST). Animals then received a second microinjection of improgan (Imp, 30 μg) into the DPAG. Results are shown in the format of Figs. 2–4
3. Discussion
Extensive characterization of improgan antinociception in rats shows the pre-clinical profile of a highly effective analgesic. However, the lack of this drug’s brain penetration and the poor understanding of improgan’s analgesic mechanism presently limit plans for further development. Antagonism of improgan antinociception by rimonabant (Hough et al., 2002) and other high-affinity CB1 ligands (Gehani et al., 2007) suggests that improgan somehow acts through a brain cannabinoid mechanism, yet extensive in vitro testing found no affinity of this drug for human or rodent CB1 or CB2 receptors (Hough et al., 2002; Hough et al., 2006). In mice, twice-daily THC treatments produced tolerance to cannabinoids which was accompanied by cross-tolerance to improgan, also suggestive of a cannabinoid mechanism (Nalwalk et al., 2006). Most recently, congeners of rimonabant lacking CB1 affinity were shown to reduce improgan and WIN antinociception, suggesting the importance of additional, non-CB1 (possibly cannabinoid) receptors relevant to pain relief (Gehani et al., 2007). Brain CB2 receptors do not produce analgesia when activated, and thus cannot account for brain improgan activity (Ibrahim et al., 2005). It is possible that improgan acts directly, or indirectly (e.g. through endocannabinoid release) on such receptors, but the identity of these targets remains unknown.
Improgan produces antinociception following icv as well as i.c. administration. Extensive i.c. mapping studies found that the drug acts in the RM, the VLPAG and DPAG (all confirmed in the present results), but not elsewhere in the brain or spinal cord (Nalwalk et al., 2004). The lack of antinociceptive activity of intrathecal improgan is in clear contrast to the intrathecal analgesic properties of cannabinoids, and adds further in vivo evidence for a lack of direct CB1 activation by this drug. Improgan actions in the PAG and/or RM are thought to stimulate descending non-opioid analgesic circuits which utilize brain stem GABAergic (Hough et al., 2001) and spinal adrenergic mechanisms (Svokos et al., 2001). Inactivation of RM neural activity with muscimol abolished icv improgan antinociception, showing that the RM is critical for the pain-relieving action of improgan (Nalwalk et al., 2004). Like improgan, cannabinoids also act in the PAG and RM to produce antinociception (Martin et al., 1995). Since icv improgan antinociception is blocked by the cannabinoid antagonist rimonabant (Hough et al., 2002), it was presently of interest to determine the extent to which cannabinoid mechanisms might be relevant to improgan actions in these brain stem areas. The dose of i.c. improgan used presently (30 μg) achieved large, but not supra-maximal antinociceptive responses (Figs. 2–5), consistent with previous i.c. and ivt dose studies (Nalwalk et al., 2004; Hough et al., 2006).
The elimination of nociceptive responses produced by improgan administered into the RM (Fig. 2) confirms the significance of this brain region in improgan action (Nalwalk et al., 2004). Furthermore, the virtually complete antagonism of improgan antinociception by rimonabant given into the RM (Fig. 2) suggests the relevance of cannabinoid mechanisms for improgan’s actions in this brain structure. The antinociceptive activity of WIN, and its antagonism by rimonabant pretreatment in the RM (Fig. 2) confirm similar earlier findings (Rinaldi-Carmona et al., 1995), and show the suitability of these experiments as positive controls.
The present studies also confirm (Nalwalk et al., 2004) that improgan elicits antinociception following i.c. injections into either the dorsal or ventrolateral PAG (Fig. 3). Similar to the results from the double-RM microinjections, antagonism of improgan in the PAG by rimonabant suggests the possible relevance of cannabinoid mechanisms (Fig. 3). Unlike the RM results, however, the 20 μg dose of rimonabant produced incomplete antagonism of improgan in the PAG (Fig. 3). In the TF test, the finding that a higher dose of rimonabant (40 μg) tended to further reverse improgan effects suggests that the PAG-RM difference may simply be related to the dose of rimonabant used.
The i.c. doses of rimonabant used presently (20–40 μg) to block improgan antinociception are somewhat lower than those previously used in i.c. studies to block exogenous cannabinoid antinociception in the PAG (50 μg: Finn et al., 2003) and RM (50 μg: Martin et al., 1998). It is clear, however, that these are large doses as compared with i.c. doses of rimonabant which are effective in other behavioral and physiological studies. For example 1 – 4 μg of i.c. rimonabant modulates drug self-administration (Caille and Parsons, 2006) and evokes penile erection (Succu et al., 2006). Even lower doses (ca. 0.8 μg) significantly reduce non-opioid (endocannabinoid-mediated) stress-induced antinociception when administered into the PAG (Hohmann et al., 2005) or RM (Monhemius et al., 2001;Suplita et al., 2005). Icv/i.c. dose-response studies with exogenous cannabinoids and rimonabant are limited, but suggest that larger doses of the latter are needed to antagonize exogenous cannabinoids as compared with those doses which inhibit endocannabinoid-mediated processes. For example, 20 and 50 μg icv rimonabant produced dose-dependent inhibition of icv WIN55,212 antinociception; the larger dose (50 μg) was needed to completely block maximal cannabinoid antinociception (Hough et al., 2002). Thus, the doses of rimonabant used presently match closely with those required to block exogenous cannabinoid antinociception (also supported in Fig. 2). The requirement for these larger doses may be due to differences in the mechanisms of action of exogenous vs. endogenous cannabinoids, or due to differences in actions produced by widespread delivery of exogenous cannabinoids vs. locally-delivered, physiologically-released cannabinoids. As pointed out, antagonism of improgan antinociception by rimonabant (taken with other recent results, e.g. Nalwalk et al., 2006) may implicate some type of cannabinoid analgesic mechanism, but this mechanism may not be via the CB1 receptor (Gehani et al., 2007) and may or may not utilize endocannabinoids.
The ability of improgan to occasionally elicit wild running and other symptoms of EMB from the VLPAG was observed previously (Nalwalk et al., 2004) but is not well understood. Excitation of the PAG by electrical stimulation (Morgan et al., 1987), amino acids (Finn et al., 2003), or GABAA antagonists (Schmitt et al., 1986) can elicit this syndrome, which may be part of coordinated defensive/escape behavior. Behavioral studies with brain stimulation suggest that it is aversive. Morphine administered into the PAG in moderate to high doses is well-known to elicit EMB symptoms (Yaksh et al., 1976); the lateral PAG (not the VLPAG) is the site which most reliably produces these symptoms (Morgan et al., 1998). Several earlier papers (Blair et al., 1978; Jacquet et al., 1977; LaBella et al., 1979) suggest the possibility that morphine-induced EMB is mediated by an unknown receptor distinct from the analgesia-producing (mu opioid) receptor, findings which await confirmation. NMDA activation via release of excitatory amino acids may be important in morphine-induced EMB (Jacquet and Squires, 1988). Interestingly, no literature has been found suggesting that i.c. cannabinoids can produce EMB-like symptoms. In fact, when administered into the dorsal PAG, cannabinoids block excitatory amino acid-induced EMB (Finn et al., 2003), and reduce contextual fear conditioning (Resstel et al., 2008). Our findings that rimonabant pre-treatment inhibited improgan antinociception in the PAG (Fig. 3), but did not reduce the incidence of improgan-induced EMB seems consistent with the conclusion that cannabinoid receptors do not mediate EMB, and further suggest that improgan-induced antinociception and improgan-induced EMB may have separate mechanisms.
There is substantial evidence indicating the existence of multiple, descending pain-relieving circuits involving the PAG, connections to the RM and nearby nuclei, and subsequent projections to the spinal dorsal horn. Activity in these circuits can exert bidirectional influences on spinal nociceptive transmission (Heinricher and Ingram, 2008). Both opioid and non-opioid mechanisms are subserved by these circuits. Most relevant to the present studies are findings demonstrating that activation of PAG opioid receptors produces antinociception which is attenuated by opioid antagonist injections into the RM (Kiefel et al., 1993; Pan and Fields, 1996). These findings suggest that opioid-mediated activation of PAG-RM transmission results in the release of opioids within the RM, implying the existence of an opioid (PAG)-opioid (RM) circuit (but see Taylor and Basbaum, 2003 for a different interpretation). Because much less is known about the mechanisms of endogenous non-opioid analgesic circuits in the brain stem, it was of interest to investigate the possible existence of a comparable cannabinoid (PAG)-cannabinoid (RM) mechanism elicited by improgan. The present results (Figs 4, 5), showing that rimonabant injections into the RM did not attenuate improgan antinociception elicited from the PAG, find no evidence for such a PAG-RM circuit. Thus, PAG cannabinoid release may accompany improgan actions in the PAG, and a similar local mechanism may exist for improgan actions in the RM, but no evidence was obtained for a cross-regional cannabinoid mechanism. This conclusion seems consistent with the current view that endocannabinoids can participate in retrograde synaptic signaling (Chevaleyre et al., 2006). Further work is needed to precisely identify the improgan receptor and to delineate the relevant endogenous cannabinoid mechanisms.
4. Experimental Procedures
4.1 Animals
Male Sprague-Dawley rats (240 – 340 g, Taconic Farms, Germantown, NY) were maintained on a 12-h light/dark cycle (lights on from 0700 to 1900) with food and water ad libitum. Rats were housed in groups of three or four until the time of surgery and individually thereafter. All animal experiments were approved by the Institutional Animal Care and Use Committee of Albany Medical College.
4.2 Drugs and solutions
Rimonabant base (National Institute on Drug Abuse, Bethesda, MD) was dissolved in 100% DMSO. Several labs (including ours, e.g. Gehani et al., 2007; Hough et al., 2002) have used DMSO as a diluent for icv/i.c. studies without adverse effects. WIN (dosed as mesylate salt; RBI/Sigma, Natick, MA) was dissolved in 60% DMSO/40% distilled, deionized water. Improgan (synthesized as described previously, Hough et al., 2000) was dissolved in 60% DMSO/40% water in experiments including WIN (Fig. 2); otherwise it was dissolved in dilute HCl, neutralized to pH 5.5–6.0, and diluted with saline. Concentrations of these drugs for i.c. microinjections (total volume =0.5 μl) were: improgan (60 μg/μl), WIN (16 μg/μl), and rimonabant (40 μg/μl), resulting in doses of 30 μg, 8 μg, and 20 μg, respectively. In Fig. 3, a higher concentration of rimonabant was also studied (80 μg/μl), resulting in a dose of 40 μg. The i.c. dose of improgan used presently was chosen to produce antinociceptive effects between 80–100% (Nalwalk et al., 2004). I.c. doses of rimonabant and WIN are based on extrapolation from earlier icv dose-response studies (Hough et al., 2002).
4.3 Surgery
Animals were anesthetized with pentobarbital (25 mg/kg, i.p.) and supplemented with isoflurane. Unilateral chronic cannulas were stereotaxically (Paxinos and Watson, 1986) implanted into RM (−11.0, 0.0, −8.5; AP, ML and DV, mm from bregma), DPAG (−7.8, 0.0, −2.5) and VLPAG (−7.8, 1.8, −2.7, 14° angle). In the studies shown in Figs. 2 and 3, single cannulas were implanted into each subject aimed at either the RM or the PAG, respectively. In the remaining experiments (Figs. 4 and 5), individual subjects were implanted with two cannulas: one in the RM, and the other in either the VLPAG (Fig. 4) or DPAG (Fig. 5). Cannulas were anchored to the skull with three stainless steel screws and dental cement (Crane and Glick, 1979). After surgery animals were housed individually with food and water freely available and allowed to recover for at least 5 to 7 days before testing. Each animal was used for a single experiment.
4.4 I.c. injections and nociceptive testing
Two nociceptive tests were used. For the HP test (Eddy and Leimbach, 1953), animals were placed on a 52° C surface and the latency to a hind paw lift or lick was recorded with a maximal exposure of 60 sec. Baseline latencies were 8 to 16 sec. For the TF test (D’Amour and Smith, 1941), the ventral surface of the tail (a randomly selected location 2–5 cm from the tip) was exposed to radiant heat, and the latency for tail movement was recorded. The heat source was set so that baseline latencies were generally between 3 and 4 sec with a 15-sec cutoff; the heat source was not adjusted for individual animals. Subjects were tested with a single, baseline HP test, followed by three TF tests performed at one min intervals, with the third test used as the baseline score. Animals were then gently secured by wrapping with a laboratory pad and the first i.c. injection made. The stylet within the guide cannula was removed, and replaced by an injection cannula extending 1 mm (RM) or 2 mm (PAG) beyond the guide to penetrate the desired areas of the brain. Injections were delivered by a hand-operated Hamilton syringe in a volume of 0.5 μl over a one min period. One min later, wire cutters were used to cut off and seal the injection cannula approximately 2 mm above the juncture with the guide cannula. Single HP and TF latencies were recorded 4.5 min later and a second i.c. injection was made into either the same (Figs. 2, 3) or a different (Figs. 4, 5) site. HP and TF latencies were then recorded at the specified times following the second i.c. injection. Successful i.c. injections were confirmed by movement of an air bubble in the tubing between the syringe and the cannula and by the absence of leakage. Upon completion of experiments, animals received pentobarbital sodium (100 mg/kg, i.p.) followed by an i.c. injection of (0.5 μl) India ink. Brains were removed, frozen, sectioned and dye placement recorded. Data from animals whose placements were outside of those shown in Fig. 1 were discarded. In all cases, the investigator was blinded to the identity of the drug solutions being injected and to the treatment group to which a particular subject belonged.
4.5 Data analysis
Results are expressed as latencies (s, mean ± SEM). One- and two-way analyses of variance (ANOVA)s with one level of repeated measures were used as appropriate. If indicated, Bonferroni post-hoc tests were performed to determine significant differences between groups (Statistica, CSS Inc., Tulsa, OK; and Prism 4.03, Graphpad, Inc., San Diego, CA).
Acknowledgments
This work was supported by a grant (DA-03816) from the National Institute on Drug Abuse.
Abbreviations
- ANOVA
analysis of variance
- DPAG
dorsal periaqueductal gray
- EMB
explosive motor behavior
- HP
hot plate
- i.c
intracerebral
- icv
intracerebroventricular
- PAG
periaqueductal gray
- RM
raphe magnus
- TF
tail flick
- VLPAG
ventrolateral periaqueductal gray
- WIN
WIN55,212
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature References
- Blair R, Cytryniak P, Shizgal P, Amit Z. Naloxone’s antagonism of rigidity, but not explosive motor behavior: possible evidence for two types of mechanisms underlying the actions of opiates and opioids. Behav Biol. 1978;24:24–31. doi: 10.1016/s0091-6773(78)92851-1. [DOI] [PubMed] [Google Scholar]
- Caille S, Parsons LH. Cannabinoid modulation of opiate reinforcement through the ventral striatopallidal pathway. Neuropsychopharmacology. 2006;31:804–813. doi: 10.1038/sj.npp.1300848. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- Crane LA, Glick SD. Simple cannula for repeated intracerebral drug administration in rats. Pharmacol Biochem Behav. 1979;10:799–800. doi: 10.1016/0091-3057(79)90336-8. [DOI] [PubMed] [Google Scholar]
- D’Amour FE, Smith DL. A method for determining loss of pain sensation. J Pharmacol Exp Ther. 1941;72:74–79. [Google Scholar]
- Eddy NB, Leimbach D. Synthetic analgesics, II Dithienylbutenyl and dithienylbutylamines. J Pharmacol Exp Ther. 1953;107:385–393. [PubMed] [Google Scholar]
- Finn DP, Jhaveri MD, Beckett SR, Roe CH, Kendall DA, Marsden CA, Chapman V. Effects of direct periaqueductal grey administration of a cannabinoid receptor agonist on nociceptive and aversive responses in rats. Neuropharmacology. 2003;45:594–604. doi: 10.1016/s0028-3908(03)00235-1. [DOI] [PubMed] [Google Scholar]
- Gehani NC, Nalwalk JW, Razdan RK, Martin BR, Sun X, Wentland M, Abood ME, Hough LB. Significance of Cannabinoid CB(1) Receptors in Improgan Antinociception. J Pain. 2007;8:850–860. doi: 10.1016/j.jpain.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinricher MM, Ingram SL. The Brainstem and Nociceptive Modulation. In: Pain AI, Basbaum MC, Bushnell D, Julius, editors. The Senses: A Comprehensive Reference. Vol. 3. Elsevier; New York: 2008. [Google Scholar]
- Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, Krey JF, Walker JM, Holmes PV, Crystal JD, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–1112. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- Hough LB. Improgan-Like Analgesics: A Family of Compounds Derived From Histamine Antagonists. Med Chem Res. 2004;13:78–87. [Google Scholar]
- Hough LB, De Esch IJ, Janssen E, Phillips J, Svokos K, Kern B, Trachler J, Abood ME, Leurs R, Nalwalk JW. Antinociceptive activity of chemical congeners of improgan: Optimization of side chain length leads to the discovery of a new, potent, non-opioid analgesic. Neuropharmacology. 2006;51:447–456. doi: 10.1016/j.neuropharm.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Hough LB, Menge WM, van de Stolpe AC, Nalwalk JW, Leurs R, De Esch IJ. Antinociceptive activity of furan-containing congeners of improgan and ranitidine. Bioorg Med Chem Lett. 2007;17:5715–5719. doi: 10.1016/j.bmcl.2007.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough LB, Nalwalk JW, Chen Y, Schuller A, Zhu Y, Zhang J, Menge WMPB, Leurs R, Timmerman H, Pintar JE. Improgan, a cimetidine analog, induces morphine-like antinociception in opioid receptor-knockout mice. Brain Res. 2000;880:102–108. doi: 10.1016/s0006-8993(00)02776-1. [DOI] [PubMed] [Google Scholar]
- Hough LB, Nalwalk JW, Lu Q, Shan Z, Svokos K, Wentland MP, Montero MJ. Antinociceptive, brain-penetrating derivatives related to improgan, a non-opioid analgesic. Eur J Pharmacol. 2005;522:38–46. doi: 10.1016/j.ejphar.2005.08.040. [DOI] [PubMed] [Google Scholar]
- Hough LB, Nalwalk JW, Menge WM, Leurs R, Timmerman H. Significance of GABAergic systems in the action of improgan, a non-opioid analgesic. Life Sci. 2001;68:2751–2757. doi: 10.1016/s0024-3205(01)01080-3. [DOI] [PubMed] [Google Scholar]
- Hough LB, Nalwalk JW, Stadel R, Timmerman H, Leurs R, Paria BC, Wang X, Dey SK. Inhibition of improgan antinociception by the cannabinoid (CB)(1) antagonist N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-p yrazole-3-carboxamide (SR141716A): lack of obligatory role for endocannabinoids acting at CB(1) receptors. J Pharmacol Exp Ther. 2002;303:314–322. doi: 10.1124/jpet.102.036251. [DOI] [PubMed] [Google Scholar]
- Ibrahim MM, Porreca F, Lai J, Albrecht PJ, Rice FL, Khodorova A, Davar G, Makriyannis A, Vanderah TW, Mata HP, Malan TP., Jr CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc Natl Acad Sci U S A. 2005;102:3093–3098. doi: 10.1073/pnas.0409888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquet YF, Klee WA, Rice KC, Iijima I, Minamikawa J. Stereospecific and nonstereospecific effects of (+)- and (−)- morphine: evidence for a new class of receptors? Science. 1977;198:842–845. doi: 10.1126/science.199942. [DOI] [PubMed] [Google Scholar]
- Jacquet YF, Squires RF. Excitatory amino acids: role in morphine excitation in rat periaqueductal gray. Behav Brain Res. 1988;31:85–88. doi: 10.1016/0166-4328(88)90161-1. [DOI] [PubMed] [Google Scholar]
- Kiefel JM, Rossi GC, Bodnar RJ. Medullary μ and δ opioid receptors modulate mesencephalic morphine analgesia in rats. Brain Res. 1993;624:151–161. doi: 10.1016/0006-8993(93)90073-v. [DOI] [PubMed] [Google Scholar]
- LaBella FS, Pinsky C, Havlicek V. Morphine derivatives with diminished opiate receptor potency show enhanced central excitatory activity. Brain Res. 1979;174:263–271. doi: 10.1016/0006-8993(79)90849-7. [DOI] [PubMed] [Google Scholar]
- Li BY, Nalwalk JW, Barker LA, Cumming P, Parsons ME, Hough LB. Characterization of the antinociceptive properties of cimetidine and a structural analog. J Pharmacol Exp Ther. 1996;276:500–508. [PubMed] [Google Scholar]
- Li BY, Nalwalk JW, Finkel JM, Glick SD, Hough LB. SKF92374, a cimetidine analog, produces mechanical and thermal antinociception in the absence of motor impairment. Analgesia. 1997;3:15–20. [Google Scholar]
- Martin WJ, Patrick SL, Coffin PO, Tsou K, Walker JM. An examination of the central sites of action of cannabinoid- induced antinociception in the rat. Life Sci. 1995;56:2103–2109. doi: 10.1016/0024-3205(95)00195-c. [DOI] [PubMed] [Google Scholar]
- Martin WJ, Tsou K, Walker JM. Cannabinoid receptor-mediated inhibition of the rat tail-flick reflex after microinjection into the rostral ventromedial medulla. Neurosci Lett. 1998;242:33–36. doi: 10.1016/s0304-3940(98)00044-5. [DOI] [PubMed] [Google Scholar]
- Mobarakeh JI, Nalwalk JW, Watanabe T, Sakurada S, Hoffman M, Leurs R, Timmerman H, Silos-Santiago I, Yanai K, Hough LB. Improgan antinociception does not require neuronal histamine or histamine receptors. Brain Res. 2003;974:146–152. doi: 10.1016/s0006-8993(03)02572-1. [DOI] [PubMed] [Google Scholar]
- Monhemius R, Azami J, Green DL, Roberts MH. CB1 receptor mediated analgesia from the Nucleus Reticularis Gigantocellularis pars alpha is activated in an animal model of neuropathic pain. Brain Res. 2001;908:67–74. doi: 10.1016/s0006-8993(01)02605-1. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Depaulis A, Leibeskind JC. Diazepam dissociates the analgesic and aversive effects of periaqueductal gray stimulation in the rat. Brain Res. 1987;423:395–398. doi: 10.1016/0006-8993(87)90870-5. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Whitney PK, Gold MS. Immobility and flight associated with antinociception produced by activation of the ventral and lateral/dorsal regions of the rat periaqueductal gray. Brain Res. 1998;804:159–166. doi: 10.1016/s0006-8993(98)00669-6. [DOI] [PubMed] [Google Scholar]
- Nalwalk JW, Svokos K, Hough LB. Cannabinoid-improgan cross-tolerance: Improgan is a cannabinomimetic analgesic lacking affinity at the cannabinoid CB(1) receptor. Eur J Pharmacol. 2006;549:79–83. doi: 10.1016/j.ejphar.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Nalwalk JW, Svokos K, Taraschenko O, Leurs R, Timmerman H, Hough LB. Activation of brain stem nuclei by improgan, a non-opioid analgesic. Brain Res. 2004;1021:248–255. doi: 10.1016/j.brainres.2004.06.066. [DOI] [PubMed] [Google Scholar]
- Pan ZZ, Fields HL. Endogenous opioid-mediated inhibition of putative pain-modulating neurons in rat rostral ventromedial medulla. Neurosci. 1996;74:855–862. doi: 10.1016/0306-4522(96)00179-0. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; Sydney: 1986. [Google Scholar]
- Resstel LB, Lisboa SF, Aguiar DC, Correa FM, Guimaraes FS. Activation of CB1 cannabinoid receptors in the dorsolateral periaqueductal gray reduces the expression of contextual fear conditioning in rats. Psychopharmacology (Berl) 2008;198:405–411. doi: 10.1007/s00213-008-1156-1. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Heaulme M, Alonso R, Shire D, Congy C, Soubrie P, Breliere JC, Le Fur G. Biochemical and pharmacological characterisation of SR141716A, the first potent and selective brain cannabinoid receptor antagonist. Life Sci. 1995;56:1941–1947. doi: 10.1016/0024-3205(95)00174-5. [DOI] [PubMed] [Google Scholar]
- Salussolia CL, Nalwalk JW, Hough LB. Improgan-induced hypothermia: A role for cannabinoid receptors in improgan-induced changes in nociceptive threshold and body temperature. Brain Res. 2007;1152:42–48. doi: 10.1016/j.brainres.2007.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt P, Carrive P, Di Scala G, Jenck F, Brandao M, Bagri A, Moreau J-L, Sandner G. A neuropharmacological study of the periventricular neural substrate involved in flight. Behav Brain Res. 1986;22:181–190. doi: 10.1016/0166-4328(86)90039-2. [DOI] [PubMed] [Google Scholar]
- Succu S, Mascia MS, Melis T, Sanna F, Boi A, Melis MR, Argiolas A. Morphine reduces penile erection induced by the cannabinoid receptor antagonist SR 141617A in male rats: role of paraventricular glutamic acid and nitric oxide. Neurosci Lett. 2006;404:1–5. doi: 10.1016/j.neulet.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Suplita RL, Farthing JN, Gutierrez T, Hohmann AG. Inhibition of fatty-acid amide hydrolase enhances cannabinoid stress-induced analgesia: sites of action in the dorsolateral periaqueductal gray and rostral ventromedial medulla. Neuropharmacology. 2005;49:1201–1209. doi: 10.1016/j.neuropharm.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Svokos K, Nalwalk JW, Leurs R, Menge WM, Timmerman H, Hough LB. A role for spinal, but not supraspinal, alpha2 adrenergic receptors in the actions of improgan, a powerful, non-opioid analgesic. Brain Res. 2001;921:12–19. doi: 10.1016/s0006-8993(01)03191-2. [DOI] [PubMed] [Google Scholar]
- Taylor BK, Basbaum AI. Systemic morphine-induced release of serotonin in the rostroventral medulla is not mimicked by morphine microinjection into the periaqueductal gray. J Neurochem. 2003;86:1129–1141. doi: 10.1046/j.1471-4159.2003.01907.x. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Yeung JC, Rudy TA. Systematic examination in the rat of brain sites sensitive to the direct application of morphine: observation of differential effects within the periaqueductal gray. Brain Res. 1976;114:83–103. doi: 10.1016/0006-8993(76)91009-x. [DOI] [PubMed] [Google Scholar]