Abstract
We aimed to test the feasibility of detecting gliosis in living brains when the blood-brain barrier (BBB) is disrupted. We designed a novel magnetic resonance (MR) probe that contains superparamagnetic iron oxide nanoparticles (SPION, a T2 susceptibility contrast agent) linked to a short DNA sequence complementary to the cerebral mRNA of glial fibrillary acidic protein (GFAP) found in glia and astrocytes. As a control, we also used a sequence complementary to the mRNA of β-actin. Our objectives are to demonstrate that this new probe, SPION-gfap, could be delivered to the brain when administered by eyedrop solution to the conjunctival sac. We induced BBB leakage by puncture wound, global cerebral ischemia, and cortical spreading depression in C57BL6 mice; 1 day after probe delivery we acquired T2* MR images and R2* (R2*=1/T2*) maps using a transcription MRI technique in live mice. We found that the SPION-gfap probe reported foci with elevated signal in subtraction R2* maps and that these foci matched areas identified as having extensive glial network (gliosis) in postmortem immunohistochemistry. Similarly, animals administered the control probe exhibited foci of R2* elevation that matched β-actin-expressing endothelia in the vascular wall. We conclude that our modular MR probe, delivered in an eyedrop solution, effectively reports gliosis associated with acute neurological disorders in living animals. As BBB leakage is often observed in acute neurological disorders, this study also served to validate noninvasive delivery of MR probes to the brains of live animals after acute neurological disorders.
Keywords: angiogenesis, antisense technology, blood-brain barrier, bulbar conjunctiva, gene expression, molecular imaging
Gliosis, the outgrowth of a fibrous network of glia in the central nervous system (CNS), is a permanent feature of many human neurological disorders and is especially prevalent in glioma, multiple sclerosis, viral encephalitis, traumatic brain injury, stroke, and cardiac arrest (1–5). Detection of gliosis or cells of abnormal growth has traditionally involved immunohistochemistry methods to detect elevated antigen of glial fibrillary acidic protein (GFAP) levels in postmortem tissue samples (6–8). However, brain biopsy to acquire tissue for these conventional assays is by itself invasive, and the procedure often results in the removal of important tissue.
MRI is a powerful and noninvasive tool for in vivo imaging of soft tissue (9–12) but is somewhat limited by the unavailability of suitable probes for the CNS. We have designed modular MR probes capable of reporting specific cells based on gene transcription in living brains of ordinary subjects (8, 13, 14). In several proof-of-concept studies, we have developed and validated the delivery and neuronal uptake of modular MR probes in the cerebrospinal fluid after intracerebroventricular (i.c.v.) infusion. Although this method of delivery is invasive, we have demonstrated its effectiveness for mRNA reporting and probe distribution through the Virchow-Robins space. Others have reported that the lymphatic system assists the circulation of peritoneal fluid (15) and preferentially retains superpara-magnetic iron oxide nanoparticles (SPION) (16). Lymphatic vessels are present in the eyelid and bulbar conjunctiva (17–20), and the lymphatic fluids eventually merge with circulating blood in the vena cava for redistribution in the arterial vessels. Given that MR contrast agent can be delivered to the brain cells via arterial injection when the BBB is disrupted (21), we hypothesize that an MR probe can be distributed via the lymphatic system, either by intraperitoneal (i.p.) injection or by eyedrop solution to the conjunctival sac, in C57BL6 mice that experience BBB leakage. Here, we demonstrate noninvasive delivery of MR contrast agent to the brains of live mice, using a modular MR probe that targets the gene transcript of GFAP in glia and astrocytes and a control complementary to the mRNA of β-actin. The ability to deliver an MR contrast probe noninvasively and to label cells based on their intracellular gene transcripts will open the door to myriad applications in the brain and throughout the body.
MATERIALS AND METHODS
All procedures and animal care practices adhered strictly to Association for Assessment and Accreditation of Laboratory Animal Care, Society for Neuroscience, and institutional guidelines for experimental animal health, safety, and comfort.
General surgical preparation
Male C57BL6 mice (24±3 g; Taconic Farms, Germantown, NY, USA) were housed under diurnal lighting conditions and allowed food and tap water ad libitum. The mice were anesthetized with ketamine (80 mg/kg, i.p.) and xylazine (12 mg/kg, i.p.) prior to surgical procedures. We induced BBB leakage by one of three methods: by cortical spreading depression, by inflicting a puncture wound and administering an i.c.v. injection of 2 μl saline in a Hamilton syringe with a 26S gauge needle to the left cerebral ventricle (14), or by performing bilateral carotid artery occlusion (BCAO) for 60 min (8, 13). Another group of animals underwent a sham operation (the same surgical procedure without the puncture wound or vessel occlusion for cerebral ischemia) at the same time. Animals that underwent the BCAO procedure typically experienced a 25% drop in body weight within the first 3 days and gradually recovered to pre-BCAO weight in 5 days (unpublished observations); we therefore delayed the probe delivery and procedures for gene activity assays using transcription MRI (tMRI) until 5 days after surgery.
Cortical spreading depression
Under isoflurane anesthesia (2.5% induction, 1% maintenance, in 70% N2O/30% O2), we used a slightly modified topical KCl application method (22, 23) to assess cortical spreading depression (CSD) susceptibility in spontaneously breathing mice. With the anesthetized mouse positioned in a stereotactic frame (David Kopf Instruments, Tujunga, CA, USA), we drilled 2 burr holes under saline cooling at the noted coordinates to apply KCl to the occipital cortex (anterior-posterior=−1.5 mm; left-right=2 mm from bregma; diameter=2 mm) and at recording site 1 (anterior-posterior=1 mm; left-right=−2 mm from bregma; diameter=1 mm). The dura was kept intact to minimize trauma. We placed a glass microelectrode at a depth of 300 μm to record extracellular steady potential (DC) and electrocorticogram (ECoG). The reference (Ag/AgCl) electrode was placed subcutaneously in the neck. After surgical preparation, we irrigated the cortex with saline for 20 min, allowing it to recover. A cotton ball (2-mm diameter) soaked with 300 mM KCl was placed on the dura to induce CSD, after which we gently washed the cortex with saline. After 8 min, we induced another CSD using the same method. A total of 3 CSDs were elicited in each mouse. Mice in the sham group underwent the same surgical procedure, using the same anesthesia protocol; however, the cortex was exposed to saline only, so as not to elicit CSD. Data were continuously recorded using a data acquisition system for off-line analysis (Power Lab Instruments, Colorado Springs, CO, USA). We measured the DC shift amplitude, duration at half-maximal amplitude, and maximum onset slope. Potassium chloride-evoked CSDs were detected by a characteristic slow DC potential shift and ECoG suppression.
Conjugation of biotinylated sODN to SPION-NA and immunohistochemistry
We synthesized 5′-biotin-labeled antisense phosphorothioate-modified oligodeoxynucleotides (sODNs) for glia and astrocytes (sODN-gfap; 5′-gtctccgctccatcctgccc-3′) to GFAP mRNA of the mouse (24), using methods previously reported for sODN-cfos, sODN-β-actin, and sODN-Ran; the sequences of these probes have also been reported previously (8, 13). We used phosphorothioate modification of all nucleotide bridges to protect the single-stranded ODNs from nonspecific nucleases, and the resulting sODNs were purified by polyacrylamide gel electrophoresis. For histological evidence of uptake, we labeled the sODNs with fluorescein isothiocyanate (FITC). We counterstained postmortem brain tissue with murine monoclonal antibodies against neurin- and Cy-3-labeled rabbit anti-mouse IgG to reduce green autofluorescence. We detected cells expressing GFAP or β-actin using rabbit IgG against GFAP or β-actin and Cy-3-labeled polyclonal anti-rabbit IgG (8, 13), and cerebral vascular endothelia using Griffonia simplicifolia lectin 1-conjugated FITC (Vector Laboratories, Burlingame, CA, USA) (25).
Preparation of the SPION-sODN
Preparation of the probe complex and MRI acquisition were performed using methods previously described (8, 13), except freshly synthesized SPION was functionalized with chloroethylamine (2 M) in 1.5N NaOH solution, and linked to NeutrAvidin (NA) in the presence of 1 M sodium cyanoboro-hydride (both from Pierce Biotechnology, Rockford, IL, USA). The resulting covalently linked product, SPION-NA, was filtered and dialyzed against a 20× volume of sodium citrate buffer solution (25 mM, pH 8.0), using a Centricon Plus-100 filter (100 kD cutoff, Millipore Corp., Bedford, MA, USA). The activated SPION (SPION-NA) was stored in an amber-colored bottle at 4°C, at a concentration of 3–4 mg Fe/ml sodium citrate buffer. We mixed 100 μl SPION-NA (3 mg Fe/ml or 9 nmol SPION/ml) with sODN or sODN-FITC (1.8 nmol) on ice for 1 h. Immediately before application, we added 4 μl lipofectamine 2000 (1 mg/ml; Invitrogen Life Sciences, Carlsbad, CA, USA). A total of 0.1 ml of the MR probe solution was i.p. injected into each mouse.
Ophthalmic route of delivery
We delivered SPION-sODN-FITC (4 mg Fe/kg in 0.1 ml and lipofectamine, 0.05 mg/ml) by eyedropper (10 μl every 10 min under ketamine (100 mg/kg) and xylazine (10 mg/kg) anesthesia i.p.).
MRI protocol
MRI assessment of SPION retention in the regions of interest (ROIs) had been reported (8, 13). To evaluate the level of probe retention, we calculated the mean R2* values in the cortex, hippocampus, and striatum. The average R2* values and SEM from each of these groups were obtained and analyzed statistically (14). To identify ROI with cell expressing elevated GFAP or actin antigen (MR cell typing), we identified regions where SPION retention was significantly above the preinfusion baseline of the same mouse using R2* maps. The hotspots were identified by referencing the stereotactic coordinates of hippocampus in C57BL6 mouse brains (26) and confirmed by immunohistochemistry examinations. We acquired diffusion-weighted MRI (DWI) with parameters as follows: TR/TE = 3000/25 ms, two b values of 154 and 1294 s/mm2 along the z direction, 8 repetitions, Δ = 12 ms, δ = 8 ms, 180 × 180 μm2 in-plane resolution, and 1 mm slice thickness for assessment of tissue injury. Maps of the apparent diffusion coefficient (ADC) were calculated by fitting the DWI images to the equation M = Mo × exp (−b ADC) using MRVision (Winchester, MA, USA). For the detection of BBB leakage, animals were scanned before the injection of Gd-DTPA (Magnevist; Shering, Berlin, Germany) using T1-weighted three-dimensional spin echo images (TR/TE=400/11 ms, 120×120 μm2 in-plane resolution and 0.5 mm thickness, NA=2). Gd-DTPA was administered to the jugular vein (0.1 mM/kg) and mice were imaged within 10 min. Extensive areas of enhanced T1 signals compared to the pre-Gd scans were considered to be areas of the brain which were leaky.
Statistical analysis
We performed a power calculation on the data gathered from the first set of animals, using in-house software to calculate the number of animals required in each group to achieve 90% power for a P value of 0.01 (8, 13). If the minimum number of animals calculated was greater than 15, we revised the hypothesis and the protocol. We repeated each study using at least one mouse more in each treatment group than indicated by the power calculation. After we had acquired data from the minimum number of animals, we computed the mean and SEM from the averaged values in each group and compared the statistical significance of these values using a t test (one-tail, type II or equal variant, GraphPad Prism IV, GraphPad Software, San Diego, CA, USA). A value of P < 0.05 was considered statistically significant.
RESULTS
We aimed to develop a novel assay using MRI and modular MR probes to image and target cells with specific mRNA transcript. When probes were delivered to the cerebrospinal fluid via i.c.v. injection in our proof-of-concept study, we observed distribution of the probe to the brain cells via the perivascular (Virchow-Robins) space (8, 13), suggesting the lymphatic system may participate in the distribution of MR probe in the brain. MR contrast agent can be delivered to the brain by intravascular injection when the BBB is disrupted (21). Knowing that neurological disorders induce BBB leakage and that the intraperitoneal fluid in the lymphatic vessels merges with blood in the vascular system, we hypothesized that our MR contrast probe could be effectively and noninvasively distributed in the living brains of C57BL6 mice if BBB leakage occurred after cerebral ischemia. Figure 1A shows the protocol for inducing BBB leakage that mimics conditions of acute neurological disorders. Figure 1B demonstrates that 60-min BCAO in one of three C57BL6 mice induces BBB leakage within 3 h of reperfusion in the cortex and striatum in both hemispheres. In a separate group of animals, we detected hyperintense DWI at 1 or 2 days after BCAO (Fig. 2A); mice that underwent sham operation did not exhibit such hyperintense DWI (Fig. 2B). Although hyperintense DWI is reversible, BBB leakage developed in the regions showing hyperintense DWI after BCAO in C57BL6 mice (Savitz et al., unpublished data).
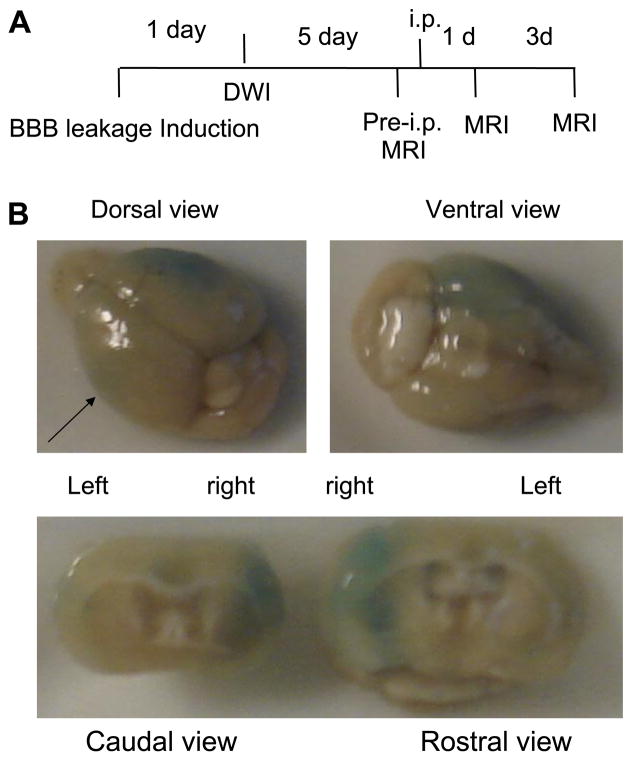
Figure 1.
Leakage of the BBB after acute neurological disorders as determined by Evans blue extravasation. A) Protocol using noninvasive delivery of SPION-sODN after BBB leakage induction (induced by puncture wound, CSD, or 60-min cerebral ischemia using BCAO). B) Brain injury demonstrated by Evans blue (1%, 0.1 ml, i.v.) at 30 min reperfusion following 60 min of BCAO. Postmortem brains were excised for photography 3 h later (n=3). Arrow points to the line of cutting to reveal intraparenchymal Evans blue extravasation, shown in the bottom panel.
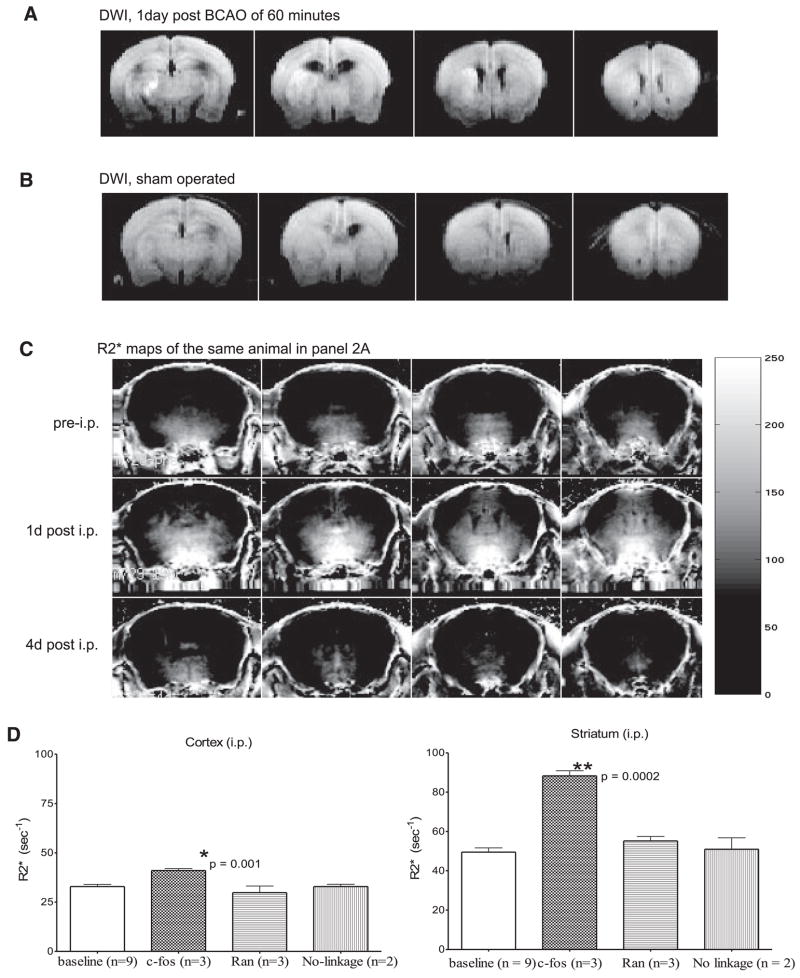
Figure 2.
Noninvasive delivery of SPION-cfos and SPION-Ran by i.p. injection. The caudal view of ischemia-induced abnormal water diffusion resulting in DWI hyperintensity 1 day after BCAO (A) compared to a sham-operated animal (B). SPION-sODN (10 mg Fe/kg in 0.1 ml, i.p.) was delivered on the 5th day after BCAO, and tMRI was acquired the next day. Temporal retention (R2* maps) of SPION-cfos at baseline (pre-i.p.) and 1 and 4 days after i.p. (1d and 4d post i.p., respectively) are shown (C). We also tested the uptake of other tMRI probes. The baseline R2* maps were statistically analyzed with those of SPION-cfos retention in the striatum and cortex (D) and revealed no statistical difference in SPION retention after SPION-Ran or mixed SPION/sODN (1 day after i.p.) and pre-i.p. R2* values (expressed as s−1 or R2*=1/T2*).
To test SPION-sODN delivery to the brain in subjects that experienced BBB leakage, we conjugated NA-labeled SPION to biotinylated and phosphorothioate-modified oligodeoxynucleotides (biosODN) complementary to c-fos mRNA (sODN-cfos) or with a random sequence (sODN-Ran, no cellular target). We delivered SPION-cfos (≤10 mg Fe/kg) by i.p. injection 5 days later. We acquired T2* MRI the day after injection and, because R2* is positively correlated with intracellular iron oxide in the mouse brain, constructed R2* maps of SPION-cfos retention (14). As shown in Fig. 2C, R2* levels in the striatum increased 1 day after delivery and then diminished 4 days later to a level not different from that measured prior to i.p. injection. We found significant retention of SPION-cfos in the striatum and cortex, but retention of SPION-Ran was not significant when compared to the baseline R2* maps (Fig. 2D). We also tested the retention of an MR probe without linkage between sODN-cfos and SPION (SPION and sODN-cfos were mixed, but not linked); we observed no uptake.
These data suggest that the fluid in the peritoneal cavity most likely distributes SPION-sODN by virtue of the eventual merging of the lymphatic and vascular circulations, aiding the MR probe in reaching the brain cells through BBB leakage. Given that lymphatic vessels exist in the stroma under the bulbar conjunctiva (17–20), we chose to examine the feasibility of imaging and targeting intracellular mRNA using our novel contrast agent and MRI after administering the probe via an ophthalmic route of delivery (OTRD). We selected an MR probe targeting the mRNA of β-actin for the distribution assay because its transcripts are not drastically altered immediately after brain injury. We aimed to apply MRI with OTRD to evaluate probe delivery after cortical spreading depression (n=4 pairs), which has been shown to induce microleakage in the BBB (22). Figure 3A shows SPION-β-actin retention in the brains of live mice 5 days after CSD episode. Retention of SPION–sODN was observed only for mice with CSD; no retention was observed in sham-operated mice. Figure 3C shows subtraction R2* maps of one representative animal from 3A, revealing elevated R2* using a computer-generated pixel scale (0–150% with an increment of 25%). The ROI in the cortex, hippocampus, and striatum show a significant increase in R2* (t test, Fig. 3B). Validating the retention of SPION–sODN, Fig. 3D shows histological evidence of sODN–β-actin-FITC uptake in the neuron where β-actin is expressed. Probe uptake occurred in mice that underwent CSD induction, but not in those that had the sham operation. We have demonstrated a novel noninvasive technique for MRI and gene transcription in living animals.
Figure 3.
Ophthalmic route of delivery of MR contrast agent for transcription MRI. Targeting agents were applied to both eye sacs in anesthetized animals 5 days after CSD induction using an Eppendorf pipette (5 μl per eye every 10 min). A) Caudal view of SPION-β-actin uptake in the brain. Four contiguous brain slices from posterior regions to anterior regions are shown. B) Significant uptake of SPION-β-actin in animals with CSD. Power calculation using the mean and SE (90% power at P=0.05) indicated that we would need at least one pair of animals (n=2) for this analysis, we chose to use 4 animals for two-paired analysis. C) One representative animal with elevated R2* in subtraction R2* maps (post-OTRD MR minus pre-OTRD MR of CSD-1 in A times 100%) with a computer-generated pixel scale. Postmortem brain samples were obtained after tMRI and stained red using Cy-3 mouse monoclonal IgG to provide enhanced contrast for FITC with SPION-β-actin (yellow). D, E) Histology of CA1 neuronal formation in the hippocampus of one representative mouse 1 day after SPION-β-actin-FITC delivery to CSD-1 (D) and sham-operated (E) mice. Color scale = 0–150 s−1 (C).
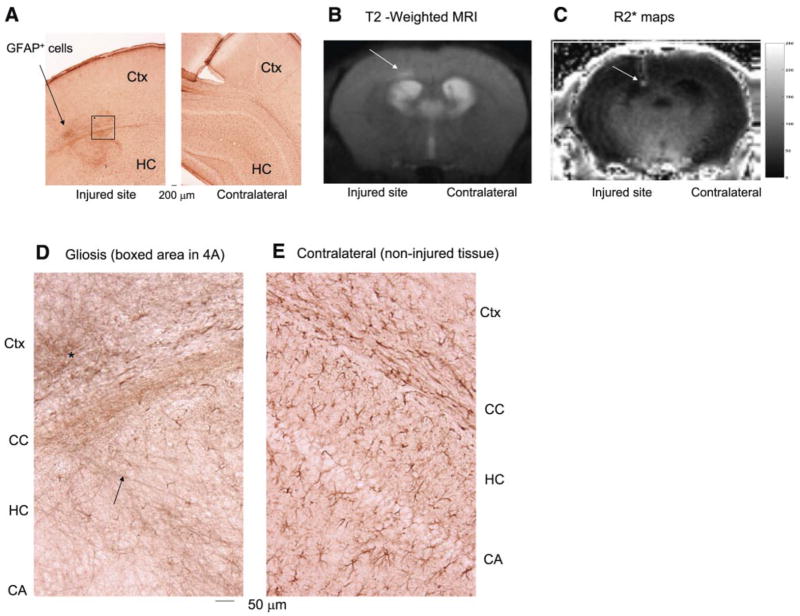
Having demonstrated the uptake of the SPION-β-actin probe in neurons after CSD, we chose to examine the feasibility of using OTRD and transcription MRI (tMRI) to target and image intracellular mRNA a few weeks after acute neurological disorder. We induced BBB leakage in the left (ipsilateral) hemisphere using i.c.v. puncture in six mice; 3 days later, we sacrificed three of the animals for histological examination of gliosis around the injured site. We found that all had gliosis around the injured site (arrow, Fig. 4A). This model allows comparisons of injured ROI and noninjured hemispheres in the same brain. In the remaining three mice, we acquired T2-weighted MRI 8 wk after i.c.v. puncture and observed no remarkable lesion in the left cortex compared to the right, except possible water retention at the injured site (arrow, Fig. 4B). We waited for 8 weeks after injury so as to avoid detecting reactive gliosis or enhanced macrophages at the injured site, which may elevate R2*. We made a short DNA sequence complementary to GFAP mRNA of the mouse (17–20) and delivered the MR probe (SPION-gfap) in an eyedrop solution via the eyelid and bulbar conjunctiva. R2* maps 1 day after OTRD of SPION-gfap revealed regions with hyperintense R2* at the injury site in all three animals (arrows, Fig. 4C). This hyperintense focal R2* region after SPION-gfap was consistent with immunohistochemistry results showing genuine gliosis (arrow, Fig. 4A) and tangles of GFAP-positive cells in the tissue surrounding the wound in the ipsilateral cortex (asterisk, Fig. 4D), but normal patterns of GFAP-positive cells in the contralateral hemisphere (Fig. 4E). Hyperintense R2* maps were no longer detectable in MRI repeated 1 wk later. Our data presented here support our assertion that OTRD can effectively deliver SPION-sODN to the brain that experiences BBB disruption.
Figure 4.
SPION-gfap detects gliosis after ophthalmic route of delivery. A) Histology of gliosis in this puncture-wound model: we used alkaline phosphotase-labeled antibodies against GFAP and nitro blue tetrazolium/5-bromo-4-chloro-3′-indolylphosphate p-toluidine (NBT/BCIP) stains to detect glia and astrocytes. Postmortem histology shows gliosis in the tissue surrounding an intracranial puncture site (arrow) in the cortex (Ctx) and hippocampus (HC). B) T2-weighted MRI (Spin echo RARE, TR/TE=3000/27 ms, 120×120 μm2, 0.5 mm thickness) 8 wk after puncture wound. C) R2* maps of the MRI in B. D, E) Comparison of puncture wound-induced gliosis and normal distribution of glia, respectively, in the Ctx, corpus callosum (CC), HC and pyramidal neuronal layer (CA). Color scale = 0–250 s−1 (C).
To apply OTRD for detecting gliosis after neurological disorders, we investigated gliosis reporting using SPION-gfap in two groups of animals that had experienced either cerebral ischemia (n=7) or sham operation (n=4). We observed hyperintense DWI/ADC drop 1 day after cerebral ischemia in all seven of the ischemic mice; hyperintense DWI/ADC drop was bilateral in five of the mice, unilateral in two. Because C57BL6 mice have defective posterior communicating arteries, unilateral hyperintense DWI in this strain of mice is considered to arise from unilateral deficit in this communicating circulation. Investigating this link will be a focus of our future work. One representative mouse with unilateral hyperintense DWI was selected for illustration purposes, so that we could compare injured and less-injured hemispheres in the same mouse (Fig. 5Ai).
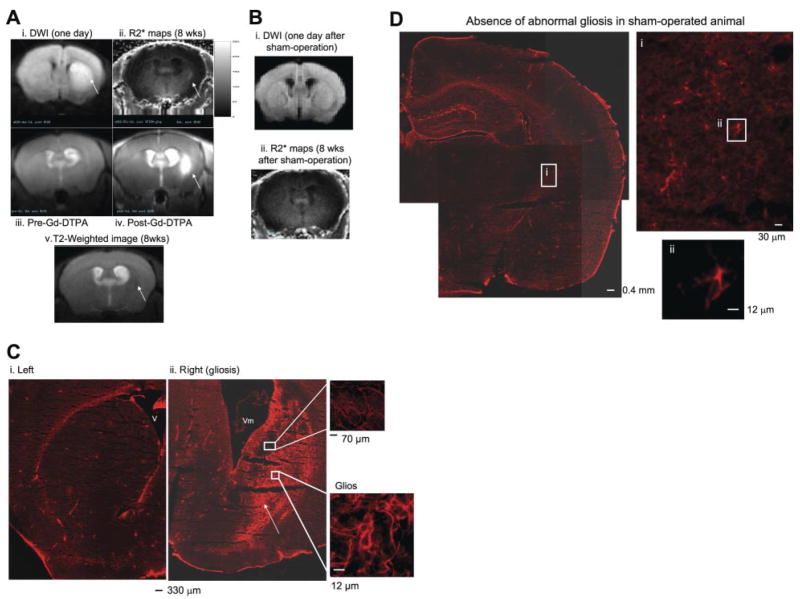
Figure 5.
SPION-gfap detects gliosis in live mice after acute neurological disorder using transcription MRI. We treated C57BL6 mice with BCAO (60 min) to induce BBB leakage. The control group was similarly treated, except there was no vessel occlusion (sham operation). DWI/ADC MRI was obtained at 1 day of reperfusion (Ai, Bi). Eight weeks later, we applied SPION-gfap in an eyedrop solution and acquired T2-weighted MRI from the same mouse (Av), and R2* maps were obtained the next day for the same mouse (Aii) and a sham-operated mouse (Bii). Evidence of BBB leakage is shown by pre- and post Gd-DTPA (0.1 mmol/kg, i.v.) and T1-weighted MRI in vivo (Aiii, iv) 9 wk after BCAO. In the postmortem samples obtained after all in vivo data acquisitions in the same mouse, a cohort of GFAP-expressing cells (Cy-3-labeled anti-rabbit IgG and rabbit anti-GFAP) is located in the right (Cii), but not in the left striatum of postmortem samples from the same mouse (Ci). A normal pattern of GFAP-expressing cells in a sham-operated mouse (n=4) is shown in D. Arrows indicate DWI hyperintensity (Ai), possibly injured tissue (Av), elevated R2* signal (Aii), BBB leakage (Aiv), and gliosis (Cii). Color scale = 0–250 s−1 (A).
Again, to avoid possible complications of elevated GFAP expression during reactive gliosis and enhanced macrophages, which may adversely affect probe delivery immediately following acute neurological disorders, we delivered SPION-GRAP 8 wk after 60 min BCAO. DWIs obtained 1 day postischemia show metabolic disturbance in expanded areas of the striatum of all seven animals; we observed no obvious abnormal T2-weighted MR images at this time point, although we did observe ventriculomegaly in the hemisphere ipsilateral to hyperintense DWI (arrow in Fig. 5Av). After delivering SPION-gfap in an eyedrop solution and allowing 1 day for uptake and distribution, we found not only hyperintense R2* maps in the brains of all animals with BCAO, but also foci of elevated retention in the ipsilateral striatum (arrow, Fig. 5Aii), where BBB leakage was validated using Gd-DTPA 1 wk later (Fig. 5Aiii, iv). We observed matching locations of hyperintense DWI, focal SPION retention, and BBB leakage in the ROI. However, neither ventriculomegaly nor hyperintense DWI was observed in any of the age- and sex-matched sham-operated animals (Fig. 5Bi). We observed no anomalies in the whole-brain R2* maps after similar SPION-gfap application to these controls (Fig. 5Bii). We obtained postmortem samples from four mice to validate gliosis by immunohistochemistry. As shown in Fig. 5Ci, there was an absence of gliosis in the left striatum where we observed normal DWI (Fig. 5Ai) and an absence of elevated SPION retention (Fig. 5Aii). However, we did observe gliosis in the right striatum ipsilateral to the enlarged ventricle in the same mouse (Fig. 5Cii). Matching patterns of gliosis were found in postmortem samples of all mice that showed hyperintense foci in R2* maps (n=4, 100%). The R2* values or retention of SPION in the brain were not statistically analyzed because each of the four animals with BCAO had different shapes and locations of hyperintense R2* foci. When the duration of cerebral ischemia was reduced from 60 to 30 min, we observed no hyperintense DWI, BBB leakage, or focal SPION retention after similar SPION-gfap delivery (n=4, 100%). In addition, the absence of an abnormal pattern of glia in sham-operated mice was validated in Fig. 5D (MRI: n=4; histology, n=2). Glia in the striatum of sham-operated mice were noted as having a shorter axon (Fig. 5Di, ii) than those in the same regions of BCAO-induced gliosis (Fig. 5Cii, insets), suggesting few or no abnormal fibrous glial network in the sham-operated animals.
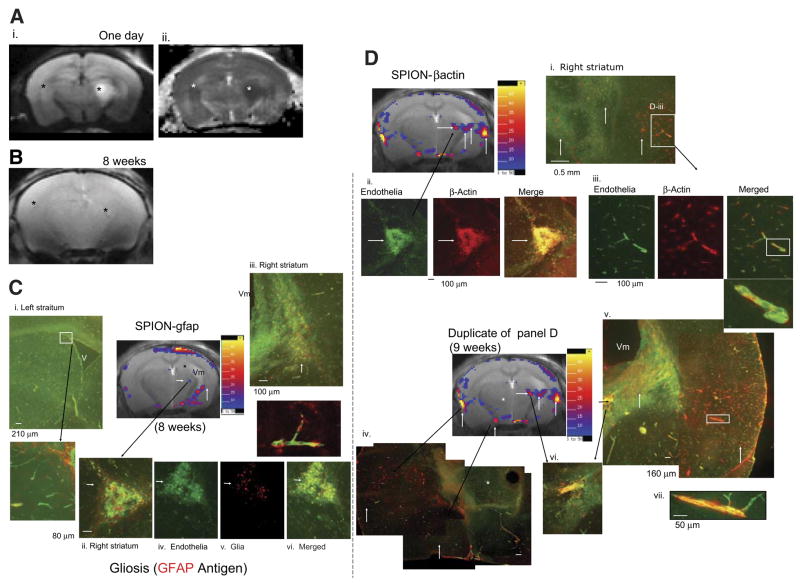
We used tMRI to demonstrate the specificity of SPION-sODN for differentiating endogenous mRNA in sODNS at different time points to the same animals that had been previously treated with BCAO and exhibited bilateral hyperintense DWI (n=4); we selected one representative mouse. Figure 6Ai, ii shows uneven hyperintense DWI and ADC drop, that is, the injury is more severe in the right hemisphere than in the left. We acquired T2*-weighted images and selected animals with focal signal drop in the vicinity of severe injury (arrow, Fig. 6B).
Figure 6.
Temporal detections of multiple gene activities for gliosis. A) DWI (i) and ADC drop (ii) 1 day after BCAO (60 min). One from a group of six animals is presented here for illustrative purposes. B) T2*-weighted MRI (Gradient Echo, TR/TE=500/6 ms, 120×120 μm2, 0.5 mm thickness) of the same mouse 8 wk later; the asterisks indicate possible lesions of signal reduction in the ROIs where we observed an ADC drop. Arrow points to a lesion of signal reduction. Possible tissue damage matching the signal reduction in B is indicated by the arrowhead in the subtraction R2* maps (postdelivery minus predelivery) (C, D). C) Subtraction R2* maps after SPION-gfap at 8 wk, at which time we observed ventriculomegaly (Vm), hippocampal atrophy (asterisk), and focal SPION retention (short arrow) in the right hemisphere. D) Subtraction R2* maps after SPION-β-actin at 9 wk. Postmortem brain tissue sections were obtained at 11 wk and randomly assigned tissue samples for immunohistochemistry assay of GFAP or β-actin antigen using rabbit IgG anti-GFAP or rabbit anti-actin (each with Cy-3 anti-rabbit IgG), and each counterstained for endothelia using FITC-Griffonia simplicifolia lectin I. Immunohistochemistry of CNS vascular endothelia (green) is shown in postmortem samples (C, D), with GFAP expressing cells (red) (Ci–vi) and β-actin-expressing cells (red) in the separate brain tissue, but within the brain tissue that exhibited R2* maps (Di -vii). The long arrows point to matched R2* hyperintense foci in the subtraction maps. β-Actin-expressing cells are mainly associated with endothelia in the vessel (yellow) (Dii, v--vii). The dimension of this triangular aggregate in Cii or Dii was 340 × 340 × 0.5 μm2 (or 0.058 mm2), and the corresponding ROI in R2* maps (arrowhead, C) contained 4 pixels or 0.058 mm2 (0.12×0.12 mm2×4). Color scales = 5–50% (C, D).
To delineate the nature of this signal drop, we applied SPION-gfap and SPION-β-actin (4 mg Fe/kg, OTRD) in the 8th and 9th weeks, respectively. We acquired R2* maps 1 day after probe delivery. The subtraction maps showed specific focal retention of SPION-gfap in tissue below the right ventricle (arrowhead and open arrow, Fig. 6C). In addition, we observed ventriculomegaly (Vm) and hippocampal atrophy (asterisks) in the right hemisphere (Fig. 6C, D). Because the alignment of MR slices became less perfect as a result of cell death and brain tissue atrophy, cortical SPION retention near the surface coil was generally ignored in this study and is pending future investigation.
We compared patterns of SPION retention after SPION-β-actin delivery 1 wk later (Fig. 6D), at which time we observed more hyperintense foci in R2* maps and SPION-β-actin focal patterns different and more widespread than those of SPION-gfap (short upward arrows in Figs. 6C, D). The aggregate previously observed in the T2*-weighted MRI became hyperintense in the subtraction maps after SPION-β-actin (arrowheads, Fig. 6D). One focal R2* value elevation (arrowhead, Fig. 6C, D), we observed the intensity of elevation was higher in SPION-β-actin than those observed for SPION-gfap. We obtained postmortem samples in the 11th week to validate antigen expression in these foci by immunohistochemistry. The validation assay used antibodies against GFAP or β-actin (red), and counter-stained for endothelia (green) in the blood vessels. We observed normal patterns of endothelia and glia in the left hemisphere, where we detected less hyperintense DWI (Fig. 6Ci and inset). However, we observed intense gliosis (red) in the location where we also observed hyperintense R2* maps in the right hemisphere. The cells that expressed GFAP are not endothelia because GFAP-expressing cells did not merge with endothelia (inset in Fig. 6Cii–vi). On the other hand, we observed groups of β-actin-expressing cells in the vicinity of R2* foci of SPION-β-actin (matching arrows in Fig. 6Di–vi); the β-actin-expressing cells merged with the vascular endothelia (Fig. 6Dii, iii).
We observed one common hyperintense aggregate in the thalamus and below the hippocampus, corresponding to a signal drop in the T2*-weighted MRI in Fig. 6B and in all R2* maps of both SPION-gfap and SPION-β-actin (long arrows, Fig. 6C, D). The intensity of this R2* lesion in the subtraction maps of SPION-β-actin is stronger than that of SPION-gfap (arrowheads, Fig. 6C, D). The lesion appears to be an aggregate of endothelia containing β-actin antigen (Fig. 6Dii–vi), though it may also contain a few GFAP-expressing cells (Fig. 6Cii). We also observed that some endothelia did not express β-actin antigen; these cells were located at sites where we found no hyperintense R2*signal (asterisk, Fig. 6Div). We conclude that our results demonstrate the specificity of SPION-sODN for reporting specialized cells with OTRD and tMRI in live animals.
DISCUSSION
We have developed a novel assay to target and image specific mRNA transcripts using tMRI and modular MR probes delivered to the peritoneal cavity or bulbar conjunctiva sac. Three different animal models of acute neurological disorders (CSD, minute intracranial puncture wound, and GCI by 60 min BCAO) were used to induce BBB leakage in live C57BL6 mice. The results of our study suggest that 1) BBB leakage is a prerequisite for noninvasive delivery, 2) the peritoneal cavity and the conjunctival sac are connected to the brain by the lymphatic and vascular circulation, and 3) SPION-gfap and SPION-β-actin have specificity for reporting their mRNA targets in living brains. We have shown the reporting of endogenous gene transcripts in live animals using specific targeting probes (8, 13). Now, we demonstrated here the same probe can report cells that express different levels of targeted mRNA. Moreover, different targeting probes are used in the same animal at different times for mRNA-based cell typing in MRI.
Here, we have demonstrated that our modular MR probe can be delivered to live brains noninvasively when the BBB is compromised. Puncture wounds and CSD are two examples of acute neurological disorders known to have characteristic BBB leakage. While puncture wound is representative of physical disruption of brain tissue, much like traumatic brain injury, the CSD model represents minimal physical injury with micro-BBB leakage and requires microscopic examination for Evans blue extravasation (22). Although BBB leakage after CSD may be short-lived (approximately 1 wk), such minor and transient disruption is sufficient to allow SPION retention and MR probe uptake in brain cells. On the other hand, the GCI model of BCAO is an acute neurological disorder thought to simulate the neurological conditions associated with cardiac arrest; it seldom induces necrosis but elicits oxidative DNA damage and apoptotic DNA fragmentation (27–29). Several reports have described how GCI induces BBB leakage in rat and mouse brains (30; Savitz et al., unpublished data); we report here that BBB leakage in a C57BL6 mouse GCI model can be detected as early as 10 h and as late as 9 wk after BCAO when assessing Gd retention. BBB leakage allows detection of brain damage using specific MRI probes in live mice (8). When employing i.p. delivery of SPION-cfos after BCAO, R2* maps of elevated SPION retention after i.p. delivery are similar to maps acquired after i.c.v. delivery. We have obtained cortical R2* values (40 s−1) that represent levels somewhat lower than those measured within 1 day of cerebral ischemia with i.c.v. delivery (60 s−1) (8, 13). We believe the difference may result from late administration of SPION-cfos probe in this model, when the expression of immediate early gene transcript was no longer at its peak (31). Expression eventually disappears altogether as a result of neuronal death (8). Our data support the notion that the sequence in the sODN of SPION-sODN dictates retention according to the intracellular level of the target mRNA (14). This notion is also demonstrated by the results obtained when using MR probes with different intracellular targets for cells that express GFAP and β-actin antigens.
Second, we have demonstrated that SPION-sODN probe complexes can be distributed in the brain when they are introduced to either the peritoneal cavity or the conjunctival sac. The results of our study suggest that the lymphatic and vascular circulation provide a connection between the peritoneal cavity or the conjunctival sac and the brain when the BBB is disrupted. However, the exact pathway is not yet fully understood. SPION-sODN can be distributed from the peritoneal cavity (by i.p. delivery) and through ocular fluid (via OTRD) to the CNS vasculature and can enter the brain cells via BBB leakage. Evidence of these capabilities of the SPION probe includes 1) the stronger R2* lesion hyperintensity in the subtraction maps of SPION-β-actin compared to SPION-gfap (arrows, Fig. 6C, D), 2) the matching between the lesion and the signal reduction in T2*-weighted MRI (Fig. 6B), 3) the aggregate of vascular tissue in the location of endothelia expressing β-actin antigen (Fig. 6Di–vii), and 4) reporting of in vivo gliosis (Fig. 6Ciii).
The prerequisite of BBB leakage for noninvasive delivery methods may indicate that OTRD or i.p. delivery has advantages over i.c.v. delivery in all applications, especially in human subjects who experience neurological disorders and BBB disruption. In some cases, patients who have special needs can be transiently treated with osmotic shock by mannitol to open BBB. OTRD is a user-friendly method, such that patients can self-administer the probe at home before reporting for the imaging examination. In addition, we can use repetitive applications of SPION-sODN with the same or different targets in the same subject, provided a clearance time is given to the subject of detection. Our current study indicates that tMRI and our MR probes are not limited to diagnosis of gliosis, angiogenesis, and brain repair, but also have potential targeting ability for glioma or cancer cells when mRNA of specific oncogenes are known. On the other hand, one disadvantage is that sealing of the BBB over time may either diminish or completely block MR probe distribution. Therefore, the assay used to determine BBB leakage is very important. We employed two conventional methods (Evans blue extravasation and tissue retention of Gd-DTPA). Whereas Evans blue extravasation terminates experiments altogether, Gd-DTPA retention is convenient and is washed clean the next day and as such can be used for predelivery testing for eligibility of tMRI using our unique MR probe.
We have demonstrated here that SPION-gfap and SPION-β-actin give different subtraction R2* maps. Because the sODN used in this study has been shown to support PCR or in situ hybridization of mRNA transcript (13, 24, 32), the basis for the specificity of these SPION-sODN probes resides in the complementarity between the sODN sequences and mRNA transcripts. Specific patterns of SPION retention, representing special mRNA transcript expression, are reflected by different cell types expressing specific mRNA of GFAP and β-actin antigens. For example, SPION-gfap detects in vivo gliosis where immunohistochemistry of postmortem samples also reveals a fibrous network of glia or astrocytes. The cell typing using SPION-sODN is based on the ability of our MRI probe to differentiate two levels of mRNA expression: the normal pattern of GFAP-expressing cells in Figs. 4E and 5Ci and the fibrous network of glia expressing elevated signal in Figs. 4D and 5Cii. Moreover, we employed subtraction R2* maps (Fig. 6) to reduce background R2* attributable to endogenous retention of baseline glia. As such, subtraction R2*maps not only identify foci of gliosis but also report ROIs with abnormal expression of β-actin, resulting from some pathophysiological condition.
Such specificity of cell typing is also demonstrated for SPION-β-actin. Normally, β-actin mRNA is not elevated immediately after cerebral ischemia (32). Tissue remodeling that involves angiogenesis has been reported in stroke model of the rat using postmortem samples (33). Indeed, SPION-β-actin retention is elevated in areas close to regions where we identified ADC drops 1 day after GCI (Fig. 6Aii). These regions were found to contain pericytes that express β-actin antigen in the vascular wall 9 wk after GCI. SPION-β-actin retention was not elevated in regions that exhibited gliosis, except in one aggregate of endothelia (arrows, Fig. 6B–D). (At the 10th wk, we also tested the retention of SPION probe targeting matrix metalloproteinase-9 (MMP-9) mRNA, known to have the vascular origin during remodeling after stroke (33).) Although the retention of SPION-mmp9 matches those of SPION-βactin, the assay is pending for suitable polyclonal antibodies for mouse MMP-9 antigen.
Therefore, our results agree with other reports showing that β-actin-expressing cells can be detected during scarring (34) and in CNS microvascular pericytes during angiogenesis (35, 36). Future investigations will be needed to validate this reporting capability of SPION-β-actin for angiogenesis.
We have developed, validated, and applied in vivo techniques using tMRI. We have demonstrated transcript imaging of glia and cells expressing smooth muscle actin in new endothelia during the repair process. Brain repair after neurological disorders has an important role in plasticity (37). This tMRI technology thus represents a breakthrough for molecular biologists of diverse disciplines, and the availability of this method and contrast probe that targets endogenous gene activities will enable real-time investigation and longitudinal tracking of pathophysiological conditions in living systems. Noninvasive methods of delivering modular probes to the brain also lend themselves to other clinical applications such as noninvasive targeting of gene action in cells when certain mRNA transcripts are known to impact a specific pathological change.
Acknowledgments
We thank Dr. C. Ayata for consultation on the CSD model, Dr. C. M. Liu for excellent technical assistance, and Ms. N. Eusemann for excellent editing. C.H.L. contributed MRI acquisition and data analysis; J.Q.R. and Z.Y. performed immunohistochemistry; Y.R.K. synthesized SPION; K.E.H. made the CSD model; P.K.L. designed, conjugated, characterized, and delivered SPION-sODN and performed BCAO and i.c.v. procedures; C.H.L. and P.K.L. wrote the manuscript. All authors discussed the results and commented on the manuscript. This project was supported by grants from the U.S. National Institutes of Health (NS045845, NS057556, DA024235, 5T32CA009502, and P41RR14075), the Deutsche Forschungsgemeinschaft (Ha5085/1–1), and the Athinoula A. A. Martinos Center for Biomedical Imaging. Two patent applications have been submitted: 1) imaging nucleic acids (U.S. Serial No. 11/574,160) and 2) targeting brain cells via ophthalmic delivery.
Footnotes
Note added in proof: We now have performed BBB leakage determination in mice using Evans blue extravasation (n=8) or Gd-MRI at 1 (n=6), 7 (n=10), and 24 (n=2) h after GCI of 60 min. We found BBB leakage in 4 mice, or 22%, within the first day of GCI. All mice that showed hyperintense DWI (with significant ADC drop) 1 day after GCI in the striatum developed striatal BBB leakage.
References
- 1.Kaur J, Zhao Z, Klein GM, Lo EH, Buchan AM. The neurotoxicity of tissue plasminogen activator? J Cereb Blood Flow Metab. 2004;24:945–963. doi: 10.1097/01.WCB.0000137868.50767.E8. [DOI] [PubMed] [Google Scholar]
- 2.Raymond J, Lebel V, Ogoudikpe C, Metcalfe A, Chagnon M, Robledo O. Recanalization of arterial thrombus, and inhibition with beta-radiation in a new murine carotid occlusion model: MRNA expression of angiopoietins, metallo-proteinases, and their inhibitors. J Vasc Surg. 2004;40:1190–1198. doi: 10.1016/j.jvs.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 3.Oda N, Abe M, Sato Y. ETS-1 converts endothelial cells to the angiogenic phenotype by inducing the expression of matrix metalloproteinases and integrin beta3. J Cell Physiol. 1999;178:121–132. doi: 10.1002/(SICI)1097-4652(199902)178:2<121::AID-JCP1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 4.Cole G, Cowie VA. Long survival after cardiac arrest: case report and neuropathological findings. Clin Neuropathol. 1987;6:104–109. [PubMed] [Google Scholar]
- 5.Pluta R. Astroglial expression of the beta-amyloid in ischemia-reperfusion brain injury. Ann N Y Acad Sci. 2002;977:102–108. doi: 10.1111/j.1749-6632.2002.tb04803.x. [DOI] [PubMed] [Google Scholar]
- 6.Chiamulera C, Terron A, Reggiani A, Cristofori P. Qualitative and quantitative analysis of the progressive cerebral damage after middle cerebral artery occlusion in mice. Brain Res. 1993;606:251–258. doi: 10.1016/0006-8993(93)90992-v. [DOI] [PubMed] [Google Scholar]
- 7.Fahrig T. Changes in the solubility of glial fibrillary acidic protein after ischemic brain damage in the mouse. J Neurochem. 1994;63:1796–1801. doi: 10.1046/j.1471-4159.1994.63051796.x. [DOI] [PubMed] [Google Scholar]
- 8.Liu CH, Huang S, Kim YR, Rosen BR, Liu PK. Forebrain ischemia-reperfusion simulating cardiac arrest in mice induces edema and DNA fragmentation in the brain. Mol Imaging. 2007b;6:156–170. [PMC free article] [PubMed] [Google Scholar]
- 9.Moseley ME, de Crespigny AJ, Roberts TP, Kozniewska E, Kucharczyk J. Early detection of regional cerebral ischemia using high-speed MRI. Stroke. 1993;24:I60–I65. [PubMed] [Google Scholar]
- 10.Cramer SC, Moore CI, Finklestein SP, Rosen BR. A pilot study of somatotopic mapping after cortical infarct. Stroke. 2000;31:668–671. doi: 10.1161/01.str.31.3.668. [DOI] [PubMed] [Google Scholar]
- 11.Stoub TR, Bulgakova M, Leurgans S, Bennett DA, Fleischman D, Turner DA, DeToledo-Morrell L. MRI predictors of risk of incident Alzheimer disease: a longitudinal study. Neurology. 2005;64:1520–1524. doi: 10.1212/01.WNL.0000160089.43264.1A. [DOI] [PubMed] [Google Scholar]
- 12.Bell-McGinty S, Lopez OL, Meltzer CC, Scanlon JM, Whyte EM, Dekosky ST, Becker JT. Differential cortical atrophy in subgroups of mild cognitive impairment. Arch Neurol. 2005;62:1393–1397. doi: 10.1001/archneur.62.9.1393. [DOI] [PubMed] [Google Scholar]
- 13.Liu CH, Huang S, Cui J, Kim YR, Farrar CT, Moskowitz MA, Rosen BR, Liu PK. MR contrast probes that trace gene transcripts for cerebral ischemia in live animals. FASEB J. 2007c;21:3004–3015. doi: 10.1096/fj.07-8203com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu CH, Kim YR, Ren JQ, Eichler F, Rosen BR, Liu PK. Imaging cerebral gene transcripts in live animals. J Neurosci. 2007;27:713–722. doi: 10.1523/JNEUROSCI.4660-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adkins ES, Salman FT, Fonkalsrud EW. Triglyceride absorption in transperitoneal alimentation. Am J Surg. 1990;159:237–240. doi: 10.1016/s0002-9610(05)80270-3. [DOI] [PubMed] [Google Scholar]
- 16.Lind K, Kresse M, Debus NP, Muller RH. A novel formulation for superparamagnetic iron oxide (SPIO) particles enhancing MR lymphography: comparison of physicochemical properties and the in vivo behaviour. J Drug Target. 2002;10:221–230. doi: 10.1080/10611860290022651. [DOI] [PubMed] [Google Scholar]
- 17.Johnston M, Zakharov A, Papaiconomou C, Salmasi G, Armstrong D. Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res. 2004;1:2. doi: 10.1186/1743-8454-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doughty MJ, Bergmanson JP. New insights into the surface cells and glands of the conjunctiva and their relevance to the tear film. Optometry. 2003;74:485–500. [PubMed] [Google Scholar]
- 19.Killer HE, Laeng HR, Groscurth P. Lymphatic capillaries in the meninges of the human optic nerve. J Neuroophthalmol. 1999;19:222–228. [PubMed] [Google Scholar]
- 20.Dickinson AJ, Gausas RE. Orbital lymphatics: do they exist? Eye. 2006;20:1145–1148. doi: 10.1038/sj.eye.6702378. [DOI] [PubMed] [Google Scholar]
- 21.Muldoon LL, Sandor M, Pinkston KE, Neuwelt EA. Imaging, distribution, and toxicity of superparamagnetic iron oxide magnetic resonance nanoparticles in the rat brain and intracerebral tumor. Neurosurgery. 2005;57:785–796. doi: 10.1093/neurosurgery/57.4.785. [DOI] [PubMed] [Google Scholar]
- 22.Gursoy-Ozdemir Y, Qiu J, Matsuoka N, Bolay H, Bermpohl D, Jin H, Wang X, Rosenberg GA, Lo EH, Moskowitz MA. Cortical spreading depression activates and upregulates MMP-9. J Clin Invest. 2004;113:1447–1455. doi: 10.1172/JCI21227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ayata C, Shimizu-Sasamata M, Lo EH, Noebels JL, Moskowitz MA. Impaired neurotransmitter release and elevated threshold for cortical spreading depression in mice with mutations in the alpha1A subunit of P/Q type calcium channels. Neuroscience. 2000;95:639–645. doi: 10.1016/s0306-4522(99)00446-7. [DOI] [PubMed] [Google Scholar]
- 24.Lewis SA, Balcarek JM, Krek V, Shelanski M, Cowan NJ. Sequence of a cDNA clone encoding mouse glial fibrillary acidic protein: structural conservation of intermediate filaments. Proc Natl Acad Sci U S A. 1984;81:2743–2746. doi: 10.1073/pnas.81.9.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.You Z, Yang J, Takahashi K, Yager PH, Kim HH, Qin T, Stahl GL, Ezekowitz RA, Carroll MC, Whalen MJ. Reduced tissue damage and improved recovery of motor function after traumatic brain injury in mice deficient in complement component C4 (Online) J Cereb Blood Flow Metab. 2007 doi: 10.1038/sj.jcbfm.9600497. [DOI] [PubMed] [Google Scholar]
- 26.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Academic Press; London: 2001. [Google Scholar]
- 27.Huang D, Shenoy A, Cui J, Huang W, Liu PK. In situ detection of AP sites and DNA strand breaks bearing 3′-phosphate termini in ischemic mouse brain. FASEB J. 2000;14:407–417. doi: 10.1096/fasebj.14.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin LH, Cao S, Yu L, Cui J, Hamilton WJ, Liu PK. Up-regulation of base excision repair activity for 8-hy-droxy-2′-deoxyguanosine in the mouse brain after forebrain ischemia-reperfusion. J Neurochem. 2000;74:1098–1105. doi: 10.1046/j.1471-4159.2000.741098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu PK, Hsu CY, Dizdaroglu M, Floyd RA, Kow YW, Karakaya A, Rabow LE, Cui JK. Damage, repair, and mutagenesis in nuclear genes after mouse forebrain ischemia-reperfusion. J Neurosci. 1996;16:6795–6806. doi: 10.1523/JNEUROSCI.16-21-06795.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mossakowski MJ, Lossinsky AS, Pluta R, Wisniewski HM. Abnormalities of the blood-brain barrier in global cerebral ischemia in rats due to experimental cardiac arrest. Acta Neurochir Suppl (Wien) 1994;60:274–276. doi: 10.1007/978-3-7091-9334-1_73. [DOI] [PubMed] [Google Scholar]
- 31.Cui J, Liu PK. Neuronal NOS inhibitor that reduces oxidative DNA lesions and neuronal sensitivity increases the expression of intact c-fos transcripts after brain injury. J Biomed Sci. 2001;8:336–341. doi: 10.1007/BF02258375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cui J, Holmes EH, Liu PK. Oxidative damage to the c-fos gene and reduction of its transcription after focal cerebral ischemia. J Neurochem. 1999;73:1164–1174. doi: 10.1046/j.1471-4159.1999.0731164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SR, Kim HY, Rogowska J, Zhao BQ, Bhide P, Parent JM, Lo EH. Involvement of matrix metalloproteinase in neuroblast cell migration from the subventricular zone after stroke. J Neurosci. 2006;26:3491–3495. doi: 10.1523/JNEUROSCI.4085-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Costa AM, Peyrol S, Porto LC, Comparin JP, Foyatier JL, Desmouliere A. Mechanical forces induce scar remodeling. Study in non-pressure-treated versus pressure-treated hypertrophic scars. Am J Pathol. 1999;155:1671–1679. doi: 10.1016/S0002-9440(10)65482-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dore-Duffy P, Katychev A, Wang X, Van Buren E. CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab. 2006;26:613–624. doi: 10.1038/sj.jcbfm.9600272. [DOI] [PubMed] [Google Scholar]
- 36.Korn J, Christ B, Kurz H. Neuroectodermal origin of brain pericytes and vascular smooth muscle cells. J Comp Neurol. 2002;442:78–88. doi: 10.1002/cne.1423. [DOI] [PubMed] [Google Scholar]
- 37.Liu PK. Ischemia-reperfusion-related repair deficit after oxidative stress: implications of faulty transcripts in neuronal sensitivity after brain injury. J Biomed Sci. 2003;10:4–13. doi: 10.1159/000068080. [DOI] [PMC free article] [PubMed] [Google Scholar]