Abstract
A pressurized liquid extraction-based method for the simultaneous extraction and in situ clean-up of polychlorinated biphenyls (PCBs), hydroxylated (OH)-PCBs and methylsulfonyl (MeSO2)-PCBs from small (< 0.5 gram) tissue samples was developed and validated. Extraction of a laboratory reference material with hexane:dichloromethane:methanol (48:43:9, v/v) and Florisil as fat retainer allowed an efficient recovery of PCBs (78–112%; RSD: 13–37%), OH-PCBs (46±2%; RSD: 4%) and MeSO2-PCBs (89±21%; RSD: 24%). Comparable results were obtained with an established analysis method for PCBs, OH-PCBs and MeSO2-PCBs.
Keywords: Polychlorinated biphenyls (PCBs), methylsulfonated biphenyls (MeSO2-PCBs), hydroxylated biphenyls (OH-PCBs), pressurized liquid extraction (PLE)
1. Introduction
Polychlorinated biphenyls (PCBs) are an important class of persistent environmental contaminants with the general structure C12HnCl10-n (n = 1 to 10). They were initially manufactured by batch chlorination of biphenyl. The resulting mixtures had desirable physicochemical properties, such as high chemical and thermal stability and high dielectric constants, and were used in a large number of technical and consumer applications. Their production was phased out in the United States in the 1970s due to environmental and public health concerns; however, the US Environmental Protection Agency still permits the use of PCBs in enclosed systems, such as transformers and capacitors. Significant quantities of PCBs have been released into the environment and transported to remote areas because of their semi-volatile character, thus resulting in worldwide contamination [1]. PCBs can bioaccumulate and biomagnify in the food web, which has resulted in concern about human exposures and adverse human health effect [2].
PCBs can be metabolized by cytochrome P-450 enzyme systems to hydroxylated PCB derivatives (polychlorinated biphenylols, OH-PCBs) [3]. This oxidation can occur either via a PCB epoxide or by direct insertion of oxygen into a CAr-H bond. Alternatively, the PCB epoxide can react with glutathione, and the resulting glutathione adduct is converted via the mercapturic acid pathway to methylsulfonyl derivatives (MeSO2-PCBs) [3]. These metabolites are retained in both humans and animals, for example in blood [4–7] and in liver and lung [3,8–11]. Recent studies have shown that the levels of MeSO2-PCBs and OH-PCBs are similar or even higher than the levels of the parent compounds [3]. These findings suggest that, in addition to PCBs themselves, their metabolites may play an important, but frequently overlooked role in adverse human health effects. Therefore, laboratory as well as epidemiological studies should assess tissue and/or serum levels of PCBs and their metabolites. Unfortunately, the analysis of PCBs and their metabolites in blood and tissue samples involves complex and time and solvent consuming extraction, separation and clean-up steps [12].
Pressurized liquid extraction (PLE) is an established sample preparation technique for the extraction of PCBs and many other compounds from a variety of environmental samples including sediments, plants and tissues [13–17]. PLE combines both elevated temperatures and high pressures to achieve a fast, efficient and highly automated extraction of the analytes from a solid matrix. The high temperature (typically 100°C) used during PLE facilitates the distribution of the solvent into the matrix due to a decrease in solvent viscosity, assists in disrupting analyte-matrix interactions and increases both solubility and mass transfer [18]. The use of pressures between 6.9 and 17.2 MPa (1000 to 2500 psi) allows extractions at temperatures above the boiling point of the extraction solvent and facilitates the isolation of the analyte. In addition to these advantages, PLE reduces the extraction time, allows the automatization of extraction procedures and requires lower solvent volumes compared to traditional Soxhlet extractions.
PLE not only allows an efficient extraction of analytes from a variety of matrices, but also the in-situ (or in cell) clean-up of the extract [14,19–26]. This significantly reduces the need for exhaustive post-clean-up procedures, such as column and/or gel-permeation chromatography, and allows the automatization of clean-up steps. Especially the post-extraction removal of lipids and other co-extractable materials represents a challenge for PCB and PCB metabolite analyses. In-situ removal of these co-extractable materials can be achieved by placing a fat retainer, such as Florisil or silica gel, in the bottom of the PLE cell. This prevents the elution of co-extractable materials from the PLE cell.
The present study describes the development of a PLE method that allows the extraction and in-situ clean-up on PCBs, MeSO2-PCBs and OH-PCBs from small tissue samples using PLE.
2. Experimental
2.1. Chemicals
Florisil (60–100 mesh), silica gel (70–230 mesh), absolute ethanol (200 proof, 99.5%), dimethylsulfoxide (anhydrous, 99.9%), hydrochloric acid, potassium hydroxide, sodium sulfite, sulfuric acid, tetrabutylammonium sulfite and pesticide grade solvents were purchased from Fisher Scientific (Pittsburg, PA, US). Alumina (acidic, about 150 mesh) was purchased from Sigma-Aldrich (St Louis, MO, US). Diatomaceous earth (DE) was obtained from Dionex (Sunnyvale, CA, US).
3,5-Dichlorobiphenyl (PCB 14), 2,4,6-trichlorobiphenyl (PCB 30), 2,3,5,6-tetrachlorobiphenyl (PCB 65), 2,3,4,5,6,4′-hexachlorobiphenyl (PCB 166), 2,3,4,5,6,2′,4′,6′-octachlorobiphenyl (PCB 204), 2,5,2′,5′-tetrachloro-3-methanesulfonyl-biphenyl (3-MeSO2-CB 52), 2,5,2′,5′-tetrachloro-4-methanesulfonyl-biphenyl (4-MeSO2-CB 52), 2,3,4,2′,5′-pentachloro-4′-methanesulfonyl-biphenyl (4′-MeSO2-CB 87), 2,3,6,2′,4′-pentachloro-4-metahnesulfonyl-biphenyl (4-MeSO2-CB 91), 2,3,6,2′,4′-pentachloro-5-methanesulfonyl-biphenyl (5-MeSO2-CB 91), 2,5,2′,3′,6′-pentachloro-3-methanesulfonyl-biphenyl (3′-MeSO2-CB 95), 2,3,6,2′,5′-pentachloro-4′-methanesulfonyl-biphenyl (4′-MeSO2-CB 95), 2,3,5,2′,5′-pentachloro-4′-methanesulfonyl-biphenyl (4′-MeSO2-CB 101), 2,3,6,2′,3′,4′-hexachloro-4-methanesulfonyl-biphenyl (4′-MeSO2-CB 132), 2,3,6,2′,3′,4′-hexachloro-5-methanesulfonyl-biphenyl (5′-MeSO2-CB 132), 2,3,6,2′,4′,5′-hexachloro-4-methanesulfonyl-biphenyl (4-MeSO2-CB 149), 2,5,6,2′,4′,5′-hexachloro-3-methanesulfonyl-biphenyl (5-MeSO2-CB 149), and 3,5,2′,3′,4′,5′-hexachloro-biphenyl-4-ol (4′-OH-CB 159), 2,3,4,5,2′,3′,6′-heptachloro-4′-methanesulfonyl-biphenyl (4′-MeSO2-CB 174), 2,3,4,5,2′,3′,6′-heptachloro-5′-methanesulfonyl-biphenyl (5′-MeSO2-CB 174), were purchased from Accustandard (New Haven, CT, US). 2,3,5,3′,4′-pentachloro-4-methoxy-biphenyl (4-MeO-CB 107), 2,4,5,2′,3′,4′-hexachloro-3-methoxy-biphenyl (3′-MeO-CB 138),2,3,5,2′,4′,5′-hexachloro-4-methoxy-biphenyl (4-MeO-CB 146), 2,3,5,6,2′,4′,5′-heptachloro-4-methoxy-biphenyl (4-MeO-CB 187) were purchased from Wellington Laboratories (Guelph, Ontario, Canada). 2,3,4,5,3′-Pentachloro-5′-methanesulfonyl-4′-methyl-biphenyl (4′-Me-5′-MeSO2-CB 106) was purchased from Cambridge Isotope Laboratories (Andover, MA, US). 2,4,2′-Trichlorobiphenyl (PCB 28), 2,5,2′,5′-tetrachlorobiphenyl (PCB 52), 3,4,3′,4′-tetrachlorobiphenyl (PCB 77), 2,4,5,2′,4′,5′-hexachlorobiphenyl (PCB 153), 4,5,3′,4′-tetrachloro-biphenyl-3-ol (5-OH-CB 77) and 4,5,3′,4′-tetrachloro-biphenyl-2-ol (6-OH-CB 77) were synthesized in our laboratory with > 99% purity [27]. PCB congeners were numbered according to the corrected Ballschmiter and Zell system [28] and the metabolites are abbreviated as described by Maevoert and co-workers [29]. The chemical structure and the concentration of the stock solutions of all compounds are summarized in Tables S1 to S3 in the Supplementary Material.
Diazomethane was synthesized from N-methyl-N-nitroso-p-toluenesulfonamide (Diazald) using an Aldrich mini Diazald apparatus (Milwaukee, WI, US) following established procedures [30]. CAUTION: Because of the highly toxic and explosive nature of diazomethane, its preparation and use should be carried out in an efficient chemical fume hood and behind a safety shield. All glass tubing used to handle diazomethane solutions should have fire polished ends.
2.2. Method development
2.2.1. Pressurized liquid extraction (PLE) of PCBs and their metabolites
A pressurized liquid extraction system ASE 200 (Dionex, Sunnyvale, CA, US) was used for the combined extraction and in-situ cleanup of PCBs and their MeSO2- and OH-metabolites. In short, PLE cells containing 10 g of fat retainer (Florisil or silica gel) and 2 g of diatomaceous earth were pre-extracted to avoid cell memory effects. In case of tissue extractions, the pre-extracted diatomaceous earth was removed from the PLE cell, thoroughly mixed with approximately 0.3 g of liver from non-PCB exposed rats and placed back into the PLE cell. The PLE cells were then spiked with a mixture of standards (either PCB, OH-PCB or MeSO2-PCB standards alone or a mixture of all three standards) and extracted twice using the same extraction condition employed for the pre-extraction. The extraction temperature, pressure and length of cycle were changed systematically as described under Results and Discussion. All results are summarized in Figures S1 to S7 in the Supplementary Material. A heating time of 6 min and a 60% cell volume flush were used for every extraction. Both parameters do not affect the extraction efficiency of PCBs and are frequently used for PLE of environmental samples [18].
2.2.2. Separation of PCBs and metabolites
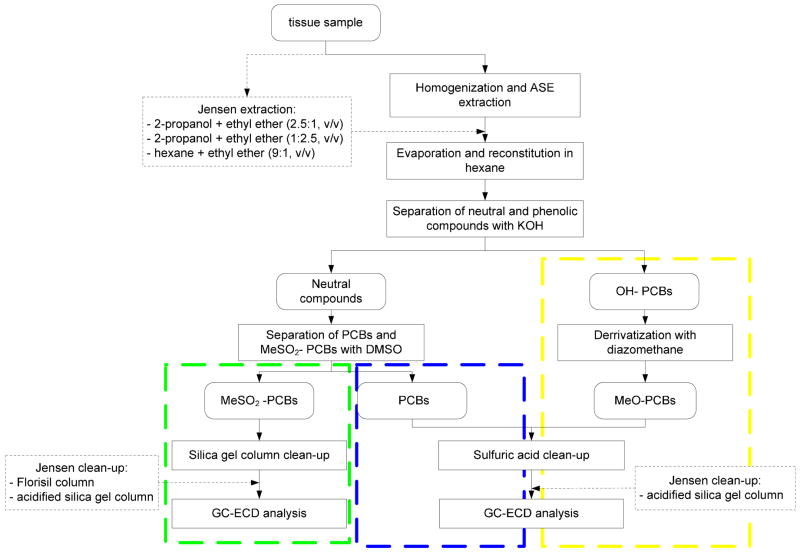
Tissue extracts were separated into PCBs, OH-PCBs and MeSO2-PCBs using their different physicochemical properties based on the procedure described by Hovander et al. [5,31] (Fig. 1). In short, the phenolic fraction containing the OH-PCBs was separated from the neutral fraction containing PCBs and MeSO2-PCBs by partitioning into an aqueous-ethanolic solution of potassium hydroxide (2ml, 0.5 M, 50% ethanol). The phenolic fraction was then acidified with hydrochloric acid (0.5 ml of 2M aqueous solution) and the OH-PCBs were extracted twice with hexane-MTBE (3ml, 9:1 v/v). The OH-PCBs were derrivatized with diazomethane in diethyl ether (0.5 ml, about 5 mmol) to form MeO-PCBs. The MeSO2-PCBs were separated from PCBs by partitioning into anhydrous DMSO (0.5 ml, traces of water may prevent the partitioning). Water (1 ml) was added to the MeSO2-PCB fraction in DMSO and the metabolites were extracted twice with hexane. The MeSO2-PCBs were purified on a silica gel column (1 g) and eluted with dichloromethane (15 ml). The dichloromethane was exchanged to hexane prior to GC analysis. Traces of sulfur containing impurities were removed from the PCB and MeO-PCB-containing extracts according to standard EPA protocols [32,33].
Fig. 1.
Scheme of separation of PCBs, MeSO2-PCBs and OH-PCBs using PLE, Additional extraction and clean-up steps required by the Jensen method are shown in boxes with a dotted border.
2.2.3. Gas chromatography analysis (GC-ECD)
All gas chromatographic analyses were performed on an Agilent 6890N gas chromatograph equipped with a 63Ni μ-ECD (electron capture) detector. A SLB5MS column was used (60 m x 0.25 mm ID; 0.25 μm film thickness; Supelco, Bellafonte, PA, US). Detector and injector temperatures were 280°C and 300°C respectively. PCBs, MeO-PCBs (derivatized OH-PCBs) and MeSO2-PCBs were analyzed using temperature program 1, 2 and 3, respectively (Temperature program 1: 80°C for 1 min, 25°/min to 280°C, hold for 15 min; temperature program 2: 100°C hold for 1 min, 25°/min to 250°C, 1°/min to 280°C, hold for 20 min; temperature program 3: 80°C for 2 min, 10°/min to 280°C, than 1°/min to 300°C, hold for 5 min). The internal standard (same for all three groups of compounds) consisting of PCB 30 and PCB 204, was added to each sample before the GC analysis [32,33]. PCB 30 and PCB 204 were used to quantify PCBs, whereas PCB 204 was used to quantify the MeSO2-PCBs and MeO-PCBs. The detector was linear over the entire concentration range under investigation (Table S4, Supplementary Material).
2.3. Method validation
2.3.1. Preparation of laboratory reference materials
The PLE-based method was validated by comparison with the established extraction method described by Jensen and co-workers [34,35] using tissues from PCB-treated rats as laboratory reference materials. The animal experiments used to generate these tissue samples were approved by the Institutional Animal Care and Use Committee at University of Iowa.
Rat liver tissue samples were prepared by administering an environmentally relevant PCB mixture to rats. In short, six male Sprague Dowley rats, 4 weeks old, average weight 98 ± 8 g were purchased from Harlan (Indianapolis, IN, US). The animals were allowed to acclimatize for one week. Five randomly selected animals received a single oral dose of 20 mg/kg body weight of a mixture of Aroclor 1242 and Aroclor 1254 (65:35, w/w) in corn oil (5 ml/kg body weight). The profile of this PCB mixture resembles the mean PCB profile in Chicago air, as reported by Integrated Atmospheric Depostition Network from 1996 to 2002 [36]. The remaining animal received a single oral dose of 5 ml/kg body weight of corn oil and was used as a sentinel. Animals were euthanized on day 7 by asphyxiation with carbon dioxide followed by cervical dislocation. Liver samples were excised en bloc and stored at −20°C.
2.3.2. PLE of rat liver tissue (optimized method)
Rat liver (0.50 ± 0.01 g) samples were mixed thoroughly with pre-extracted diatomaceous earth (as described above) and placed on the top of 10 g of pre-extracted Florisil. The samples were spiked with surrogate standards PCB 14, PCB 65, PCB 166, 4′-Me-5′-MeSO2-CB 106 and 4′-OH-CB 159 and extracted with hexane-dichloromethane-methanol (48:43:9) at 100°C, 1500 psi and one static cycle of 5 min. Afterwards, extracts were concentrated to dryness, reconstituted in hexane and separated into PCBs, OH-PCBs and MeSO2-PCBs as described in Section 2.2.2 above.
2.3.3. Tissue extraction using the “Jensen method”
The modified lipid extraction method described by Jensen and co-workers [34,35] was scaled down and used to compare the extraction efficiency of both methods for the laboratory reference material. In short, liver (0.56 ± 0.05 g) samples were placed in a 10 ml glass tube, weighted and spiked with the surrogate standards as described above. The sample was then homogenized twice in 2-propanol (2.5 ml) and diethyl ether (1 ml) mixture with homogenizer (IKA, Wilmington, NC, US). The organic extracts were combined and placed over phosphoric acid solution (5 ml, 0.1 M solution in 0.9% aqueous sodium chloride). After inverting for 3 min, the organic phase was separated, and the aqueous phase was re-extracted with hexane-diethyl ether mixture (1 ml, 9:1 v/v). Afterwards, extracts were concentrated to dryness, reconstituted in hexane and separated into PCBs, OH-PCBs and MeSO2-PCBs as outlined above. Sulfur impurities were removed from the PCB and MeO-PCB fractions as described in Section 2.2.2 above [32,33], followed by column chromatography on acidified silica gel column (0.5 g of silica gel : concentrated H2SO4 = 2:1 w/w, with 0.1g silica gel at the bottom of the column; eluted with 10 ml of dichloromethane). The MeSO2-PCB fraction was purified using a Florisil column (1g of Florisil, eluted with 10 ml of hexane-acetone 4:1 v/v), followed by an acidified silica gel column as described for PCBs and MeO-PCBs. The GC-ECD analysis was performed as described in Section 2.2.3, with the exception that temperature program 4 and 5 were used to analyze PCBs and MeSO2-PCBs (temperature program 4: 100°C for 1 min, 1°/min to 240°C, 10°/min to 280°C hold for 20 min; temperature program 5: 80°C for 2 min, 10°/min to 280°C, than 1°/min to 300°C, hold for 5 min). Representative chromatograms are shown in the Supporting Material.
2.4. Statistical methods
The data are presented as mean ± standard deviation (SD) or standard error (SE), where appropriate. The differences between “Jensen” and PLE methods were analyzed by two sample t-test, α=0.05. R open source statistical software (version 2.6.0, http://www.r-project.org/index.html) was used for statistical analyses.
3. Results and discussion
3.1. Study outline
We herein investigate the simultaneous extraction and in-situ clean-up of PCBs and their metabolites using PLE to facilitate the concurrent analysis of PCBs and their metabolites in small tissue samples. Based on our previous experience with the extraction of PCBs using PLE [33,37–40], we first investigated the effect of PLE parameters (e.g. length and number of cycles, pressure and temperature), solvents and fat retainers on the recoveries of PCBs, MeSO2-PCBs or OH-PCBs. Subsequently, different fat retainers were studied for their effectiveness to simultaneously extract and clean-up these three groups of compounds. In this step, spiked liver tissue was subjected to PLE extraction in the presence of a fat retainer, followed by the separation of PCBs, MeSO2-PCBs and OH-PCBs as shown in Fig. 1. Finally, the optimized PLE method was validated by comparing it to the established method reported by Jensen and co-workers (“Jensen method”) [34].
3.1.1. Selection of model compounds
Several representative PCBs, MeSO2-PCBs and OH-PCBs were selected for the method development based on their use as analytical standards or their environmental and/or toxicological relevance. PCB 14, PCB 65 and PCB 166 were included in our study because they are commonly used as surrogate standard for PCB determinations [32,33], whereas PCB 28, PCB 52 and PCB 153 were selected as important components of commercial and environmental PCB mixtures [32]. 4′-MeSO2-PCB 87 and 4′-MeSO2-PCB 101 were chosen as representative MeSO2-PCB metabolites that have been detected in human tissue samples [4,8,41–43], whereas 3-MeSO2-CB 52 and 4-MeSO2-PCB 52 are metabolites of PCB 52 [44]. 4′-Me-5′-MeSO2-CB 106 was included in this study because of its use as a surrogate standard for the analysis of methylsulfonated compounds [8,42,43]. Finally, 5-OH-CB 77 and 6-OH-CB 77 were included in our study because they are hydroxylated metabolites of PCB 77 [45], a PCB congener that has been extensively studied in our laboratory [40,46].
3.1.2. Selection of tissue
The PLE method development outlined herein focused on the liver. The liver is not only the major site of PCB metabolism, but also a storage site for PCBs and MeSO2-PCBs, both in laboratory animal models [10,11] and in wildlife [3]. Although OH-PCBs are mostly retained in blood, they have also been found in the liver [9].
3.2. Effect of solvent and PLE parameters on recoveries from spiked PLE cells
3.2.1. Effect of solvent
In general, the choice of solvent is important for the extraction of environmental contaminants from the matrix of interest. The development of PLE methods is not different in this regard [13]. The major challenge in the present study was the selection of a solvent system that allows the simultaneous extraction and clean-up of PCBs, MeSO2-PCBs and OH-PCBs. Hexane-acetone (1:1, v/v) [47–51], dichloromethane-acetone (1:1) [18,52], dichloromethane [48,49,53,54] and hexane-dichloromethane (1:1, v/v) [55,56] were selected because these solvent systems have been frequently used to extract PCBs from abiotic (e.g., sediments) and biotic samples (e.g., fish and mussels) using PLE. The hexane-acetone mixture was also successfully used for the extraction of PCBs from tissue samples in our laboratory [33,40]. In addition, other combinations of the above mentioned solvents, such as hexane-dichloromethane-methanol (50:45:5, v/v) were initially investigated. Based on an earlier investigation [57], methanol was of particular interest as a polar modifier to facilitate the extraction of polar OH-PCBs from fat retainers, such as Florisil.
PLE with all five solvent combinations resulted in comparably good recoveries of all PCBs from spiked PLE cells (Fig. 2A), with no PCBs detectable in the second, independent extraction step. Similarly, recoveries of all MeSO2-PCBs were close to 100% for most solvent combinations investigated (Fig. 2B). The only exception was hexane-dichloromethane (1:1, v/v), which had recoveries of only 30–50% in the first extraction step. Additional 9–13% of the respective MeSO2-PCBs were extracted during the second, independent extraction step (Fig. S1 C and D, Supplementary Material), which suggests that the extraction of MeSO2-PCBs was not complete under these PLE conditions.
Fig. 2.
Effect of solvent mixture used for PLE (120°C, 10.3 MPa, 1 static cycle of 5 min) on recoveries of model compounds (n = 5). Recoveries of (A) PCBs, (B) MeSO2-PCBs and (C) OH-PCBs.
The recoveries of the two model OH-PCBs were significantly lower compared to PCBs and MeSO2-PCBs (Fig. 2C) because the extraction was incomplete with hexane-acetone (1:1, v/v), dichloromethane, hexane-dichloromethane (1:1, v/v) and dichloromethane-acetone (1:1, v/v). Only hexane-dichloromethane-methanol (50:45:5, v/v) gave higher recoveries (i.e., 30% for 5-OH-CB77 and 58% for 6-OH-CB77). Independent of the extraction solvent, recoveries followed the order 5-OH-CB77 < 6-OH-CB77, which suggests that the recoveries of OH-PCBs may depend on their chemical structure.
Because the hexane-dichloromethane-methanol solvent system gave the best OH-PCB recoveries in all experiments investigating solvents, PLE parameters and fat retainers (see below), we revisited the role of different amounts of methanol on PCB, MeSO2-PCB and OH-PCB recoveries. An increase in methanol content from 0 to 30% had no effect on the recoveries of PCBs (Fig. 3A). A methanol content of 5% improved the recovery of MeSO2-PCBs to approximately 100%; however, residue extraction from the PLE cell was observed with a methanol content of 10% (Fig. 3B), followed by decreasing recoveries (80–100%) at methanol concentrations of 20–30%. Also, an improvement of the recoveries of both model OH-PCBs was observed (Fig. 3C), from 33–34% at 5% methanol to 48–58% at 30% methanol. Similar recoveries of OH-PCBs from different animal tissues were reported previously by Saito et al. in their PLE method for extraction of PCBs and their metabolites [58]. Ultimately, a methanol content of 10% (hexane-dichloromethane-methanol = 48:43:9, v/v) was selected for the method validation experiments because it provided optimum recoveries for PCBs, MeSO2-PCBs and OH-PCBs.
Fig. 3.
The effect of the methanol content of the hexane-dichloromethane mixture (50:45 v/v) on recovery of model compounds in PLE (120°C, 10.3 MPa, 1 static cycle of 5 min, n ≥ 2). Recoveries of (A) PCBs, (B) MeSO2-PCBs and (C) OH-PCBs.
3.2.2. Effect of PLE parameters
In addition to different solvent systems, several PLE parameters were studied in order to optimize the recovery of selected target analytes (Fig. 4). Specifically, temperature (60 to 120°C), pressure (6.9–13.8 MPa, 1000–2000 psi) and the length of the static cycle (1 to 9 min) were investigated using hexane-dichloromethane-methanol (50:45:5, v/v) as extraction solvent. In addition, the number of extraction steps required to quantitatively recover each model compounds was assessed by extracting each PLE cell twice using the same extraction condition.
Fig. 4.
The effect of extraction temperature, pressure and duration of extraction cycle on the recoveries of PCBs, MeSO2-PCBs and OH-PCBs. Effect of extraction temperature on the recoveries of (A) PCBs, (B) MeSO2-PCBs and (C) OH-PCBs. Effect of pressure on the recoveries of (D) PCBs, (E) MeSO2-PCBs and (F) OH-PCBs. Effect of the duration of the extraction cycle on the recoveries of (G) PCBs, (H) MeSO2-PCBs and (I) OH-PCBs. All extractions were performed with hexane-dichloromethane-methanol (50:45:5, v/v) at 120°C and 10.3 MPa using one static cycle of 5 min (n ≥ 2).
The extraction temperature is a critical parameter for PLE extractions because it greatly improves the extraction efficiency by increasing the solubility of the analyte in the solvent, improving mass transfer from the matrix to the solvent and disrupting analyte-matrix interactions [18]. Several studies have shown that PLE recoveries for PCBs are typically very good. However, the optimal extraction temperature depends on the nature of the matrix [20,52,59,60]. In the present study, recoveries of PCBs were excellent (85–122%) over the entire temperature range investigated and appeared to be independent of the extraction temperature (Fig. 4A). PCB recoveries above 100% are probably due to minor, co-eluting impurities present in some sample extracts.
In contrast, the recoveries of MeSO2-PCBs reached a maximum at temperatures ranging from 90–110°C (Fig. 4B) and decreased both at lower (70–80°C) and higher temperatures (120°C). There were some differences in the recoveries of OH-PCBs with increasing temperature, but there was no obvious trend between 60–100°C (Fig. 4C); however, the recoveries of OH-PCBs decreased slightly at 120°C. Overall, an extraction temperature of 100°C appears to be optimal for the extraction of PCBs and their metabolites.
There was no effect of pressure on recoveries of PCBs (Fig. 4D), which is also in agreement with earlier studies [60,61]. This is not surprising because the primary function of the high pressure is to keep the solvent in the liquid state in the PLE cell [12,13,61]. However, the lowest (6.9MPa, 1000 psi) and highest (13.8 MPa, 2000 psi) pressure investigated had a negative effect on the recoveries of MeSO2-PCBs (Fig. 4E). In both cases, an additional 10–20% of the model compounds was recovered with the second extraction (i.e., the extraction was incomplete at the lowest and highest pressure investigated). The biggest effect of pressure was observed for OH-PCBs (Fig. 4F), with no model compounds recovered at 6.9 MPa (1000 psi) and 13.8MPa (2000 psi). Together, these results suggest that a pressure of 10.3 MPa (1500 psi) is optimal for the extraction of PCBs and their metabolites from tissue samples. Similarly, other studies have used pressures ≥1500 psi to extract PCBs and their metabolites from complex environmental matrices [18,25,48,49,52,54,55].
Extraction times up to 7 min had no effect on the recoveries of PCBs, MeSO2-PCBs or OH-PCBs (Fig. 4G to 4I); however, at 9 min an increase of residue extraction from the PLE cell was observed for PCBs and MeSO2 (recoveries > 100%). Overall, a 5 min extraction time appeared to be optimal because it provides good recoveries in reasonable length of time [13]. Finally, one static cycle was found to be generally sufficient for the extraction of PCBs and their metabolites in this and other studies [51].
3.3. Effect of fat retainers on PLE recoveries in the presence and absence of clean tissue samples
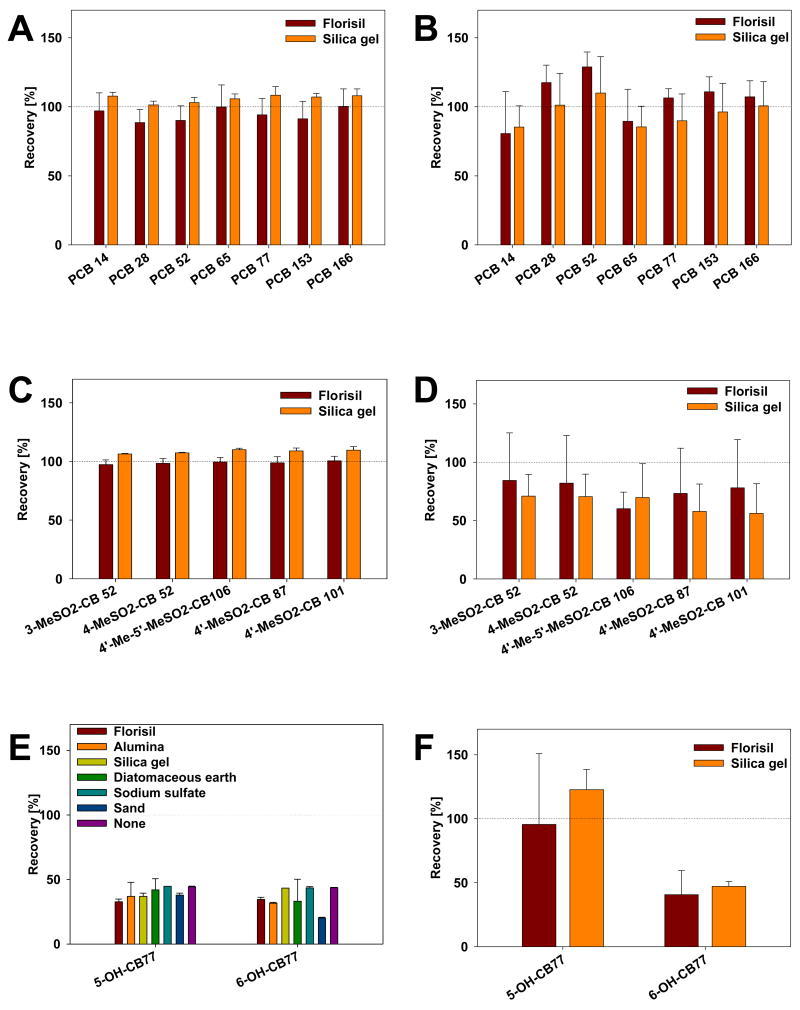
PLE offers to advantage that the extraction of the analytes can be combined with an in-situ clean-up step by placing the sample on top of a suitable fat retainer [19–26]. This approach typically requires a fat-to-fat retainer ratio of 0.025 [19,25] and is, therefore, suitable for the simultaneous extraction and clean-up of small tissue samples (< 0.5 gram) using 33 mL PLE cells. Here we investigated the effect of Florisil, silica gel, silica gel acidified with concentrated H2SO4 (1:0.66, w/w) and Alumina as fat retainers on the recovery of target analytes in the presence and absence of rat liver tissue spiked with PCBs, MeSO2-PCBs and OH-PCBs. Florisil has been successfully used as fat retainer for the simultaneous extraction and clean-up of PCBs from animal tissues by PLE in our [33,40] and other laboratories [20]. Silica gel, especially acidified silica gel, has been found to be an excellent fat retainer for the clean-up of fish and other foodstuffs using PLE [19,25].
In preliminary experiments, acidified silica gel (but not silica gel alone) caused the degradation of the extraction solvent (i.e., hexane-dichloromethane-methanol 50:45:5, v/v). Furthermore, extraction of Alumina with the same solvent mixture resulted in yellowish extracts. Similar observations have been reported by Bjorklund and coworkers for PLE extractions with Alumina [19]. Therefore, only Florisil and silica gel were systematically investigated as fat retainers.
Recoveries of PCBs in the presence of Florisil and silica gel were excellent using hexane:dichloromethane:methanol (50:45:5, v/v) as extraction solvent, with no notable difference between both fat retainers (Fig. 5A). Furthermore, PCB recoveries were comparable when the PLE extraction was performed in the presence or absence of rat liver tissue (Fig.5A and 5B). Similarly, recoveries of MeSO2-PCBs extracted in the presence of Florisil and silica gel were excellent and independent of the fat retainer employed (Fig. 5C); however, recoveries in the presence of rat liver samples during extraction were lower for both fat retainers compared to the extraction of MeSO2-PCB standards alone (Fig. 5D).
Fig. 5.
The effect of fat retainer on recoveries of model compounds after extraction with hexane-dichloromethane-methanol mixture (50:45:5, v/v) using PLE (120°C, 10.3 MPa, 1 static cycle of 5 min) with and without liver tissue present (n ≥ 2). Recoveries of (A) PCBs, standards only; (B) PCBs, with tissue; (C) MeSO2-PCBs, standards only; (D) MeSO2-PCBs, with tissue; (E) OH-PCBs, standards only; (F) OH-PCBs, with tissue.
In contrast to PCBs and MeSO2-PCBs, recoveries of OH-PCB standards were systematically lower (30–45%) during PLE extraction, regardless of the fat retainer employed (Fig. 5E and 5F). Furthermore, recoveries of OH-PCBs extracted from empty PLE cells or in the presence of diatomaceous earth, sodium sulfate or sand were comparably low. These findings suggest that the PLE extraction of OH-PCBs generally gives poor-to-moderate recoveries [31,58,62], probably due to the chemical instability of OH-PCBs.
3.4. Method validation
The optimized PLE conditions (i.e., hexane:dichloromethane:methanol (48:43:9, v/v), 100°C, 1500 psi with a 6 min heating time, one static cycle of 5 minutes and a 60% cell volume flush) were employed to validate the PLE extraction method by verifying its linearity and repeatability. In addition, the PLE method was compared with the “Jensen method” [34], an established method for the analysis of PCBs and their metabolites in tissue samples. As shown in Fig. 1, the Jensen method requires more extraction and clean-up steps than the PLE method described herein. Specifically, the Jensen method involves three labor-intensive extraction steps with different solvent combinations of increasing polarity, the PLE method only requires a single, automated PLE extraction step that, as discussed above, combines an extraction and an in-situ clean-up step. In addition, the Jensen method requires additional column clean-up steps for both PCBs and MeSO2PCBs.
3.4.1. Method linearity and repeatability
The linearity of the method was studied over a range of concentrations (from 10–1000 ng) for selected target analytes, with and without liver tissue present in the samples. The method was linear, with correlation coefficients >0.999 for most model compounds (Table S5, Supplementary Material). At the same time, the repeatability of recoveries of surrogate standards was assessed with and without liver tissue present in the PLE cell. The RSD was ≤16% for PCBs (PCB 14, 65 and 166) and 4′-Me-5′-MeSO2-CB 106. The only exception was the extraction of 4′-Me-5′-MeSO2-CB 106 in the absence of liver tissues (RSD = 34%).
3.4.2. Comparison with the “Jensen method”
Since no Standard Reference Material is certified for PCBs and their hydroxylated and methylsulfonyl metabolites, a well established reference method was used to verify the performance of the method. In an initial experiment, rat liver tissue from PCB treated animals was extracted in parallel with the optimized PLE method and the “Jensen method” [34]. Seven PCB congeners (out of twelve congeners analyzed) were detected in the livers of PCB treated rats, with mean tissue concentrations ranging from 2.9 to 254 ng/g liver tissue (Table 1). The major OH-PCB detected in the liver was 4-MeO-CB107. Similarly, Bergman and co-workers have reported that 4-MeO-CB107 is the major OH-PCBs formed in rats treated with Aroclor 1254 [6]. Small quantities of 3′-MeO-CB138 were also detected. The tissue concentrations of all fourteen MeSO2-PCBs analyzed were below the respective detection limit in all samples (Table S4 in the Supplementary Material), independent of the method employed. However, 4-MeSO2-CB 52, 5-MeSO2-CB91 and 5-MeSO2-CB149 were detected in pooled extracts from the PLE method, and 4-MeSO2-CB 52 and 5-MeSO2-CB91 were detected in extracts from the “Jensen method”. These metabolites were also reported in livers of rats exposed to Clophen A50 [63]. However, in this earlier study the 4-substituted methylsulfonyl metabolites of PCB 132 and PCB 149 were preferentially formed over the 5-substituted metabolites, and the ratio changed with time of exposure.
Table 1.
Precision and accuracy of PLE method compared to “Jensen method” [34].
| PLE method | “Jensen method” | ||||
|---|---|---|---|---|---|
| A. Surrogate standards for PCBs (n=6) | |||||
| Compound | Recovery ± SD[%] | RSD [%] | Recovery ± SD[%] | RSD [%] | |
| PCB 14 | 84 ± 31 | 37 | 83 ± 18 | 22 | |
| PCB 65 | 112± 37 | 33 | 119 ± 29 | 22 | |
| PCB 166 | 78 ± 10 | 13 | 68 ± 9 | 13 | |
|
B. PCB concentrations in liver tissue (n=5) | |||||
| Compound | Mean tissue concentration[ng/g] | Mean tissue concentration [ng/g] | |||
| PCB 28 | 151 ± 24 | 148 ± 49 | |||
| PCB 52 | 24 ± 14 | 12 ± 3 | |||
| PCB 77(+PCB 110)a | ND | ND | |||
| PCB 91 | ND | ND | |||
| PCB 95 | 254 ± 77 | 250 ± 92 | |||
| PCB 132(+105)a | ND | ND | |||
| PCB 136 | ND | ND | |||
| PCB 149(+PCB 123)a | 26 ± 12 | 23 ± 11 | |||
| PCB 153 | 61 ± 24 | 66 ± 28 | |||
| PCB 174 | ND | ND | |||
| PCB 176(+PCB137+PCB130)a | 13 ± 5 | 17 ± 6 | |||
| PCB 183 | 2.8 ± 1.0 | 3.2 ± 0.8 | |||
|
C. Surrogate standard for OH-PCBs (n=6) | |||||
| Compound | Recovery ± SD[%] | RSD [%] | Recovery ± SD[%] | RSD [%] | |
| 4′-OH-CB159 | 46 ± 2* | 4 | 52 ± 5 | 10 | |
|
D. OH-PCB concentrations in liver tissue (n=5) | |||||
| Compound | Mean tissue concentration[ng/g] | Mean tissue concentration[ng/g] | |||
| 4-MeO-CB107 | 3.70 ± 1.40 | 4.90 ± 2.10 | |||
| 3′-MeO-CB138 | 0.14 ± 0.06** | 0.28 ± 0.10 | |||
| 4-MeO-CB146 | ND | ND | |||
| 4-MeO-CB187 | ND | ND | |||
|
E. Surrogate standard for MeSO2-PCB (n=6) | |||||
| Compound | Recovery ± SD[%] | RSD [%] | Recovery ± SD[%] | RSD [%] | |
| 4′-Me-5′-MeSO2-CB106 | 89 ± 21 | 24 | 103 ± 30 | 30 | |
|
F. MeSO2-PCB concentrations in liver tissue | |||||
| Compound | Mean tissue concentrationb[ng/g] | Mean tissue concentrationb[ng/g] | |||
| 3-MeSO2-CB 52 | ND | ND | |||
| 4-MeSO2-CB 52 | 1.4 | 0.57 | |||
| 4′-MeSO2-CB 87 | ND | ND | |||
| 4-MeSO2-CB91 | ND | ND | |||
| 5-MeSO2-CB91 | 4.0 | 1.2 | |||
| 3′-MeSO2-CB95 | ND | ND | |||
| 4′-MeSO2-CB95 | ND | ND | |||
| 4′-MeSO2-CB 101 | ND | ND | |||
| 4′-MeSO2-CB132 | ND | ND | |||
| 5′-MeSO2-CB132 | ND | ND | |||
| 4-MeSO2-CB149 | ND | ND | |||
| 5-MeSO2-CB149 | 0.8 | ND | |||
| 4′-MeSO2-CB174 | ND | ND | |||
| 5′-MeSO2-CB174 | ND | ND | |||
The target PCB congeners co-eluted with congeners in parentheses as described in [32].
Pooled samples.
Significantly different between the PLE and the “Jensen” method. Two sample t-test, =0.05, P<0.01.
Significantly different between the PLE and the “Jensen” method. Two sample t-test, =0.05, P<0.05.
Comparison of the recoveries of surrogates standards as well as selected PCBs revealed no differences between the two methods (two sample t-test, α=0.05, p value <0.05; Table 1). This observation is not surprising because the recovery of PCBs is typically excellent, independent of the extraction and clean-up methods employed. Recovery of the OH-PCB surrogate standard, 4′-OH-PCB159, and the two OH-PCBs detected in liver samples tended to be slightly lower with the PLE method compared to the “Jensen method”. This difference was statistically significant for surrogate standard and 3′-MeO-CB138. In contrast, mean tissue concentrations of MeSO2-PCBs (determined in pooled samples) were higher with the PLE method compared to the “Jensen method”.
Supplementary Material
Acknowledgments
We thank Dr. Wei Xie, Dr. Sanjay Telu and Mr. Bartlomiej Milanowski for their help with the animal studies. The research was supported by grants ES05605, ES013661 and ES012475 from the National Institute of Environmental Health Sciences, NIH, and Major Research Instrumentation grant BES-0420378 from the National Science Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hornbuckle KC, Carlson DL, Swackhamer DL, Baker JE, Eisenreich SJ. In: Handbook of Environmental Chemistry. Hites R, editor. Springer Verlag; Berlin Heidelberg: 2006. p. 13. [Google Scholar]
- 2.Robertson LW, Hansen LG. PCBs: Recent Advances in Environmental Toxicology and Health Effects. University Press of Kentucky; Lexington, KY: 2001. [Google Scholar]
- 3.Letcher RJ, Klasson-Wehler E, Bergman A. In: The handbook of environmental chemistry - New types of persistent halogenated compounds. Passivirta J, editor. Springer-Verlag; Berlin Heidelberg: 2000. p. 315. [Google Scholar]
- 4.Weistrand C, Noren K, Nilsson A. Environ Sci Pollut Res. 1997;4:2. doi: 10.1007/BF02986256. [DOI] [PubMed] [Google Scholar]
- 5.Hovander L, Linderholm L, Athanasiadu M, Bignert A, Fangstrom B, Kocan A, Petrick J, Trnovec T, Bergman A. Environ Sci Technol. 2006;40:3696. doi: 10.1021/es0525657. [DOI] [PubMed] [Google Scholar]
- 6.Bergman A, Klasson-Wehler E, Kuroki H. Environ Health Perspect. 1994;102:464. doi: 10.1289/ehp.94102464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Letcher RJ, Li HX, Chu SG. J Anal Toxicol. 2005;29:209. doi: 10.1093/jat/29.4.209. [DOI] [PubMed] [Google Scholar]
- 8.Weistrand C, Noren K. Environ Health Perspect. 1997;105:644. doi: 10.1289/ehp.97105644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guvenius DM, Hassanzadeh P, Bergman A, Noren K. Environ Toxicol Chem. 2002;21:2264. [PubMed] [Google Scholar]
- 10.Norström K, Eriksson J, Haglund J, Silvari V, Bergman A. Environ Sci Technol. 2006;40:7649. doi: 10.1021/es061584t. [DOI] [PubMed] [Google Scholar]
- 11.Larrson C, Ellerichmann T, Huhnerfuss H, Bergman A. Environ Sci Technol. 2002;36:2833. doi: 10.1021/es025512n. [DOI] [PubMed] [Google Scholar]
- 12.Fidalgo-Used N, Blanco-Gonzalez E, Sanz-Medel A. Anal Chim Acta. 2007;590:1. doi: 10.1016/j.aca.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Bjorklund E, Nilsson T, Bowadt S. Trends Anal Chem. 2000;19:434. [Google Scholar]
- 14.Carabias-Martinez R, Rodriguez-Gonzalo E, Revilla-Ruiz P, Hernandez-Mendez J. J Chromatogr A. 2005;1089:1. doi: 10.1016/j.chroma.2005.06.072. [DOI] [PubMed] [Google Scholar]
- 15.Ramos L, Kristenson EM, Brinkman UAT. J Chromatogr A. 2002;975:3. doi: 10.1016/s0021-9673(02)01336-5. [DOI] [PubMed] [Google Scholar]
- 16.Schantz MM. Anal Bioanal Chem. 2006;386:1043. doi: 10.1007/s00216-006-0648-2. [DOI] [PubMed] [Google Scholar]
- 17.Giergielewicz-Mozajska H, Dabrowski L, Namiesnik J. Crit Rev Anal Chem. 2001;31:149. [Google Scholar]
- 18.Richter BE, Jones BA, Ezzell JL, Porter NL. Anal Chem. 1996;68:1033. [Google Scholar]
- 19.Bjorklund E, Muller A, von Holst C. Anal Chem. 2001;73:4050. doi: 10.1021/ac010178j. [DOI] [PubMed] [Google Scholar]
- 20.Wenzel KD, Hubert A, Manz M, Weissflog L, Engewald W, Schuurmann G. Anal Chem. 1998;70:4827. doi: 10.1021/ac991005l. [DOI] [PubMed] [Google Scholar]
- 21.McCant DD, Inouye LS, McFarland VA. Bull Environ Contam Toxicol. 1999;63:282. doi: 10.1007/s001289900978. [DOI] [PubMed] [Google Scholar]
- 22.Sporring S, Bjorklund E. J Chromatogr A. 2004;1040:155. doi: 10.1016/j.chroma.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 23.Sporring S, van Holst C, Bjorklund E. Chromatographia. 2006;64:533. [Google Scholar]
- 24.Gomez-Ariza JL, Bujalance M, Giraldez I, Velasco A, Morales E. J Chromatogr A. 2002;946:209. doi: 10.1016/s0021-9673(01)01534-5. [DOI] [PubMed] [Google Scholar]
- 25.Muller A, Bjorklund E, von Holst C. J Chromatogr A. 2001;925:197. doi: 10.1016/s0021-9673(01)01028-7. [DOI] [PubMed] [Google Scholar]
- 26.Bjorklund E, Sporring S, Wiberg K, Haglund P, Holst Cv. Trends Anal Chem. 2006;25:318. doi: 10.1021/ac0624501. [DOI] [PubMed] [Google Scholar]
- 27.Kania-Korwel I, Parkin S, Robertson LW, Lehmler HJ. Chemosphere. 2004;56:735. doi: 10.1016/j.chemosphere.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 28.Ballschmiter K, Bacher R, Mennel A, Fischer R, Riehle U, Swarev M. J High Resol Chromatogr. 1992;15:260. [Google Scholar]
- 29.Maervoet J, Covaci A, Schepens P, Sandau CD, Letcher R. Environ Health Perspect. 2004;112:291. doi: 10.1289/ehp.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Black TH. Aldrichim Acta. 1982;15:3. [Google Scholar]
- 31.Hovander L, Athanasiadu M, Asplund L, Jensen S, Klasson-Wehler E. J Anal Toxicol. 2000;24:696. doi: 10.1093/jat/24.8.696. [DOI] [PubMed] [Google Scholar]
- 32.Kania-Korwel I, Hornbuckle KC, Peck A, Ludewig G, Robertson LW, Sulkowski WW, Espandiari P, Gairola CG, Lehmler HJ. Environ Sci Technol. 2005;39:3513. doi: 10.1021/es047987f. [DOI] [PubMed] [Google Scholar]
- 33.Kania-Korwel I, Shaikh N, Hornbuckle KC, Robertson LW, Lehmler HJ. Chirality. 2007;19:56. doi: 10.1002/chir.20342. [DOI] [PubMed] [Google Scholar]
- 34.Jensen S, Haggberg L, Jorundsdottir H, Odham G. J Agric Food Chem. 2003;51:5607. doi: 10.1021/jf0301201. [DOI] [PubMed] [Google Scholar]
- 35.Jensen S, Johnels A, Olsson M, Otterlind G. Ambio Spec Rep No 1.1. 1972:71. [Google Scholar]
- 36.Sun P, Basu I, Hites RA. Environ Sci Technol. 2006;40:1178. doi: 10.1021/es051725b. [DOI] [PubMed] [Google Scholar]
- 37.Kania-Korwel I, Hornbuckle KC, Robertson LW, Lehmler HJ. Environ Toxicol Chem. 2007;27:299. doi: 10.1897/07-359R.1. [DOI] [PubMed] [Google Scholar]
- 38.Kania-Korwel I, Hornbuckle KC, Robertson LW, Lehmler HJ. Food Chem Toxicol. 2008;46:637. doi: 10.1016/j.fct.2007.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kania-Korwel I, Xie W, Hornbuckle KC, Robertson LW, Lehmler HJ. Arch Environ Contam Toxicol. 2008;55:510. doi: 10.1007/s00244-007-9111-4. [DOI] [PubMed] [Google Scholar]
- 40.Bunaciu PR, Tharappel JC, Lehmler HJ, Kania-Korwel I, Robertson LW, Srinivasan C, Spear BT, Glauert HP. Toxicology. 2007;239:147. doi: 10.1016/j.tox.2007.06.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linderholm L, Park JS, Kocan A, Trnovec T, Athanasiadou M, Bergman K, Hertz-Picciotto I. Chemosphere. 2007;69:403. doi: 10.1016/j.chemosphere.2007.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noren K, Lunden A, Pettersson E, Bergman A. Environ Health Persp. 1996;104:766. doi: 10.1289/ehp.104-1469405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newsome WH, Davies D. Chemosphere. 1996;33:559. doi: 10.1016/0045-6535(96)00199-3. [DOI] [PubMed] [Google Scholar]
- 44.Bergman A, Brandt I, Darnerud PO, Wachtmeister CA. Xenobiotica. 1982;12:1. doi: 10.3109/00498258209052449. [DOI] [PubMed] [Google Scholar]
- 45.Klasson-Wehler E, Bergman A, Brandt I, Darnerud PO, Wachtmeister CA. Drug Metab Disp. 1989;17:441. [PubMed] [Google Scholar]
- 46.van den Hurk P, Kubiczak GA, Lehmler HJ, James MO. Environ Health Perspect. 2002;110:343. doi: 10.1289/ehp.02110343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martens D, Gfrerer M, Wenzl T, Zhang A, Gawlik BM, Schramm KW, Lankmayr E, Kettrup A. Anal Bioanal Chem. 2002;372:526. doi: 10.1007/s00216-001-1120-y. [DOI] [PubMed] [Google Scholar]
- 48.Numata M, Yarita T, Aoyagi Y, Tsuda Y, Yamazaki M, Takatsu A, Ishikawa K, Chiba K, Okamaoto K. Anal Bioanal Chem. 2007;387:2313. doi: 10.1007/s00216-006-0691-z. [DOI] [PubMed] [Google Scholar]
- 49.Schantz MM, Nichols JJ, Wise SA. Anal Chem. 1997;69:4210. [Google Scholar]
- 50.Sporring S, Bowadt S, Svensmark B, Bjorklund E. J Chromatogr A. 2005;1090:1. doi: 10.1016/j.chroma.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 51.Bjorklund E, Bowadt S, Nilsson T, Mathiasson L. J Chromatogr A. 1999;836:285. [Google Scholar]
- 52.Bandh C, Bjorklund E, Mathiasson L, Naf C, Zebuhr Y. Environ Sci Technol. 2000;34:4995. [Google Scholar]
- 53.Dabrowski L, Giergielewicz-Mozajska H, Biziuk M, Gaca J, Namiesnik J. J Chromatogr A. 2002;957:59. doi: 10.1016/s0021-9673(02)00287-x. [DOI] [PubMed] [Google Scholar]
- 54.Brubaker WW, Schantz MM, Wise SA. Fres J Anal Chem. 2000;367:401. doi: 10.1007/s002160000438. [DOI] [PubMed] [Google Scholar]
- 55.Wang D, Atkinson S, Hoover-Miller A, Li QX. Rapid Commun Mass Spectrom. 2005;19:1815. doi: 10.1002/rcm.1990. [DOI] [PubMed] [Google Scholar]
- 56.Suchan P, Pulkrabova J, Hajslova J, Kocourek V. Anal Chim Acta. 2004;520:193. [Google Scholar]
- 57.Dumas P, Sandanger TM, Sandau CD, Sjodin A, Ayotte P. Organohalogen Compd. 2006;68:1593. [Google Scholar]
- 58.Saito K, Sjodin A, Sandau CD, Davis MD, Nakazawa H, Matsuki Y, Patterson DG., Jr Chemosphere. 2004;57:373. doi: 10.1016/j.chemosphere.2004.04.050. [DOI] [PubMed] [Google Scholar]
- 59.von Holst C, Muller A, Serrano F, Sporring S, Bjorklund E. Chromatographia. 2005;61:391. [Google Scholar]
- 60.Hubert A, Wenzel KD, Manz M, Weissflog L, Engewald W, Schuurmann G. Anal Chem. 2000;72:1294. doi: 10.1021/ac991005l. [DOI] [PubMed] [Google Scholar]
- 61.Ramos JJ, Dietz C, Gonzalez MJ, Ramos L. J Chromatogr A. 2007;1152:254. doi: 10.1016/j.chroma.2006.11.097. [DOI] [PubMed] [Google Scholar]
- 62.Houde M, Pacepavicius G, Wells RS, Fair PA, Letcher RJ, Alaee M, Bossart GD, Hohn AA, Sweeney J, Solomon KR, Muir DCG. Environ Sci Technol. 2006;40:5860. doi: 10.1021/es060629n. [DOI] [PubMed] [Google Scholar]
- 63.Larsson C, Ellerichmann T, Hühnerfuss H, Bergman A. Environ Sci Technol. 2002;36:2833. doi: 10.1021/es025512n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.