Abstract
Purpose
Glaucoma is a leading cause of irreversible visual impairment and blindness in the world. A major risk factor for glaucoma is elevated intraocular pressure due to increased resistance of aqueous humor outflow through the trabecular meshwork (TM). In the glaucomatous TM, there is increased accumulation of extracellular matrix (ECM) material due to a disruption of the normal balance between ECM deposition and degradation. Tissue transglutaminase (TGM2) belongs to a family of calcium-dependent enzymes that catalyze the posttranslational modification of the ECM by cross-linking proteins, thus making these proteins resistant to enzymatic and physical degradation. It is possible that the increase in ECM proteins in the glaucomatous TM is due to increased cross-linking activity of TGM2. The purpose of this study was to determine whether there are differences in expression and activity of TGM2 between normal and glaucoma TM cells and tissues.
Methods
Normal (n = 3 NTM) and glaucomatous (n = 3 GTM) human TM cell lines were grown until confluent. Western immunoblot analysis of cell lysates was used to compare TGM2 protein levels in NTM and GTM cells. TGM2 enzyme activity between NTM and GTM cells was studied by using a biotin cadaverine assay. In addition, immunohistochemistry of three normal and three glaucomatous TM tissues was used to evaluate the in vivo expression of TGM2, fibronectin (FN) and ε-(γ-glutamyl) lysine (GGEL) proteins.
Results
Western blot analysis and immunohistochemistry demonstrated the presence of TGM2 protein in both NTM and GTM cells. There was an increase in TGM2 protein in GTM cells compared with NTM cells, and GTM cells also had increased in TGM2 enzyme activity compared with NTM cells. Immunohistochemical results demonstrated increased expression of TGM2 and FN in GTM tissues. FN and GGEL proteins were colocalized in GTM tissues, indicating significant cross-linking of FN by TGM2.
Conclusions
This study demonstrated that both NTM and GTM cells express TGM2. In addition, TGM2 protein levels and enzyme activities were elevated in GTM cells. There was also an increase in colocalization of FN and GGEL protein in GTM tissues. These results indicate that TGM2 may play an important role in the pathogenesis of glaucoma by cross-linking ECM proteins such as FN and thus making the ECM more resistant to degradation.
A major cause of irreversible blindness in the world is primary open-angle glaucoma (POAG). Elevated intraocular pressure (IOP) is an important risk factor in the development of POAG1 and the progression of glaucomatous damage.2,3 Elevated IOP is due to increased aqueous humor (AH) outflow resistance4 and appears to be associated with several morphologic and biochemical changes in the trabecular meshwork (TM). In the normal human TM, a balance exists between extracellular matrix protein (ECM) deposition and degradation. However in POAG, there is increased accumulation of cross-linked ECM proteins in the TM that is believed to result in increased AH outflow resistance and elevated IOP.5,6
Several studies have focused on regulators of the ECM in the TM including matrix metalloproteinases (MMPs), plasminogen activators, and growth factors. Reports have indicated that MMPs assist in maintaining AH outflow and this effect can be blocked by synthetic MMP inhibitors or by activating tissue inhibitors of matrix metalloproteinases (TIMPs).7–9 Plasminogen activators (PAs) are serine proteases involved in degrading fibrin and the ECM; however, the effects of PAs can be blocked by plasmin activator inhibitor (PAI)-1.10,11
The transglutaminase enzyme family (EC 2.3.2.13) contains several members that are widely distributed in various tissues. Transglutaminase enzymes are encoded by a group of genes that are structurally and functionally related.12 Family members include keratinocyte transglutaminase (TGM1), tissue transglutaminase (TGM2), epidermal transglutaminase (TGM3), prostate transglutaminase (TGM4), TGM X (TGM5), TGM Y (TGM 6), TGM Z (TGM 7), and factor XIII-A subunit (fibrin-stabilizing factor).13,14 Although identified at the gene level, TGM5, TGM6, and TGM7 have unknown functions.12 Transglutaminase enzymes are known to catalyze the posttranslational modification of proteins via formation of isopeptide bonds.15 The enzymatic reaction occurs by forming bonds between ε-(γ-glutamyl) lysine or (γ-glutamyl) polyamine.16 The resultant cross-linked proteins are highly resistant to both physical and enzymatic degradation.16
TGM2 is a unique transglutaminase family member. Within the TGM family, TGM2 is the most ubiquitous enzyme and is found in numerous tissues, including the human peripheral and central nervous system.17 It has been reported that TGM2 expression is mostly detected in the cytoplasm of cells,18 but it is also found in the plasma membrane19 and the nucleus.20 In addition, TGM2 can be secreted into the ECM. The membrane-bound form of TGM2 binds GTP and may function as a G protein.19 This enzyme incorporates amines into proteins, regulates site-specific deamination and isopeptidase activity, and promotes cell–matrix interactions.21 TGM2 is involved in apoptosis,22 cell adhesion,23 and cell matrix interactions resulting in cross-linking of ECM proteins including fibronectin.24 TGM2 can be upregulated and/or activated in a variety of diseases associated with enhanced accumulation of cross-linked proteins in the ECM and therefore may also be involved in the pathophysiology of glaucoma.
Welge-Lüssen et al.25 reported increased mRNA expression and protein levels of TGM2 in cultured human TM cells after exogenous treatment with either transforming growth factor (TGF) β-1 or TGF β-2. In addition to TGM2 expression, they showed that TGM2 enzymatic activity was also stimulated by TGF-β1 and -β2 in normal cultured TM cells. TGF-β2 treatment of cultured optic nerve head (ONH) astrocytes also upregulated TGM2 expression.26 Interestingly, Fuchshofer et al.26 also demonstrated TGF-β2 induced TGM2 expression was mediated through the action of connective tissue growth factor.
These reports are very insightful, since several studies have demonstrated that patients with glaucoma have elevated TGF-β2 levels in both the AH27–29 and the ONH.30,31 This finding has led to the concept that elevated TGF-β2 levels may increase cross-linked proteins in the ECM of the glaucomatous TM and ONH. However, we are not aware of any studies that have compared TGM2 expression or enzyme activity in normal and glaucomatous TM cells and/or tissues. The purpose of this study was to expand our knowledge of TGM2 in the pathophysiology of POAG by examining differences in both protein expression and enzyme activity of TGM2 between normal and glaucomatous TM cells and tissues.
Materials and Methods
TM Dissection and Cell Culture
Human TM cells were isolated from carefully dissected human TM tissue explants and characterized as previously described.32–34 Isolated TM cells were grown in Dulbecco’s modified Eagle’s medium (low glucose) supplemented with 10% fetal bovine serum (FBS; both from HyClone Laboratories, Logan, UT), L-glutamine (0.292 mg/mL), penicillin (100 U/mL), streptomycin (0.1 mg/mL), and amphotericin B (4 μg/mL). Antibiotics were purchased from Invitrogen-Gibco (Grand Island, NY). Medium was exchanged every 2 to 3 days and near-confluent cultures were trypsinized and seeded in 25-cm2 cell culture flasks (Corning Inc., Corning, NY) and maintained at 37°C in 5% CO2 and 95% air. Three normal (aged 2 [HTM 2], 54 [HTM10A], and 72 [NTM115-01] years) and three glaucomatous (aged 60 [GTM75A], 72 [GTM60A], and 87 [GTM152-99]) years human TM cell lines were used in the experiments.
Protein Extraction and Western Blot Analysis of TGM2 Protein Levels in Normal and Glaucomatous TM Cell Lines
Total cellular protein was isolated from cultured cells using M-PER extraction buffer (78501; Pierce Biotech, Rockford, IL) and protease inhibitor cocktail (78415; Pierce Biotech) or a lysis buffer containing 10 mM Tris-HCl, 0.5% NP40, 150 mM NaCl, 0.5% sodium deoxycholate, 0.1% SDS, 0.2 mM PMSF in ethanol, 1 μg/mL aprotinin, 4 μg/mL pepstatin, 10 μg/mL leupeptin, and 1 mM sodium orthovanadate (10 μL/mL).35 Protein concentration was determined by protein assay (Dc Protein Assay System; Bio-Rad, Hercules CA), as described by the manufacturer. A standard curve was generated using bovine serum albumin (BSA) and absorbance at 750 nm was read within 15 minutes.
A total of 20 μg of protein was loaded per well and separated on an SDS-PAGE denaturing polyacrylamide gel and then transferred by electrophoresis to PVDF membranes. The PVDF membranes were incubated in 5% milk in Tris-buffered saline Tween (TBST; 20 mM Tris, 0.5M NaCl, and 0.05% Tween 20 [pH 7.4]) for approximately 60 minutes to block nonspecific binding. Blots were processed with a mouse anti-TGM2 monoclonal primary antibody (MS-300-P1; Neomarkers, Fremont, CA) and the secondary consisted of goat anti-mouse antibody (SC 2005; Santa Cruz Biotechnology, Santa Cruz, CA). Chemiluminescent substrate (Super Signal West Dura Extended Duration Substrate, 34075; Pierce Biotech) was used for detection and blots were exposed to an imager (Fluorchem 8900; Alpha Innotech, San Leandro, CA).
TGM2 Enzyme Activity
Cell Enzyme Activity Assay
TGM2 enzyme activity was measured using a microscopic assay as previously described.36,37 Briefly, TGM2 activity was assessed by exposing cells to 1 mM biotin cadaverine, a pseudosubstrate of TGM2, for 48 hours. Cells were fixed for 30 minutes in 3.7% (vol/vol) formaldehyde in PBS and permeabilized with 0.2% (vol/vol) Triton X-100 in PBS for 30 minutes at room temperature. Cells were further incubated with an AlexaFluor 488 streptavidin-conjugate (1:1000, vol/vol) in PBS for 1 hour at room temperature, washed, and mounted. The cells were observed with a trinocular research microscope (model BX51; Olympus, Tokyo, Japan). TGM2 activity was seen as increased fluorescence labeling.
Western Blot Analysis
Details of the TGM2 activity assay have been published previously38 with slight modifications. N-(5-aminopentyl)biotinamide, trifluoroacetic acid salt (biotin cadaverine, 1 mM; Invitrogen-Molecular Probes, Eugene, OR), a pseudosubstrate for TGM2, was added to TM cells in serum-free medium for 48 hours. Cell lysate and conditioned medium were collected, separated by SDS-PAGE, and transferred to PVDF membranes. TGM2-mediated incorporation of biotin cadaverine into proteins was detected by streptavidin-HRP with chemiluminescent substrate (Super Signal West Dura Extended Substrate; Pierce Biotech). After the detection of the proteins, the blots were then stripped and reprobed with anti-fibronectin (FN).
Immunolocalization of TGM2, FN, and GGEL in Normal and Glaucomatous TM Tissues
Details of the immunocytochemistry of TGM2 in TM tissues have been published.39 To document the presence of TGM2, FN, and cross-linked GGEL isopeptide in the TM, three sets of normal and glaucomatous age-matched human eyes were used (e.g., normal donors of 72, 88, and 94 years of age; glaucomatous donors of 76, 87, and 92 years of age). Briefly, eyes were obtained from regional eye banks within 6 hours of death and fixed in 10% formalin. The eyes were obtained and managed in compliance with the Declaration of Helsinki. Tissues were dehydrated and embedded in paraffin and stored until further use. TM tissues were deparaffinized in xylene, treated with 0.02 M glycine for 15 minutes and dehydrated twice with 100%, 95%, 70%, 50% ethanol for 5 minutes each. After boiling TM sections in 10 mM citrate buffer, (pH 6.0), the TM tissues were incubated with normal serum and 3% BSA in PBS for 30 minutes. TM sections were washed and processed with primary antibodies for TGM2 (MS-300-P1, Neomarkers), FN (F-3648; Sigma-Aldrich, St. Louis, MO), and GGEL (ab424; Abcam, Cambridge MA). The primary antibodies were detected with appropriate secondary antibodies (AlexaFluor 488, 564, and/or 633; Invitrogen-Molecular Probes) for 45 minutes. The colocalization of FN and GGEL protein indicates transamidation of FN by TGM2 in TM tissues. The visualization of cell nuclei was performed by staining tissue sections with DAPI (300 μm) for 10 minutes. Controls consisted of omission of primary antibodies. Images were captured using a confocal imaging system (model 410; Carl Zeiss, Thornwood, NY).
Statistics
The average values for NTM and GTM are stated as means ± SD. Statistical analysis was performed using Microsoft Excel software, and the data were analyzed by t-test (two-sample assuming unequal variances). All differences were considered statistically significant at P < 0.005 between NTM and GTM.
Results
TGM2 Protein Levels in Normal and Glaucomatous Trabecular Meshwork Cells
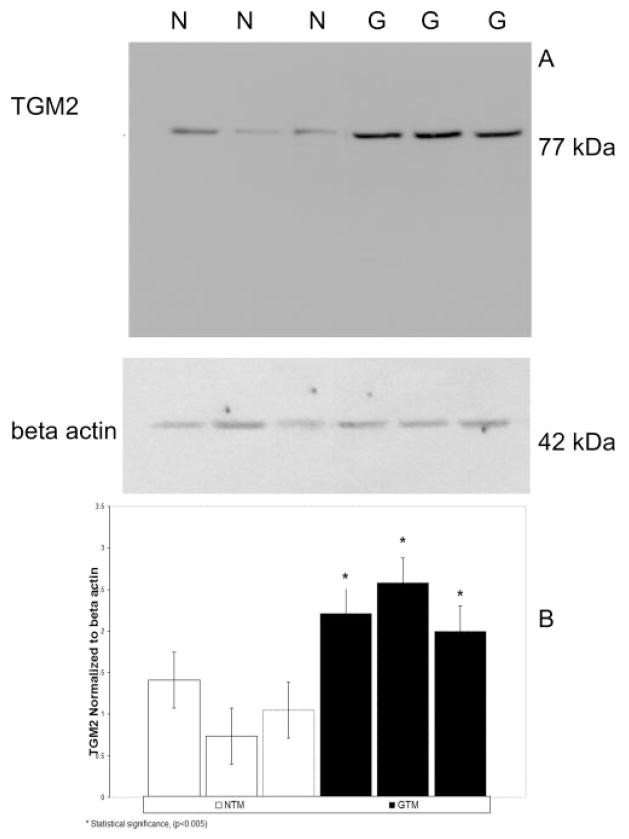
We examined TGM2 protein expression in the lysates of three normal and three glaucomatous TM cell lines. TGM2 was expressed in all six cell lines as a 77-kDa protein band on the Western blots (Fig. 1A). The 3 GTM cell lines appeared to have increased TGM2 protein levels compared with the NTM cell lines. All Western blots were reprobed for β-actin (Fig. 1), which served as a loading control. Relative TGM2 intensity levels were measured via densitometry (Fig. 1B). These data indicate that both NTM and GTM cell lines contain TGM2 protein, and that the protein levels of TGM2 were significantly elevated (P < 0.005) in the GTM cell lines.
Figure 1.
Chemiluminescent detection of TGM2 protein in normal and glaucomatous human TM cells. (A) Total protein was collected from three normal (N) and three glaucomatous (G) cell lines and electrophoresed in SDS-PAGE gels followed by Western immunoblot analysis for TGM2 (77 kDa). All cell lines express TGM2 proteins. Protein levels of TGM2 were higher in GTM than in the NTM cell lines. β-Actin was used as an internal loading control. (B) Densitometric readings of TGM2 normalized to β-actin for three NTM and three GTM cell lines. *Statistical difference at the P < 0.005 level (±SD).
Immunohistochemical Localization of TGM2 Protein in Normal and Glaucomatous Human TM Tissue
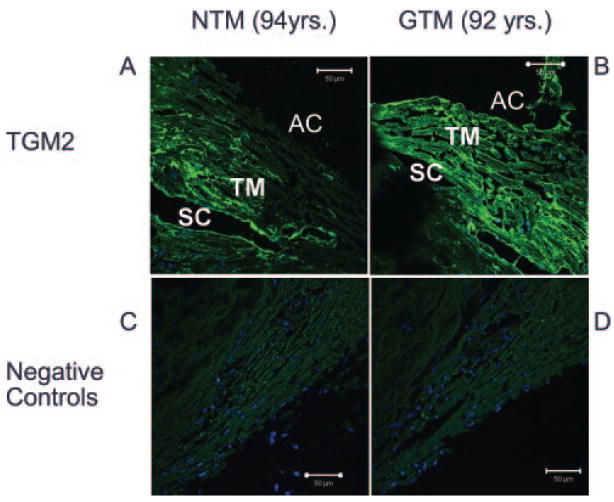
We next examined the protein levels of TGM2 in three normal and three glaucomatous TM tissues from human donors. TGM2 was present in all six human TM samples. Figure 2 is a representative example of one set of age-matched eyes. In agreement with TM cell lysate Western blot data, we found that TGM2 appeared elevated in the TM of glaucomatous donor eyes (Fig. 2B) compared with the age-matched control (Fig. 2A), and this increase was seen in all three sets of glaucomatous donor eyes.
Figure 2.
Immunofluorescent staining of TGM2 in normal and glaucomatous TM tissues. Six different human eyes, three NTM (72, 88, and 94 years of age) and three GTM (76, 87, and 92 years of age), were fixed, sectioned, and stained with antibodies for TGM2. The negative control experiments consisted of PBS-BSA without primary antibody, normal IgG, and mouse ascites. TGM2 expression appeared to be higher in GTM (G) than in NTM (N) tissues (A, B); no-primary-antibody control (C), a representative of an ascites control (D). TGM2 was localized to the cytoplasm, in agreement with previous reports. DAPI staining was used for nuclear staining. AC, anterior chamber; TM, trabecular meshwork; SC, Schlemm’s Canal. Magnification, ×400.
TGM2 Enzyme Activity in Normal and Glaucomatous TM Cells
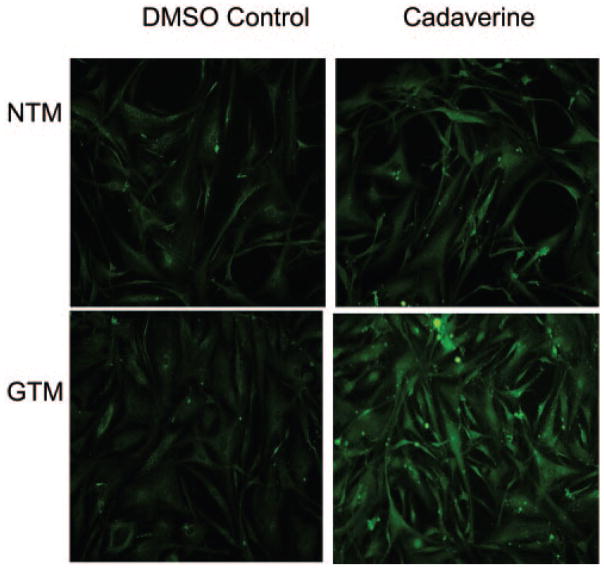
We next examined TGM2 enzyme activity in both normal and glaucomatous cultured TM cells. To analyze TGM2 activity, a biotin labeled cadaverine-streptavidin immunohistochemical staining assay was performed in NTM and GTM cells. The cells were labeled with biotin cadaverine for 48 hours before fixation and staining, and TGM2 enzyme activity was detected by the AlexaFluor 488 streptavidin conjugate. GTM cells contained higher TGM2 activity than did NTM cells (Fig. 3). Control experiments included incubation of both cell types in the dimethylsulfoxide (DMSO) carrier in the absence of biotin cadaverine.
Figure 3.
Transglutaminase activity in NTM and GTM cells. The cells were incubated with vehicle (DMSO) control or biotin-labeled cadaverine (1 mM). Transamidated and cross-linked cadaverine was detected by AlexaFluor 488 streptavidin-conjugate (green).
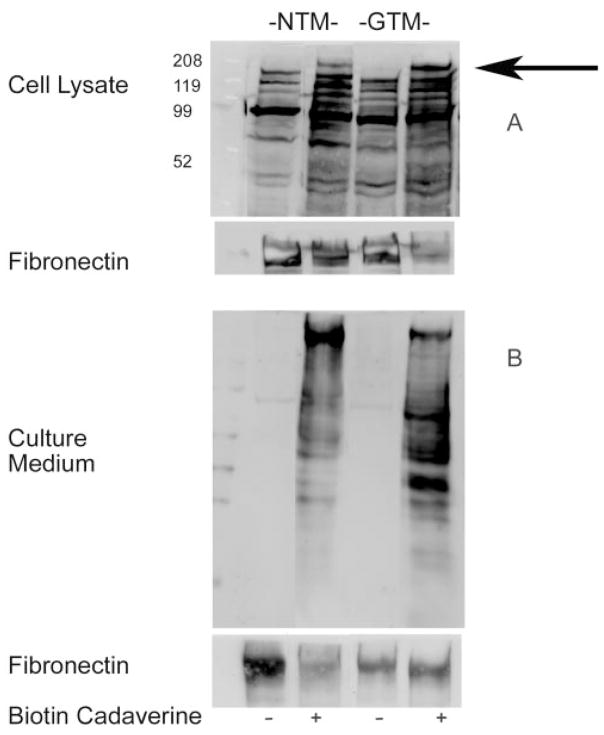
TGM2 activity was also assessed by Western immunoblot analysis in three independent experiments comparing normal and glaucomatous cell lines by supplementing serum-free medium with biotin cadaverine (1 mM), a pseudosubstrate for TGM2. Samples were extracted, processed together, separated by SDS-PAGE, and, transferred to a PVDF membrane. TGM2 activity was detected with streptavidin-HRP. TGM2 activity was seen in the cell lysates (Fig. 4A) and in the culture medium (Fig. 4B) of both NTM and GTM cells incubated with biotin cadaverine, resulting in the appearance of 220-kDa bands on Western blots (Fig. 4). The blots were then stripped and reprobed with an anti-FN antibody that detected an identical 220-kDa band, suggesting that the cross-linked product was FN.
Figure 4.
Western immunoblot of TGM2 activity in NTM and GTM cells. TGM2 enzyme activity was present in cell lysate (A) and culture medium (B) of NTM and GTM cells. The cells were incubated with vehicle (DMSO) control or biotin-labeled cadaverine (1 mM). TGM2-mediated incorporation of biotin cadaverine into cells was detected by Western immunoblot analysis using streptavidin-HRP–conjugated secondary antibody. The blots were then stripped and reprobed with an anti-FN antibody, corresponding to the 220-kDa band.
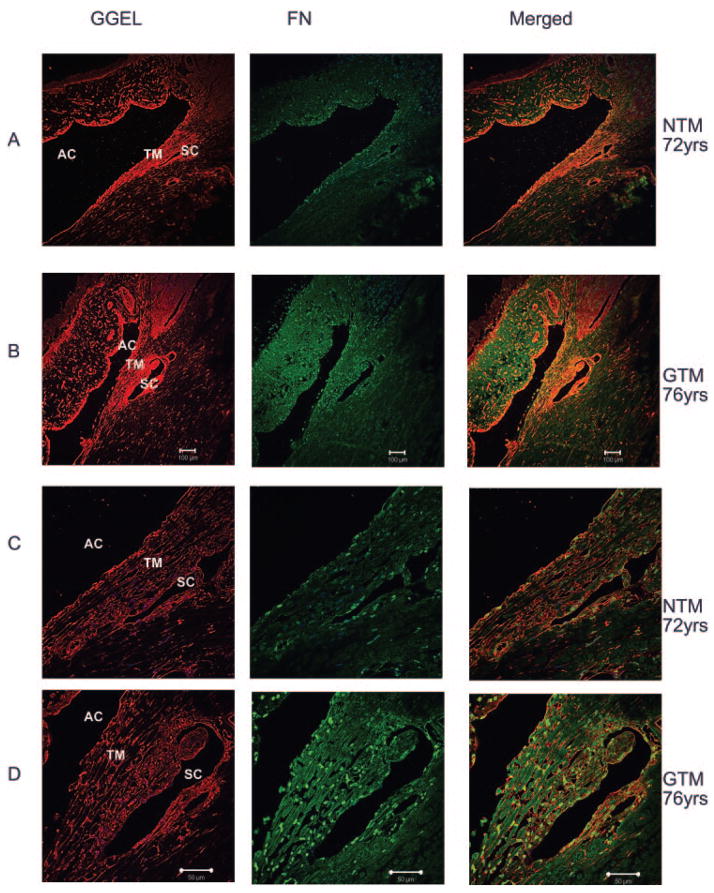
Immunohistochemical Staining of FN and GGEL Isopeptide in Normal and Glaucomatous TM Tissue
To verify TGM2 activity in TM tissues, we examined the expression of fibronectin along with the cross-linked GGEL isopeptide in eyes of three normal and three glaucomatous human donors. FN and GGEL protein were colocalized in both NTM and GTM tissues (Fig. 5), suggesting the transamidation of FN by TGM2 in these tissues. In two of the three GTM tissues, fibronectin was clearly expressed at higher levels compared with NTM. Of interest, colocalization of FN and GGEL isopeptide was increased surrounding Schlemm’s canal (Fig. 5).
Figure 5.
Immunofluorescent staining to GGEL and FN in normal and glaucomatous TM tissues. Six human eyes, three NTM (72, 88, and 94 years of age) and three GTM (76, 87, and 92 years of age), were fixed, sectioned, and stained with antibodies for GGEL and FN. Negative controls consisted of PBS-BSA without primary antibody, IgG, and mouse ascites (data not shown). GTM tissues had increased expression of GGEL, increased FN expression, and colocalization of GGEL and FN: (A) NTM, (B) GTM, (C) NTM, and (D) GTM. Both GGEL and FN were localized to the cytoplasm in TM tissues. AC, anterior chamber; TM, trabecular mesh-work; SC, Schlemm’s canal. Magnification: (A, B) ×100; (C, D) ×400.
Discussion
Elevated IOP is a primary risk factor for the development and progression of glaucoma.1–3 Glaucomatous elevated IOP is caused by increased AH outflow resistance4 and is closely associated with morphologic and biochemical changes in the TM. There is an increased accumulation of ECM proteins in the glaucomatous TM.4,40 The increased deposition of ECM proteins in the glaucomatous TM may be due to increased ECM synthesis and/or decreased degradation. ECM metabolism within the human TM is regulated by MMPs,7,41 PAI-1,11,42 and growth factors.39,40,43 Human TM cells express numerous growth factor receptors and respond to exogenous growth factors.32 Growth factors within the AH also appear to be involved in regulating ECM metabolism in the TM and aqueous outflow pathway.
Numerous studies have reported elevated levels of TGF-β2 in the AH of patients with glaucoma.27–29 Members of the TGFβ superfamily, including TGF-β2, regulate ECM synthesis and deposition. Welge-Lüssen et al.25 have reported that TGM2 mRNA expression and protein levels of TGM2 are increased in TGF-β1 or -β2 treated cultured human TM cells. In addition to TGM2 expression, TGM2 enzymatic activity was also increased in TGF-β-treated TM cells. TGM2 can also increase the conversion of latent TGF-β to its biologically active form,44–46 providing a potential feedback mechanism in the glaucomatous TM and leading to further TGM2 induction.
Transglutaminase enzymes catalyze the posttranslational modification of proteins via formation of isopeptide bonds.15 The resultant cross-linked proteins are highly resistant to both physical and enzymatic degradation.16 Of the various members of the transglutaminase family of enzymes, TGM2 has been implicated in numerous fibrotic disorders such as pulmonary fibrosis, renal fibrosis, and atherosclerosis.47–55 Although TGM2 can be induced by TGF-β1 and -β2 in TM cells,25 little is known about the role for TGM2 in glaucoma pathogenesis. Therefore, the purpose of this study was to determine whether there are any differences in TGM2 protein levels and activity between NTM and GTM cells and tissues.
Western immunoblot and immunohistochemical analyses showed that TGM2 is present in both NTM and GTM cells and tissues. Our results support the previous study by Welge-Lüssen et al.25 who first reported the presence of TGM2 in cultured TM cells. More important, we demonstrated significantly increased protein levels of TGM2 in cultured TM cells and TM tissues obtained from patients with glaucoma. We believe that this is the first report that TGM2 is upregulated in glaucomatous TM cells and tissues.
However, the presence of TGM2 protein in cells or tissues does not necessarily mean that the enzyme is biologically active. Therefore, the enzymatic activity of TGM2 was measured by the incorporation of biotin-cadaverine, a pseudosubstrate for TGM2, into cells. We looked for the presence of GGEL bonds in the TM, since TGM2 inserts these bonds between proteins that it cross-links, such as FN. This action makes ECM proteins more resistant to proteolytic degradation. Because FN can serve as a substrate in the TGM2 catalyzed reactions, we looked at colocalization of both FN and the GGEL proteins in normal and glaucomatous cultured TM cells and tissues. Our immunohistochemical results indicated increased colocalization of FN and GGEL proteins associated with Schlemm’s canal in glaucomatous human tissues. These results suggest that TGM2 may render the ECM resistant to proteolytic degradation at the site of AH outflow.
In conclusion, our results confirm the presence of TGM2 in normal cultured human TM cells. In addition we demonstrated that cultured glaucomatous TM cells and tissues from human donors have elevated levels of TGM2. We also showed that TGM2 enzymatic activity is elevated in glaucomatous cells and tissues. Last, there is colocalization of GGEL proteins with FN in the area of Schlemm’s canal. This area of the human TM is known to be involved in AH outflow resistance. Proteins cross-linked by TGM2 exhibit resistance to proteolytic degradation and thus may restrict AH outflow from the TM. The normal function of the TM is dependent on a balance between ECM deposition and degradation. Increased cross-linked proteins in the TM would result in the ECM’s being more resistant to enzymatic degradation and turnover. Taken together, these findings suggest that TGM2 protein and enzyme activity are elevated in the glaucomatous TM and may play a pathogenic role in increased outflow resistance and glaucomatous ocular hypertension.
Acknowledgments
Supported by Alcon Research, Ltd., and the National Science Foundation (Project SCORE).
The authors thank Ann-Marie Brun and I-fen Chang of the Department of Cell Biology and Genetics, University of North Texas Health Science Center, for technical assistance and Paula Billman (Alcon Research, Ltd.) and the Central Florida Eye and Tissue Bank for acquisition of donor eyes.
Footnotes
This study is taken in part from a dissertation submitted to the University of North Texas Health Science Center in partial fulfillment of the requirements for the degree Doctor of Philosophy for TT-V.
Disclosure: T. Tovar-Vidales, None; R. Roque, None; A.F. Clark, Alcon Research, Ltd. (F); R.J. Wordinger, None
References
- 1.Gordon MO, Beiser JA, Brandt JD, et al. The ocular hypertension treatment study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–720. doi: 10.1001/archopht.120.6.714. discussion 829–830. [DOI] [PubMed] [Google Scholar]
- 2.Kass MA, Heuer DK, Higginbotham EJ, et al. The ocular hypertension treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. discussion 829–830. [DOI] [PubMed] [Google Scholar]
- 3.AGIS Investigators. The advanced glaucoma intervention study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS investigators. Am J Ophthalmol. 2000;130:429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 4.Rohen JW. Why is intraocular pressure elevated in chronic simple glaucoma?—anatomical considerations. Ophthalmology. 1983;90:758–765. doi: 10.1016/s0161-6420(83)34492-4. [DOI] [PubMed] [Google Scholar]
- 5.Rohen JW, Witmer R. Electron microscopic studies on the trabecular meshwork in glaucoma simplex. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1972;183:251–266. doi: 10.1007/BF00496153. [DOI] [PubMed] [Google Scholar]
- 6.Lütjen-Drecoll E. Functional morphology of the trabecular mesh-work in primate eyes. Prog Retin Eye Res. 1999;18:91–119. doi: 10.1016/s1350-9462(98)00011-1. [DOI] [PubMed] [Google Scholar]
- 7.Bradley JM, Vranka J, Colvis CM, et al. Effect of matrix metalloproteinases activity on outflow in perfused human organ culture. Invest Ophthalmol Vis Sci. 1998;39:2649–2658. [PubMed] [Google Scholar]
- 8.Pang IH, Hellberg PE, Fleenor DL, Jacobson N, Clark AF. Expression of matrix metalloproteinases and their inhibitors in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2003;44:3485–3493. doi: 10.1167/iovs.02-0756. [DOI] [PubMed] [Google Scholar]
- 9.Fleenor DL, Pang IH, Clark AF. Involvement of AP-1 in interleukin-1alpha-stimulated MMP-3 expression in human trabecular mesh-work cells. Invest Ophthalmol Vis Sci. 2003;44:3494–3501. doi: 10.1167/iovs.02-0757. [DOI] [PubMed] [Google Scholar]
- 10.Eitzman DT, Ginsburg D. Of mice and men. The function of plasminogen activator inhibitors (PAIs) in vivo. Adv Exp Med Biol. 1997;425:131–141. [PubMed] [Google Scholar]
- 11.Fuchshofer R, Welge-Lüssen U, Lütjen-Drecoll E. The effect of TGF-beta2 on human trabecular meshwork extracellular proteolytic system. Exp Eye Res. 2003;77:757–765. doi: 10.1016/s0014-4835(03)00220-3. [DOI] [PubMed] [Google Scholar]
- 12.Esposito C, Caputo I. Mammalian transglutaminases: identification of substrates as a key to physiological function and physiopathological relevance. FEBS Lett. 2005;272:615–631. doi: 10.1111/j.1742-4658.2004.04476.x. [DOI] [PubMed] [Google Scholar]
- 13.Lesort M, Tucholski J, Miller ML, Johnson GV. Tissue transglutaminase: a possible role in neurodegenerative diseases. Prog Neurobiol. 2000;61:439–463. doi: 10.1016/s0301-0082(99)00052-0. [DOI] [PubMed] [Google Scholar]
- 14.Aeschlimann D, Koeller MK, Allen-Hoffmann BL, Mosher DF. Isolation of a cDNA encoding a novel member of the transglutaminase gene family from human keratinocytes: detection and identification of transglutaminase gene products based on reverse transcription-polymerase chain reaction with degenerate primers. J Biol Chem. 1998;273:3452–3460. doi: 10.1074/jbc.273.6.3452. [DOI] [PubMed] [Google Scholar]
- 15.Beninati S, Piacentini M. The transglutaminase family: an overview (minireview) Amino Acids. 2004;26:367–372. doi: 10.1007/s00726-004-0091-7. [DOI] [PubMed] [Google Scholar]
- 16.Folk JE, Finlayson JS. The epsilon-(gamma-glutamyl)lysine crosslink and the catalytic role of transglutaminases. Adv Protein Chem. 1977;31:1–133. doi: 10.1016/s0065-3233(08)60217-x. [DOI] [PubMed] [Google Scholar]
- 17.Tucholski J, Johnson GV. Tissue transglutaminase directly regulates adenylyl cyclase resulting in enhanced cAMP-response element-binding protein (CREB) activation. J Biol Chem. 2003;278:26838–26843. doi: 10.1074/jbc.M303683200. [DOI] [PubMed] [Google Scholar]
- 18.Fesus L, Szondy Z, Uray I. Probing the molecular program of apoptosis by cancer chemopreventive agents. J Cell Biochem Suppl. 1995;22:151–161. doi: 10.1002/jcb.240590820. [DOI] [PubMed] [Google Scholar]
- 19.Nakaoka H, Perez DM, Baek KJ, et al. Gh: A GTP-binding protein with transglutaminase activity and receptor signaling function. Science. 1994;264:1593–1596. doi: 10.1126/science.7911253. [DOI] [PubMed] [Google Scholar]
- 20.Piredda L, Farrace MG, Lo Bello M, et al. Identification of ‘tissue’ transglutaminase binding proteins in neural cells committed to apoptosis. FASEB J. 1999;13:355–364. doi: 10.1096/fasebj.13.2.355. [DOI] [PubMed] [Google Scholar]
- 21.Fesus L, Piacentini M. Transglutaminase 2: an enigmatic enzyme with diverse functions. Trends Biochem Sci. 2002;27:534–539. doi: 10.1016/s0968-0004(02)02182-5. [DOI] [PubMed] [Google Scholar]
- 22.Fesus L, Davies PJ, Piacentini M. Apoptosis: molecular mechanisms in programmed cell death. Eur J Cell Biol. 1991;56:170–177. [PubMed] [Google Scholar]
- 23.Cai D, Ben T, De Luca LM. Retinoids induce tissue transglutaminase in NIH-3T3 cells. Biochem Biophys Res Commun. 1991;175:1119–1124. doi: 10.1016/0006-291x(91)91681-2. [DOI] [PubMed] [Google Scholar]
- 24.Greenberg CS, Birckbichler PJ, Rice RH. Transglutaminases: multifunctional cross-linking enzymes that stabilize tissues. FASEB J. 1991;5:3071–3077. doi: 10.1096/fasebj.5.15.1683845. [DOI] [PubMed] [Google Scholar]
- 25.Welge-Lüssen U, May CA, Lütjen-Drecoll E. Induction of tissue transglutaminase in the trabecular meshwork by TGF-beta1 and TGF-beta2. Invest Ophthalmol Vis Sci. 2000;41:2229–2238. [PubMed] [Google Scholar]
- 26.Fuchshofer R, Birke M, Welge-Lüssen U, Kook D, Lütjen-Drecoll E. Transforming growth factor-beta 2 modulated extracellular matrix component expression in cultured human optic nerve head astrocytes. Invest Ophthalmol Vis Sci. 2005;46:568–578. doi: 10.1167/iovs.04-0649. [DOI] [PubMed] [Google Scholar]
- 27.Tripathi RC, Li J, Chan WF, Tripathi BJ. Aqueous humor in glaucomatous eyes contains an increased level of TGF-beta 2. Exp Eye Res. 1994;59:723–727. doi: 10.1006/exer.1994.1158. [DOI] [PubMed] [Google Scholar]
- 28.Inatani M, Tanihara H, Katsuta H, Honjo M, Kido N, Honda Y. Transforming growth factor-beta 2 levels in aqueous humor of glaucomatous eyes. Graefes Arch Clin Exp Ophthalmol. 2001;239:109–113. doi: 10.1007/s004170000241. [DOI] [PubMed] [Google Scholar]
- 29.Picht G, Welge-Lüssen U, Grehn F, Lütjen-Drecoll E. Transforming growth factor beta 2 levels in the aqueous humor in different types of glaucoma and the relation to filtering bleb development. Graefes Arch Clin Exp Ophthalmol. 2001;239:199–207. doi: 10.1007/s004170000252. [DOI] [PubMed] [Google Scholar]
- 30.Pena JD, Taylor AW, Ricard CS, Vidal I, Hernandez MR. Transforming growth factor beta isoforms in human optic nerve heads. Br J Ophthalmol. 1999;83:209–218. doi: 10.1136/bjo.83.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hernandez MR. The optic nerve head in glaucoma: role of astrocytes in tissue remodeling. Prog Retin Eye Res. 2000;19:297–321. doi: 10.1016/s1350-9462(99)00017-8. [DOI] [PubMed] [Google Scholar]
- 32.Wordinger RJ, Clark AF, Agarwal R, et al. Cultured human trabecular meshwork cells express functional growth factor receptors. Invest Ophthalmol Vis Sci. 1998;39:1575–1589. [PubMed] [Google Scholar]
- 33.Wordinger RJ, Lambert W, Agarwal R, Talati M, Clark AF. Human trabecular meshwork cells secrete neurotrophins and express neurotrophin receptors (trk) Invest Ophthalmol Vis Sci. 2000;41:3833–3841. [PubMed] [Google Scholar]
- 34.Clark AF, Steely HT, Dickerson JE, Jr, et al. Glucocorticoid induction of the glaucoma gene MYOC in human and monkey trabecular meshwork cells and tissues. Invest Ophthalmol Vis Sci. 2001;42:1769–1780. [PubMed] [Google Scholar]
- 35.Lambert W, Agarwal R, Howe W, Clark AF, Wordinger RJ. Neurotrophin and neurotrophin receptor expression by cells of the human lamina cribrosa. Invest Ophthalmol Vis Sci. 2001;42:2315–2323. [PubMed] [Google Scholar]
- 36.Zhang J, Lesort M, Guttmann RP, Johnson GV. Modulation of the in situ activity of tissue transglutaminase by calcium and GTP. J Biol Chem. 1998;273:2288–2295. doi: 10.1074/jbc.273.4.2288. [DOI] [PubMed] [Google Scholar]
- 37.Kim SW, Lee ZW, Lee C, Im KS, Ha KS. The role of tissue transglutaminase in the germinal vesicle breakdown of mouse oocytes. Biochem Biophys Res Commun. 2001;286:229–234. doi: 10.1006/bbrc.2001.5381. [DOI] [PubMed] [Google Scholar]
- 38.Beck KE, De Girolamo LA, Griffin M, Billett EE. The role of tissue transglutaminase in 1-methyl-4-phenylpyridinium (MPP+)-induced toxicity in differentiated human SH-SY5Y neuroblastoma cells. Neurosci Lett. 2006;405:46–51. doi: 10.1016/j.neulet.2006.06.061. [DOI] [PubMed] [Google Scholar]
- 39.Wordinger RJ, Fleenor DL, Hellberg PE, et al. Effects of TGF-beta2, BMP-4, and gremlin in the trabecular meshwork: implications for glaucoma. Invest Ophthalmol Vis Sci. 2007;48:1191–1200. doi: 10.1167/iovs.06-0296. [DOI] [PubMed] [Google Scholar]
- 40.Lütjen-Drecoll E. Morphological changes in glaucomatous eyes and the role of TGFbeta2 for the pathogenesis of the disease. Exp Eye Res. 2005;81:1–4. doi: 10.1016/j.exer.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 41.Pang IH, Fleenor DL, Hellberg PE, Stropki K, McCartney MD, Clark AF. Aqueous outflow-enhancing effect of tert-butylhydroquinone: involvement of AP-1 activation and MMP-3 expression. Invest Ophthalmol Vis Sci. 2003;44:3502–3510. doi: 10.1167/iovs.02-0758. [DOI] [PubMed] [Google Scholar]
- 42.Fleenor DL, Shepard AR, Hellberg PE, Jacobson N, Pang IH, Clark AF. TGFbeta2-induced changes in human trabecular meshwork: implications for intraocular pressure. Invest Ophthalmol Vis Sci. 2006;47:226–234. doi: 10.1167/iovs.05-1060. [DOI] [PubMed] [Google Scholar]
- 43.Bradley JM, Anderssohn AM, Colvis CM, et al. Mediation of laser trabeculoplasty-induced matrix metalloproteinase expression by IL-1beta and TNFalpha. Invest Ophthalmol Vis Sci. 2000;41:422–430. [PubMed] [Google Scholar]
- 44.Kojima S, Nara K, Rifkin DB. Requirement for transglutaminase in the activation of latent transforming growth factor-beta in bovine endothelial cells. J Cell Biol. 1993;121:439–448. doi: 10.1083/jcb.121.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kojima S, Vernooy R, Moscatelli D, Amanuma H, Rifkin DB. Lipo-polysaccharide inhibits activation of latent transforming growth factor-beta in bovine endothelial cells. J Cell Physiol. 1995;163:210–219. doi: 10.1002/jcp.1041630124. [DOI] [PubMed] [Google Scholar]
- 46.Nunes I, Gleizes PE, Metz CN, Rifkin DB. Latent transforming growth factor-beta binding protein domains involved in activation and transglutaminase-dependent cross-linking of latent transforming growth factor-beta. J Cell Biol. 1997;136:1151–1163. doi: 10.1083/jcb.136.5.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verderio EA, Johnson T, Griffin M. Tissue transglutaminase in normal and abnormal wound healing (review) Amino Acids. 2004;26:387–404. doi: 10.1007/s00726-004-0094-4. [DOI] [PubMed] [Google Scholar]
- 48.Boisvert WA, Rose DM, Boullier A, et al. Leukocyte transglutaminase 2 expression limits atherosclerotic lesion size. Arterioscler Thromb Vasc Biol. 2006;26:563–569. doi: 10.1161/01.ATV.0000203503.82693.c1. [DOI] [PubMed] [Google Scholar]
- 49.Ikee R, Kobayashi S, Hemmi N, et al. Involvement of transglutaminase-2 in pathological changes in renal disease. Nephron Clin Pract. 2007;105:c139–c146. doi: 10.1159/000098646. [DOI] [PubMed] [Google Scholar]
- 50.Johnson TS, Griffin M, Thomas GL, et al. The role of transglutaminase in the rat subtotal nephrectomy model of renal fibrosis. J Clin Invest. 1997;99:2950–2960. doi: 10.1172/JCI119490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Griffin M, Smith LL, Wynne J. Changes in transglutaminase activity in an experimental model of pulmonary fibrosis induced by paraquat. Br J Exp Pathol. 1979;60:653–661. [PMC free article] [PubMed] [Google Scholar]
- 52.Wiebe RI, Tarr AH, Bowness JM. Increased transglutaminase in the aortas of cholesterol-fed rabbits: occurrence of buffer soluble and insoluble forms and an inhibitor. Biochem Cell Biol. 1991;69:821–827. doi: 10.1139/o91-122. [DOI] [PubMed] [Google Scholar]
- 53.Bowness JM, Tarr AH. Lipoprotein binding of crosslinked type III collagen aminopropeptide and fractions of its antigen in blood. Biochem Biophys Res Commun. 1990;170:519–525. doi: 10.1016/0006-291x(90)92122-g. [DOI] [PubMed] [Google Scholar]
- 54.Bowness JM, Tarr AH, Wiebe RI. Transglutaminase-catalysed cross-linking: a potential mechanism for the interaction of fibrinogen, low density lipoprotein and arterial type III procollagen. Thromb Res. 1989;54:357–367. doi: 10.1016/0049-3848(89)90094-7. [DOI] [PubMed] [Google Scholar]
- 55.Bowness JM, Venditti M, Tarr AH, Taylor JR. Increase in epsilon-(gamma-glutamyl)lysine crosslinks in atherosclerotic aortas. Atherosclerosis. 1994;111:247–253. doi: 10.1016/0021-9150(94)90099-x. [DOI] [PubMed] [Google Scholar]