Abstract
DNA strand breaks trigger marked phosphorylation of histone H2AX (i.e. γ-H2AX). While DNA double-strand breaks (DSBs) provide a strong stimulus for this event, the accompanying structural alterations in chromatin may represent the actual signal that elicits γ-H2AX. Our data show that changes in chromatin structure are sufficient to elicit extensive γ-H2AX formation in the relative absence of DNA strand breaks. Cells subjected to hypotonic (0.05 M) treatment exhibit γ-H2AX levels that are equivalent to those found after the induction of 80–200 DNA DSBs (i.e. 2–5 Gy). Despite this significant increase in phosphorylation, cell survival remains relatively unaffected (<10% cytotoxicity), and there is no significant increase in apoptosis. Nuclear staining profiles indicate that γ-H2AX-positive cells induced under altered tonicity exhibit variable levels of staining, ranging from uniform pan staining to discrete punctate foci more characteristic of DNA strand breakage. The capability to induce significant γ-H2AX formation under altered tonicity in the relative absence of DNA strand breaks suggests that this histone modification evolved in response to changes in chromatin structure.
Introduction
The nuclear histones play a major role in defining chromatin structure, and covalent modification of these proteins can trigger a wide range of biological responses. One such modification that has received a great deal of attention involves the phosphorylation of histone H2AX to form γ-H2AX. The formation of γ-H2AX was first found to be dependent on DNA strand-breaking agents, particularly those that produce DNA double-strand breaks (DSBs) (1,2). Since then a great deal of effort has been focused on understanding the significance of this phosphorylation event (3–7). While there is little doubt that this histone modification can be stimulated by DNA strand breaks, it is less clear whether phosphorylation of H2AX is entirely dependent on DNA strand breakage.
Phosphorylation of H2AX has been reported in the relative absence of DNA damage, but usually under circumstances involving the remodeling and/or segregation of chromosomes (8–10). Generally, studies analyzing γ-H2AX formation utilize agents or conditions that cause DNA strand breaks directly (e.g. ionizing radiation) or indirectly (via replication arrest and fork breakdown). Another inherent feature of these studies is that regardless of the mode of strand break production, the local structure of the chromatin is changed. The inability to separate the inevitable changes in chromatin structure that accompany overt DNA strand breaks may have obscured a more fundamental role for H2AX phosphorylation, one that evolved in response to structural changes in chromatin.
To address the nature of the γ-H2AX-stimulating signal in the present study, we relied on a strategy designed to produce structural changes in chromatin in the relative absence of DNA breaks. Considerable data exist showing that γ-H2AX is formed in response to DSBs (1–3), and numerous studies have shown that cells subjected to irradiation (6,11,12), replication arrest (4,13,14) or apoptosis (15) exhibit significant increases in γ-H2AX. Immunohistochemical detection of γ-H2AX in single cells is characterized by distinct intranuclear foci (4,6,16). Presumably, these foci are first formed in close proximity to the sites of DSBs, but can correspond to much larger megabase regions of the chromatin, as local changes in chromatin structure expand to impact larger chromatin domains (1). The size and distribution of radiation-induced γ-H2AX foci can be altered in response to changing salt conditions (17,18), implying that once γ-H2AX foci are formed, their size and/or distribution can be altered further in response to changing chromatin conformations. In the current study, we adopted a distinctly different approach, determining whether exposure to hypotonic salt conditions designed to alter chromatin structure were sufficient to induce γ-H2AX alone, rather than alter its immunohistochemical appearance once formed after irradiation (17,18).
There has been a previous effort to induce γ-H2AX in the absence of DNA strand breaks using low salt, but that investigation was not able to demonstrate any real change in the status of γ-H2AX (19). One reason for this finding might be due to a salt concentration (100 mM) that was insufficiently low to elicit the altered chromatin topology needed to trigger H2AX phosphorylation. Thus, we contend that conditions able to alter chromatin structure without causing significant DNA strand breakage are in principal sufficient to elicit phosphorylation of histone H2AX. Here we describe our studies showing that incubation under relatively non-toxic conditions of hypotonic salt is indeed sufficient to induce significant levels of γ-H2AX.
Materials and methods
Cell culture
The following cell lines were used: 1) immortalized neural precursor cells isolated from the mouse cerebellum (20), 2) primary neural precursor cells isolated from the mouse subventricular or hippocampal dentate subgranular zones (21), 3) immortalized human fibroblasts (HCA) (22) and 4) primary human fibroblasts (IMR90). Cerebellar precursor cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Carlsbad, CA) supplemented with 10% foetal bovine serum (FBS; Hyclone, Logan, UT), 5% horse serum (Hyclone), 100 units/ml penicillin, 100 μg/ml streptomycin and 1.25 μg/ml fungizone (Invitrogen). Subventricular precursor cells were grown in Dulbecco's Modified Eagle's Medium: Nutrient Mixture F-12 (1:1) (DME/F12; Invitrogen), 0.4% bovine serum albumin (BSA; Sigma, St Louis, MO), 0.76 units/ml heparin (Sigma), 200 ng/ml epidermal growth factor (Biomedical Technologies, Stoughton, MA) and 40 ng/ml fibroblast growth factor (FGF; Peprotech, Rocky Hills, NJ). Hippocampal precursor cells were grown in DME/F12 supplemented with 100 units/ml penicillin, 100 μg/ml streptomycin, 20 ng/ml FGF and N2 supplement (Invitrogen). HCA cells were grown in Minimal Essential Medium (MEM; Invitrogen) supplemented with 10% FBS, 2 mM glutamine (Invitrogen), 100 units/ml penicillin, 100 μg/ml streptomycin and MEM non-essential amino acids (Invitrogen). IMR90 cells were grown in DMEM supplemented with 15% FBS, 2 mM glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin. All cell lines were grown at 37°C anchored to flasks, except for the subventricular neural precursor cells that are cultured as neurospheres in suspension (23). Under these conditions, all cell types exhibited doubling times of 24–36 h. To analyze the impact of hypotonic treatments on confluence-arrested cerebellar cultures, cells were maintained as confluent monolayers for 5 days in the presence of 1% serum.
Salt/irradiation treatments
For hypotonic treatments, a 0.05 M salt solution was made using divalent cation-free phosphate-buffered saline (PBS, pH 7.4) diluted in water. Cells were then incubated in these solutions at 37°C for the specified period of time. For cellular exposures to γ-rays, a 137Cs irradiator (J.L. Shepard and Associates, San Fernando, CA; Mark I) was used at a dose rate of 1.17 Gy/min.
Fluorescent-activated cell sorting analysis
Fluorescent-activated cell sorting (FACS) was used to quantify the levels of H2AX phosphorylation following hypotonic treatments. Cells subjected to altered tonicity (with or without recovery in isotonic medium) were counted and then fixed and permeabilized in preparation for FACS analysis following the instructions provided in the H2AX FACS kit (Upstate, NY). The appropriate amount of anti-H2AX antibody (Millipore, Billerica, MA) was added to each sample and incubated overnight at 4°C. The next day, cells were stained with propidium iodide and left at 4°C for 4 h before FACS analysis. Negative controls (untreated) and positive controls (γ-irradiated) for γ-H2AX were routinely included for comparison. To compute the relative levels of H2AX phosphorylation, the ratio of ungated fluorescent means (test cells/untreated controls) was used.
Cell cycle analysis of cerebellar cells after hypotonic incubation and recovery was accomplished as previously described (21). Cells were analysed for DNA content by FACS analysis based on propidium iodide fluorescence and data were analysed using the CellQuest™ algorithm. Cell cycle distributions were derived from a minimum of 3000 gated events under set parameters that minimized reverse chi-square values.
Immunohistochemistry
Immunohistochemistry was performed to determine the nuclear distribution of γ-H2AX in individual cells. Cells were grown on chambered slides 1 day prior to irradiation or hypotonic salt treatments. All treatments were carried out while cells were still attached to slides, after which cells were fixed in 4% paraformaldehyde and permeabilized in a 0.2% solution of Triton X-100 in PBS. Subsequent detection was accomplished after blocking in 10% FBS/1% BSA for 1 h, using a 1:1000 dilution of the flourescein isothiocyanate (FITC)-labelled mouse monoclonal antibody against γ-H2AX (Upstate) in the background reducing antibody diluent (DAKO plus S3022; Upstate). Following overnight incubation at 4°C, slides were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) in MP Prolong GOLD antifade (Invitrogen-Molecular Probes, Carlsbad, CA). Nuclei staining positive for γ-H2AX were scored double blind using a subjective scale based on the intensity of the fluorescent nuclear signal. Brightly and moderately staining nuclei visible against a DAPI counterstain were scored as either strong or medium staining cells, respectively. Cells that could be unambiguously identified as γ-H2AX positive only after eliminating the nuclear DAPI signal (via a FITC bandpass filter) were classified as weakly staining cells.
Survival measurements
Apoptosis was measured as described by us previously (18). Briefly, apoptosis was assayed using the annexin V−FITC apoptosis kit (BD Biosiences, San Jose, CA) following the manufacturer's recommendations. Immediately following specific hypotonic treatments (30−60 min) and recovery in isotonic media (30−120 min), cells were re-suspended in binding buffer, incubated with the annexin−FITC conjugate and analysed by FACS.
To determine how the salt treatments impacted survival, clonogenic assays were performed using the two immortalized cell lines (cerebellar precursors, HCA fibroblasts). Cells subjected to hypotonicity were diluted, plated in triplicate and stained (25% crystal violet in ethanol) after visible colonies developed after 10–14 days. Surviving fraction was calculated as the number of colonies counted divided by the number seeded corrected for plating efficiency. Plating efficiencies for the immortalized neural precursors and fibroblasts were 52 (±6) and 76 (±8)%, respectively.
Results
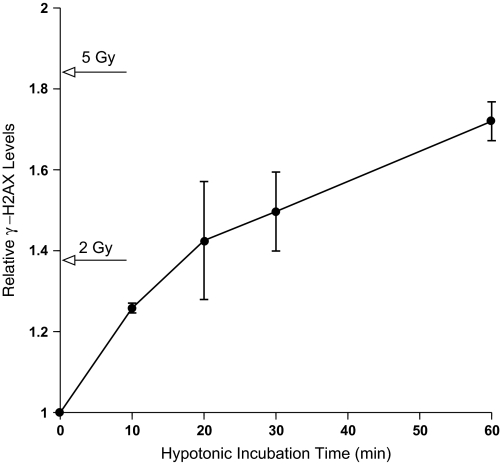
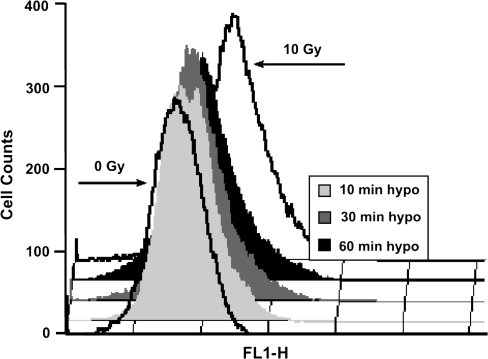
To test the hypothesis that γ-H2AX could be induced independent of DNA strand breaks, cerebellar precursor cells were subjected to a range of incubation times under hypotonic conditions and allowed to recover in isotonic medium for a fixed period of 30 min before processing for FACS analysis. These initial studies showed that relatively short exposures (10 min) to low salt were sufficient to elicit significant increases in γ-H2AX levels over background (Figure 1). Hypotonic treatments up to 1 h resulted in progressively higher levels of γ-H2AX, with the most rapid rise occurring during the initial 20 min (Figure 1). Hypotonic treatment of other cells suggested that changes in chromatin structure provide a general signal for triggering H2AX phosphorylation across different tissues (i.e. neural precursor cells, fibroblasts) and species (i.e. rodent, human). Analysis of primary neural precursors (hippocampal and subventricular) and primary (IMR90) and immortalized (HCA) human fibroblasts indicated that all cells analysed responded to reduced tonicity (30 and 60 min) by increasing γ-H2AX levels (Table I). Overall, hypotonic treatments were found to increase γ-H2AX levels by 1.3–2.3 fold. For cells subjected to hypotonic treatments, FACS histograms showed that increasing treatment times led to a progressive rightward shift in a single fluorescent peak, representing increased levels of γ-H2AX (Figure 2). Extended hypotonic treatments were also associated with a broadening of the single fluorescent peak, which likely reflected an increased heterogeneity of H2AX phosphorylation within the population of cells exposed to low salt.
Fig. 1.
H2AX phosphorylation induced by hypotonic exposure in cerebellar precursor cells. Cells were subjected to hypotonic (0.05 M) treatments for various lengths of time then allowed to recover in isotonic medium for 30 min before processing for γ-H2AX levels by FACS. Hypotonic treatments were found to progressively increase γ-H2AX levels over 60 min of incubation. The levels of γ-H2AX quantified in cells subjected to x-irradiation are indicated along the ordinate for comparative purposes. Relative γ-H2AX levels were all normalized to untreated controls set to unity and represent the average of at least three experiments (±standard deviation).
Table I.
H2AX phosphorylation induced in mammalian cells exposed to hypotonic conditions
| Cell type | Hypotonic incubation (min) | Recovery time (min)a | Relative change in γ-H2AXb |
| Cerebellarc | 30 | 30 | 1.5 ± 0.1 |
| 60 | 30 | 1.7 ± 0.05 | |
| Subventricularc | 30 | 30 | 2.0 ± 0.2 |
| 60 | 30 | 2.3 ± 0.3 | |
| Hippocampalc | 30 | 30 | 1.2 ± 0.1 |
| 60 | 30 | 1.9 ± 0.1 | |
| HCAd | 30 | 30 | 1.3 ± 0.07 |
| IMR90d | 30 | 30 | 1.4 ± 0.1 |
Time in isotonic medium before assay.
±Standard deviation.
Neural precursor cells from different regions of the brain.
Fibroblasts.
Fig. 2.
FACS histograms of cerebellar precursor cells. Fluorescent histograms of cells subjected to hypotonic conditions show how increasing incubation times lead to progressively higher levels of γ-H2AX (rightward shift along x-axis).
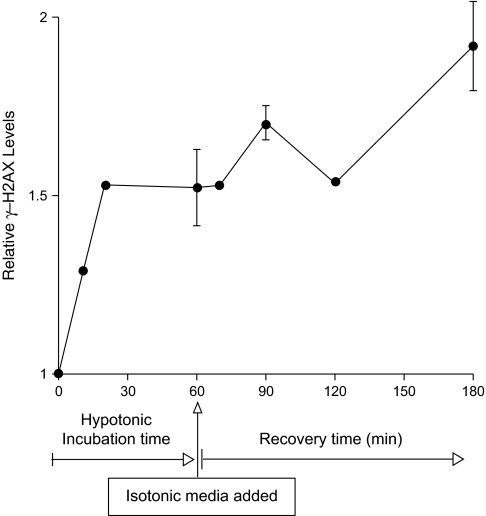
To determine the persistence of salt-induced changes in γ-H2AX, cerebellar precursor cells were exposed (up to 1 h) in hypotonic salt, before switching to isotonic medium, where cells were then allowed to recover over various times (up to 2 h). Under hypotonic conditions, cells remained competent to phosphorylate H2AX, where the level of γ-H2AX increased (50%) over the initial 20 min and remained constant thereafter (Figure 3, solid line). Upon return to isotonic media, little change in γ-H2AX levels were observed in these cells until 2 h, where γ-H2AX increased another 30% (Figure 3).
Fig. 3.
Persistence of H2AX phosphorylation induced by hypotonic treatments in cerebellar precursor cells. Cells were incubated under hypotonic conditions for up to 60 min, then returned to isotonic media for various lengths of time. Cells assayed before return to isotonic media showed that γ-H2AX was formed soon after hypotonic treatment. Upon return to isotonic media, cells previously subjected to hypotonic treatment showed relatively little change in γ-H2AX levels, until 2 h where γ-H2AX levels were found to increase.
The formation γ-H2AX after hypotonic exposure was inhibited completely by wortmannin (20 μM, data not shown), suggesting that these treatments were activating a member of the phosphoinositide 3-kinases (PI3 kinase) family that could phosphorylate the histone target. Wortmannin had a similar inhibitory effect on γ-H2AX formation in control cells kept under isotonic conditions but irradiated with 10 Gy. Several other experiments were run to determine whether DNA intercalators could elicit γ-H2AX formation, and despite repeated attempts using ethidium bromide or chloroquine at 50 and 250 ng/ml, results were routinely negative (data not shown). To determine whether hypotonic conditions might perturb replication in S-phase cells and elicit γ-H2AX and/or interfere with cell cycle progression, cells were subjected to hypotonic conditions and analysed by FACS for any changes in the distribution of cells throughout the cell cycle. Cells incubated under hypotonic conditions for 30 or 60 min and allowed to recover for various lengths of time in isotonic medium did not show any major changes in cell cycle distribution (Table II). S-phase percentages were minimally changed compared to untreated controls, suggesting that the majority of H2AX phosphorylation resulting from hypotonic exposure was not restricted to the fraction of S-phase cells. To more conclusively rule out the possibility that γ-H2AX following hypotonic treatments was primarily due to an effect on S-phase cells, cerebellar precursor cells subjected to confluence arrest were analysed for changes in H2AX phosphorylation. Hypotonic exposures of 30 and 60 min followed by 30 min of isotonic recovery increased γ-H2AX levels by 1.4 ± 0.1-fold and 1.8 ± 0.04-fold, respectively, over untreated controls. These values are in close agreement with those for log-phase cells (Figure 1) and suggest that hypotonic-induced changes in H2AX phosphorylation are not limited to replicating cells.
Table II.
Cell cycle distribution of cerebeiiar precursor cells subjected to hypotonic conditions
| Hypotonic incubation (min) | Recovery time (min)a | G1 %b | S %b | G2/M %b |
| 0c | 30 | 40 | 45 | 15 |
| 180 | 52 | 38 | 10 | |
| 30 | 30 | 41 | 44 | 15 |
| 120 | 46 | 44 | 10 | |
| 60 | 30 | 36 | 47 | 17 |
| 120 | 41 | 41 | 18 |
Time in isotonic medium before assay.
Percentages calculated using the ModFit™ algorithm.
Controls kept in isotonic suspension for the minimum and maximum treatment times.
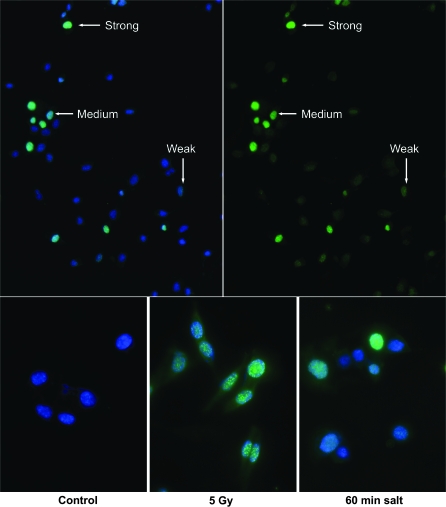
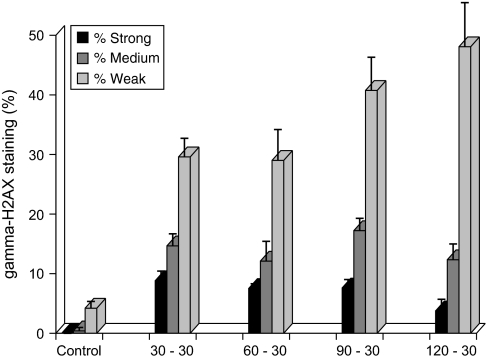
Immunohistochemistry was performed to ascertain the impact of hypotonic conditions on the nuclear staining profiles of γ-H2AX in neural precursor cells. To qualitatively assess differences in nuclear staining profiles, cells were subjected to hypotonic incubations and the yields of strong, moderate and weakly staining cells were compared to negative (isotonic treatment) and positive (γ-irradiated) controls (Figure 4, Table III). Untreated controls showed weak γ-H2AX staining (4.2%) and were not found to exhibit the more robust nuclear staining profiles (i.e. moderate or strong staining) (Figure 4, lower left panel). In contrast, nearly all cells (≥99%) subjected to γ-irradiation were found to exhibit strong nuclear staining with numerous brightly staining γ-H2AX foci (Figure 4, lower center panel). Compared to irradiated samples, cells subjected to hypotonic salt showed qualitative different staining patterns for γ-H2AX (Figure 4, upper and lower right panels). In general, hypotonic-treated cells showed a more homogeneous ‘pan-nuclear’ staining profile that was not evident in γ-irradiated controls. Cells treated under hypotonic conditions did, however, exhibit a spectrum of different staining profiles varying from uniform (pan staining) to more punctate (visible foci). Examples of these different types of γ-H2AX staining are shown in Figure 4. The yield of γ-H2AX-positive cells (Table III), plotted for each of the various hypotonic treatments, clearly shows an increase in the number of weakly staining cells with increasing incubation time (Figure 5). The yield of strongly and moderately staining cells was found to be less dependent on hypotonic incubation time.
Fig. 4.
Immunohistochemistry of γ-H2AX in neural precursor cells subjected to hypotonic conditions. Neural precursor cells were subjected to hypotonic salt (60 min) and allowed to recover (30 min) before fixation and immunohistochemical analysis of γ-H2AX nuclear staining patterns. Upper panels (×40) show examples of strong, medium and weakly staining cells. The dual bandpass image (left) shows the signals for both γ-H2AX (FITC) and DAPI nuclear counterstains, while a single bandpass (right) used to eliminate DAPI fluorescence reveals more weakly staining cells from the same field. Lower panels (×60) show examples of the minimal γ-H2AX staining found in isotonic controls (left), the maximal punctate staining in irradiated (5 Gy) cells fixed 20 min later (center) and the more uniform γ-H2AX staining found in cells subjected to hypotonic salt (right).
Table III.
Immunohistochemical staining of γ-H2AX and analysis of apoptosis in cells exposed to hypotonic salt
| Hypotonic incubation (min) | Recovery time (min) | Nuclear H2AX staininga,b |
Fold change in annexinV (+) cellsc,d | ||
| % Strong | % Medium | % Weak | |||
| 0 | 30–120 | 0 | 0 | 4.2 ± 1.1 | 0.00 |
| 30 | 30 | 8.8 ± 1.6 | 14.7 ± 2.0 | 29.6 ± 3.1 | 0.66 ± 0.12 |
| 120 | 0.86 ± 0.06 | ||||
| 60 | 30 | 7.5 ± 0.8 | 12.1 ± 3.3 | 29.01 ± 5.2 | 0.87 ± 0.24 |
| 120 | 1.0 ± 0.40 | ||||
| 90 | 30 | 7.6 ± 1.4 | 17.2 ± 2.1 | 40.8 ± 5.6 | n/d |
| 120 | 30 | 3.75 ± 2.0 | 12.3 ± 2.6 | 48.1 ± 7.4 | n/d |
n/d, not deteremined.
≥1000 total cells were analysed after each treatment.
Total background staining was 4.2% (weak) from 0.5 to 2 h in isotonic medium.
Data averaged from cerebellar and subventricular neural precursor cells.
Fold change in the number of annexin V-positive cells compared to controls.
Fig. 5.
Immunohistochemical analyses of γ-H2AX-positive cells. Bar charts derived from the data shown in Table III, plot the percentage of γ-H2AX-positive cells scored as strong, moderate or weakly staining as described (see Materials and methods). Control cells were incubated in isotonic medium (30–120 min), while test cells were incubated in hypotonic salt (30–120 min) followed by recovery (30 min) in isotonic media. Numbers along x-axis refer to ‘hypotonic–isotonic’ treatment times (min).
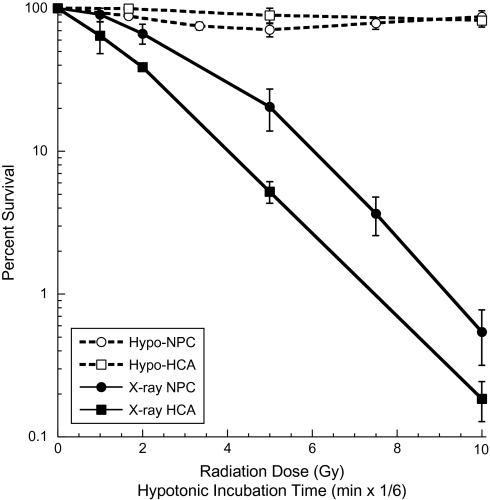
To assess the toxicity of the hypotonic treatments, clonogenic assays were performed using immortalized neural precursors and fibroblasts. Cells were also subjected to irradiation to assess their response to an agent known to produce DNA strand breaks. Both cell lines showed typical responses to irradiation, having shouldered survival curves and exponential killing with D0 values of 1.56 (fibroblasts) and 1.91 Gy (neural precursors) (Figure 6). In comparison, hypotonic treatments lasting up to 60 min resulted in virtually no cell kill (Figure 6). Analysis of non-clonogenic cells (i.e. primary subventricular and hippocampal precursors) for viability using the 2,3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino) carbonyl]-2H-tetrazolium hydroxide (XTT) assay (functionally equivalent to the MTT assay) also showed no reduction in survival after hypotonic treatments (data not shown).
Fig. 6.
Cell survival after hypotonic exposure or γ-irradiation. Gamma-irradiated cerebellar precursor cells (NPC, circles) and immortalized human fibroblasts (HCA, squares) show typical shouldered survival curves over a dose range of 1–10 Gy (solid lines). Hypotonic incubation of these same cells over the course of 60 min leads to relatively little toxicity (dashed lines). For comparison, hypotonic data are plotted at 1/6 scale (i.e. 60 min = 10 Gy). Experiments repeated in triplicate (±standard error).
Cerebellar and subventricular neural precursor cells subjected to hypotonic treatments were also evaluated for apoptosis using annexin V staining. Following 30- or 60-min treatments in hypotonic salt, cells were allowed to recover for either 30 or 120 min in isotonic medium before annexin V binding and FACS analysis. Both cell types gave qualitatively similar results, showing that the percentage of apoptotic cells actually decreased (although not significantly) after hypotonic treatments (Table III).
Discussion
The primary objective of these studies was to determine if we could detect the formation of γ-H2AX under conditions that did not induce overt changes in chromatin structure associated with DNA strand breaks. Here we present evidence that γ-H2AX can be formed in significant yield under minimally toxic conditions of altered tonicity. Cells subjected to hypotonic conditions rapidly form γ-H2AX in a manner dependent on the time of exposure (Figure 1). This effect appears to be general, at least, for neural precursors and fibroblasts, cells that routinely showed elevated γ-H2AX levels following hypotonic treatments.
Cells retain the requisite kinase activity necessary to phosphorylate histone H2AX under hypotonic conditions (Figure 3). While not the focus of this investigation, the capability of wortmannin to inhibit the formation of γ-H2AX suggests that the PI3 kinases (e.g. ataxia telangiectasia mutated, ataxia telangiectasia and Rad3 related and/or DNA-dependent protein kinase) are likely to be involved in mediating H2AX phosphorylation after hypotonic exposure (18,24). This idea is also supported by a number of past studies analyzing the substrate specificities of this kinase family after different types of cellular stress (25–27). The formation of γ-H2AX in response to hypotonic treatment persists well after return to isotonic medium (Figure 3), suggesting that certain disruptions to chromatin structure persist, and/or the ability of phosphatases to return H2AX to the unphosphorylated state are compromised. It may also be possible that hypotonic conditions compromise cellular metabolism and energy pools, potentially impacting H2AX phosphorylation by altering the response of DNA damage and chromatin sensing pathways. While different salt levels are likely to have many effects in cells, the impact of the resultant structural alterations to chromatin induced under hypotonic conditions are still likely to play a contributory if not causal role in eliciting γ-H2AX.
Past studies have tried to quantify the cellular response to DNA damage through immunohistochemical approaches aimed at analyzing the nuclear staining of γ-H2AX (4,6,28,29). Such efforts have clearly demonstrated that agents and/or conditions capable of causing DNA DSBs directly (e.g. ionizing radiation) or indirectly (e.g. via replication fork breakdown) lead to the formation of brightly staining γ-H2AX foci of widely varying size. However, efforts to quantify fluorescent γ-H2AX foci within nuclei are often hampered by the marked heterogeneity in the level of background γ-H2AX staining. If, as we suggest, γ-H2AX levels are sensitive to chromatin change, then much of the heterogeneity in nuclear γ-H2AX staining profiles may simply reflect the multiple structural conformations of DNA actively undergoing replication and/or repair. In support of this possibility, past studies from us and others have found that background H2AX staining is highest during S-phase (4,30,31).
For the reasons alluded to above, we chose to focus on the quantification of γ-H2AX levels via FACS analysis. Immunohistochemical analyses undertaken for comparative purposes demonstrated unequivocally the capability of hypotonic treatments to elicit γ-H2AX nuclear staining. The different nuclear staining profiles evident after various treatments did, however, reveal marked variations in γ-H2AX signal intensity and distribution in the nucleus. Overall, hypotonic treatments led to more uniform pan-nuclear staining while γ-irradiated cells all showed very discrete punctate patterns of nuclear staining (Figure 4). The number of weakly staining nuclei increased from 30 to 50% as hypotonic incubations increased from 30 to 120 min (Table III, Figure 5). Similar hypotonic treatments had a smaller impact on the fraction of cells staining more intensely, where the yield of positive cells scored as strong or moderate fluctuated around mean values of 6.9 ± 2% and 14 ± 2%, respectively (Table III, Figure 5).
Clearly, there are caveats associated with this type of subjective immunohistochemical analyses, which limits the types of conclusions that can be drawn. We reiterate that these types of studies were included with the intent of highlighting qualitative differences between γ-H2AX staining patterns found after irradiation and those found after hypotonic salt. It is possible that the most brightly staining cells were those destined to die (possibly via apoptosis), but at the time at which our H2AX measurements were performed, annexin V staining was minimal (Table III). If in fact pan-nuclear staining was a marker for early apoptosis, then it also did not impact clonogenic kill. Based on the increased H2AX phosphorylation observed after hypotonic treatments, it is unlikely that apoptosis could account for the majority of the increased H2AX phosphorylation we report in this study. Despite inherent differences between the quantification of γ-H2AX-positive cells by FACS or immunohistochemistry, each technique did show that exposure to hypotonic conditions increased the number of cells positive for γ-H2AX.
Further support that γ-H2AX is a response sensitive to chromatin disruptions comes in a recent report showing that UV light induces significant increases in γ-H2AX levels (30,32), a response that was muted in cells deficient for nucleotide excision repair (NER). UVC light does not produce DNA DSBs, but at the fluences used, hundreds of thousands of thymine dimers and 6–4 photoproducts are formed throughout the genome (33,34). These UV-induced lesions are excised during NER, a repair process that involves the formation of intermediate D-loop structures and gap-filling synthesis in the DNA. If γ-H2AX acts to respond to structural change in chromatin, then activation of NER would be predicted to increase γ-H2AX levels. Repair competent cells exposed to UVC light not only exhibited marked increases in γ-H2AX but also showed pan-nuclear staining patterns for γ-H2AX (i.e. similar to that shown in Figure 4) (30). These results lend support to the idea that more subtle changes in chromatin structure can elicit γ-H2AX formation.
One of the critical issues these investigations sought to clarify was if γ-H2AX formation was not entirely (if at all) dependent on DNA strand breakage. Because measuring low-level yields of DNA DSBs in mammalian cells (i.e. 1 Gy equivalent, ∼40/cell) presents a considerable technical challenge (35,36), it becomes difficult to conclusively determine whether a cell at any given time contains only a few or no DSBs. Consequently, to unequivocally substantiate that the hypotonic treatments used here do not create any DNA DSBs would be difficult. However, the survival curves shown (Figure 6) do provide an unambiguous assessment of survival and allow one to estimate relative DSB yields. Thus, at a dose of 5 Gy, ∼200 DSBs elicit 80% cell kill and a 1.8-fold increase in γ-H2AX levels (Figure 1) in neural precursor cells. In comparison, a 60-min hypotonic treatment elicits equivalent γ-H2AX levels (∼1.7-fold over background) but only results in 13% cell kill, survival levels typically found at doses <1 Gy (i.e. <40 DSBs). Therefore, based on the survival data shown, the level of γ-H2AX induced under hypotonic conditions cannot be explained solely by the induction of DNA DSBs, even if such treatments were in fact found to induce low yields (≤1 Gy) of these lesions.
In summary, our investigation was initiated not to dispute the role of the DSB-dependent formation of γ-H2AX but rather to provide evidence that the phosphorylation of this histone is responsive to alterations in chromatin structure that are not strictly dependent upon strand break formation. Changes in tonicity elicit predictable changes governing the interactions between macromolecules in cells, and based on these expectations, it is difficult to reconcile how the hypotonic conditions used here would not elicit at least minimal changes in chromatin structure. While we did not attempt to measure such structural alterations, salt-induced changes were in fact sufficient to elicit the phosphorylation of histone H2AX. We maintain that γ-H2AX formation is principally responsive to changes in chromatin structure and that such changes likely provided the selective pressure for the adaptation of γ-H2AX into a DNA damage-responsive niche.
Funding
National Institutes of Health/National Institute of Neurological Disorders and Stroke (1R01NS052781 to J.E.C.); American Cancer Society (RSG-00-036-04-CNE to C.L.L.).
Acknowledgments
We would also like to express our gratitude to Katherine Tran for the immunohistochemical staining and scoring of γ-H2AX-positive cells. Conflict of interest statement: None declared.
References
- 1.Rogakou EP, Boon C, Redon C, Bonner WM. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J. Cell Biol. 1999;146:905–916. doi: 10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 3.Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr. Biol. 2000;10:886–895. doi: 10.1016/s0960-9822(00)00610-2. [DOI] [PubMed] [Google Scholar]
- 4.Limoli CL, Giedzinski E, Bonner WM, Cleaver JE. UV-induced replication arrest in the xeroderma pigmentosum variant leads to double strand breaks, γ-H2Ax formation, and Mre11 relocalization. Proc. Natl Acad. Sci. USA. 2002;99:233–238. doi: 10.1073/pnas.231611798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Celeste A, Fernandez-Capetillo O, Kruhlak MJ, Pilch DR, Staudt DW, Lee A, Bonner RF, Bonner WM, Nussenzweig A. Histone H2AX phosphorylation is dispensable for the initial recognition of DNA breaks. Nat. Cell Biol. 2003;5:675–679. doi: 10.1038/ncb1004. [DOI] [PubMed] [Google Scholar]
- 6.Rothkamm K, Lobrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc. Natl Acad. Sci. USA. 2003;100:5057–5062. doi: 10.1073/pnas.0830918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez-Capetillo O, Lee A, Nussenzweig M, Nussenzweig A. H2AX: the histone guardian of the genome. DNA Repair (Amst.) 2004;3:959–967. doi: 10.1016/j.dnarep.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Capetillo O, Mahadevaiah SK, Celeste A, Romanienko PJ, Camerini-Otero RD, Bonner WM, Manova K, Burgoyne P, Nussenzweig A. H2AX is required for chromatin remodeling and inactivation of sex chromosomes in male mouse meiosis. Dev. Cell. 2003;4:497–508. doi: 10.1016/s1534-5807(03)00093-5. [DOI] [PubMed] [Google Scholar]
- 9.McManus KJ, Hendzel MJ. ATM-dependent DNA damage-independent mitotic phosphorylation of H2AX in normally growing mammalian cells. Mol. Biol. Cell. 2005;16:5013–5025. doi: 10.1091/mbc.E05-01-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ichijima Y, Sakasai R, Okita N, Asahina K, Mizutani S, Teraoka H. Phosphorylation of histone H2AX at M phase in human cells without DNA damage response. Biochem. Biophys. Res. Commun. 2005;336:807–812. doi: 10.1016/j.bbrc.2005.08.164. [DOI] [PubMed] [Google Scholar]
- 11.Olive PL, Banath JP. Phosphorylation of histone H2AX as a measure of radiosensitivity. Int. J. Radiat. Oncol. Biol. Phys. 2004;58:331–335. doi: 10.1016/j.ijrobp.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 12.Stiff T, O'Driscoll M, Rief N, Iwabuchi K, Lobrich M, Jeggo PA. ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res. 2004;64:2390–2396. doi: 10.1158/0008-5472.can-03-3207. [DOI] [PubMed] [Google Scholar]
- 13.Ward IM, Chen J. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J. Biol. Chem. 2001;276:47759–47762. doi: 10.1074/jbc.C100569200. [DOI] [PubMed] [Google Scholar]
- 14.Furuta T, Takemura H, Liao ZY, et al. Phosphorylation of histone H2AX and activation of Mre11, Rad50, and Nbs1 in response to replication-dependent DNA-double-strand breaks induced by mammalian DNA topoisomerase I cleavage complexes. J. Biol. Chem. 2003;278:20303–20312. doi: 10.1074/jbc.M300198200. [DOI] [PubMed] [Google Scholar]
- 15.Rogakou EP, Nieves-Neira W, Boon C, Pommier Y, Bonner WM. Initiation of DNA fragmentation during apoptosis induces phosphorylation of H2AX histone at serine 139. J. Biol. Chem. 2000;275:9390–9395. doi: 10.1074/jbc.275.13.9390. [DOI] [PubMed] [Google Scholar]
- 16.Olive PL. Detection of DNA damage in individual cells by analysis of histone H2AX phosphorylation. Methods Cell Biol. 2004;75:355–373. doi: 10.1016/s0091-679x(04)75014-1. [DOI] [PubMed] [Google Scholar]
- 17.Reitsema TJ, Banath JP, MacPhail SH, Olive PL. Hypertonic saline enhances expression of phosphorylated histone H2AX after irradiation. Radiat. Res. 2004;161:402–408. doi: 10.1667/rr3153. [DOI] [PubMed] [Google Scholar]
- 18.Reitsema T, Klokov D, Banath JP, Olive PL. DNA-PK is responsible for enhanced phosphorylation of histone H2AX under hypertonic conditions. DNA Repair (Amst.) 2005;4:1172–1181. doi: 10.1016/j.dnarep.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 20.Snyder EY, Deitcher DL, Walsh C, Arnold-Aldea S, Hartwieg EA, Cepko CL. Multipotent neural cell lines can engraft and participate in development of mouse cerebellum. Cell. 1992;68:33–51. doi: 10.1016/0092-8674(92)90204-p. [DOI] [PubMed] [Google Scholar]
- 21.Limoli CL, Giedzinski E, Rola R, Otsuka S, Palmer TD, Fike JR. Radiation response of neural precursor cells: linking cellular sensitivity to cell cycle checkpoints, apoptosis and oxidative stress. Radiat. Res. 2004;161:17–27. doi: 10.1667/rr3112. [DOI] [PubMed] [Google Scholar]
- 22.Pirzio LM, Freulet-Marriere MA, Bai Y, Fouladi B, Murnane JP, Sabatier L, Desmaze C. Human fibroblasts expressing hTERT show remarkable karyotype stability even after exposure to ionizing radiation. Cytogenet. Genome Res. 2004;104:87–94. doi: 10.1159/000077470. [DOI] [PubMed] [Google Scholar]
- 23.Pacey L, Stead J, Gleave A, Tomczyk K, Doering L. Neural stem cell culture: neurosphere generation, microscopical analysis and cryopreservation. Nat. Protoc. 2006;215:1–14. [Google Scholar]
- 24.Limoli CL, Laposa R, Cleaver JE. DNA replication arrest in XP variant cells after UV exposure is diverted into an Mre11-dependent recombination pathway by the kinase inhibitor wortmannin. Mutat. Res. 2002;510:121–129. doi: 10.1016/s0027-5107(02)00257-9. [DOI] [PubMed] [Google Scholar]
- 25.Giaccia AJ, Kastan MB. The complexity of p53 modulation:emerging patterns from divergent signals. Genes Dev. 1998;12:2973–2983. doi: 10.1101/gad.12.19.2973. [DOI] [PubMed] [Google Scholar]
- 26.Dent P, Yacoub A, Contessa J, Caron R, Amorino G, Valerie K, Hagan MP, Grant S, Schmidt-Ullrich R. Stress and radiation-induced activation of multiple intracellular signaling pathways. Radiat. Res. 2003;159:283–300. doi: 10.1667/0033-7587(2003)159[0283:sariao]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 27.Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001;15:2177–2196. doi: 10.1101/gad.914401. [DOI] [PubMed] [Google Scholar]
- 28.Sedelnikova OA, Rogakou EP, Panyutin IG, Bonner WM. Quantitative detection of (125)IdU-induced DNA double-strand breaks with gamma-H2AX antibody. Radiat. Res. 2002;158:486–492. doi: 10.1667/0033-7587(2002)158[0486:qdoiid]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 29.MacPhail SH, Banath JP, Yu Y, Chu E, Olive PL. Cell cycle-dependent expression of phosphorylated histone H2AX: reduced expression in unirradiated but not X-irradiated G1-phase cells. Radiat. Res. 2003;159:759–767. doi: 10.1667/rr3003. [DOI] [PubMed] [Google Scholar]
- 30.Marti TM, Hefner E, Feeney L, Natale V, Cleaver JE. H2AX phosphorylation within the G1 phase after UV irradiation depends on nucleotide excision repair and not DNA double-strand breaks. Proc. Natl Acad. Sci. USA. 2006;103:9891–9896. doi: 10.1073/pnas.0603779103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Feraudy S, Limoli CL, Giedzinski E, Karentz D, Marti TM, Feeney L, Cleaver JE. Pol eta is required for DNA replication during nucleotide deprivation by hydroxyurea. Oncogene. 2007;26:5713–5721. doi: 10.1038/sj.onc.1210385. [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto M, Yaginuma K, Igarashi A, et al. Perturbed gap-filling synthesis in nucleotide excision repair causes histone H2AX phosphorylation in human quiescent cells. J. Cell Sci. 2007;120:1104–1112. doi: 10.1242/jcs.03391. [DOI] [PubMed] [Google Scholar]
- 33.Trosko JE, Chu EHY, Carrier WL. The induction of dimers in ultraviolet-irradiated mammalian cells. Radiat. Res. 1965;24:667–672. [PubMed] [Google Scholar]
- 34.Mitchell DL, Haipe CA, Clarkson JM. (6-4) Photoproducts are removed from the DNA of UV-irradiated mammalian cells more efficiently than cyclobutane dimers. Mutat. Res. 1985;143:109–112. doi: 10.1016/s0165-7992(85)80018-x. [DOI] [PubMed] [Google Scholar]
- 35.Dewey WC, Wong RS, Albright N. Pulsed-field gel electrophoretic migration of DNA broken by X irradiation during DNA synthesis: experimental results compared with Monte Carlo calculations. Radiat. Res. 1997;148:413–420. [PubMed] [Google Scholar]
- 36.Wojewodzka M, Buraczewska I, Kruszewski M. A modified neutral comet assay: elimination of lysis at high temperature and validation of the assay with anti-single-stranded DNA antibody. Mutat. Res. 2002;518:9–20. doi: 10.1016/s1383-5718(02)00070-0. [DOI] [PubMed] [Google Scholar]