Abstract
Rationale: Idiopathic pulmonary fibrosis (IPF) is characterized by progressive dyspnea, impaired gas exchange, and ultimate mortality.
Objectives: To test the hypothesis that maximal oxygen uptake during cardiopulmonary exercise testing at baseline and with short-term longitudinal measures would predict mortality in patients with idiopathic pulmonary fibrosis.
Methods: Data from 117 patients with IPF and longitudinal cardiopulmonary exercise tests were examined retrospectively. Survival was calculated from the date of the first cardiopulmonary exercise test.
Measurements and Main Results: Patients with baseline maximal oxygen uptake less than 8.3 ml/kg/min had an increased risk of death (n = 8; hazard ratio, 3.24; 95% confidence interval, 1.10–9.56; P = 0.03) after adjusting for age, gender, smoking status, baseline forced vital capacity, and baseline diffusion capacity for carbon monoxide. We were unable to define a unit change in maximal oxygen uptake that predicted survival in our cohort.
Conclusions: We conclude that a threshold maximal oxygen uptake of 8.3 ml/kg/min during cardiopulmonary exercise testing at baseline adds prognostic information for patients with IPF.
Keywords: idiopathic pulmonary fibrosis, exercise test, mortality
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Prognosis in idiopathic pulmonary fibrosis (IPF) is poor. Predicting prognosis by examining exercise performance with the six-minute-walk test has led to conflicting results.
What This Study Adds to the Field
This study shows that  o2max predicts mortality in patients with IPF. Patients with
o2max predicts mortality in patients with IPF. Patients with 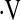 o2max less than 8.3 ml/kg/min at baseline had an increased risk of death.
o2max less than 8.3 ml/kg/min at baseline had an increased risk of death.
Idiopathic pulmonary fibrosis (IPF) is a disease of unknown etiology characterized by progressive dyspnea and ultimate mortality (1). Mean survival from time of diagnosis to death is 3 years (1). However, the disease course is variable: some patients progress rapidly and others remain stable for many years (2). There is no effective treatment and many patients, if eligible, are referred for lung transplantation. Identification of surrogate short-term measures of mortality is critical to the management and study of patients with IPF.
Several factors have been identified that predict poor survival in patients with IPF, including age, sex, smoking history, diffusion capacity for carbon monoxide (DlCO), FVC, degree of fibrosis on high-resolution computerized tomography of the chest, and number of fibroblastic foci on histopathology (3–11). Longitudinal changes in FVC or DlCO have been found to have important prognostic value. A decrease in FVC of at least 10% or DlCO of at least 15% over 6 or 12 months is associated with decreased survival (10–15).
Gas exchange worsens with exercise in IPF (1, 16, 17). Several studies have examined this feature using either cardiopulmonary exercise tests (CPET) or the 6-minute-walk test (6MWT). A decrease in PaO2 during CPET in patients with IPF contributes up to 10.5% of the total clinical, radiographic, and physiologic (CRP) score (5) used to estimate prognosis in IPF. Desaturation during CPET has been shown to predict mortality in some (16) but not all (18, 19) studies. Desaturation below 88% during 6MWT is a more consistent marker of increased risk for mortality (7, 9, 10), whereas shorter walk distance is less predictive (15). Longitudinal change in 6MWT data predicts mortality in patients who do not desaturate less than 88% at baseline (15).
Patients with IPF have impaired ventilatory and cardiovascular responses to exercise (20) due to multiple abnormalities, including low tidal volume; a failure to decrease ventilatory dead space; a rapid, shallow breathing pattern; impaired gas exchange due to interstitial fibrosis; pulmonary hypertension; ventilation/perfusion mismatching; and low mixed venous O2.
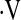 o2max is an integrated measure of cardiovascular, respiratory, and neuromuscular function (21). In prior studies of patients with interstitial lung disease,
o2max is an integrated measure of cardiovascular, respiratory, and neuromuscular function (21). In prior studies of patients with interstitial lung disease, 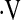 o2max correlated poorly with measures of lung volume, suggesting that it more accurately reflects derangements in hemodynamics as well as ventilation during exercise (20). Although change in FVC is a good surrogate for subsequent mortality, it is imperfect as some patients die without a 10% decline in FVC, whereas others can live for prolonged periods even after a 10% decline in FVC (2, 22). Therefore we chose to examine longitudinal change in
o2max correlated poorly with measures of lung volume, suggesting that it more accurately reflects derangements in hemodynamics as well as ventilation during exercise (20). Although change in FVC is a good surrogate for subsequent mortality, it is imperfect as some patients die without a 10% decline in FVC, whereas others can live for prolonged periods even after a 10% decline in FVC (2, 22). Therefore we chose to examine longitudinal change in 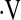 o2max a priori because it is an integrated measure of cardiovascular, respiratory, and neuromuscular function (21). We tested the hypothesis that a decrease in
o2max a priori because it is an integrated measure of cardiovascular, respiratory, and neuromuscular function (21). We tested the hypothesis that a decrease in 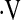 o2max during baseline and short-term longitudinal CPETs predicts mortality in patients with IPF.
o2max during baseline and short-term longitudinal CPETs predicts mortality in patients with IPF.
METHODS
Patient Selection
This is a retrospective analysis of 117 patients in the University of Michigan Specialized Center of in the Pathobiology of Fibrotic Lung Disease Database. Patients in this database were referred for enrollment in study protocols for suspected IPF based on typical symptoms, physiologic findings, and radiographic findings (1). Patients with a high-resolution computerized tomography scan showing a definite pattern of usual interstitial pneumonitis (23) were not required to undergo surgical lung biopsy (n = 42) (24, 25). Patients with underlying connective tissue disease, occupational or environmental exposures, or histopathologic pattern on surgical lung biopsy other than usual interstitial pneumonitis were excluded. Patients were treated with varied regimens, including no therapy, immunosuppression (prednisone ± azathioprine or cyclophosphamide), colchicine, and experimental protocols. The lack of a standardized treatment regimen prevented an analysis of the data based on therapy. Approval for the use of these data was provided by our Institutional Review Board. Subgroups of these patients have been previously described (7, 15, 26).
Pulmonary Function, 6-Minute Walk, and Cardiopulmonary Exercise Tests
Pulmonary function and exercise tests, including FVC, DlCO, 6MWT, and CPET, were performed as described (7, 18). Desaturation during a 6MWT was defined a priori as less than 88% based on published data (7).
Statistical Analysis
The date of the first CPET was used as the start date for survival analysis. Death date was supplemented by searching the Social Security Death Index (27); patients not listed in this index were censored 3 months prior to the analysis date to account for potential lags in reporting. Multivariate Cox proportional hazard models (28) adjusted for age, sex, baseline forced vital capacity percent predicted, baseline DlCO percent predicted, and smoking status were constructed for potential 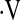 o2max thresholds ranging from 4.4 ml/kg/min to 25.1 ml/kg/min, in increments of 0.3 ml/kg/min. Resulting hazard ratios were plotted against
o2max thresholds ranging from 4.4 ml/kg/min to 25.1 ml/kg/min, in increments of 0.3 ml/kg/min. Resulting hazard ratios were plotted against 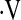 o2max to determine if a threshold
o2max to determine if a threshold 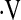 o2max could be identified that correlated with increased risk of mortality. Similar secondary analyses were performed on resting PaO2. Baseline characteristics between patients with
o2max could be identified that correlated with increased risk of mortality. Similar secondary analyses were performed on resting PaO2. Baseline characteristics between patients with 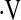 o2max above and below the thresholds were compared using t tests (29) for continuous measures and chi-square (30) tests for categorical measures. Data are expressed as mean ± standard deviation (SD) or frequency (%). In secondary analyses, index of concordance (31) was used compare the
o2max above and below the thresholds were compared using t tests (29) for continuous measures and chi-square (30) tests for categorical measures. Data are expressed as mean ± standard deviation (SD) or frequency (%). In secondary analyses, index of concordance (31) was used compare the 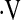 o2max threshold, desaturation less than 88% during a 6MWT, and resting PaO2 to determine which was the strongest predictor of survival. Survival between patients with baseline
o2max threshold, desaturation less than 88% during a 6MWT, and resting PaO2 to determine which was the strongest predictor of survival. Survival between patients with baseline 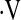 o2max above and below thresholds was examined with unadjusted Kaplan Meier survival curves (32) and log-rank tests (33, 34). Multivariate Cox proportional hazard models studied the predictive value of the
o2max above and below thresholds was examined with unadjusted Kaplan Meier survival curves (32) and log-rank tests (33, 34). Multivariate Cox proportional hazard models studied the predictive value of the 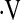 o2max threshold adjusted for age, gender, smoking status, baseline FVC percent predicted (FVC%), and baseline DlCO percent predicted (DlCO%). Statistical significance was set at P = 0.05. Statistical analysis was performed with R software (http://www.r-project.org/index.html) and SPSS (version 14.0; SPSS Inc., Chicago, IL).
o2max threshold adjusted for age, gender, smoking status, baseline FVC percent predicted (FVC%), and baseline DlCO percent predicted (DlCO%). Statistical significance was set at P = 0.05. Statistical analysis was performed with R software (http://www.r-project.org/index.html) and SPSS (version 14.0; SPSS Inc., Chicago, IL).
RESULTS
Data from 117 patients with at least two cardiopulmonary exercise tests were analyzed. Seventy-five (64%) patients were diagnosed with a surgical lung biopsy. Patients who did not undergo a lung biopsy were significantly older (66.73 ± 8.50 vs. 63.07 ± 8.23 y, P = 0.024) but otherwise not different than those with a lung biopsy (data not shown).
We explored the predictive value of 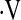 o2max on survival.
o2max on survival. 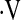 o2max did not predict survival when examined as a continuous variable (hazard ratio [HR], 0.969; 95% confidence interval [CI], 0.88–1.07; P = 0.55). Exploratory analyses revealed a threshold
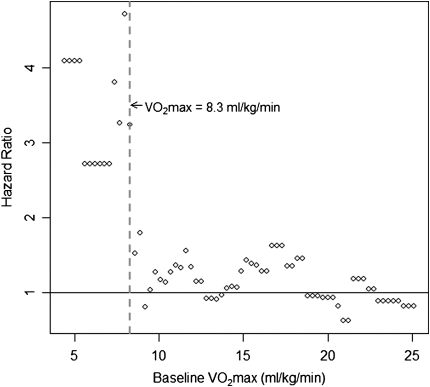
o2max did not predict survival when examined as a continuous variable (hazard ratio [HR], 0.969; 95% confidence interval [CI], 0.88–1.07; P = 0.55). Exploratory analyses revealed a threshold 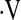 o2max of 8.3 ml/min/kg that was associated with an increased risk of subsequent mortality (Figure 1). This threshold effectively captures patients with a higher risk of mortality and discriminates these patients from those with a lower risk of mortality.
o2max of 8.3 ml/min/kg that was associated with an increased risk of subsequent mortality (Figure 1). This threshold effectively captures patients with a higher risk of mortality and discriminates these patients from those with a lower risk of mortality.
Figure 1.
Determination of a 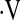 o2max threshold of 8.3 ml/kg/min. Multivariate Cox proportional hazard models adjusted for age, sex, baseline FVC percent predicted, baseline diffusion capacity of carbon monoxide percent predicted, and smoking status were constructed for potential
o2max threshold of 8.3 ml/kg/min. Multivariate Cox proportional hazard models adjusted for age, sex, baseline FVC percent predicted, baseline diffusion capacity of carbon monoxide percent predicted, and smoking status were constructed for potential 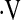 o2max thresholds ranging from 4.4 ml/kg/min to 25.1 ml/kg/min, in increments of 0.3 ml/kg/min. The vertical axis gives hazard ratios comparing risk of patients above and below the corresponding threshold along the horizontal axis. A threshold baseline
o2max thresholds ranging from 4.4 ml/kg/min to 25.1 ml/kg/min, in increments of 0.3 ml/kg/min. The vertical axis gives hazard ratios comparing risk of patients above and below the corresponding threshold along the horizontal axis. A threshold baseline 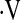 o2max of 8.3 ml/min/kg was determined (dashed line).
o2max of 8.3 ml/min/kg was determined (dashed line).
Demographic data and baseline physiologic data for patients with an initial 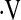 o2max above or below 8.3 ml/min/kg are presented in Table 1. Patients whose baseline
o2max above or below 8.3 ml/min/kg are presented in Table 1. Patients whose baseline 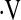 o2max was below threshold were more often female (P = 0.04), had significantly lower FVC% (P = 0.01) and DlCO% (P = 0.006), shorter 6-minute walk distance (P = 0.002), and significantly lower exercise capacity during baseline CPET compared with those with baseline
o2max was below threshold were more often female (P = 0.04), had significantly lower FVC% (P = 0.01) and DlCO% (P = 0.006), shorter 6-minute walk distance (P = 0.002), and significantly lower exercise capacity during baseline CPET compared with those with baseline 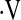 o2max above threshold (Table 1). Surprisingly, not all patients with
o2max above threshold (Table 1). Surprisingly, not all patients with 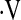 o2max below threshold at baseline desaturated during a baseline 6MWT.
o2max below threshold at baseline desaturated during a baseline 6MWT.
TABLE 1.
BASELINE CHARACTERISTICS OF PATIENTS WITH BASELINE 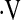 o2max ABOVE OR BELOW THE 8.3 ml/kg/min THRESHOLD
o2max ABOVE OR BELOW THE 8.3 ml/kg/min THRESHOLD
| Above Threshold | Below Threshold | P Value | |
|---|---|---|---|
| n | 109 | 8 | |
| Age, yr | 64.0 ± 8.4 | 69.1 ± 8.6 | 0.10 |
| Sex | 0.044 | ||
| Male | 78 (78.8%) | 3 (37.5%) | |
| Female | 31 (31.3%) | 5 (62.5%) | |
| Diagnosed with lung biopsy | 70 (70.7%) | 5 (62.5%) | 0.92 |
| Smokers | 0.30 | ||
| Never smokers | 28 (28.3%) | 4 (50%) | |
| Former smokers | 76 (76.7%) | 4 (50%) | |
| Current smokers | 5 (5.0%) | 0 | |
| Pack-years | 39.2 ± 24.9 | 42.0 ± 44.4 | 0.84 |
| Spirometry at baseline | |||
| FVC, L | 2.8 ± 0.9 | 1.8 ± 0.6 | 0.002 |
| FVC% | 69.0 ± 17.8 | 53.0 ± 9.6 | 0.01 |
| DlCO, ml/min/mm Hg | 12.3 ± 4.4 | 7.1 ± 1.3 | 0.002 |
| DlCO% | 47.8 ± 14.9 | 31.9 ± 7.7 | 0.006 |
| Exercise at baseline | |||
| Exercise PaO2 | 67.4 ± 15.0 | 59.4 ± 12.6 | 0.25 |
| Exercise Aa gradient | 41.0 ± 14.7 | 42.2 ± 13.3 | 0.85 |
| Exercise saturation | 89.5 ± 4.9 | 88.0 ± 5.7 | 0.41 |
| Minutes of exercise | 7.4 ± 1.9 | 4.2 ± 1.5 | <0.001 |
| Work, W | 95.2 ± 35.0 | 40.0 ± 11.6 | <0.001 |
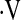 o2max, ml/kg/min o2max, ml/kg/min |
14.0 ± 4.2 | 6.9 ± 1.4 | <0.001 |
| Exercise gas exchange score | 12.4 ± 9.8 | 20.0 ± 14.2 | 0.04 |
| 6MWT at baseline | |||
| Desaturation < 88% | 38 (38.4%) | 5 (62.5%) | 0.12 |
| Distance, ft | 1,116.6 ± 456.0 | 582.2 ± 386.1 | 0.002 |
Definition of abbreviations: Aa gradient = alveolar-arterial gradient; DlCO% = diffusion capacity for carbon monoxide percent predicted; FVC% = forced vital capacity percent predicted; 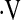 o2max = maximal oxygen consumption during cardiopulmonary exercise testing; 6MWT = 6-min-walk test.
o2max = maximal oxygen consumption during cardiopulmonary exercise testing; 6MWT = 6-min-walk test.
Data are shown as mean ± standard deviation (SD) or frequency (%).
Multivariate relationships between 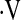 o2max and baseline demographic and pulmonary function variables were explored (Table 2). In multivariate linear regression models, age, male gender, history of smoking, and baseline FVC were predictors of
o2max and baseline demographic and pulmonary function variables were explored (Table 2). In multivariate linear regression models, age, male gender, history of smoking, and baseline FVC were predictors of 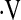 o2max. A linear predictor incorporating these variables significantly predicted whether a patient's baseline
o2max. A linear predictor incorporating these variables significantly predicted whether a patient's baseline 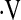 o2max would be below threshold (Table 3). With this model, patients who are younger, male, never smokers, with higher FVC% and DlCO% are more likely to have a
o2max would be below threshold (Table 3). With this model, patients who are younger, male, never smokers, with higher FVC% and DlCO% are more likely to have a 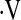 o2max above 8.3 ml/min/kg, and thus are predicted to have overall improved survival than older, female, ever smokers with lower FVC% and DlCO%.
o2max above 8.3 ml/min/kg, and thus are predicted to have overall improved survival than older, female, ever smokers with lower FVC% and DlCO%.
TABLE 2.
MULTIVARIATE LINEAR REGRESSION MODEL DETECTING ASSOCIATIONS WITH 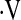 o2max
o2max
| β | SE | P Value | |
|---|---|---|---|
| Age, yr | −0.23 | 0.04 | <0.001 |
| Male sex | 2.53 | 0.79 | 0.002 |
| Ever smoker | −1.61 | 0.72 | 0.028 |
| Baseline FVC% | 0.82 | 0.28 | 0.004 |
| Baseline DlCO% | 0.30 | 0.31 | 0.332 |
Definition of abbreviations: DlCO% = diffusion capacity for carbon monoxide percent predicted; FVC% = forced vital capacity percent predicted; SE = standard error; 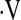 o2max = maximal oxygen consumption during cardiopulmonary exercise testing.
o2max = maximal oxygen consumption during cardiopulmonary exercise testing.
n = 103.
TABLE 3.
LOGISTIC REGRESSION MODEL OF PREDICTORS OF 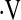 o2max < 8.3 ml/kg/min
o2max < 8.3 ml/kg/min
| OR | 95% CI | P Value | |
|---|---|---|---|
| Linear predictor | 0.57 | 0.37–0.87 | 0.01 |
Linear predictor = −0.232(age) + 2.53(male gender) − 1.6(ever smoker) + 0.82(FVC%) + 0.30(DlCO%).
Definition of abbreviations: CI = confidence interval; DlCO% = baseline diffusion capacity for carbon monoxide percent predicted; FVC% = baseline forced vital capacity percent predicted; OR = odds ratio; 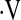 o2max = maximal oxygen consumption during cardiopulmonary exercise testing.
o2max = maximal oxygen consumption during cardiopulmonary exercise testing.
n = 103.
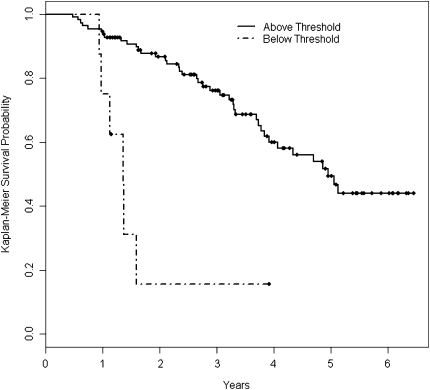
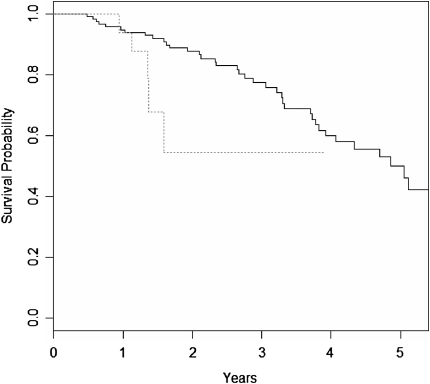
Survival in patients with baseline 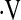 o2max below the 8.3 ml/kg/min threshold was lower than that of patients above the threshold over time (log rank P < 0.001, Figure 2). This difference was maintained in multivariate Cox proportional hazard survival models adjusting for patient age, smoking status, male sex, baseline FVC%, and baseline DlCO% (HR for being below threshold, 3.24; 95% CI, 1.10–9.56; P = 0.03) (Figure 3 and Table 4).
o2max below the 8.3 ml/kg/min threshold was lower than that of patients above the threshold over time (log rank P < 0.001, Figure 2). This difference was maintained in multivariate Cox proportional hazard survival models adjusting for patient age, smoking status, male sex, baseline FVC%, and baseline DlCO% (HR for being below threshold, 3.24; 95% CI, 1.10–9.56; P = 0.03) (Figure 3 and Table 4).
Figure 2.
Unadjusted Kaplan-Meier survival curves for patients whose baseline 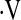 o2max was greater than 8.3 ml/min/kg (Above Threshold, n = 99) or less than 8.3 ml/min/kg (Below Threshold, n = 8). Survival time was calculated from the date of the first cardiopulmonary exercise test.
o2max was greater than 8.3 ml/min/kg (Above Threshold, n = 99) or less than 8.3 ml/min/kg (Below Threshold, n = 8). Survival time was calculated from the date of the first cardiopulmonary exercise test.
Figure 3.
Cox proportional hazard survival curves for patients with baseline 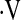 o2max greater than 8.3 ml/kg/min (solid line) and less than 8.3 ml/kg/min (dashed line) adjusted for age, sex, smoking status, baseline FVC percent predicted, and baseline DlCO percent predicted. Survival time was calculated from the date of the first cardiopulmonary exercise test.
o2max greater than 8.3 ml/kg/min (solid line) and less than 8.3 ml/kg/min (dashed line) adjusted for age, sex, smoking status, baseline FVC percent predicted, and baseline DlCO percent predicted. Survival time was calculated from the date of the first cardiopulmonary exercise test.
TABLE 4.
MULTIVARIATE COX PROPORTIONAL HAZARD SURVIVAL MODEL ASSESSING THE PREDICTIVE VALUE OF 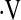 o2max < 8.3 ml/min/kg
o2max < 8.3 ml/min/kg
| HR | 95%CI | P Value | |
|---|---|---|---|
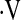 o2max below threshold o2max below threshold |
3.24 | 1.10–9.56 | 0.03 |
| Age, yr | 1.04 | 1.00–1.08 | 0.08 |
| Male sex | 1.29 | 0.62–2.69 | 0.50 |
| Ever smoker | 1.33 | 0.71–2.48 | 0.37 |
| Baseline FVC% | 0.93 | 0.72–1.21 | 0.58 |
| Baseline DlCO% | 0.66 | 0.49–0.90 | 0.01 |
Definition of abbreviations: CI = confidence interval; DlCO% = diffusion capacity for carbon monoxide percent predicted; FVC% = forced vital capacity percent predicted; HR = hazard ratio; 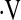 o2max = maximal oxygen consumption during cardiopulmonary exercise testing.
o2max = maximal oxygen consumption during cardiopulmonary exercise testing.
n = 103.
In the majority of patients studied, 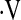 o2max did not change between baseline and 6 months (n = 99). Of the 109 patients with
o2max did not change between baseline and 6 months (n = 99). Of the 109 patients with 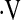 o2max above threshold at baseline, 5 had a further decline in
o2max above threshold at baseline, 5 had a further decline in 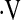 o2max below the threshold with longitudinal measures. However, we were unable to define a unit change in
o2max below the threshold with longitudinal measures. However, we were unable to define a unit change in 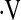 o2max that predicted survival in our cohort (data not shown).
o2max that predicted survival in our cohort (data not shown).
Prior studies have shown that resting PaO2 predicts survival in IPF. In secondary analyses, resting PaO2 was a significant predictor of mortality (HR, 0.934; 95% CI, 0.88–0.99; P = 0.02), when adjusted for age, sex, smoking status, and baseline FVC% and DlCO%. A clear threshold for resting PaO2 could not be determined (data not shown). An adjusted multivariate Cox model with the  o2max threshold (HR, 2.66; 95% CI, 0.74–9.5; P = 0.13) and resting PaO2 (HR, 0.95; 95% CI, 0.89–1.00; P = 0.09) did not show significance of either predictor. When the
o2max threshold (HR, 2.66; 95% CI, 0.74–9.5; P = 0.13) and resting PaO2 (HR, 0.95; 95% CI, 0.89–1.00; P = 0.09) did not show significance of either predictor. When the 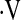 o2max threshold, resting PaO2, and desaturation below 88% during a 6MWT were included in an adjusted Cox model, the
o2max threshold, resting PaO2, and desaturation below 88% during a 6MWT were included in an adjusted Cox model, the 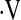 o2max threshold was a significant predictor of mortality (HR, 3.48; 95% CI, 1.16–10.43; P = 0.03), whereas desaturation less than 88% was not (HR, 1.49; 95% CI, 0.617–3.62; P = 0.37). Index of concordance analysis demonstrated that the
o2max threshold was a significant predictor of mortality (HR, 3.48; 95% CI, 1.16–10.43; P = 0.03), whereas desaturation less than 88% was not (HR, 1.49; 95% CI, 0.617–3.62; P = 0.37). Index of concordance analysis demonstrated that the 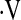 o2max threshold is a more robust predictor of survival than resting PaO2 or desaturation less than 88% during a 6MWT (Table 5).
o2max threshold is a more robust predictor of survival than resting PaO2 or desaturation less than 88% during a 6MWT (Table 5).
TABLE 5.
INDEX OF CONCORDANCE ANALYSIS COMPARING THE STRENGTH OF 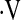 o2max < 8.3 ml/kg/min, DESATURATION < 88% DURING A 6-MINUTE WALK TEST, AND RESTING PaO2 AS PREDICTORS OF SURVIVAL IN IDIOPATHIC PULMONARY FIBROSIS
o2max < 8.3 ml/kg/min, DESATURATION < 88% DURING A 6-MINUTE WALK TEST, AND RESTING PaO2 AS PREDICTORS OF SURVIVAL IN IDIOPATHIC PULMONARY FIBROSIS
| Model | Ratio | Concordance |
|---|---|---|
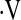 o2max < 8.3 ml/kg/min o2max < 8.3 ml/kg/min |
0.716 | 2342.5 |
| Desaturation < 88% | 0.708 | 2316.5 |
| Resting PaO2 | 0.702 | 2322.7 |
Definition of abbreviations: DlCO% = diffusion capacity for carbon monoxide percent predicted; FVC% = forced vital capacity percent predicted; PaO2 = arterial partial pressure of oxygen; 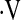 o2max = maximal oxygen consumption during cardiopulmonary exercise testing; 6MWT = 6-min-walk test.
o2max = maximal oxygen consumption during cardiopulmonary exercise testing; 6MWT = 6-min-walk test.
Covariates in each model were: age, male sex, smoking status, FVC%, and DlCO%. Number of paired observations: 3,271.
DISCUSSION
In this study, we examined the relationship between maximal oxygen uptake during cardiopulmonary exercise testing and mortality. We hypothesized that 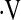 o2max measured during cardiopulmonary exercise testing predicts mortality in patients with IPF. We found that
o2max measured during cardiopulmonary exercise testing predicts mortality in patients with IPF. We found that 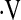 o2max examined as a continuous variable does not predict mortality in IPF. However, baseline threshold
o2max examined as a continuous variable does not predict mortality in IPF. However, baseline threshold 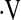 o2max of 8.3 ml/kg/min predicts mortality in these patients. This threshold is a robust predictor of survival when compared with desaturation less than 88% during a 6MWT and resting PaO2. Demographic and pulmonary function data can be used to estimate whether
o2max of 8.3 ml/kg/min predicts mortality in these patients. This threshold is a robust predictor of survival when compared with desaturation less than 88% during a 6MWT and resting PaO2. Demographic and pulmonary function data can be used to estimate whether 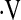 o2max is above or below the 8.3 ml/kg/min threshold.
o2max is above or below the 8.3 ml/kg/min threshold.
We found a threshold baseline 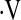 o2max less than 8.3 ml/kg/min predicts mortality in patients with IPF. A similar threshold value has not been reported in fibrotic lung disease. In a study of 86 patients with primary pulmonary hypertension, Wensel and colleagues (35) found a
o2max less than 8.3 ml/kg/min predicts mortality in patients with IPF. A similar threshold value has not been reported in fibrotic lung disease. In a study of 86 patients with primary pulmonary hypertension, Wensel and colleagues (35) found a 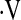 o2max threshold of 10.4 ml/kg/min predicts mortality. Various measures of exercise capacity have been examined for their ability to predict survival in IPF. A CRP score was developed by Watters and colleagues (36) as a tool to assess and follow patients' clinical impairment from IPF. The score used an exercise gas exchange score, which assigned points based on change in saturation during exercise, change in Vo2, and predicted
o2max threshold of 10.4 ml/kg/min predicts mortality. Various measures of exercise capacity have been examined for their ability to predict survival in IPF. A CRP score was developed by Watters and colleagues (36) as a tool to assess and follow patients' clinical impairment from IPF. The score used an exercise gas exchange score, which assigned points based on change in saturation during exercise, change in Vo2, and predicted 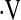 o2max. The maximum points attributable to exercise gas exchange in the CRP score was 30, greater than the radiological, symptoms, and pulmonary function components of the score, reflecting the importance of exercise gas exchange in clinical impairment in IPF. More recently, Miki and colleagues (37) calculated the change in PaO2 per change in Vo2 during CPET (ΔPaO2/ΔVo2 or PaO2 slope) and found this relationship to predict mortality in patients with IPF. However, not all studies show that CPET measurements of gas exchange predict survival (18, 19). Studies that have used multistep scores (CRP score) or slope calculations (PaO2 slope) to define the risk of mortality attributable to exercise gas exchange in IPF may be too cumbersome for routine use outside of clinical trials. Our data suggest that a simple threshold for
o2max. The maximum points attributable to exercise gas exchange in the CRP score was 30, greater than the radiological, symptoms, and pulmonary function components of the score, reflecting the importance of exercise gas exchange in clinical impairment in IPF. More recently, Miki and colleagues (37) calculated the change in PaO2 per change in Vo2 during CPET (ΔPaO2/ΔVo2 or PaO2 slope) and found this relationship to predict mortality in patients with IPF. However, not all studies show that CPET measurements of gas exchange predict survival (18, 19). Studies that have used multistep scores (CRP score) or slope calculations (PaO2 slope) to define the risk of mortality attributable to exercise gas exchange in IPF may be too cumbersome for routine use outside of clinical trials. Our data suggest that a simple threshold for 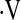 o2max of 8.3 ml/kg/min predicts mortality in patients with IPF, without the need for lengthy calculations.
o2max of 8.3 ml/kg/min predicts mortality in patients with IPF, without the need for lengthy calculations.
Several authors have examined the prognostic value of the 6MWT or other walk tests in IPF. Desaturation during 6MWT (7, 9), distance walked (9), and progressive impairment in longitudinal 6MWTs (15) have been found to predict mortality in IPF. One criticism of the 6MWT is that it is a patient-driven, symptom- and effort-limited test. This may explain the controversy in the literature about whether distance walked or desaturation is a better predictor of mortality. It may also explain why not all patients in this study with a baseline 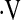 o2max below threshold had desaturation during 6MWT: these patients might not have walked sufficiently fast or far enough to produce desaturation.
o2max below threshold had desaturation during 6MWT: these patients might not have walked sufficiently fast or far enough to produce desaturation.
Using index of concordance techniques, we compared the ability of desaturation less than 88% during a 6MWT and the 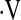 o2max threshold to predict mortality in IPF. Despite the small number of patients with
o2max threshold to predict mortality in IPF. Despite the small number of patients with 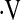 o2max below threshold at baseline, this variable was a stronger predictor than desaturation less than 88% in our cohort in multivariate Cox models. The
o2max below threshold at baseline, this variable was a stronger predictor than desaturation less than 88% in our cohort in multivariate Cox models. The 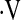 o2max threshold is also more robust than resting PaO2 and desaturation less than 88% during a 6MWT in concordance analyses.
o2max threshold is also more robust than resting PaO2 and desaturation less than 88% during a 6MWT in concordance analyses.
We were unable to identify a unit change in 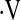 o2max per time that predicts survival in short-term follow-up. This may be due to the small number of patients who had a decline in
o2max per time that predicts survival in short-term follow-up. This may be due to the small number of patients who had a decline in 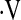 o2max during the follow-up period. This could suggest that
o2max during the follow-up period. This could suggest that 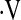 o2max is more stable over time compared with other measures, such as FVC or DlCO. It could also reflect a selection bias in that as patients became sicker they may have declined exercise testing but still been able to perform pulmonary function testing. Further prospectively collected data are needed to explore the changes in
o2max is more stable over time compared with other measures, such as FVC or DlCO. It could also reflect a selection bias in that as patients became sicker they may have declined exercise testing but still been able to perform pulmonary function testing. Further prospectively collected data are needed to explore the changes in 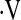 o2max over time and their impact on survival.
o2max over time and their impact on survival.
There are several limitations to this study. Patients in this study were not evaluated for the presence of pulmonary hypertension. Pulmonary hypertension has been shown to be an important predictor of mortality in IPF (38–40), although its presence does not universally portend a poor outcome (40). Patients included in this study were enrolled in a number of treatment protocols; the varied nature of the protocols prevents an analysis of the data based on therapy. However, none of the therapies in use in these protocols has been found to slow, halt, or reverse pulmonary fibrosis in this population, and prior analyses of the effects of therapy on this population have shown no benefit (7, 18, 41). In our cohort of 117 patients, 8 had a baseline 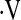 o2max below threshold and 5 had a decrease in
o2max below threshold and 5 had a decrease in 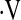 o2max to below threshold over the course of follow-up, yet 46% of the patients died during follow-up. Other measures that demonstrate significant change over time, such as serial measures of FVC%, may be more sensitive predictors of mortality in this population. Alternately, deaths could have been due to acute exacerbations of IPF or other acute events, which we did not measure.
o2max to below threshold over the course of follow-up, yet 46% of the patients died during follow-up. Other measures that demonstrate significant change over time, such as serial measures of FVC%, may be more sensitive predictors of mortality in this population. Alternately, deaths could have been due to acute exacerbations of IPF or other acute events, which we did not measure.
In this study, we examined the prognostic value of CPET in IPF. A baseline 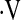 o2max less than 8.3 ml/kg/min threshold was identified, below which the risk of death was greatly increased. This study provides an easy-to-use threshold for
o2max less than 8.3 ml/kg/min threshold was identified, below which the risk of death was greatly increased. This study provides an easy-to-use threshold for 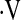 o2max for patients with IPF that predicts an increased risk of death. An unexpected finding was that not all patients with
o2max for patients with IPF that predicts an increased risk of death. An unexpected finding was that not all patients with 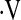 o2max below threshold desaturated during a 6MWT. Direct comparison of baseline
o2max below threshold desaturated during a 6MWT. Direct comparison of baseline 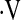 o2max less than 8.3 ml/kg/min during CPET and desaturation less than 88% during a 6MWT shows that the
o2max less than 8.3 ml/kg/min during CPET and desaturation less than 88% during a 6MWT shows that the 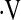 o2max threshold is a better predictor of survival in IPF. CPET and the 8.3 ml/kg/min threshold provide additional information to clinicians and their patients to help guide therapeutic decisions in IPF.
o2max threshold is a better predictor of survival in IPF. CPET and the 8.3 ml/kg/min threshold provide additional information to clinicians and their patients to help guide therapeutic decisions in IPF.
Supported by National Institute of Health NHLBI grant P50HL-56402, NHLBI, 2 K24 HL04212, 1 K23 HL68713, and 1K23 HL077719. C.D.F. was supported by the Alberta Heritage Foundation for Medical Research.
Originally Published in Press as DOI: 10.1164/rccm.200802-241OC on December 12, 2008
Conflict of Interest Statement: C.D.F. has been compensated for serving on an advisory board in 2007 for Actelion. L.X.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. E.A.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. B.H.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. W.D.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. T.V.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. G.B.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. F.J.M. is a consultant for Altana Pharma and has received compensation greater than $10K. F.J.M. has been a member of several Advisory Boards, CME committees, and the Speaker's Bureau for Boehringer Ingelheim, Pfizer, and GlaxoSmithKline. His total compensation per company is greater than $10K. In addition, F.J.M. is on the advisory board for Novartis and the Speaker's Bureau for Sepracor, Schering Plough, and Astra, receiving less than $10K per company. F.J.M. has been an investigator for industry-sponsored studies for GlaxoSmithKline, Boehringer Ingelheim, and Actelion. K.R.F. has served as a consultant for companies evaluating novel treatments for idiopathic pulmonary fibrosis, including Genzyme, Intermune, and Boehringer Ingelheim.
References
- 1.King TE Jr, Costabel U, Cordier J-F, DoPico GA, du Bois RM, Lynch D, Lynch JP, Myers J, Panos R, Raghu G, et al. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. Am J Respir Crit Care Med 2000;161:646–664. [DOI] [PubMed] [Google Scholar]
- 2.Martinez FJ, Safrin S, Weycker D, Starko KM, Bradford WZ, King TE Jr, Flaherty KR, Schwartz DA, Noble PW, Raghu G, et al. The clinical course of patients with idiopathic pulmonary fibrosis. Ann Intern Med 2005;142:963–967. [DOI] [PubMed] [Google Scholar]
- 3.Hubbard R, Johnston I, Britton J. Survival in patients with cryptogenic fibrosing alveolitis: a population-based cohort study. Chest 1998;113:396–400. [DOI] [PubMed] [Google Scholar]
- 4.King T Jr, Schwarz M, Brown K, Tooze J, Colby T, Waldron J Jr, Flint A, Thurlbeck W, Cherniack R. Idiopathic pulmonary fibrosis: Relationship between histopathologic features and mortality. Am J Respir Crit Care Med 2001;164:1025–1032. [DOI] [PubMed] [Google Scholar]
- 5.King T Jr, Tooze J, Schwarz M, Brown K, Cherniack R. Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. Am J Respir Crit Care Med 2001;164:1171–1181. [DOI] [PubMed] [Google Scholar]
- 6.Flaherty K, Toews G, Travis W, Colby T, Kazerooni E, Gross B, Jain A, Strawderman R III, Paine R III, Flint A, et al. Clinical significance of histological classification of idiopathic interstitial pneumonia. Eur Respir J 2002;19:275–283. [DOI] [PubMed] [Google Scholar]
- 7.Lama VN, Flaherty KR, Toews GB, Colby TV, Travis WD, Long Q, Murray S, Kazerooni EA, Gross BH, Lynch JP III, et al. Prognostic value of desaturation during a 6-minute walk test in idiopathic interstitial pneumonia. Am J Respir Crit Care Med 2003;168:1084–1090. [DOI] [PubMed] [Google Scholar]
- 8.Mogulkoc N, Brutsche MH, Bishop PW, Greaves SM, Horrocks AW, Egan JJ, Consortium GMPF. Pulmonary function in idiopathic pulmonary fibrosis and referral for lung transplantation. Am J Respir Crit Care Med 2001;164:103–108. [DOI] [PubMed] [Google Scholar]
- 9.Hallstrand TS, Boitano LJ, Johnson WC, Spada CA, Hayes JG, Raghu G. The timed walk test as a measure of severity and survival in idiopathic pulmonary fibrosis. Eur Respir J 2005;25:96–103. [DOI] [PubMed] [Google Scholar]
- 10.Eaton T, Young P, Milne D, Wells AU. Six-minute walk, maximal exercise tests: reproducibility in fibrotic interstitial pneumonia. Am J Respir Crit Care Med 2005;171:1150–1157. [DOI] [PubMed] [Google Scholar]
- 11.Jegal Y, Kim DS, Shim TS, Lim CM, Do Lee S, Koh Y, Kim WS, Kim WD, Lee JS, Travis WD, et al. Physiology is a stronger predictor of survival than pathology in fibrotic interstitial pneumonia. Am J Respir Crit Care Med 2005;171:639–644. [DOI] [PubMed] [Google Scholar]
- 12.Latsi PI, du Bois RM, Nicholson AG, Colby TV, Bisirtzoglou D, Nikolakopoulou A, Veeraraghavan S, Hansell DM, Wells AU. Fibrotic idiopathic interstitial pneumonia: the prognostic value of longitudinal functional trends. Am J Respir Crit Care Med 2003;168:531–537. [DOI] [PubMed] [Google Scholar]
- 13.Collard H, King T, Bartelson B, Vourlekis J, Schwarz M, Brown K. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2003;168:538–542. [DOI] [PubMed] [Google Scholar]
- 14.Flaherty K, Mumford J, Murray S, Kazerooni E, Gross B, Colby T, Travis W, Flint A, Toews G, Lynch J, et al. Prognostic implications of physiologic and radiographic changes in idiopathic interstitial pneumonia. Am J Respir Crit Care Med 2003;168:543–548. [DOI] [PubMed] [Google Scholar]
- 15.Flaherty KR, Andrei A-C, Murray S, Fraley C, Colby TV, Travis WD, Lama V, Kazerooni EA, Gross BH, Toews GB, et al. Idiopathic pulmonary fibrosis: prognostic value of changes in physiology and six minute hallwalk. Am J Resp Crit Care Med 2006;174:803–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lama VN, Martinez FJ. Resting and exercise physiology in interstitial lung diseases. Clin Chest Med 2004;25:435–453. [DOI] [PubMed] [Google Scholar]
- 17.Agusti AG, Roca J, Rodriguez-Roisin R, Xaubet A, Agusti-Vidal A. Different patterns of gas exchange response to exercise in asbestosis and idiopathic pulmonary fibrosis. Eur Respir J 1988;1:510–516. [PubMed] [Google Scholar]
- 18.Gay SE, Kazerooni EA, Toews GB, Lynch JP III, Gross BH, Cascade PN, Spizarny DL, Flint A, Schork MA, Whyte RI, et al. Idiopathic pulmonary fibrosis: predicting response to therapy and survival. Am J Respir Crit Care Med 1998;157:1063–1072. [DOI] [PubMed] [Google Scholar]
- 19.Erbes R, Schaberg T, Loddenkemper R. Lung function tests in patients with idiopathic pulmonary fibrosis: are they helpful for predicting outcome? Chest 1997;111:51–57. [DOI] [PubMed] [Google Scholar]
- 20.Hansen JE, Wasserman K. Pathophysiology of activity limitation in patients with interstitial lung disease. Chest 1996;109:1566–1576. [DOI] [PubMed] [Google Scholar]
- 21.American Thoracic Society/American College of Chest Physicians. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003;167:211–277. [DOI] [PubMed] [Google Scholar]
- 22.King TE Jr, Safrin S, Starko KM, Brown KK, Noble PW, Raghu G, Schwartz DA. Analyses of efficacy end points in a controlled trial of interferon-gamma1b for idiopathic pulmonary fibrosis. Chest 2005;127:171–177. [DOI] [PubMed] [Google Scholar]
- 23.Lynch DA, David Godwin J, Safrin S, Starko KM, Hormel P, Brown KK, Raghu G, King TE Jr, Bradford WZ, Schwartz DA, et al. High-resolution computed tomography in idiopathic pulmonary fibrosis: diagnosis and prognosis. Am J Respir Crit Care Med 2005;172:488–493. [DOI] [PubMed] [Google Scholar]
- 24.Hunninghake G, Zimmerman M, Schwartz D, King T Jr, Lynch J, Hegele R, Waldron J, Colby T, Muller N, Lynch D, et al. Utility of a lung biopsy for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2001;164:193–196. [DOI] [PubMed] [Google Scholar]
- 25.Flaherty K, Thwaite E, Kazerooni E, Gross B, Toews G, Colby T, Travis W, Mumford J, Murray S, Flint A, et al. Radiological versus histological diagnosis in UIP and NSIP: survival implications. Thorax 2003;58:143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fraley C, Martinez F, Lama V, Colby T, Travis W, Toews G, Flint A, Chang A, Flaherty K. Distance walking during a six minute walk test (6MWT) relative to the quantity of desaturation predicts mortality in patients with idiopathic pulmonary fibrosis (IPF). Proc Am Thorac Soc 2005;2:A316. [Google Scholar]
- 27.Social Security Death Index (SSDI) Interactive Search [Internet]. Provo, UT: The Generations Network, Inc. c1998– [updated 2008 Dec 23; accessed 2008 Dec 22]. Available from: http://ssdi.rootsweb.ancestry.com. (SSDI is generated from the U.S. Social Security Administration's Death Master File.)
- 28.Cox D. Regression models and life tables (with discussion). J R Stat Soc [Ser A] 1972;B34:187–220. [Google Scholar]
- 29.Gosset WSS. The probable error of a mean. Biometrika 1908;6:1–25. [Google Scholar]
- 30.K. Pearson. On the criterion that a given system of deviations from the probable in the case of a correlated system of variables is such that it can be reasonably supposed to have arisen from random sampling. Philosophical Magazine, Series 5 1900;50:157–175. [Google Scholar]
- 31.Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361–387. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–481. [Google Scholar]
- 33.Savage IR. Contributions to the theory of rank-order statistics—the two sample case. Ann Math Stat 1956;27:590–615. [Google Scholar]
- 34.Mantel N. Evaluation of survival data and two new rank-order statistics arising in its consideration. Cancer Chemother Rep 1966;50:163–170. [PubMed] [Google Scholar]
- 35.Wensel R, Opitz CF, Anker SD, Winkler J, Hoffken G, Kleber FX, Sharma R, Hummel M, Hetzer R, Ewert R. Assessment of survival in patients with primary pulmonary hypertension: importance of cardiopulmonary exercise testing. Circulation 2002;106:319–324. [DOI] [PubMed] [Google Scholar]
- 36.Watters L, King T, Schwarz M, Waldron J, Stanford R, Cherniack R. A clinical, radiographic, and physiologic scoring system for the longitudinal assessment of patients with idiopathic pulmonary fibrosis. Am Rev Respir Dis 1986;133:97–103. [DOI] [PubMed] [Google Scholar]
- 37.Miki K, Maekura R, Hiraga T, Okuda Y, Okamoto T, Hirotani A, Ogura T. Impairments and prognostic factors for survival in patients with idiopathic pulmonary fibrosis. Respir Med 2003;97:482–490. [DOI] [PubMed] [Google Scholar]
- 38.Lettieri CJ, Nathan SD, Barnett SD, Ahmad S, Shorr AF. Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis. Chest 2006;129:746–752. [DOI] [PubMed] [Google Scholar]
- 39.Nadrous HF, Pellikka PA, Krowka MJ, Swanson KL, Chaowalit N, Decker PA, Ryu JH. Pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Chest 2005;128:2393–2399. [DOI] [PubMed] [Google Scholar]
- 40.Hamada K, Nagai S, Tanaka S, Handa T, Shigematsu M, Nagao T, Mishima M, Kitaichi M, Izumi T. Significance of pulmonary arterial pressure and diffusion capacity of the lung as prognosticator in patients with idiopathic pulmonary fibrosis. Chest 2007;131:650–656. [DOI] [PubMed] [Google Scholar]
- 41.Flaherty KR, Brewer GJ, Andrei A, Murray S, Toews GB, Martinez FJ. A phase I/II trial of tetrathiomolybdate for patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2007;175:A497. [Google Scholar]