Abstract
Background
Ischaemic heart disease (IHD) can be excluded in the majority of patients with unspecific chest pain. The remainder have what is generally referred to as non-cardiac chest pain, which has been associated with gastrointestinal, neuromusculoskeletal, pulmonary, and psychiatric causes.
Aim
To assess morbidity and mortality following a new diagnosis of non-specific chest pain in patients without established IHD.
Design of study
Population-based cohort study with nested case-control analysis.
Setting
UK primary care practices contributing to the General Practice Research Database.
Method
Patients aged 20–79 years with chest pain who had had no chest pain consultation before 2000 and no IHD diagnosis before 2000 or within 2 weeks after the index date were selected from the General Practice Research Database. The selected 3028 patients and matched controls were followed-up for 1 year.
Results
The incidence of chest pain in patients without established IHD was 12.7 per 1000 person-years. In the year following the index date, patients who had chest pain but did not have established IHD were more likely than controls to receive a first IHD diagnosis (hazard ratio [HR] = 18.2, 95% confidence interval [CI] = 11.6 to 28.6) or to die (HR = 2.3, 95% CI = 1.3 to 4.1). Patients with chest pain commonly had a history of gastro-oesophageal reflux disease (GORD; odds ratio [OR] = 2.0, 95% CI = 1.5 to 2.7) or went on to be diagnosed with GORD (risk ratio 4.5, 95% CI = 3.1 to 6.4).
Conclusion
Patients with chest pain but without established IHD were found to have an increased risk of being diagnosed with IHD. Chest pain in patients without established IHD was also commonly associated with GORD.
Keywords: chest pain, gastro-oesophageal reflux disease, mortality, myocardial ischaemia, primary healthcare
INTRODUCTION
There is a well-known association between chest pain and heart disease, and this is a major cause of concern to both patients and physicians. However, ischaemic heart disease (IHD) can be excluded in the majority of patients presenting with unspecific chest pain while the remainder are considered to have what is generally called non-cardiac chest pain. Non-specific chest pain is common in the community; a recent Australian population-based study evaluating non-cardiac chest pain in 1000 individuals using a validated questionnaire reported that it affected up to 32% of men and 33% of women.1 Two other large population-based studies reported similar prevalence estimates.2,3
Non-cardiac chest pain presents a diagnostic challenge; the application of a range of diagnostic techniques to patients with non-specific chest pain has shown that it is associated with gastrointestinal, neuromusculoskeletal, pulmonary, and psychiatric diseases.4 Population-based studies have identified a similarly wide range of associated factors.4 Gastrooesophageal reflux disease (GORD) has been reported to be present in up to 60% of people with chest pain,1,5 musculoskeletal conditions have a prevalence of 25–35% in individuals with non-cardiac chest pain,1,6 and associations with anxiety and depression have also been observed.6,7
The large Australian population-based study by Eslick et al revealed that only 23% of individuals with non-cardiac chest pain had consulted a physician within the previous year, compared with 48% of individuals with cardiac chest pain.1 Given the low consultation rate for non-specific chest pain, it is unclear how the prevalence estimates and comorbidities reported by previous population-based studies relate to primary care. Here, the General Practice Research Database was utilised, a large longitudinal database of UK primary care data, to describe the characteristics, treatment patterns, and comorbidities of a cohort of patients newly diagnosed with chest pain, but without established IHD, in comparison with a cohort of individuals without a diagnosis of chest pain. Further aims were evaluating the 1-year risk of IHD and other specific comorbidities, as well as estimating mortality in both cohorts.
METHOD
The data for this population-based study were extracted from the UK General Practice Research Database. This large longitudinal primary care database contains information entered by some 1500 primary care physicians covering a population of three million individuals representative of the UK's general population.8 The primary care physicians hold the complete medical records of all individuals registered with them, including demographics, diagnoses, prescriptions, and referrals. This information is anonymised and sent to the Medicines and Healthcare products Regulatory Agency for use in research projects. The accuracy and completeness of the database has been validated by previous studies9,10 and mortality data have been used successfully in several analyses.11–13
Study population and design
The source population for this study was made up of individuals aged 20–79 years during 2000, who had been enrolled with their primary care physician for at least 2 years before the start date, and had had at least one healthcare consultation in the preceding 3 years. From this source population, patients who had had an IHD diagnosis, chest pain consultation, cardiac surgery, or cancer before 2000 were excluded, as well as women who were pregnant during the study period.
To identify cases of non-specific chest pain, all patients in the source population were followed-up from 1 January 2000 until the first occurrence of one of these endpoints:
register code of chest pain recorded using Oxford Medical Information Systems/Read codes that did not specify a type or location for the chest pain (Appendix 1);
diagnosis of cancer;
80 years of age;
death; or
the end of study period (31 December 2000).
How this fits in
There is a well-known association between chest pain and heart disease. However, ischaemic heart disease (IHD) can be excluded clinically in the majority of patients with chest pain. The remaining patients are a diagnostic challenge, as many conditions are believed to be associated with non-cardiac chest pain. This article highlights that chest pain should be considered a marker of IHD even in patients without established IHD. It is also a marker for gastro-oesophageal reflux disease and other diseases.
To avoid selecting patients in whom IHD was immediately obvious at inclusion, patients who were diagnosed with IHD within 2 weeks of the first consultation for chest pain were excluded. Individuals were also excluded if they were current users of nitrates with at least three prescriptions before the first consultation. To ensure access to all the required information, only patients registered in practices with at least 1 year of follow-up data were included in the final analysis.
The same source population and eligibility criteria were used to select an age- and sex-matched control cohort who did not have any record of chest pain. An index date in 2000 was randomly assigned to each of these individuals. Patients in both cohorts were followed for 1 year after the index date, and the risk of mortality, incident IHD, and other specific morbidities were estimated.
Data collection
Data regarding demographics, risk factors (smoking status, body mass index [BMI], and alcohol intake), and comorbidities were collected from computer records, as were the number of consultations, admissions, and referrals in the year before and the year after the index date. New prescriptions of the following drugs in the month were assessed after the index date among patients who were not prescribed them in the previous year:
non-steroidal anti-inflammatory drugs (NSAIDs);
paracetamol;
proton-pump inhibitors (PPIs);
histamine2 receptor antagonists;
antidepressants;
anxiolytics;
beta2-agonists; and
oral steroids.
The cause of death as registered by the physician was ascertained for all deaths occurring during the first year of follow-up. Patients who die while registered with a practice have their registration status changed in the database. If the cause of death is known, it is entered together with the date of death. In cases where the cause of death is unknown, the code for ‘cause of death unknown’ is entered, and the physician is asked to enter any relevant indications and remarks in the comments section. In the present study, the cause of death registered by the physician was confirmed through manual review of the computer patient profiles for all deaths that occurred within the first year of follow-up.
Statistical analyses
The incidence of non-specific chest pain was estimated according to age and sex among patients without prior IHD as the number of patients identified with a first record of chest pain divided by the total number of person-years in each stratum.
The occurrence of various health-related factors were analysed, including prescribed drug use and diagnoses of chronic diseases in the previous year, in patients with a diagnosis of non-specific chest pain and in a control cohort with no chest pain diagnosis. Logistic regression analysis was used to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) of the studied factors in association with non-specific chest pain. OR estimates were adjusted by age, sex, and number of visits to the primary care physician in the previous year. The risk of onset of a new comorbidity in the year following the first consultation for non-specific chest pain was estimated as a risk ratio, comparing the risk of patients in the non-specific chest pain group developing a new condition (proportion of new cases of a specific outcome among individuals free of this diagnosis before the index date) with the same risk for individuals in the control group. One-year mortality was estimated from the number of patients who died in the year following the index date. Proportional hazards regression analysis was used to estimate the hazard ratio (HR) and 95% CI for death and IHD diagnosis associated with non-specific chest pain, adjusting for age, sex, and other health-related factors.
To avoid potential investigation bias, a sensitivity analysis on the follow-up of IHD was also performed. Patients who received a diagnosis of IHD within the 3 months following the non-specific chest pain consultation were excluded.
RESULTS
A cohort of 6343 potential cases were identified from the source population of 628 449 individuals, from which the patients who had non-specific chest pain during 2000 were identified and registered with a primary care physician, with at least 1 year of follow-up. This gave a cohort of 3028 incident cases with non-specific chest pain, defined as a first ever consultation for non-specific chest pain not related to IHD. An age- and sex-matched control cohort who did not have any record of chest pain (n = 9000) was selected from the same source population using the same eligibility criteria.
The incidence of non-specific chest pain believed to be non-cardiac in 2000 was estimated to be 12.7 per 1000 person-years. The incidence was slightly higher in males (13.1 per 1000 person-years) than in females (12.6 per 1000 person-years) and increased from 10.6 per 1000 person-years at age 20–39 years to 15.6 per 1000 person-years at age of 70–79 years. In comparison with an age- and sex-matched control cohort, patients with non-specific chest pain were significantly more likely to be smokers (OR = 1.3, 95% CI = 1.2 to 1.4; Table 1). Alcohol intake and BMI were not significantly associated with non-specific chest pain. In the year before diagnosis, patients newly diagnosed with non-specific chest pain consulted their physician and were hospitalised significantly more frequently than individuals without non-specific chest pain.
Table 1.
Demographic characteristics and healthcare utilisation and their association with a first consultation for non-specific chest pain.
| Patients with chest pain (n = 3028), n (%) | Controlsa (n = 9000), n (%) | Odds ratiob (95% CI) | |
|---|---|---|---|
| Age, years | |||
| 20–39 | 865 (28.6) | 2534 (28.2) | n/a |
| 40–59 | 1355 (44.7) | 4024 (44.7) | |
| 60–80 | 808 (26.7) | 2442 (27.1) | |
| Sex | |||
| Male | 1527 (50.4) | 4536 (50.4) | n/a |
| Female | 1501 (49.6) | 4464 (49.6) | |
| Smoking statusc | |||
| Non-smoker | 1456 (48.1) | 4551 (50.6) | 1 |
| Smoker | 999 (33.0) | 2283 (25.4) | 1.3 (1.2 to 1.4) |
| Ex-smoker | 278 (9.2) | 829 (9.2) | 1.0(0.8 to 1.1) |
| Body mass index,d kg/m2 | |||
| <20.0 | 181 (6.0) | 498 (5.5) | 1.0(0.9 to 1.3) |
| 20.0–24.9 | 998 (33.0) | 2936 (32.6) | 1 |
| 25.0–29.9 | 887 (29.3) | 2456 (27.3) | 1.0(0.9 to 1.1) |
| ≥30.0 | 452 (14.9) | 1110 (12.3) | 1.1 (0.9 to 1.2) |
| Alcohol use,e units/wee! | |||
| None | 1018 (33.6) | 2592 (28.8) | 1 |
| 1–15 | 1153 (38.1) | 3445 (38.3) | 0.9 (0.8 to 1.0) |
| 16–42 | 264 (8.7) | 773 (8.6) | 0.9 (0.7 to 1.0) |
| >42 | 137 (4.5) | 276 (3.1) | 1.1 (0.9 to 1.4) |
| Primary care consultations in previous year | |||
| None | 328 (10.8) | 2149 (23.9) | 1 |
| 1–4 | 1287 (42.5) | 4505 (50.1) | 2.0 (1.7 to 2.2) |
| ≥5 | 1413 (46.7) | 2346 (26.1) | 4.3 (3.8 to 5.0) |
| Referrals and hospitalisation in previous year | |||
| None | 1528 (50.5) | 5717 (63.5) | 1 |
| ≥1 | 1500 (49.5) | 3283 (36.5) | 1.2 (1.1 to 1.3) |
Controls were age and sex matched to the non-specific chest pain cases.
Odds ratio estimates and 95% CIs were adjusted by age and sex and consultations in the prior year, using logistic regression.
In 14.9% of controls and 9.7% of patients, smoking status was not recorded.
In 22.2% of controls and 16.8% of cases, body mass index could not be calculated.
In 21.3% of controls and 15.1% of cases, alcohol use was not recorded.
Comorbidities in the year before non-specific chest pain consultation
Patients with non-specific chest pain were observed to be more likely than controls to have had an upper gastrointestinal complaint in the year before diagnosis. A prior consultation for GORD (OR = 2.0, 95% CI = 1.5 to 2.7) or dyspepsia (OR = 1.7, 95% CI = 1.4 to 2.2) was significantly associated with an increased risk of non-specific chest pain (Table 2). Psychological conditions such as anxiety (OR = 1.6, 95% CI = 1.2 to 2.0) were also significantly associated with a new diagnosis of non-specific chest pain, and an association with sleep disorders and depression was also observed (Table 2). Non-specific chest pain was also significantly associated with chronic obstructive pulmonary disease (COPD; OR = 1.7, 95% CI = 1.1 to 2.7), musculoskeletal disease (OR = 1.2, 95% CI = 1.1 to 1.4), and a range of painful conditions (OR = 1.5, 95% CI = 1.3 to 1.6) (Table 2).
Table 2.
Comorbidity in the year before first consultation for non-specific chest pain.
| Patients with chest pain n = 3028, n (%) | Controlsan = 9000, n (%) | ORb (95% CI) | |
|---|---|---|---|
| GORD | 90 (3.0) | 94 (1.0) | 2.0 (1.5 to 2.7) |
| Dyspepsia | 170 (5.6) | 214 (2.4) | 1.7 (1.4 to 2.2) |
| Peptic ulcer | 9 (0.3) | 12 (0.1) | 1.7 (0.7 to 4.1) |
| IBS | 35 (1.2) | 84 (0.9) | 0.8 (0.6 to 1.3) |
| IBD | 11 (0.4) | 12 (0.1) | 1.9 (0.8 to 4.4) |
| Anxiety | 135 (4.5) | 175 (1.9) | 1.6 (1.2 to 2.0) |
| Depression | 191 (6.3) | 302 (3.4) | 1.2 (1.0 to 1.5) |
| Stress | 57 (1.9) | 113 (1.3) | 1.0 (0.7 to 1.4) |
| Sleep disorders | 84 (2.8) | 122 (1.4) | 1.3 (1.0 to 1.8) |
| COPD | 39 (1.3) | 46 (0.5) | 1.7 (1.1 to 2.7) |
| Asthma | 124 (4.1) | 210 (2.3) | 1.2 (0.9 to 1.5) |
| Musculoskeletal | 777 (25.7) | 1507 (16.7) | 1.2 (1.1 to 1.4) |
| Painful conditionsc | 953 (31.5) | 1636 (18.2) | 1.5 (1.3 to 1.6) |
Controls were age- and sex-matched to those patients with chest pain.
OR estimates with their 95% CIs were adjusted by age and sex and consultations in the previous year, using logistic regression. The reference category in each case was the absence of the studied diagnosis.
Painful conditions included headache, neuralgia, menstrual and inter-menstrual pain, female genital pain, eye pain, earache, nose pain, throat pain, mouth pain, respiratory pain, abdominal pain, rectal pain, urinary-tract pain, penile and testicular pain, skin pain, breast pain, limb pain, muscular and joint pain, neck pain, and generalised pain. COPD = chronic obstructive pulmonary disease. GORD = gastro-oesophageal reflux disease. IBD = inflammatory bowel disease. IBS = irritable bowel syndrome. OR = odds ratio.
New treatment and new comorbidities in the following year
Among those untreated at the index date, patients with non-specific chest pain were much more likely than controls to begin treatment with NSAIDs or paracetamol (Table 3). It was found that 3.6% of patients with non-specific chest pain started treatment with PPIs, compared with 0.1% of controls.
Table 3.
Proportion of patients starting new drug treatment in the month after the index date.a
| Non-specific chest pain cases | Controlsb | |||
|---|---|---|---|---|
| n | new users (%) | n | new users (%) | |
| H2-RAs | 2432 | 68 (2.8) | 7871 | 8 (0.1) |
| PPIs | 2590 | 93 (3.6) | 8277 | 7 (0.1) |
| Antidepressants | 2136 | 30 (1.4) | 7329 | 11 (0.2) |
| Anxiolytics | 2572 | 10 (0.4) | 8152 | 6 (0.1) |
| NSAIDs | 1069 | 80 (7.5) | 4267 | 21 (0.5) |
| Paracetamol | 1357 | 83 (6.1) | 5364 | 15 (0.3) |
| Beta2-agonists | 2358 | 17(0.7) | 7637 | 7(0.1) |
| Oral steroids | 2437 | 11 (0.5) | 7835 | 9 (0.1) |
New drug use was defined as a prescription during the first month following the index date among those not receiving the drug before the index date.
Controls were age- and sex-matched to patients. H2-RAs = histamine2 receptor antagonists. NSAIDs = non-steroidal anti-inflammatory drugs. PPIs = proton pump inhibitors.
In the year after the index date, patients in the non-specific chest pain cohort were significantly more likely than controls to be newly diagnosed with GORD (risk ratio = 4.5, 95% CI = 3.1 to 6.4; Table 4). In addition, they had at least a twofold significantly increased risk of being diagnosed with dyspepsia, asthma, pneumonia, stress, or anxiety compared with individuals without non-specific chest pain. Weaker significant associations were observed with depression and musculoskeletal conditions, and an association of non-specific chest pain with a new diagnosis of sleep disorders was observed (Table 4).
Table 4.
Risk of new onset of specific comorbidity in the cohort with non-specific chest pain compared with controls.
| Cohort with chest pain | Controls | ||||
|---|---|---|---|---|---|
| na | New cases | na | New cases | Risk ratiob of a new diagnosis (95% CI) | |
| Gastrointestinal | |||||
| GORD | 2612 | 80 | 8287 | 55 | 4.5 (3.1 to 6.4) |
| Dyspepsia | 2297 | 103 | 7681 | 79 | 4.1 (3.0 to 5.6) |
| Peptic ulcer disease | 2910 | 7 | 8786 | 7 | 2.7 (0.9 to 8.1) |
| IBS | 2775 | 17 | 8540 | 22 | 1.8 (0.9 to 3.4) |
| Psychological | |||||
| Anxiety | 2469 | 66 | 7910 | 86 | 2.0 (1.4 to 2.8) |
| Depression | 2300 | 75 | 7585 | 115 | 1.7 (1.2 to 2.3) |
| Stress | 2739 | 46 | 8477 | 58 | 2.0 (1.4 to 3.0) |
| Sleep disorders | 2614 | 40 | 8265 | 64 | 1.5 (1.0 to 2.3) |
| Respiratory | |||||
| Asthma | 2641 | 27 | 8182 | 17 | 3.9 (2.1 to 7.4) |
| COPD | 2922 | 9 | 8833 | 25 | 0.9 (0.4 to 2.0) |
| Pneumonia | 2961 | 14 | 8834 | 9 | 3.6 (1.5 to 8.7) |
| Musculoskeletal | 1115 | 170 | 4538 | 353 | 1.9 (1.6 to 2.3) |
Number of patients without the specific diagnosis in the 12 months before chest pain consultation.
Risk ratio of the risk of first-time diagnosis of specific outcomes in the group with non-specific chest pain compared to controls. Estimates are adjusted forage, sex, visits to the GP in the previous year, BMI, alcohol use, smoking, and chest pain diagnosis, using logistic regression. COPD = chronic obstructive pulmonary disease; GORD = gastroesophageal reflux disease; IBS = irritable bowel syndrome.
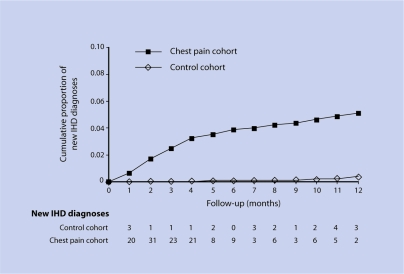
In the first year of follow-up, 137 patients in the non-specific chest pain group and 23 in the control group received a first diagnosis of IHD (Figure 1). The HR of an IHD diagnosis during the year following the index date was 18.2 (95% CI = 11.6 to 28.6) for patients with non-specific chest pain compared with controls. Among those developing IHD, an episode of acute myocardial infarction was confirmed in 12 patients in the non-specific chest pain group and eight controls, corresponding to an HR of a first myocardial infarction associated with non-specific chest pain of 4.1 (95% CI = 1.6 to 10.3). Of the patients with non-specific chest pain who developed IHD, most were males and were significantly older than those who did not develop IHD. These patients also had several known cardiovascular risk factors such as hypertension and hyperlipidaemia, and had experienced repeated consultations for chest pain (data not shown). Indeed, a sensitivity analysis using the cut-off criteria of the first 3 months of follow-up showed that the association with chest pain decreases in strength, but not significance (HR 11.3; 95% CI = 6.7 to 19.1).
Figure 1.
Time to new diagnosis of IHD during the 1-year follow-up period.
One-year mortality among patients with non-specific chest pain (9.8 per 1000 persons) was higher than for controls (3.9 per 1000 persons). The HR of death associated with non-specific chest pain in the first year was 2.3 (95% CI = 1.3 to 4.1). There were several causes of death in the group of patients with non-specific chest pain, including cancer, cerebrovascular disease, and ischaemic disease, while cancer was the main cause of death in the control group (33% of patients). Crude and adjusted HRs are presented in Appendix 2.
DISCUSSION
Summary of main findings
A total of 3028 patients were identified without established IHD who had their first ever consultation for chest pain in UK primary care during 2000. The chest pain was considered to be non-cardiac due to the fact that patients did not have a prior IHD diagnosis or receive one within the 2 weeks following the chest pain consultation. On this basis, the incidence of non-specific chest pain was shown to be 12.7 per 1000 person-years. Patients with non-specific chest pain were more likely to be smokers and to have consulted their physician more frequently than those individuals who had no chest pain.
In the first year of follow-up, patients with chest pain but no history of IHD were more likely than controls to receive a first diagnosis of IHD. The results also show that, not only are patients with non-specific chest pain, that is believed to be non-cardiac in origin, more likely to have a history of GORD, but that, after IHD, GORD is the most strongly associated comorbidity diagnosed in the year following chest pain consultation. Furthermore, it was found that 1-year mortality was higher for patients with non-specific chest pain than for controls. This increased risk of death was consistent with previous findings,14 and was mainly due to IHD-and cancer-related deaths.
Strengths and limitations of this study
The use of the General Practice Research Database allowed data for this study to be collected prospectively from a large representative sample of the UK general population registered with a primary care physician. Consultation rates for non-specific chest pain are known to be low,7 but the actual incidence of non-specific chest pain is likely to be higher than was assessed. In order to avoid as much potential consultation bias as possible, it was requested that both groups of patients (those with and without chest pain) had undergone at least one healthcare consultation within the previous 3 years.
As is common in epidemiological studies, non-specific chest pain was defined as unexplained chest pain in the absence of prior IHD. Although the results are not necessarily generalisable to the broad population of patients diagnosed as having non-cardiac chest pain in primary care, they are representative of the broader population of patients consulting with chest pain in whom no underlying cause is immediately reached. The study relied on the diagnosis entered by the primary care physicians and, therefore, included potential misdiagnoses. Indeed, a high incidence of IHD was found in the year following non-specific chest pain consultation among patients who were free of IHD before the index date. This suggests that a cardiovascular diagnosis was missed in some cases. A 2-week period was allocated following the first consultation for chest pain to allow for a diagnosis of IHD to occur. Although this time frame was arbitrary, data from Figure 1 showing that IHD diagnoses occurred gradually over the 1-year follow-up period suggest that these results would not be markedly affected by extending this period of time. Indeed, a sensitivity analysis using a wider cut-off criterion of 3 months showed that the association with chest pain decreases in strength, but not significance.
It should also be noted that the database does not definitively link subsequent diagnoses to chest pain; as such, although many patients did not receive any diagnosis during the study period, others may have received more than one. Indeed, the data shows that, at most, only 20% of the cohort with non-specific chest pain received a new diagnosis of one of the studied conditions during the follow-up period.
Comparison with existing literature
A recent review reported that up to 39% of patients presenting with chest pain for the first time to emergency care are ultimately diagnosed with coronary artery disease.15 It has also been shown that approximately 4% of patients presenting in emergency care with chest pain are diagnosed with acute myocardial infarction.16 A multicentre cohort study of patients diagnosed with non-cardiac chest pain attending rapid-access chest pain clinics reported that 2.73% were subsequently diagnosed with angina.17 As the data are based on recorded diagnoses rather than patient recall, as were previous studies,1,6 the results are not completely comparable. The data reveal that, among patients presenting with unspecific chest pain and without a history of coronary disease, over 5% were diagnosed with IHD in the year following their consultation, a rate 18 times higher than in the control population.
GORD
Previous studies that investigated patients with non-cardiac chest pain in secondary care using oesophageal endoscopy and pH monitoring have reported the oesophagus to be a potential origin of non-cardiac chest pain in up to two-thirds of patients, with GORD being the most likely cause.18–21 A study in UK primary care reported that IHD was ruled out in half of all patients consulting with chest pain, and that the chest pain had an oesophageal origin in 10% of patients.22 Similarly, in this study it was found that 10% of patients with non-specific chest pain reported gastrointestinal complaints such as GORD, dyspepsia, or peptic disease during the year preceding the index date, and that 3% of patients with non-specific chest pain had previously received a GORD diagnosis.
In the year following a first consultation for non-specific chest pain, the strongest new association with chest pain after IHD was GORD. According to the recent Montreal Definition of GORD, non-cardiac chest pain is considered an ‘atypical’ GORD symptom.23 However, confirmation of GORD as the cause of non-specific chest pain in individual patients is often difficult and may need expensive, prolonged pH-monitoring or manometry.15,24,25
In this study, among those patients not previously consulting for reflux symptoms, there was a greater than fourfold increased risk of developing GORD or dyspepsia after the initial consultation for non-specific chest pain. Furthermore, it has been shown that, among those untreated at the index date, patients with non-specific chest pain were much more likely than controls to begin new treatment with PPIs, which is likely to be a reflection of new GORD diagnoses. The new PPI prescriptions may also indicate their usage as a diagnostic tool for GORD; a recent systematic review supports the use of a PPI as a cost-effective aid in the diagnosis of GORD in patients with non-cardiac chest pain.26
Psychological conditions
It has been shown that there is an association between non-specific chest pain and diagnoses of anxiety or depression in the year before the index date. Other studies have shown that up to 30% of patients with non-cardiac or non-specific chest pain experience panic disorder and anxiety.27–29 The magnitude of the association observed with anxiety is similar to that reported previously in cross-sectional studies.1 It was also found that patients with non-specific chest pain had an up to twofold increased risk of receiving a new diagnosis of stress, anxiety, or depression, which is in line with cross-sectional data from a previous population-based study.6
Other conditions
Non-specific chest pain was associated with a diagnosis of COPD in the year before the index date. It was also found that patients consulting for non-specific chest pain had an approximate threefold increased risk of receiving a diagnosis of respiratory conditions such as asthma or pneumonia in the year following the index date. There is very little published evidence of an association between asthma and non-cardiac chest pain; however, it is possible that the chest tightness associated with asthma may be diagnosed as chest pain. Another possibility is that, as with non-cardiac chest pain, asthma has been associated with GORD, which perhaps further implicates GORD as an underlying factor in non-cardiac or non-specific chest pain.23
Musculoskeletal disorders and trauma could also be a cause of non-specific chest pain. A close to twofold increased risk of a new diagnosis of musculoskeletal diseases among patients consulting for non-specific chest pain, was also found which is similar to previously reported values.30,31
Implications for future research and clinical practice
The data here highlight the need for a firm diagnosis to be reached for patients presenting with chest pain in primary care. The data show that at least 80% of patients who consulted for the first time for non-specific chest pain did not receive a diagnosis in the 1-year follow-up period that could explain this symptom. Data here have shown that, compared with controls, patients with chest pain that is believed to be non-cardiac in origin are more likely to receive a diagnosis of IHD in the following year. Therefore, it is imperative that caution is exercised when excluding a cardiac cause for chest pain; monitoring is essential for these patients, particularly for those with established risk factors for IHD such as hypertension and hyperlipidaemia.
Chest pain remains a diagnostic challenge in primary care, and patients who do not receive a definitive diagnosis accumulate substantial healthcare costs through frequent primary care and emergency hospital visits, as well as inappropriate drug therapies.32 These patients also report a poorer quality of life.1,33,34 Prompt diagnosis and treatment are, therefore, essential to optimise the management of these patients.
This study highlights the need for improved methods of differentiating between cardiac and non-cardiac chest pain. It also emphasises the need for a firm diagnosis, so future research should concentrate on these areas.
Acknowledgments
We thank Dr Catriona Turnbull, of Oxford PharmaGenesis, who provided medical writing support funded by AstraZeneca R&D Mölndal, Sweden.
Appendix 1 Diagnostic codes selected from the General Practice Research Database.
| Dictionary | Code | Descriptor |
|---|---|---|
| OXMIS | 7837B | Pain chest |
| OXMIS | 7837BB | Sore chest |
| OXMIS | 7837BC | Ache chest |
| READ | R065.00 | [D] Chest pain |
| READ | R065000 | [D] Chest pain, unspecified |
| READ | R065z00 | [D] Chest pain NOS |
| READ | 182..00 | Chest pain |
| READ | 182Z.00 | Chest pain NOS |
[D] = working diagnosis, where a specific diagnosis is not yet ascertained; OXMIS = Oxford Medical Information Systems.
Appendix 2 Crude and adjusted hazard ratios associated with non-specific chest pain.
| Crude HR (95% CI) | Adjusteda HR (95% CI) | |
|---|---|---|
| 1-year mortality | 2.4 (1.4 to 4.1) | 2.3 (1.3 to 4.1) |
| 1-year IHD diagnosis | 18.2 (11.7 to 28.3) | 18.2 (11.6 to 28.6) |
| 1-year MI | 4.6 (1.9 to 11.2) | 4.1 (1.6 to 10.3) |
Adjusted by age sex visits BMI, alcohol and smoke status using Cox Regression. IHD = ischaemic heart disease. MI = myocardial infarction. HR = hazard ratio.
Funding body
This study was partially supported by an unrestricted research grant from ASTRAZENECA R&D, Mölndan Sweden
Ethics committee
Protocol was approved on 21/11/02 by the GPRD Scientific and Ethical Committee (SEAG num ref: 499)
Competing interests
In addition to the posts listed, Saga Johansson and Mari-Ann Wallander are also employees of AstraZeneca R&D Mölndal, Sweden
Discuss this article
Contribute and read comments about this article on the Discussion Forum: http://www.rcgp.org.uk/bjgp-discuss
REFERENCES
- 1.Eslick GD, Jones MP, Talley NJ. Non-cardiac chest pain: prevalence, risk factors, impact and consulting—a population-based study. Aliment Pharmacol Ther. 2003;17(9):1115–1124. doi: 10.1046/j.1365-2036.2003.01557.x. [DOI] [PubMed] [Google Scholar]
- 2.Locke GR, III, Talley NJ, Fett SL, et al. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112(5):1448–1456. doi: 10.1016/s0016-5085(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 3.Lampe FC, Whincup PH, Wannamethee SG, et al. Chest pain on questionnaire and prediction of major ischaemic heart disease events in men. Eur Heart J. 1998;19(1):63–73. doi: 10.1053/euhj.1997.0729. [DOI] [PubMed] [Google Scholar]
- 4.Flook N, Unge P, Agréus L, et al. Approach to managing undiagnosed chest pain: could gastroesophageal reflux disease be the cause? Can Fam Physician. 2007;53(2):261–266. [PMC free article] [PubMed] [Google Scholar]
- 5.Wong WM, Lai KC, Lau CP, et al. Upper gastrointestinal evaluation of Chinese patients with non-cardiac chest pain. Aliment Pharmacol Ther. 2002;16(3):465–471. doi: 10.1046/j.1365-2036.2002.01217.x. [DOI] [PubMed] [Google Scholar]
- 6.Wong WM, Lam KF, Cheng C, et al. Population based study of noncardiac chest pain in southern Chinese: prevalence, psychosocial factors and health care utilization. World J Gastroenterol. 2004;10(5):707–712. doi: 10.3748/wjg.v10.i5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eslick GD. Noncardiac chest pain: epidemiology, natural history, health care seeking, and quality of life. Gastroenterol Clin North Am. 2004;33(1):1–23. doi: 10.1016/S0889-8553(03)00125-0. [DOI] [PubMed] [Google Scholar]
- 8.García Rodríguez LA, Pérez Gutthann S. Use of the UK General Practice Research Database for pharmacoepidemiology. Br J Clin Pharmacol. 1998;45(5):419–425. doi: 10.1046/j.1365-2125.1998.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jick H, Jick SS, Derby LE. Validation of information recorded on general practitioner based computerised data resource in the United Kingdom. BMJ. 1991;302(6779):766–768. doi: 10.1136/bmj.302.6779.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jick SS, Kaye JA, Vasilakis-Scaramozza C, et al. Validity of the general practice research database. Pharmacotherapy. 2003;23(5):686–689. doi: 10.1592/phco.23.5.686.32205. [DOI] [PubMed] [Google Scholar]
- 11.Watson DJ, Rhodes T, Guess HA. All-cause mortality and vascular events among patients with rheumatoid arthritis, osteoarthritis, or no arthritis in the UK General Practice Research Database. J Rheumatol. 2003;30(6):1196–1202. [PubMed] [Google Scholar]
- 12.Lawrenson R, Wyndaele JJ, Vlachonikolis I, et al. A UK general practice database study of prevalence and mortality of people with neural tube defects. Clin Rehabil. 2000;14(6):627–630. doi: 10.1191/0269215500cr371oa. [DOI] [PubMed] [Google Scholar]
- 13.Ruigómez A, Johansson S, Wallander MA, García Rodríguez LA. Predictors and prognosis of paroxysmal atrial fibrillation in general practice in the UK. BMC Cardiovasc Disord. 2005;5:20. doi: 10.1186/1471-2261-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilhelmsen L, Rosengren A, Hagman M, Lappas G. ‘Nonspecific’ chest pain associated with high long-term mortality: results from the primary prevention study in Göteborg, Sweden. Clin Cardiol. 1998;21(7):477–482. doi: 10.1002/clc.4960210706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kachintorn U. How do we define non-cardiac chest pain? J Gastroenterol Hepatol. 2005;(20 Suppl):S2–5. doi: 10.1111/j.1440-1746.2005.04164.x. [DOI] [PubMed] [Google Scholar]
- 16.Kohn MA, Kwan E, Gupta M, Tabas JA. Prevalence of acute myocardial infarction and other serious diagnoses in patients presenting to an urban emergency department with chest pain. J Emerg Med. 2005;29(4):383–390. doi: 10.1016/j.jemermed.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Sekhri N, Feder GS, Junghans C, et al. How effective are rapid access chest pain clinics? Prognosis of incident angina and non-cardiac chest pain in 8762 consecutive patients. Heart. 2007;93(4):458–463. doi: 10.1136/hrt.2006.090894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hewson EG, Sinclair JW, Dalton CB, Richter JE. Twenty-four-hour esophageal pH monitoring: the most useful test for evaluating noncardiac chest pain. Am J Med. 1991;90(5):576–583. [PubMed] [Google Scholar]
- 19.De Caestecker JS, Blackwell JN, Brown J, Heading RC. The oesophagus as a cause of recurrent chest pain: which patients should be investigated and which tests should be used? Lancet. 1985;2(8465):1143–1146. doi: 10.1016/s0140-6736(85)92676-5. [DOI] [PubMed] [Google Scholar]
- 20.Fruergaard P, Launbjerg J, Hesse B, et al. The diagnoses of patients admitted with acute chest pain but without myocardial infarction. Eur Heart J. 1996;17(7):1028–1034. doi: 10.1093/oxfordjournals.eurheartj.a014998. [DOI] [PubMed] [Google Scholar]
- 21.DeMeester TR, O'Sullivan GC, Bermudez G, et al. Esophageal function in patients with angina-type chest pain and normal coronary angiograms. Ann Surg. 1982;196(4):488–498. doi: 10.1097/00000658-198210000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nilsson S, Scheike M, Engblom D, et al. Chest pain and ischaemic heart disease in primary care. Br J Gen Pract. 2003;53(490):378–382. [PMC free article] [PubMed] [Google Scholar]
- 23.Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101(8):1900–1920. doi: 10.1111/j.1572-0241.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 24.Heading RC. Review article: diagnosis and clinical investigation of gastro-oesophageal reflux disease: a European view. Aliment Pharmacol Ther. 2004;(20 Suppl 8):9–13. doi: 10.1111/j.1365-2036.2004.02221.x. [DOI] [PubMed] [Google Scholar]
- 25.Heatley M, Rose K, Weston C. The heart and the oesophagus: intimate relations. Postgrad Med J. 2005;81(958):515–518. doi: 10.1136/pgmj.2004.029074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cremonini F, Wise J, Moayyedi P, Talley NJ. Diagnostic and therapeutic use of proton pump inhibitors in non-cardiac chest pain: a metaanalysis. Am J Gastroenterol. 2005;100(6):1226–1232. doi: 10.1111/j.1572-0241.2005.41657.x. [DOI] [PubMed] [Google Scholar]
- 27.Cayley WE., Jr Diagnosing the cause of chest pain. Am Fam Physician. 2005;72(10):2012–2021. [PubMed] [Google Scholar]
- 28.Hamer HP, McCallin AM. Cardiac pain or panic disorder? Managing uncertainty in the emergency department. Nurs Health Sci. 2006;8(4):224–230. doi: 10.1111/j.1442-2018.2006.00287.x. [DOI] [PubMed] [Google Scholar]
- 29.Fleet RP, Dupuis G, Marchand A, et al. Panic disorder in emergency department chest pain patients: prevalence, comorbidity, suicidal ideation, and physician recognition. Am J Med. 1996;101(4):371–380. doi: 10.1016/S0002-9343(96)00224-0. [DOI] [PubMed] [Google Scholar]
- 30.Mukerji B, Mukerji V, Alpert MA, Selukar R. The prevalence of rheumatologic disorders in patients with chest pain and angiographically normal coronary arteries. Angiology. 1995;46(5):425–430. doi: 10.1177/000331979504600510. [DOI] [PubMed] [Google Scholar]
- 31.Jensen S. Musculoskeletal causes of chest pain. Aust Fam Physician. 2001;30(9):834–839. [PubMed] [Google Scholar]
- 32.Fang J, Bjorkman D. A. critical approach to noncardiac chest pain: pathophysiology, diagnosis, and treatment. Am J Gastroenterol. 2001;96(4):958–968. doi: 10.1111/j.1572-0241.2001.03678.x. [DOI] [PubMed] [Google Scholar]
- 33.Atienza F, Velasco JA, Brown S, et al. Assessment of quality of life in patients with chest pain and normal coronary arteriogram (syndrome X) using a specific questionnaire. Clin Cardiol. 1999;22(4):283–290. doi: 10.1002/clc.4960220406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karlson BW, Wiklund I, Bengtson A, Herlitz J. Prognosis, severity of symptoms, and aspects of well-being among patients in whom myocardial infarction was ruled out. Clin Cardiol. 1994;17(8):427–431. doi: 10.1002/clc.4960170805. [DOI] [PubMed] [Google Scholar]