Abstract
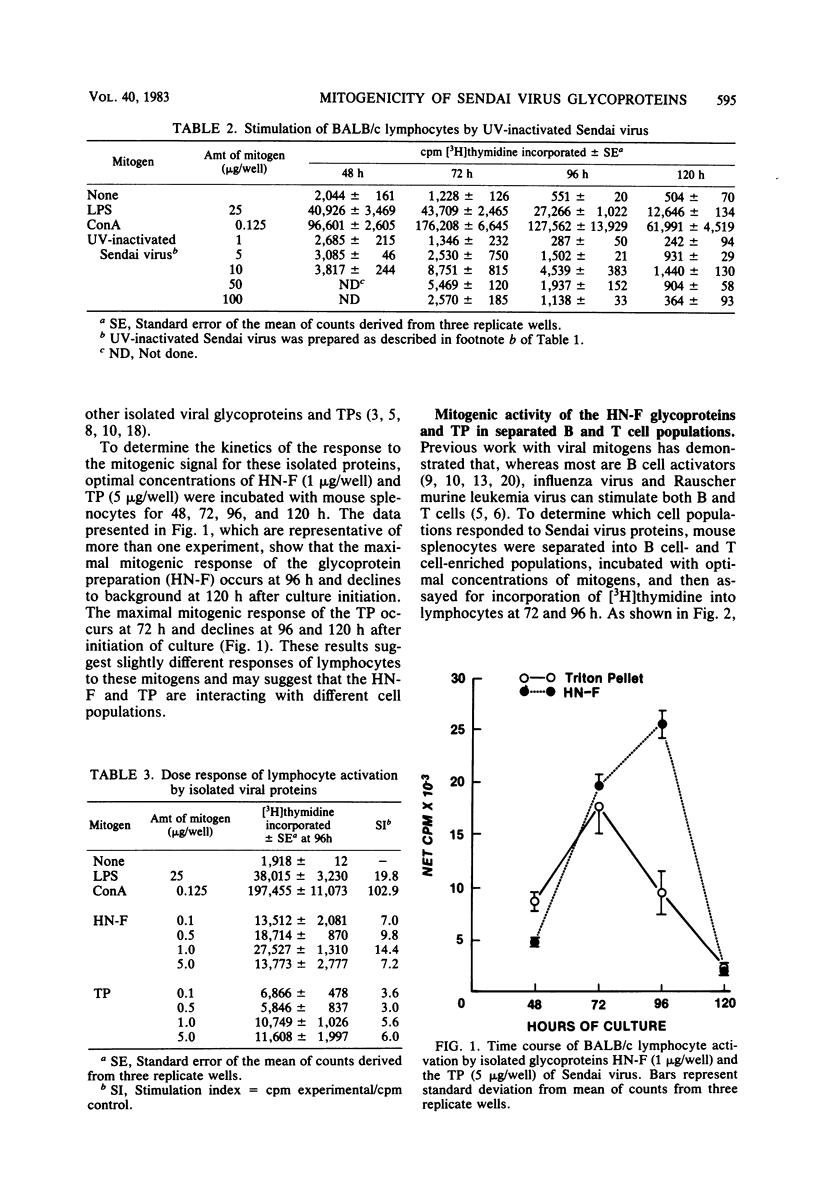
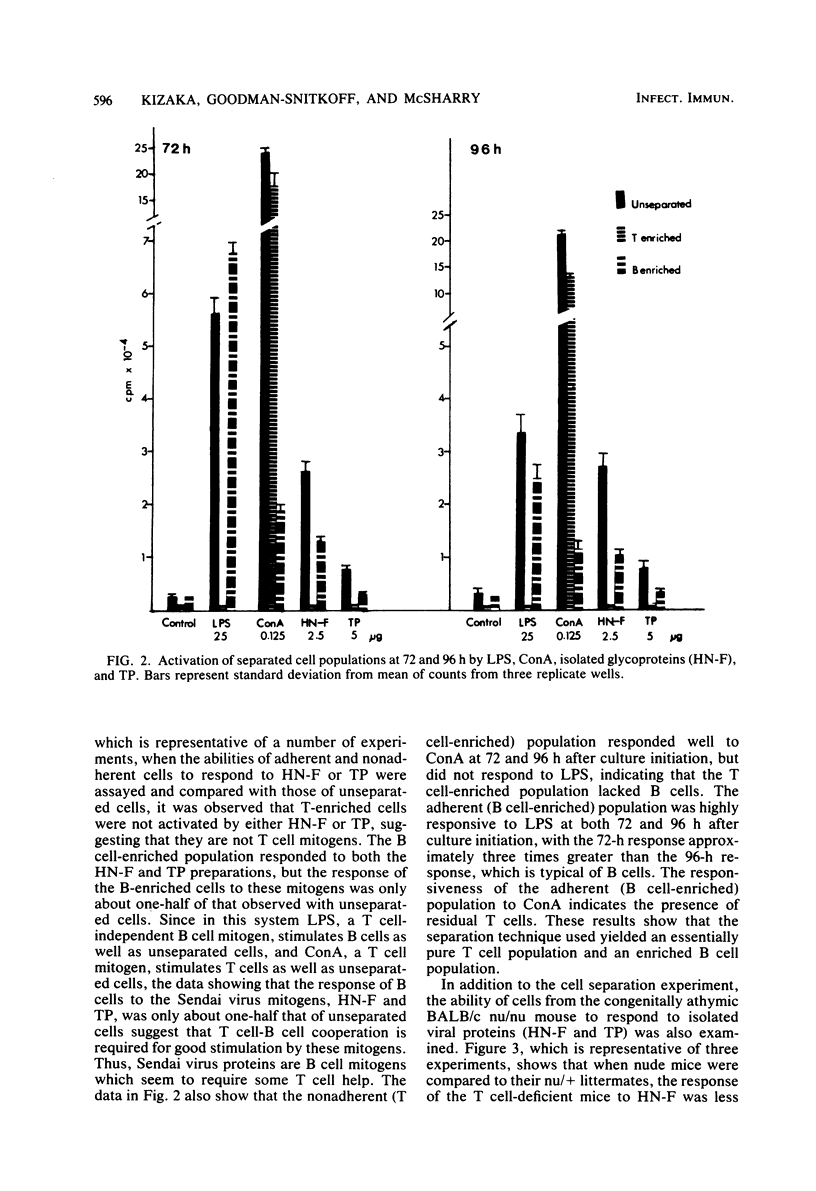
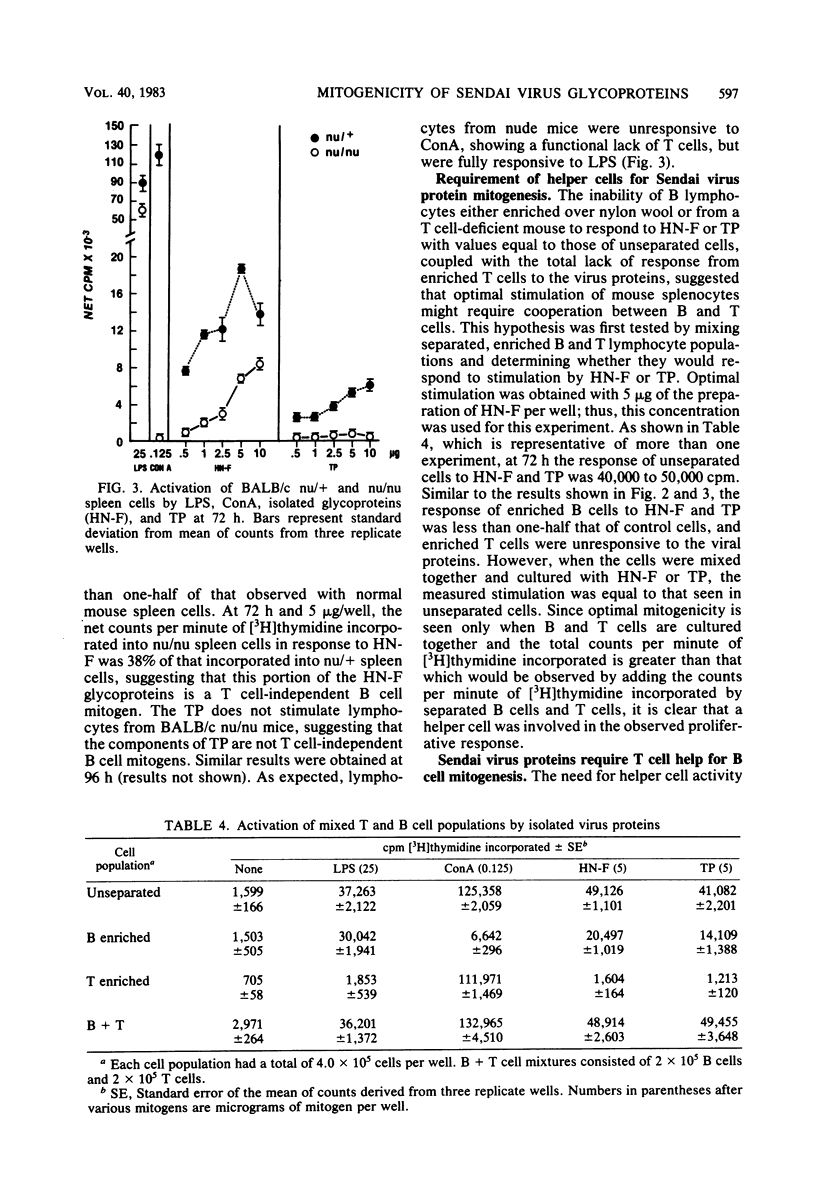
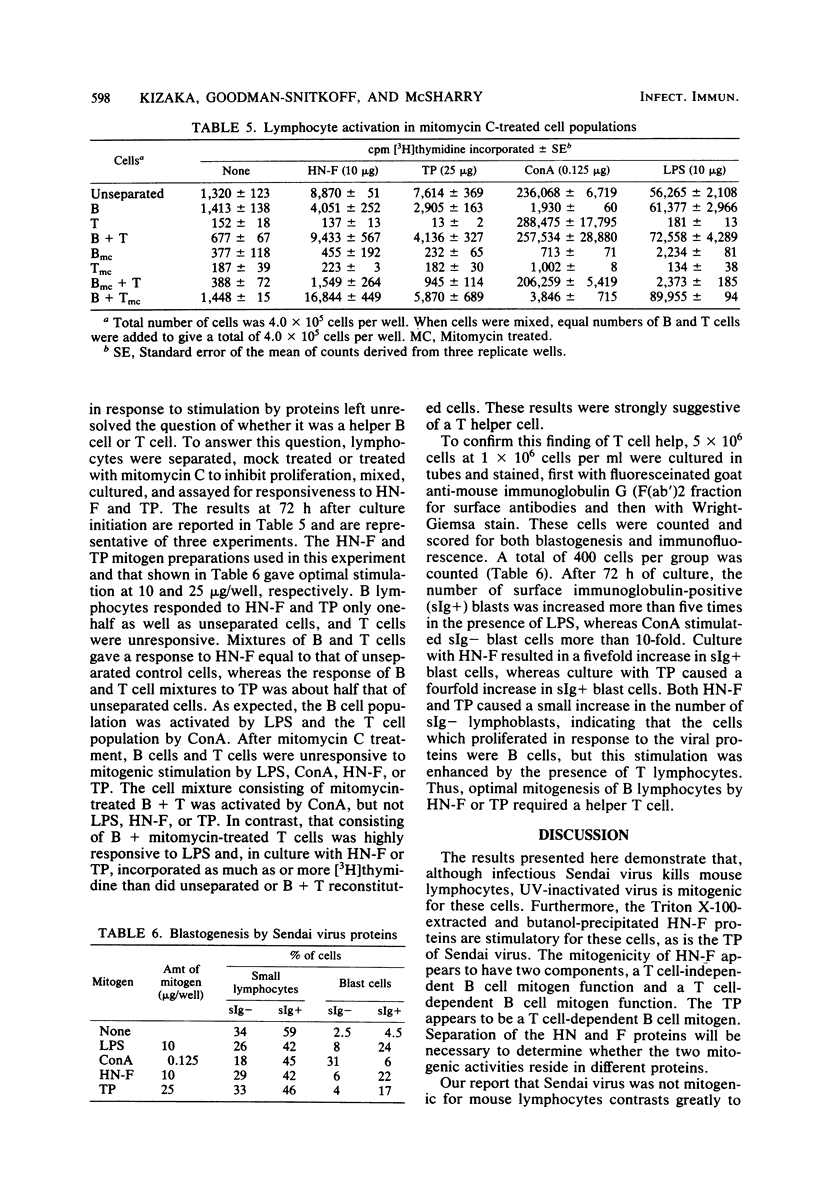
UV-inactivated Sendai virus is mitogenic for murine splenocytes, whereas infectious Sendai virus kills spleen cells in vitro. The isolated hemagglutinin-neuraminidase (HN) and fusion (F) glycoproteins of Sendai virus are also mitogenic for cultured mouse spleen cells. A mixture of these glycoproteins (1 microgram/well) gives maximum stimulation 96 h after culture initiation. Viral proteins remaining insoluble after Triton X-100 extraction are also mitogenic for mouse spleen cells, with maximum stimulation occurring at 72 h after culture initiation with 1 to 5 microgram/well. On the basis of protein concentration, the HN and F glycoproteins are approximately three times more mitogenic than the Triton X-100-insoluble material. The mitogenic response of the HN and F glycoproteins has two components, a T cell-independent B cell proliferation, which is less than one-half of the total stimulation observed, and a T cell-dependent B cell proliferation. In contrast, the Triton X-100-insoluble material is a T cell-dependent B cell mitogen. Purified T lymphocytes do not respond to the mitogenic signal of either HN-F or Triton X-100-insoluble material.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. J., Bainbridge D. R., Pattison J. R., Heath R. B. Cell-mediated immunity to Sendai virus infection in mice. Infect Immun. 1977 Jan;15(1):239–244. doi: 10.1128/iai.15.1.239-244.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M. J., Pattison J. R., Cureton R. J., Argent S., Heath R. B. The role of host responses in the recovery of mice from Sendai virus infection. J Gen Virol. 1980 Feb;46(2):373–379. doi: 10.1099/0022-1317-46-2-373. [DOI] [PubMed] [Google Scholar]
- Armstrong R. B., Butchko G. M., Kiley S. C., Phelan M. A., Ennis F. A. Mitogenicity of influenza hemagglutinin glycoproteins and influenza viruses bearing H2-hemagglutinin. Infect Immun. 1981 Oct;34(1):140–143. doi: 10.1128/iai.34.1.140-143.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braciale T. J., Yap K. L. Role of viral infectivity in the induction of influenza virus-specific cytotoxic T cells. J Exp Med. 1978 Apr 1;147(4):1236–1252. doi: 10.1084/jem.147.4.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubbers J. E., Elder J. H., Dixon F. J. Stimulation of murine lymphocytes by Rauscher leukemia virus in vitro. J Immunol. 1980 Jan;124(1):388–394. [PubMed] [Google Scholar]
- Butchko G. M., Armstrong R. B., Martin W. J., Ennis F. A. Influenza A viruses of the H2N2 subtype are lymphocyte mitogens. Nature. 1978 Jan 5;271(5640):66–67. doi: 10.1038/271066a0. [DOI] [PubMed] [Google Scholar]
- Gething M., Koszinowski U., Waterfield M. Fusion of Sendai virus with the target cell membrane is required for T cell cytotoxicity. Nature. 1978 Aug 17;274(5672):689–691. doi: 10.1038/274689a0. [DOI] [PubMed] [Google Scholar]
- Goodman-Snitkoff G. W., McSharry J. J. Activation of mouse lymphocytes by vesicular stomatitis virus. J Virol. 1980 Sep;35(3):757–765. doi: 10.1128/jvi.35.3.757-765.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman-Snitkoff G., Mannino R. J., McSharry J. J. The glycoprotein isolated from vesicular stomatitis virus is mitogenic for mouse B lymphocytes. J Exp Med. 1981 Jun 1;153(6):1489–1502. doi: 10.1084/jem.153.6.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman-Snitkoff G., McSharry J. J. Mitogenic activity of Sindbis virus and its isolated glycoproteins. Infect Immun. 1982 Dec;38(3):1242–1248. doi: 10.1128/iai.38.3.1242-1248.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale A. H., Lyles D. S., Fan D. P. Elicitation of anti-Sendai virus cytotoxic T lymphocytes by viral and H-2 antigens incorporated into the same lipid bilayer by membrane fusion and by reconstitution into liposomes. J Immunol. 1980 Feb;124(2):724–731. [PubMed] [Google Scholar]
- Hodes R. J., Handwerger B. S., Terry W. D. Synergy between subpopulations of mouse spleen cells in the in vitro generation of cell-mediated cytotoxicity: evidence for the involvement of a non-T cell. J Exp Med. 1974 Dec 1;140(6):1646–1659. doi: 10.1084/jem.140.6.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner H., Darai G., Hirt H. M., Keyssner K., Munk K. In vitro mitogenic stimulation of murine spleen cells by herpes simplex virus. J Immunol. 1978 Feb;120(2):641–645. [PubMed] [Google Scholar]
- Koszinowski U. H., Simon M. M. Generation of virus-specific cytotoxic T cells in vitro. I. Induction conditions of primary and secondary Sendai virus-specific cytotoxic T cells. Eur J Immunol. 1979 Sep;9(9):715–722. doi: 10.1002/eji.1830090910. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McGee M., Hale A. H., Panetti M. Elicitation of primary anti-Sendai virus cytotoxic T lymphocytes with purified viral glycoproteins. Eur J Immunol. 1980 Dec;10(12):923–928. doi: 10.1002/eji.1830101207. [DOI] [PubMed] [Google Scholar]
- McSharry J. J., Choppin P. W. Biological properties of the VSV glycoprotein. 1. Effects of the isolated glycoprotein on host macromolecular synthesis. Virology. 1978 Jan;84(1):172–182. doi: 10.1016/0042-6822(78)90229-5. [DOI] [PubMed] [Google Scholar]
- Miskimen J. A., Guertin D. P., Fan D. P., David C. S. Influence of H-2-linked genes on T cell proliferative and cytolytic responses to peptides of Sendai viral proteins. J Immunol. 1982 Apr;128(4):1522–1528. [PubMed] [Google Scholar]
- Mochizuki D., Hedrick S., Watson J., Kingsbury D. T. The interaction of Herpes Simplex Virus with murine lymphocytes. I. Mitogenic properties of herpes simplex virus. J Exp Med. 1977 Dec 1;146(6):1500–1510. doi: 10.1084/jem.146.6.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. C., Lewandowski L. J., Waters D. Non-infectious virus induces cytotoxic T lymphocytes and binds to target cells to permit their lysis. Nature. 1977 Oct 13;269(5629):595–597. doi: 10.1038/269595a0. [DOI] [PubMed] [Google Scholar]
- Roberts N. J., Jr Different effects of influenza virus, respiratory syncytial virus, and Sendai virus on human lymphocytes and macrophages. Infect Immun. 1982 Mar;35(3):1142–1146. doi: 10.1128/iai.35.3.1142-1146.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson T. W., Cureton R. J., Heath R. B. The effect of cyclophosphamide on Sendai virus infection of mice. J Med Microbiol. 1969 May;2(2):137–145. doi: 10.1099/00222615-2-2-137. [DOI] [PubMed] [Google Scholar]
- Robinson T. W., Cureton R. J., Heath R. B. The pathogenesis of Sendai virus infection in the mouse lung. J Med Microbiol. 1968 Aug;1(1):89–95. doi: 10.1099/00222615-1-1-89. [DOI] [PubMed] [Google Scholar]
- Wainberg M. A., Israel E. Viral inhibition of lymphocyte mitogenesis. I. Evidence for the nonspecificity of the effect. J Immunol. 1980 Jan;124(1):64–70. [PubMed] [Google Scholar]
- Wiktor T. J., Doherty P. C., Koprowski H. In vitro evidence of cell-mediated immunity after exposure of mice to both live and inactivated rabies virus. Proc Natl Acad Sci U S A. 1977 Jan;74(1):334–338. doi: 10.1073/pnas.74.1.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Althage A., Holland J. Target antigens for H-2-restricted vesicular stomatitis virus-specific cytotoxic T cells. J Immunol. 1978 Aug;121(2):744–748. [PubMed] [Google Scholar]


