Abstract
Background
Sustained agonist-promoted ubiquitination of β-arrestin has been correlated with increased stability of the GPCR – β-arrestin complex. Moreover, abrogation of β-arrestin ubiquitination has been reported to inhibit receptor internalization with minimal effects on receptor degradation.
Results
Herein we report that agonist activation of M1 mAChRs produces a sustained β-arrestin ubiquitination but no stable co-localization with β-arrestin. In contrast, sustained ubiquitination of β-arrestin by activation of M2 mAChRs does result in stable co-localization between the M2 mAChR and β-arrestin. Internalization of receptors was unaffected by proteasome inhibitors, but down-regulation was significantly reduced, suggesting a role for the ubiquitination machinery in promoting down-regulation of the receptors. Given the ubiquitination status of β-arrestin following agonist treatment, we sought to determine the effects of β-arrestin ubiquitination on M1 and M2 mAChR down-regulation. A constitutively ubiquitinated β-arrestin 2 chimera in which ubiquitin is fused to the C-terminus of β-arrestin 2 (YFP-β-arrestin 2-Ub) significantly increased agonist-promoted down-regulation of both M1 and M2 mAChRs, with the effect substantially higher on the M2 mAChR. Based on this observation, we were interested in examining the effects of disruption of potential ubiquitination sites in the β-arrestin sequence on receptor down-regulation. Agonist-promoted internalization of the M2 mAChR was not affected by expression of β-arrestin lysine mutants lacking putative ubiquitination sites, β-arrestin 2K18R, K107R, K108R, K207R, K296R, while down-regulation and stable co-localiztion of the receptor with this β-arrestin lysine mutant were significantly reduced. Interestingly, expression of β-arrestin 2K18R, K107R, K108R, K207R, K296R increased the agonist-promoted down-regulation of the M1 mAChR but did not result in a stable co-localiztion of the receptor with this β-arrestin lysine mutant.
Conclusion
These findings indicate that ubiquitination of β-arrestin has a distinct role in the differential trafficking and degradation of M1 and M2 mAChRs.
Background
There are five subtypes of muscarinic acetylcholine receptors (M1 – M5 mAChR) with distinct yet overlapping tissue distributions. Muscarinic receptors regulate a variety of physiological responses ranging from cardiac homeostasis to cholinergic signaling in the brain [1]. A common feature of mAChRs, and in fact all GPCRs, is that both their expression and activation are tightly regulated. Agonist-promoted trafficking of mAChRs, and most other GPCRs can be broken down into five distinct phases: agonist-binding promotes G protein dissociation (I) from the receptor which allows phosphorylation of specific serine and threonine residues (II) on internal loops of the receptor by G protein receptor kinases (GRKs). This phosphorylation allows the binding of β-arrestins (III) which promotes homologous desensitization and subsequent internalization of the receptor into clathrin coated pits. Following internalization, the receptor can either be dephosphorylated and recycled (IV) to the cell surface or targeted for degradation (V) in proteasomes or lysosomes [1].
β-arrestins have emerged as a central control point in the trafficking of nearly all GPCRs [2]. In addition to mediating desensitization of GPCRs, β-arrestin also participates in clathrin-dependent endocytosis of activated receptors by directly interacting with clathrin and the clathrin-associated adaptor AP-2 [3,4]. Once internalized, β-arrestins are involved in regulation of post-endocytotic trafficking and recently have been shown to function as scaffolding proteins interacting with cellular trafficking machinery [2,5].
Initial reports examining the role of β-arrestin, clathrin and dynamin indicated that agonist-promoted internalization of the M2 mAChR proceeded via both arrestin-dependent and -independent pathways [6,7]. Later work, however, indicated the essential role of β-arrestin in M2 mAChR internalization, and suggested that all M2 mAChR internalization, like other mAChR subtypes, is dynamin-dependent [8,9].
Two classes of GPCRs have been identified with respect to kinetics of receptor recycling and interaction with β-arrestins. Class A receptors, such as the β2 adrenergic (β2AR), dopamine D1A and endothelin 1A receptors recycle rapidly and dissociate from β-arrestin prior to receptor internalization. Class B receptors, such as the vasopressin 2 (V2R), angiotensin 1a and neurotensin 1 receptors, recycle slowly and internalize in a stable association with β-arrestin [10,11]. Recently, it has become clear that the classification of receptors as A or B directly correlates with patterns of β-arrestin ubiquitination and deubiquitination [12]. Stimulation of Class A β2ARs leads to transient ubiquitination of β-arrestin with deubiquitination occurring shortly (minutes) after internalization [12]. In contrast, stimulation of Class B V2Rs leads to a stable ubiquitination of β-arrestin [12].
Ubiquitination of proteins is a signal for degradation that leads to delivery to and degradation of proteins in the 26S proteasome [13]. There are a number of examples where ubiquitination has been shown to be involved in the regulation of GPCRs, including the opiod receptors [14], yeast pheromone receptor [15], human immunodeficiency virus co-receptor CXCR4 [16], and β2 ARs [17].
Class A receptors, which do not internalize with β-arrestin, display a pattern of transient β-arrestin ubiquitination. Class B receptors, on the other hand, do internalize with β-arrestin, and display a sustained β-arrestin ubiquitination pattern [12]. Shenoy et al. [17] showed that agonist stimulation led to the ubiquitination of both β-arrestin and the β2 AR. Their data suggested that it was the ubiquitination of β-arrestin that was involved in the initial receptor internalization step whereas ubiquitination of the receptor itself was required for subsequent receptor degradation.
In the present study, we wanted to address several questions regarding the role of β-arrestin ubiquitination in the agonist-promoted down-regulation of mAChRs. First we wanted to determine if there was a role for β-arrestin ubiquitination in receptor down-regulation. Collectively, our data support a role for β-arrestin in differential targeting of mAChRs towards down-regulation. In addition, the data indicate that this differential targeting may be controlled by preferential interaction of mAChR subtypes with the different β-arrestin subtypes which in turn may be mediated by specific patterns of β-arrestin ubiquitination.
Results
For a number of years there has been some controversy as to the role of β-arrestin in M2 mAChR specific internalization and down-regulation. Our recent publication [9] established the essential role of β-arrestin in the internalization of the M2 mAChR. The current study is a follow up which not only addresses the role of β-arrestin in agonist-promoted mAChR down-regulation, but also indicates a differential role for β-arrestin in M1 versus M2 mAChR down-regulation. As in the previous study, we performed our down-regulation experiments in mouse embryonic fibroblasts (MEFs) derived from β-arrestin 1/2 double knockout mice (MEF KO1/2) [18]. Experiments performed using this cell line offer a significant advantage over previous studies of the role of β-arrestin in GPCR internalization and down-regulation that used dominant-negative or knockdown strategies because they avoid any possible complications that could arise from the presence of low levels of endogenous arrestin proteins. These cells have been characterized to confirm the absence of mAChR expression using both PCR and radioligand binding assays [9].
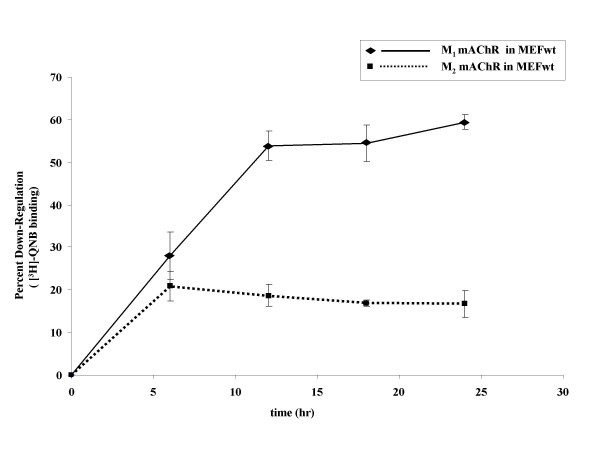
To determine whether or not MEF cells could be used in the current study, we performed agonist-promoted down-regulation time courses for both the M1 and M2 mAChR subtypes. Total receptor numbers were measured using the non-selective membrane permeable muscarinic antagonist quinuclidinyl benzilate (QNB), which binds to both surface and intracellular pools of mAChR. Wild-type MEF cells that were transfected with either eGFP-M1 mAChR or HA-M2 mAChR resulted in similar expression levels (~4000 fmol/mg). After 24 hr, cells were treated with 1 mM carbachol for the indicated time. Maximal down-regulation of the M2 mAChR occurred after 6 hr of stimulation (Figure 1). In contrast, maximal down-regulation of the M1 subtype did not occur until after 12 hr of carbachol stimulation. In addition, M2 mAChRs were maximally decreased by only 22% while the M1 subtype was decreased by 55%. These results demonstrate that exogenously expressed M1 and M2 mAChRs undergo agonist-promoted down-regulation in MEFwt cells. The different time course and extent of down-regulation suggest the possibility that down-regulation of the two subtypes may involve distinct pathways. Despite the fact that M2 mAChRs were maximally down-regulated by 6 hr of stimulation, subsequent single time point experiments used the 12 hr time point so that the M1 and M2 subtype experiments would be compared at a standardized time point.
Figure 1.
Time-course of agonist-promoted down-regulation of mAChRs in MEF. MEF wt cells were transfected with eGFP-M1 (◆) or HA-M2 (■) mAChR. 24 hr following transfection, cells were treated with 1 mM carbachol for the indicated time. Down-regulation was determined using [3H]-QNB binding (fmol/mg protein) in crude membranes as described in methods. The M1 mAChR displays nearly threefold the down-regulation of the M2 subtype over the same time course. Data are expressed as percent down-regulation compared to t = 0 control and are presented as mean ± standard error of the mean for three independent experiments with duplicate data points. Total mAChR expressed (fmol/mg) at t = 0 was 4000 for both M1 and M2 mAChRs.
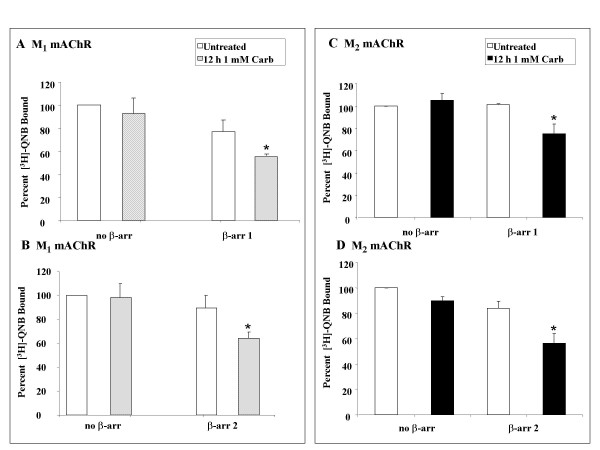
Several recent studies have reported that mAChR down-regulation occurs independently of agonist-promoted internalization [19-21]. Recently, we demonstrated that agonist-promoted internalization of M2 mAChRs in MEF cells was β-arrestin dependent [9]. To examine whether or not agonist-promoted down-regulation of M1 and M2 mAChRs is β-arrestin dependent, we performed down-regulation studies in MEF β-arrestin double knockout cells transiently expressing either M1 or M2 mAChRs. MEF KO1/2 cells were transfected with eGFP-M1 mAChR or HA-M2 mAChR and either FLAG-β-arrestin 1 or 2. After 24 hr, cells were treated with 1 mM carbachol for 12 hr. In the absence of exogenous β-arrestin, there was no down-regulation in response to agonist stimulation in the double knockout cell line (Figure 2A–D). Expression of either β-arrestin 1 or β-arrestin 2, however, rescued down-regulation of both receptor subtypes in response to agonist (Figure 2A – D). These results demonstrate that agonist-promoted down-regulation of M1 and M2 mAChRs is β-arrestin dependent.
Figure 2.
Rescue of mAChR down-regulation in MEF KO1/2 with β-arrestin 1 or 2. MEF KO1/2 cells were transfected with eGFP-M1 mAChR (A-B) or HA-M2 mAChR (C-D) and either FLAG-β-arrestin 1 (top) or 2 (bottom). 24 hr following transfection, cells were treated with 1 mM carbachol for 12 hr. Down-regulation was determined using [3H]-QNB binding (fmol/mg protein) in crude membranes as described in methods. No down-regulation occurs in the absence of β-arrestin and either isoform (β-arrestin 1 or 2) rescues both constitutive and agonist-promoted down-regulation. Data are expressed as percent of [3H]-QNB bound (fmol/mg total protein) compared to untreated, no β-arrestin control and are presented as mean ± standard error of the mean from three independent experiments with duplicate data points. Statistical analysis was performed using a paired t-test. *: p ≤ 0.05 versus the untreated, no β-arrestin control. Total M1 mAChR expressed (fmol/mg) at t = 0 was 400 – 900 (M1) and 1000 – 2000 (M2) for both the β-arrestin 1 and β-arrestin 2 rescue experiments.
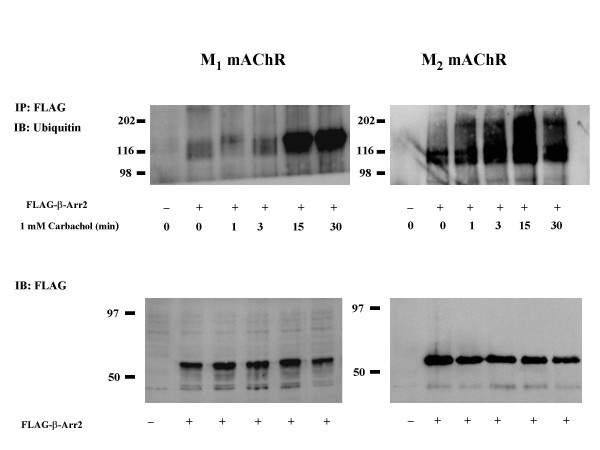
Recent studies have demonstrated that agonist stimulation of GPCRs results in ubiquitination of β-arrestin 2 [12]. This group demonstrated receptor specific agonist-promoted ubiquitination of β-arrestin 2 that was either transient (peaked at 1 min of agonist stimulation) or stable (still present after 15 min of agonist stimulation) [12]. We performed experiments in order to determine if agonist stimulation of M1 or M2 mAChRs promoted the ubiquitination of β-arrestin and if so, examine whether the observed ubiquitination was transient or stable. MEF KO1/2 cells were transfected with FLAG-β-arrestin 2 and eGFP-M1 or HA-M2 mAChR and treated with carbachol for 0, 1, 3, 15 and 30 min. Stimulation of M1 and M2 mAChRs significantly increased ubiquitination of β-arrestin 2 (Figure 3). This increase is clearly visible at both the 15 and 30 min time point for both mAChR subtypes. These results demonstrate that stimulation of M1 or M2 mAChRs promotes a stable ubiquitination of β-arrestin 2.
Figure 3.
Agonist treatment promotes ubiquitination of β-arrestin 2 in MEF KO1/2 cells expressing the mAChRs and β-arrestin 2. MEF KO1/2 cells transfected with FLAG-β-arrestin 2 and either eGFP-M1 or HA-M2 mAChR. 24 hr following transfection, cells were treated with 1 mM carbachol for the indicated times. Top panel: cells were lysed and immunoprecipitated (IP) with anti-FLAG MAb and blotted (IB) with anti-ubiquitin-MAb. There was an increase in ubiquitination of β-arrestin 2 with increasing exposure to 1 mM carbachol. Each blot is representative of two independent experiments. Bottom panel: lysates blotted with anti-FLAG MAb to demonstrate β-arrestin 2 expression levels.
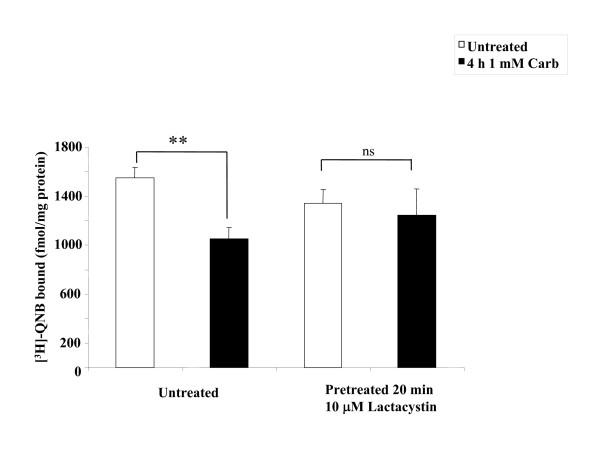
Having established that β-arrestin is required for mAChR down-regulation and that muscarinic stimulation leads to the ubiquitination of β-arrestin, we wanted to examine if there was a direct role for β-arrestin ubiquitination in receptor down-regulation. Since ubiquitination is known to serve as a signal for protein degradation [13] we were interested in determining whether disruption of the ubiquitin/proteasome pathway would affect the agonist-promoted down-regulation of the mAChRs. To confirm that β-arrestin 2 promotes down-regulation via a ubiquitin dependent pathway, we examined agonist-promoted down-regulation of the M2 mAChR in the presence of the proteosomal inhibitor lactacystin (impairs ubiquitin recycling). MEFwt cells were transfected with HA-M2 mAChR and after 24 hr cells were incubated for 20 min with or without 10 μM lactacystin prior to treatment with 1 mM carbachol for 4 hr. Lactacystin was able to completely block agonist-promoted down-regulation (Figure 4) suggesting that the ubiquitination machinery has a role in mediating agonist-promoted down-regulation of mAChRs.
Figure 4.
The proteosomal inhibitor lactacystin inhibits the β-arrestin 2 mediated agonist-promoted down-regulation of the M2 mAChR in MEFwt. MEFwt cells were transfected with HA-M2 mAChR and, after 24 hr cells were incubated for 20 min with or without 10 μM lactacystin then treated with 1 mM carbachol for 4 hr. Lactacystin inhibits the β-arrestin 2 mediated down-regulation of the M2 mAChR receptor in response to agonist. Down-regulation was determined using [3H]-QNB binding in crude membranes as described in methods. Data are expressed as [3H]-QNB bound (fmol/mg total protein) and are presented as mean ± standard deviation from three independent experiments with duplicate data points. Statistical analysis was performed using a paired t-test, ** indicates p ≤ 0.001 (compared to paired untreated control), ns indicates not significant.
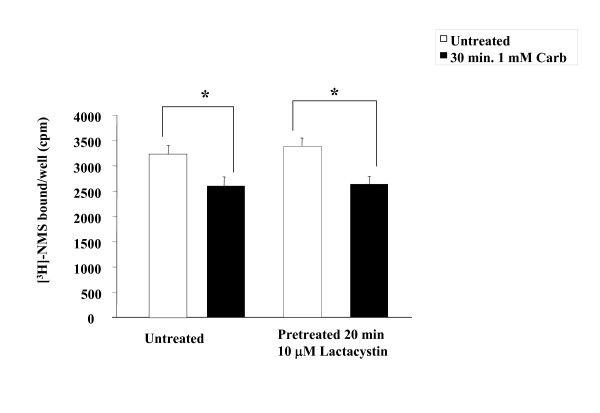
To determine whether or not the effects of lactacystin were occurring at the level of receptor internalization, we examined the effect of lactacystin on the agonist-promoted internalization of mAChRs using the membrane impermeable muscarinic antagonist N-methylscopolamine (NMS). MEFwt cells were transfected with HA-M2 mAChR, and after 24 hr cells were incubated for 20 min in the absence or presence of 10 μM lactacystin prior to treatment with 1 mM carbachol for 30 min. Pretreatment with inhibitor had no effect on agonist-promoted internalization (Figure 5). These results indicate that agonist-promoted down-regulation of mAChRs involves receptor β-arrestin ubiquitination.
Figure 5.
The proteosomal inhibitor lactacystin does not affect agonist-promoted down-regulation internalization of the M2 mAChR in MEFwt. MEFwt cells were transfected with HA-M2 mAChR and, after 24 hr cells were incubated for 20 min with or without 10 μM lactacystin then treated with 1 mM carbachol for 30 min. Lactacystin has no effect on the internalization of the M2 mAChR receptor in response to agonist. Internalization was determined using [3H]-NMS binding in whole cells as described in methods. Data are [3H]-NMS bound per well (plated at 1 × 105 cells) and are presented as mean ± standard deviation from three independent experiments with duplicate data points. Statistical analysis was performed using a paired t-test, * indicates p ≤ 0.05; (compared to paired untreated control), ns indicates not significant.
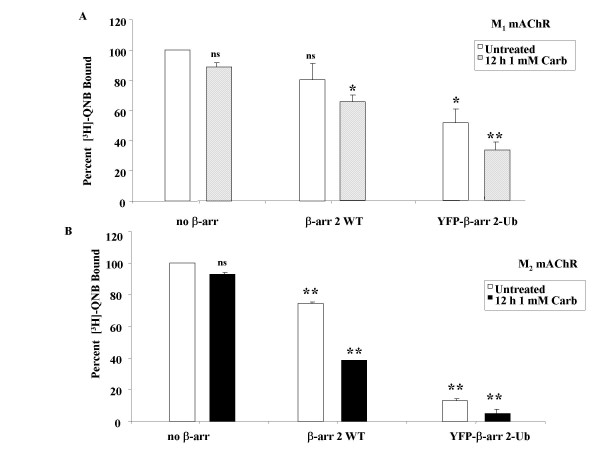
To examine the consequences of β-arrestin ubiquitination on receptor down-regulation, we expressed a yellow fluorescent protein-tagged β-arrestin 2-ubiquitin chimera that cannot be deubiquitinated by cellular deubiquitinases (YFP-β-arrestin 2-Ub) [12]. MEF KO1/2 cells were transfected with eGFP-M1 or HA-M2 mAChR and either FLAG-β-arrestin 2 or YFP-β-arrestin 2-Ub. After 24 hr, cells were treated with 1 mM carbachol for 12 hr. There was a 40% agonist-promoted down-regulation of the M1 mAChR by β-arrestin 2 alone which increased to 70% in the presence of β-arrestin 2-Ub (Figure 6). These values were 62 and 95%, respectively, for the M2 subtype (Figure7). β-arrestin 2-Ub also increased the constitutive receptor down-regulation, and again the effect was much larger for the M2 (90%) vs M1 (50%) subtype (Figure 6A and 6B). It is clear from these data that ubiquitination enhanced the ability of β-arrestin 2 to mediate both constitutive and agonist-promoted down-regulation of both the M1 and M2 mAChRs.
Figure 6.
Expression of a chimeric ubiquitinated form of β-arrestin 2 enhances the agonist-promoted down-regulation of M1 and M2 mAChRs in MEF KO1/2. MEF KO1/2 cells were transfected with eGFP-M1 (A) or HA-M2 (B) mAChRs and either no β-arrestin, FLAG-β-arrestin 2 or YFP-β-arrestin 2-Ub. 24 hr following transfection, cells were treated with 1 mM carbachol for 12 hr. Down-regulation was determined using [3H]-QNB binding (fmol/mg protein) in crude membranes as described in methods. Expression of the constitutively ubiquitinated form of β-arrestin (β-arrestin 2-Ub) had a greater effect on down-regulation of the M2 mAChR compared to the M1 subtype. Data are expressed as percent of [3H]-QNB bound compared to the untreated control with no β-arrestin and are presented as mean ± standard deviation from two independent experiments with duplicate data points. Statistical analysis was performed using a repeated measures ANOVA with Bonferroni post test; * indicates p ≤ 0.05 and ** indicates p ≤ 0.001 (compared to untreated, no β-arrestin control), ns indicates not significant. Total mAChR expressed (fmol/mg) in the absence of β-arrestin was 1000 – 2000 for both receptor subtypes.
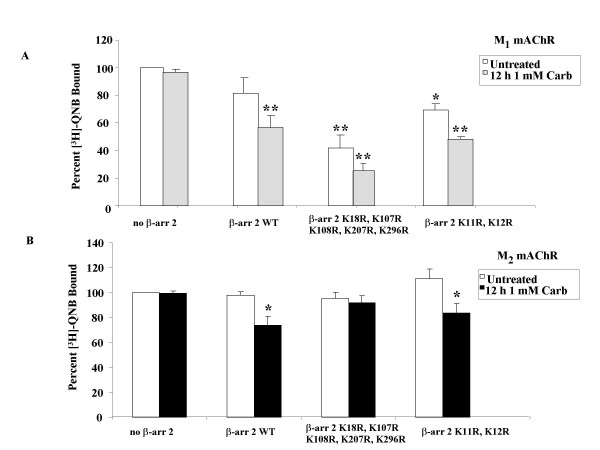
Having demonstrated a role for ubiquitin in agonist-promoted mAChR degradation, we were interested in the effects of disrupting β-arrestin 2 ubiquitination on receptor down-regulation. Several lysine residues on β-arrestin are known to be sites of ubiquitination [22]. To further confirm the essential role of ubiquitination in agonist-promoted down-regulation, we examined the ability of specific β-arrestin 2 lysine mutants to mediate agonist-promoted down-regulation of the M1 and M2 mAChR. MEF KO1/2 cells were transfected with eGFP-M1 or HA-M2 mAChR and either empty vector (control), FLAG-β-arrestin 2 (wild-type), FLAG-β-arrestin 2K18R, K107R, K108R, K207R, K296R or FLAG-β-arrestin 2K11R, K12R. After 24 hr, cells were treated with 1 mM carbachol for 12 hr. As shown previously, there was no down-regulation in the control cells in the absence of β-arrestin 2 (Figure 7). All three β-arrestin 2 constructs were able to rescue agonist-promoted down-regulation of the M1 subtype. We also noted a large constitutive effect on down-regulation with both mutant β-arrestins. In contrast, for the M2 subtype only wild-type β-arrestin 2 (24%) and β-arrestin 2K11R, K12R (27%) were able to rescue agonist-promoted down-regulation (Figure 7). β-arrestin 2K18R, K107R, K108R, K207R, K296R, had lost the ability to rescue agonist-promoted down-regulation of the M2 mAChR (Figure 7).
Figure 7.
β-arrestin 2K18R, K107R, K108R, K207R, K296Rdifferentially affects down-regulation of M1 and M2 mAChRs in MEF KO1/2. MEF KO1/2 cells were transfected with eGFP-M1 (A) mAChR or HA-M2 (B) mAChR and either empty vector (control), FLAG-β-arrestin 2 (WT), FLAG-β-arrestin 2K18R, K107R, K108R, K207R, K296R or FLAG-β-arrestin 2K11R, K12R. 24 hr after transfection, cells were treated with 1 mM carbachol for 12 hr. Down-regulation was determined in crude membranes (fmol/mg protein) as described in methods. All β-arrestin 2 constructs were able to mediate agonist-promoted down-regulation of mAChR with the exception of the β-arrestin 2K18R, K107R, K108R, K207R, K296R mutant when co-expressed with the M2 mAChR. Data are expressed as percent of [3H]-QNB bound (compared to untreated, no β-arrestin control) and presented as mean ± standard deviation from three independent experiments with duplicate or quadruplicate data points. Statistical analysis was performed using a repeated measures ANOVA with Bonferroni post test; * indicates p ≤ 0.05 ** indicates p ≤ 0.001 (compared to untreated, no β-arrestin control), ns indicates not significant. Total M1 (300–500 fmol/mg) and M2 (1500–2500 fmol/mg) mAChR expressed in the absence of β-arrestin constructs was similar.
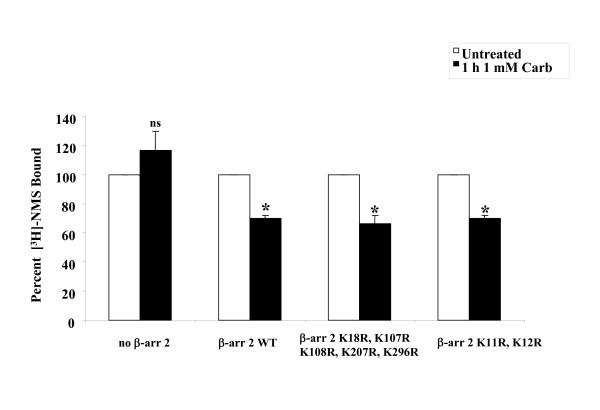
Since it was possible that the effect of β-arrestin 2K18R, K107R, K108R, K207R, K296R on M2 mAChR down-regulation seen in the previous experiment was actually occurring at the receptor internalization step, we performed receptor internalization experiments with [3H]-NMS. Transfections were identical to the previous experiment. After 24 hr, cells were treated with 1 mM carbachol for 1 hr. All β-arrestin 2 constructs (wild-type or lysine mutants) were able to rescue agonist-promoted internalization of M2 mAChRs in MEF KO1/2 (Figure 8).
Figure 8.
Agonist-promoted internalization of M2 mAChR is unaffected by β-arrestin 2 lysine mutants in MEF KO1/2. MEF KO1/2 cells were transfected with HA-M2 mAChR and either empty vector (control), FLAG-β-arrestin 2 (WT), FLAG-β-arrestin 2K18R, K107R, K108R, K207R, K296R or FLAG-β-arrestin 2K11R, K12R. 24 hr after transfection, cells were treated with 1 mM carbachol for 1 hr. Internalization was determined in whole cells (receptors/cell) as described in methods. All constructs were able to mediate agonist-promoted internalization. Data are expressed as percent of [3H]-NMS bound compared to untreated control and presented as mean ± standard deviation from three independent experiments with duplicate data points. Statistical analysis was performed using a repeated measures ANOVA with Bonferroni post test; * indicates p ≤ 0.05 (compared to untreated, no β-arrestin control), ns indicates not significant. Total receptor expressed in the presence of all four β-arrestin constructs was between 5 and 6 × 105 receptor/cell.
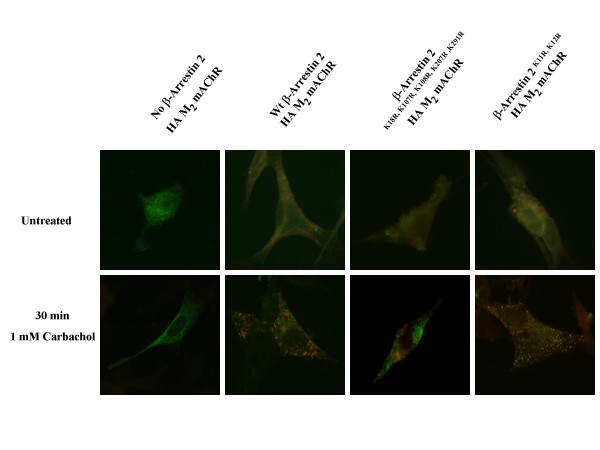
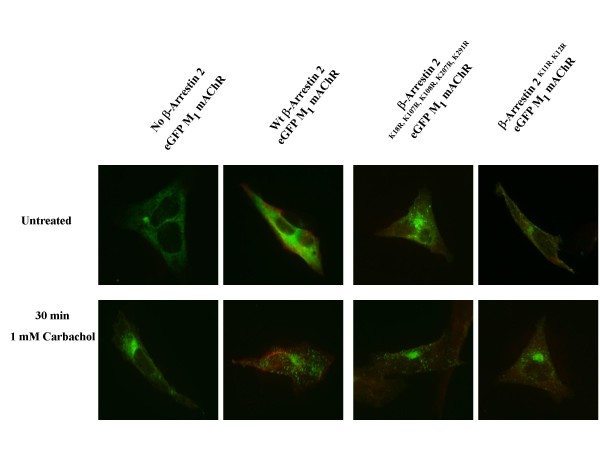
Since β-arrestin 2K18R, K107R, K108R, K207R, K296R not only appears to interfere with the agonist promoted down-regulation of the M2 mAChR but also increased the constitutive down-regulation of the M1 mAChR we examined the ability of this mutant to co-localize with the M1/M2 mAChR compared to wt and β-arrestin 2K11R, K12R following agonist stimulation. MEF KO1/2 were plated on cover slips in 6-well plates. 24 hrs after transfection with eGFP-M1 mAChR or HA-M2 mAChR and FLAG tagged β-arrestin (wt or lysine mutants) cells were treated for 30 min or 12 hrs with 1 mM carbachol. Cells were fixed and processed for indirect immunofluorescence as described in methods. After 30 minutes of agonist exposure there is clear overlap of the M2 mAChR receptor and β-arrestin signal signified by the yellow puncta (Figure 9) for the wt and β-arrestin 2K11, K12R. This overlap is absent with β-arrestin 2K18R, K107R, K108R, K207R, K296R which indicates a disrupted or impaired interaction between this β-arrestin mutant and the M2 mAChR. Surprisingly, despite our previous observation of the effects of β-arrestin 2K18R, K107R, K108R, K207R, K296R on constitutive M1 down-regulation, no co-localization was observed between β-arrestin and the M1 mAChR (Figure 10).
Figure 9.
β-arrestin 2K18R, K107R, K108R, K207R, K296Rdemonstrates impaired agonist-promoted co-localiztion with M2 mAChR in MEF KO1/2. MEF KO1/2 cells were transfected with HA-M2 mAChRs (green) and either wild-type FLAG-β-arrestin 2, FLAG-β-arrestin 2K18R, K107R, K108R, K207R, K296R or FLAG-β-arrestin 2K11R, K12R (red). 24 hr after transfection, cells were treated with 1 mM carbachol for 30 min. Cells were fixed, probed, and imaged as described in methods. Wild-type and β-arrestin 2K11R, K12R demonstrate co-localization with the M2 mAChR which is significantly diminished with β-arrestin 2K18R, K107R, K108R, K207R, K296R. Image shown is representative of two independent experiments.
Figure 10.
β-arrestin 2K18R, K107R, K108R, K207R, K296Rdemonstrates no co-localization with M1 mAChR in MEF KO1/2. MEF KO1/2 cells were transfected with eGFP-M1 (A) mAChRs (green) and either wild-type FLAG-β-arrestin 2, FLAG-β-arrestin 2K18R, K107R, K108R, K207R, K296R or FLAG-β-arrestin 2K11R, K12R (red). 24 hr after transfection, cells were treated with 1 mM carbachol for 30 min. Cells were fixed, probed, and imaged as described in methods. None of the β-arrestin constructs demonstrate co-localization with the M1 mAChR. Image shown is representative of two independent experiments.
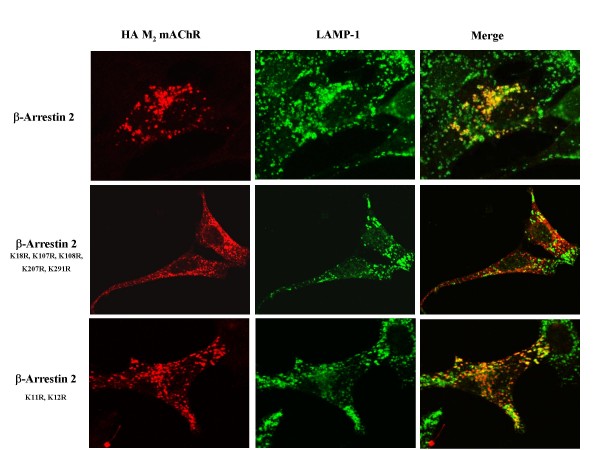
Finally we used immunocytochemistry with the lysosomal marker LAMP-1 to examine the effects of the β-arrestin 2K18R, K107R, K108R, K207R, K296R mutant on mAChR lysosomal targeting down-regulation. MEF KO1/2 were transfected with HA-M2 mAChR and either empty vector (control), FLAG-β-arrestin 2 (wild-type), FLAG-β-arrestin 2K11R, K12R or FLAGβ-arrestin 2K18R, K107R, K108R, K207R, K296R. After 24 hr, cells were treated with 1 mM carbachol for 6 hr. There was significant co-localization of the receptor with LAMP-1 in the presence of wild-type β-arrestin 2 or β-arrestin 2K11R, K12R (Figure 11). It is clear, however, that β-arrestin 2K18R, K107R, K108R, K207R, K296R shows reduced co-localization of the M2 mAChR with LAMP (Figure 11).
Figure 11.
β-arrestin 2K18R, K107R, K108R, K207R, K296Rdisrupts the co-localization of M2 mAChR and LAMP-1 in MEF KO1/2. MEF KO1/2 cells were transfected with HA-M2 mAChR and either wild-type FLAG-β-arrestin 2, FLAG-β-arrestin 2K18R, K107R, K108R, K207R, K296R or FLAG-β-arrestin 2K11R, K12R. 24 hr after transfection, cells were treated with 1 mM carbachol for 6 hr. Cells were fixed, probed, and imaged as described in methods. β-arrestin 2K18R, K107R, K108R, K207R, K296R disrupts the co-localization of M2 mAChR and LAMP-1 compared to wild-type and β-arrestin 2K11R, K12R. Image shown is representative of three independent experiments.
Collectively, these data indicate that the agonist-promoted down-regulation of the M1 and M2 mAChRs involves differential sorting of the receptor by ubiquitinated β-arrestin.
Discussion
Our recent work established the essential role of β-arrestin in the internalization of the M2 mAChR [9]. The present study extends the observations of previous work and demonstrates that the agonist-promoted down-regulation of M1 and M2 mAChRs is β-arrestin dependent, and that the ubiquitination pattern of β-arrestin has a critical role in the differential down-regulation for M1 vs M2 mAChRs.
It has been previously established in a variety of cell lines that a prolonged activation of M1 or M2 mAChRs induces receptor down-regulation. In agreement with these findings, long-term stimulation of M1 or M2 mAChRs induced receptor down-regulation with the M1 mAChR showing significantly more down-regulation than M2 subtype. This observation suggests that the two subtypes are differentially regulated by endogenous β-arrestins. No down-regulation of either mAChR subtype occurred in the absence of β-arrestin and either β-arrestin subtype was able to rescue receptor down-regulation.
The observations of Shenoy and co-workers suggest that the ubiquitination of β-arrestin is a critical control point in the trafficking of different classes of GPCRs [12,17,22]. The time course of ubiquitination of β-arrestin 2 in response to muscarinic stimulation in MEF KO1/2 cells transiently expressing the M1 or M2 mAChRs was slow in onset and stable over time. This observation suggests that both M1 and M2 mAChRs display a Class B β-arrestin ubiquitination pattern despite the fact that the M1 mAChR is known to recycle rapidly compared to M2 [23], and does not show a stable endocytotic co-localization with β-arrestin 2 [9] – both characteristics of Class A GPCRs. This observation suggests that there is some flexibility in the Class A vs B distinction. This same flexibility has been observed in the categorization of somatostatin receptor subtypes [24].
To confirm that agonist-promoted down-regulation of mAChRs was ubiquitin-dependent we performed internalization/down-regulation experiments in the presence of the proteosomal inhibitor lactacystin. Lactacystin is a specific inhibitor of the 26S proteasome that functions by covalently modifying the active site and inhibiting the enzymatic activity of the proteasome [13]. This inhibition of the enzyme would inhibit recycling and thus deplete the available ubiquitin. Lactacystin was able to completely block agonist-promoted down-regulation with no effect on agonist-promoted internalization. These results suggested that the availability of free ubiquitin has a role in agonist-promoted down-regulation of the mAChRs. This effect is most likely due to effects on ubiquitin-dependent lysosomal sorting as evidenced by a later experiment that demonstrated the co-localization of the agonist-internalized M2 mAChR with the lysosomal membrane protein LAMP-1.
Having established that β-arrestin is ubiquitinated via mAChR activation, we then examined whether the ubiquitination of β-arrestin enhances its ability to mediate agonist-promoted down-regulation. M1 mAChR levels were reduced and M2 levels were nearly completely ablated in the presence of β-arrestin 2-Ub compared to wild-type β-arrestin. The effect was seen on both constitutive and agonist promoted down-regulation.
In order to examine the role of β-arrestin ubiquitination in targeting mAChRs toward degradation, we used β-arrestin lysine mutants that have been previously shown to have a role in the endocytotic trafficking of other GPCRs. The constructs, generated by Shenoy and co-workers, contain mutations of potential ubiquitination sites near the amino terminus (β-arrestin 2K11R, K12R) and at five other sites in the protein (β-arrestin 2K18R, K107R, K108R, K207R, K296R) [22]. We were particularly interested in the possible differential effects of β-arrestin ubiquitination patterns on M1 vs M2 down-regulation given the fact that M1 and M2 mAChRs did not fit neatly into the classical Class A vs B distinction.
Both of the β-arrestin lysine mutants were able to mediate agonist-promoted down-regulation of the M1 mAChR to the same extent as wild-type β-arrestin. While our data clearly indicates an essential role for β-arrestin ubiquitination in M1 mAChR down-regulation, other lysine residues than the ones examined must be the targets for the necessary ubiquitination required for targeting the M1 mAChRs toward down-regulation. We did observe increased constitutive down-regulation of the M1 mAChR with both mutant β-arrestin constructs. It is possible that the pattern of ubiquitination of these mutant β-arrestins altered the constitutive targeting of the M1 mAChR toward lysosomal down-regulation. This observation is one of several in this study that suggests that the pattern of ubiquitination on β-arrestin is directly implicated in the downstream targeting of mAChRs. Previously it has been suggested that the only role for β-arrestin ubiquitination is to promote the internalization of GPCRs.
We obtained surprisingly different results with the effects of the β-arrestin lysine mutants on the agonist-promoted down-regulation of the M2 mAChR subtype. Mutation of the two lysine residues in β-arrestin 2K11R, K12R had no effect on either agonist-promoted internalization or down-regulation of M2 mAChRs. This result is similar to those seen with other Class B receptors such as the V2R and NK1R [22]. The results are different however from those seen with another Class B receptor, the AT1a receptor. When this receptor was co-expressed with β-arrestin 2K11R, K12R, agonist stimulation resulted in transient association of the receptor with β-arrestin 2, thus converting the receptor to a Class A type.
In contrast, the β-arrestin 2K18R, K107R, K108R, K207R, K296R mutant significantly reduced M2 mAChR degradation with no effect on receptor internalization. These results are similar to those seen with the Class B V2R, where β-arrestin 2K18R, K107R, K108R, K207R, K296R was shown to impair association of V2Rs with β-arrestin 2 on endosomes [22].
The fact that β-arrestin 2K18R, K107R, K108R, K207R, K296R was able to internalize but not down-regulate the M2 mAChR suggests that one or more of these lysines has a role in down-regulation but not internalization for this subtype. These data suggest a direct role for the ubiquitination state of β-arrestin in the lysosomal targeting of the M2 mAChR.
Unlike effects observed with the M2 mAChR, β-arrestin 2K18R, K107R, K108R, K207R, K296R was able to mediate down-regulation of M1 mAChRs. This observation clearly demonstrates that the role of these lysines in down-regulation is subtype specific and that a specific pattern of ubiquitination on β-arrestin can differentially target the two mAChR subtypes for down-regulation.
Co-localization of wild-type and β-arrestin 2K11R, K12R with the M2 mAChR, observed after 30 min of agonist treatment is absent with β-arrestin 2K18R, K107R, K108R, K207R, K296R which may indicate a disrupted or impaired interaction between this β-arrestin mutant and the M2 mAChR. β-arrestin 2K18R, K107R, K108R, K207R, K296R was also shown to disrupt the co-localization of the M2 mAChR and the lysosomal marker LAMP-1 confirming our observation that β-arrestin 2K18R, K107R, K108R, K207R, K296R does not mediate the agonist-promoted degradation of the M2 mAChR. Surprisingly, no co-localization of M1 mAChR was observed with any of the three β-arrestin constructs. Time points from 1 min to 12 hrs of carbachol treatment were performed to attempt to image co-localization between the M1 mAChR and the β-arrestins (data not shown). This observation seems to indicate that the role of β-arrestin in M1 mAChR down-regulation may involve a transient interaction between β-arrestin and the receptor which fates the receptor toward degradation.
Our observations of M2 mAChR trafficking are different from the results of work with the Class A β2 AR [17] which suggests that ubiquitination of β-arrestin is required for receptor internalization and that ubiquitination of the receptor itself is required for receptor degradation. Our data indicate that, for the M1 mAChR, the ubiquitination state of β-arrestin appears to have a role in the level of constitutive (agonist-independent) down-regulation and for the M2 mAChR the ubiquitination state of β-arrestin has a direct role in the agonist-promoted down-regulation of the receptor. Of the seven lysine residues we examined, at least one (or more) of the group 18, 107, 108, 207 and 296 are required for this M2 mAChR down-regulation. We also observed that M2 mAChR internalization was independent of β-arrestin ubiquitination at these same lysine residues that have been shown to interfere with the association of β-arrestin with other Class B receptors [22]. The possibility that other lysine residues than those we examined are ubiquitinated in order to internalize the receptor would preserve the role for β-arrestin in receptor internalization. The absence of down-regulation of M2 mAChRs with β-arrestin 2K18R, K107R, K108R, K207R, K296R however, clearly indicates a direct role for the ubiquitination of β-arrestin in receptor down-regulation.
Conclusion
We conclude that agonist-promoted down-regulation of both M1 and M2 mAChRs is β-arrestin-dependent. We further conclude that this down-regulation is specifically modulated by the ubiquitination state of β-arrestin and that this β-arrestin ubiquitination differentially targets the receptor subtypes for degradation in lysosomes. Significantly, where it has been suggested that Class A GPCR down-regulation proceeds primarily via receptor ubiquitination, these data indicate that the ubiquitination state of β-arrestin 2 has an essential role in the differential targeting the M1 and M2 mAChR toward down-regulation. Future studies will examine the role of receptor ubiquitination in M1 and M2 mAChR down-regulation. Specifically, we will examine whether expression of wild type vs the β-arrestin 2K18R, K107R, K108R, K207R, K296R mutant differentially affects M1 and M2 mAChR ubiquitination and subsequent down-regulation.
Methods
Reagents
[3H]-N-methylscopolamine -(81–84 Ci/mmol) and [3H]-L-quinuclidinyl benzilate- (43 Ci/mmol) were purchased from Amersham Corporation (Buckinghamshire, England). LipofectAMINE 2000 and Protein A agarose were purchased from Invitrogen (Carlsbad, CA). Halt protease inhibitor and BCA protein assay kit were purchased from Pierce (Rockford, Il). Atropine, N-ethyl maleimide, carbamyl choline chloride, -anti-FLAG polyclonal antibody (F7425); polyclonal anti-LAMP1 antibody (L1418) and all other chemicals were purchased from Sigma Aldrich (St. Louis, MO). Anti-FLAG monoclonal antibody (200–472) was purchased from Stratagene (La Jolla, CA). Monoclonal anti-HA antibody (MMS101R) was purchased from Covance (Berkeley, CA). Polyclonal anti-HA antibody (71–5500) was purchased from Zymed (Carlsbad, CA). Monoclonal anti-Ub antibody P4D1 (sc 8017) was purchased from Santa Cruz Biotech (Santa Cruz, CA). Alexa 594 goat anti-mouse, Alexa 488 goat anti-Rabbit and Texas red goat anti-mouse (115-075-003) were purchased from Molecular Probes (Eugene, OR). FITC goat anti-rabbit (AP132F) was purchased from Chemicon (Temecula, CA). -HRP-conjugated secondary antibodies were purchased from Bio-Rad Laboratories (Hercules, CA). Prolong Gold Antifade (P36930) was purchased from Jackson Immuno Research (West Grove, PA.). pIRESeGFP were purchased from Clontech (Palo Alto CA).
Expression constructs and cell lines
Expression constructs and cell lines were generously provided by the following: MEF wild-type cells, β-arrestin 1 and 2 double knockout cells, FLAG-tagged β-arrestin 1 and 2, YFP-β-arrestin-2-Ub, β-arrestin 2K11R, K12R, and β-arrestin 2K18R, K107R, K108R, K207R, K296R (pcDNA3.1 FLAG β-arrestin 1 or 2, pEYFP β-Arr-Ub, pcDNA3.1 FLAG β-arrestin 2K11R, K12R, and pcDNA3.1 FLAG β-arrestin 2K18R, K107R, K108R, K207R, K296R) by Dr. Robert Lefkowitz (Duke University Medical Center, Durham, NC); HA tagged human M2 mAChR (pRK HA M2 mAChR) by Dr. Audrey Claing (University of Montreal, Montreal Canada); eGFP tagged M1 mAChR (pCEP4eGFP M1 mAChR by Dr. Brigitte Ilien (University of Strasbourg, Strasbourg, France).
Cell culture and transient transfection
Mouse embryonic fibroblast (MEF) cells (wild-type and knockouts) were maintained in Dulbecco's Modified Eagle's Medium (DMEM). Media was supplemented with 10% fetal bovine serum (FBS), 100 IU/mL penicillin, and 100 μg/mL streptomycin. All cells were maintained at 37°C with 5% CO2. At 24 hr prior to transfection, cells were plated at 7.5 × 104 cells/well (12-well plate), 1.5 × 105cells/well (6-well plate) and 2 × 106 cells/dish (100 mm dish). Cells were transfected using LipofectAMINE 2000 according to the manufacturer's protocol with 1.6, 5 or 25 μg total DNA per well/plate on a 12-well plate, 6-well plate and 100 mm dish, respectively. Transfection efficiencies of 40 – 60% were routinely obtained [determined by including 10% of total DNA as eGFP construct (pIRESeGFP) and visualizing transfected cells using an Olympus 1X71 fluorescent microscope].
Crude membrane preparation
Two wells of a 6-well plate were rinsed twice with ice cold PBS and cells were scraped in 50 mM sodium phosphate pH 7.0, pooled and homogenized with 20 strokes through a Dounce homogenizer. Homogenate was spun at 10,000 rpm for 20 min at 4°C in a Sorvall Mach 1.6R fixed angle rotor. Pellet was resuspended in 50 mM sodium phosphate pH 7.0 and spun again. Pellet was resuspended in 0.55 mL 50 mM sodium phosphate pH 7.0 and used for protein assay and radioligand binding.
Immunoprecipitation
At 24 hr post transfection, cells were lysed by sonication in HEPES lysis buffer (50 mM HEPES, pH 7.5, 1% Triton X-100, 10% glycerol, 10 mM NaCl, 1 mM EDTA, 1 mM EGTA, 50 mM NaF, 10 mM N-ethyl maleimide, 1 μg/mL each leupeptin, aprotinin, and pepstatin A). Next, 500 μg (BCA) of lysate was precleared using Protein-A agarose beads for 4 hr at 4°C followed by incubation with 5 μg of monoclonal anti-FLAG antibody at 4°C overnight. The immunocomplex was incubated for 4 hr with 100 μL Protein-A beads (50% slurry) at 4°C with rotation. Beads were washed 3 × 500 μL in the same buffer and resuspended in 30 μl of Laemmli buffer and boiled for 5–10 min. The immunocomplex was resolved on a 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gradient gel.
Immunoblotting
Lysates were prepared as described above and 10 – 20 μg total protein was run on a 10% SDS-PAGE gel. Samples from immunoprecipitation or for direct immnuoblotting were transferred to nitrocellulose membranes, blocked with 5% powdered nonfat milk in Tris Buffered Saline, 0.1% Tween 20 (TBST) and probed with antibody as indicated. Immunoreactive bands were visualized with a Fuji imaging system using enhanced chemiluminescence after adding HRP conjugated secondary antibody.
Radioligand binding
Internalization
Internalization was determined by measuring the binding of – the membrane-impermeable muscarinic antagonist [3H]-N-methylscopolamine (NMS) to intact cells. Briefly, 24–42 hr after transfection, MEF cells were treated or not treated with 1 mM carbachol for 30 min or 1 h at 37°C. Cultures were washed 3 × 1 mL with serum free media and incubated with 100 nM – [3H]-NMS in 1 mL PBS for 30 min at 37°C or 4 hr at 4°C. Nonspecific binding was determined as the bound radioactivity in the presence of 1 μM atropine. Labeled cells were washed 3 × 1 mL with ice-cold PBS, solubilized in 0.5 mL 1% Triton-X-100, and combined with 3.5 mL scintillation fluid followed by measurement of radioactivity. Cpm were converted to receptors per well which were then corrected to receptors per cell by dividing by cells/well. Cells were counted using a standard haemocytometer. Receptor internalization is defined as percent of surface M2 mAChR not accessible to [3H]-NMS at each time relative to untreated or control cells.
Down-regulation
Down-regulation was determined by measuring the binding of the membrane permeable muscarinic antagonist [3H]-L-quinuclidinyl benzilate (QNB). Crude membranes (100 μL) were incubated with 30 nM [3H]-QNB in a total volume of 1 mL 50 mM sodium phosphate pH 7.0 for 2 hr at 25°C. Nonspecific binding was determined as the bound radioactivity in the presence of 1 μM atropine. Membranes were harvested on glass fiber filters using a Brandel cell harvester (Gaithersburg, MD) and washed 3 × 2 mL with ice cold 50 mM sodium phosphate pH 7.0. Filter discs were combined with 3.5 mL scintillation fluid followed by measurement of radioactivity.
Cpm per tube was converted to fmoles receptor, which was then corrected to mg of total protein per tube. Percent down-regulation was determined as percent of sites remaining compared to untreated control membranes.
Indirect immunofluorescence
LAMP-1:M2 mAChR co-localization
Cells were fixed in 4% formaldehyde in PBS for 5 minutes, and rinsed with 10% fetal bovine serum and 0.02% azide in PBS (PBS/serum). Fixed cells were incubated with primary antibodies (1:1000 anti-LAMP1 and 1:500 anti-HA MAb) diluted in PBS/serum containing 0.2% saponin for 45 minutes, and then washed with PBS/serum (3 × 5 min.). The cells were then incubated with fluorescently labeled secondary antibodies (1:800 Alexa 488 goat anti-rabbit and Alexa 594 goat anti-mouse) in PBS-serum and 0.2% saponin for 45 minutes, washed with PBS/serum (3 × 5 min.) and once with PBS, and mounted on glass slides in prolong gold antifade.
FLAG-β-Arrestin 2:HA-M2 mAChR or eGFP-M1 mAChR co-localization
Cells were fixed in 4% formaldehyde in PBS for 5 minutes. Fixed cells were permeabilized for 5 minutes with 0.1% triton in PBS and washed 3 × with PBS and blocked in rabbit pre-immune sera (1:200 in PBS). Fixed cells were incubated with primary antibodies (1:500 anti HA PAb or 1:800 anti FLAG MAb) rinsed 3 × with PBS ad incubated with fluorescent secondary antibodies (1:1000 FITC goat anti-rabbit or 1:1000 Texas red goat anti-mouse). Coverslips were mounted on slides in prolong gold antifade. Images were acquired using an Olympus 1X71 inverted fluoresecent microscope equipped with a 60x oil immersion objective. Images were obtained with Olympus Image Manager (Center Valley, PA).
Abbreviations
AT1aR: angiotensin 1a receptor; β2 AR: β2 adrenergic receptor; CHO: Chinese hamster ovary cells; GPCR: G protein-coupled receptor; MAb: monoclonal antibody; mAChR: muscarinic acetylcholine receptor; MEF: mouse embryonic fibroblast; NK1R: neurokinin 1 receptor; NMS: N-methylscopolamine; PAb: polyclonal antibody; QNB: quinuclidinyl benzilate; V2R: vasopressin 2 receptor.
Competing interest Statement
The authors declare that they have no competing interests.
Authors' contributions
VAM performed down-regulation, internalization, immunoblotting, participated in the design of the study and drafted the manuscript. KJTperformed down-regulation and immunocytochemistry. KMH performed immunocytochemistr. NAM was involved in critical revision of the manuscript. DAJ designed the study and participated in drafting and critical evaluation of the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
The authors would like to acknowledge Sean M. Seagraves for technical assistance. This research was supported by National Institutes of Health Grants NS044164, P20 RR15583 and P20 RR017670
Contributor Information
Valerie A Mosser, Email: vamosser@yahoo.com.
Kymry T Jones, Email: kymry_j@hotmail.com.
Katie M Hoffman, Email: Katie.Hoffman@umontana.edu.
Nael A McCarty, Email: nael_mccarty@oz.ped.emory.edu.
Darrell A Jackson, Email: darrell.jackson@umontana.edu.
References
- van Koppen CJ, Kaiser B. Regulation of muscarinic acetylcholine receptor signaling. Pharmacol Ther. 2003;98:197–220. doi: 10.1016/S0163-7258(03)00032-9. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ. Historical review: a brief history and personal retrospective of seven-transmembrane receptors. Trends Pharmacol Sci. 2004;25:413–422. doi: 10.1016/j.tips.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Goodman OB, Jr, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- Laporte SA, Oakley RH, Zhang J, Holt JA, Ferguson SS, Caron MG, Barak LS. The beta2-adrenergic receptor/betaarrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc Natl Acad Sci USA. 1999;96:3712–3717. doi: 10.1073/pnas.96.7.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- Pals-Rylaarsdam R, Gurevich VV, Lee KB, Ptasienski JA, Benovic JL, Hosey MM. Internalization of the m2 muscarinic acetylcholine receptor. Arrestin-independent and -dependent pathways. J Biol Chem. 1997;272:23682–23689. doi: 10.1074/jbc.272.38.23682. [DOI] [PubMed] [Google Scholar]
- Vogler O, Nolte B, Voss M, Schmidt M, Jakobs KH, van Koppen CJ. Regulation of muscarinic acetylcholine receptor sequestration and function by beta-arrestin. J Biol Chem. 1999;274:12333–12338. doi: 10.1074/jbc.274.18.12333. [DOI] [PubMed] [Google Scholar]
- Werbonat Y, Kleutges N, Jakobs KH, van Koppen CJ. Essential role of dynamin in internalization of M2 muscarinic acetylcholine and angiotensin AT1A receptors. J Biol Chem. 2000;275:21969–21974. doi: 10.1074/jbc.M001736200. [DOI] [PubMed] [Google Scholar]
- Jones KT, Echeverry M, Mosser VA, Gates A, Jackson DA. Agonist mediated internalization of M2 mAChR is beta-arrestin-dependent. J Mol Signal. 2006;1:7. doi: 10.1186/1750-2187-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. Association of beta-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J Biol Chem. 1999;274:32248–32257. doi: 10.1074/jbc.274.45.32248. [DOI] [PubMed] [Google Scholar]
- Zhang J, Barak LS, Anborgh PH, Laporte SA, Caron MG, Ferguson SS. Cellular trafficking of G protein-coupled receptor/beta-arrestin endocytic complexes. J Biol Chem. 1999;274:10999–11006. doi: 10.1074/jbc.274.16.10999. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, Lefkowitz RJ. Trafficking patterns of beta-arrestin and G protein-coupled receptors determined by the kinetics of beta-arrestin deubiquitination. J Biol Chem. 2003;278:14498–14506. doi: 10.1074/jbc.M209626200. [DOI] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- Chaturvedi K, Bandari P, Chinen N, Howells RD. Proteasome involvement in agonist-induced down-regulation of mu and delta opioid receptors. J Biol Chem. 2001;276:12345–12355. doi: 10.1074/jbc.M008054200. [DOI] [PubMed] [Google Scholar]
- Hicke L, Riezman H. Ubiquitination of a yeast plasma membrane receptor signals its ligand-stimulated endocytosis. Cell. 1996;84:277–287. doi: 10.1016/S0092-8674(00)80982-4. [DOI] [PubMed] [Google Scholar]
- Marchese A, Benovic JL. Agonist-promoted ubiquitination of the G protein-coupled receptor CXCR4 mediates lysosomal sorting. J Biol Chem. 2001;276:45509–45512. doi: 10.1074/jbc.C100527200. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science. 2001;294:1307–1313. doi: 10.1126/science.1063866. [DOI] [PubMed] [Google Scholar]
- Kohout TA, Lin FS, Perry SJ, Conner DA, Lefkowitz RJ. beta-Arrestin 1 and 2 differentially regulate heptahelical receptor signaling and trafficking. Proc Natl Acad Sci USA. 2001;98:1601–1606. doi: 10.1073/pnas.041608198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro RA, Nathanson NM. Deletion analysis of the mouse m1 muscarinic acetylcholine receptor: effects on phosphoinositide metabolism and down-regulation. Biochemistry. 1989;28:8946–8950. doi: 10.1021/bi00448a039. [DOI] [PubMed] [Google Scholar]
- Goldman PS, Nathanson NM. Differential role of the carboxyl-terminal tyrosine in down-regulation and sequestration of the m2 muscarinic acetylcholine receptor. J Biol Chem. 1994;269:15640–15645. [PubMed] [Google Scholar]
- Shockley MS, Burford NT, Sadee W, Lameh J. Residues specifically involved in down-regulation but not internalization of the m1 muscarinic acetylcholine receptor. J Neurochem. 1997;68:601–609. doi: 10.1046/j.1471-4159.1997.68020601.x. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, Lefkowitz RJ. Receptor-specific ubiquitination of beta-arrestin directs assembly and targeting of seven-transmembrane receptor signalosomes. J Biol Chem. 2005;280:15315–15324. doi: 10.1074/jbc.M412418200. [DOI] [PubMed] [Google Scholar]
- Vogler O, Bogatkewitsch GS, Wriske C, Krummenerl P, Jakobs KH, van Koppen CJ. Receptor subtype-specific regulation of muscarinic acetylcholine receptor sequestration by dynamin. Distinct sequestration of m2 receptors. J Biol Chem. 1998;273:12155–12160. doi: 10.1074/jbc.273.20.12155. [DOI] [PubMed] [Google Scholar]
- Tulipano G, Stumm R, Pfeiffer M, Kreienkamp HJ, Hollt V, Schulz S. Differential beta-arrestin trafficking and endosomal sorting of somatostatin receptor subtypes. J Biol Chem. 2004;279:21374–21382. doi: 10.1074/jbc.M313522200. [DOI] [PubMed] [Google Scholar]