Abstract
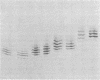
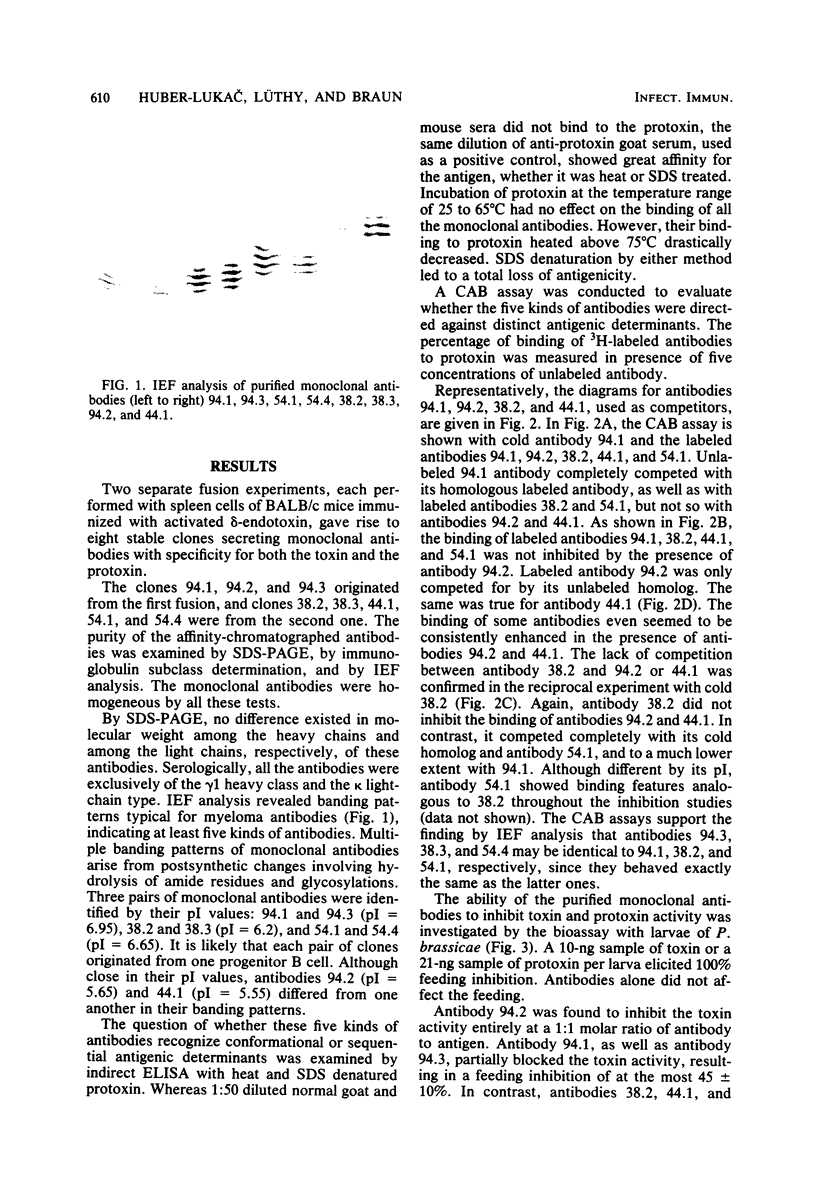
Eight hybrid cell lines secreting monoclonal antibodies directed against the activated delta-endotoxin of Bacillus thuringiensis var. thuringiensis were grown in BALB/c mice. Ascites fluids were collected, and the antibodies were purified by antigen-affinity chromatography. The specificity of each monoclonal antibody for the toxin and protoxin was established by the indirect enzyme-linked immunosorbent assay. All the antibodies consisted of gamma 1 heavy and kappa light chains. They were reactive with both the native toxin and the protoxin. In contrast to specific goat antiserum, they failed, however, to bind to heat and sodium dodecyl sulfate denatured antigen. These eight cloned cell lines gave rise to five kinds of antibodies distinguished by isoelectric focusing. Competitive antibody binding studies revealed that these five antibodies recognize at least four distinct antigenic determinants of the native toxin and the protoxin. Two of the epitopes are unrelated, whereas three antibodies compete for binding to their antigenic determinants. In the bioassay with larvae of Pieris brassicae, one antibody was found to block the toxin and protoxin activity completely. A second inhibited it partially, whereas the other three antibodies did not affect it at all.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Celada F., Strom R. Antibody-induced conformational changes in proteins. Q Rev Biophys. 1972 Aug;5(3):395–425. doi: 10.1017/s0033583500000998. [DOI] [PubMed] [Google Scholar]
- Delafield F. P., Somerville H. J., Rittenberg S. C. Immunological homology between crystal and spore protein of Bacillus thuringiensis. J Bacteriol. 1968 Sep;96(3):713–720. doi: 10.1128/jb.96.3.713-720.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Schesser J. H., Kramer K. J., Bulla L. A., Jr Bioassay for homogeneous parasporal crystal of Bacillus thuringiensis using the tobacco hornworm, Manduca sexta. Appl Environ Microbiol. 1977 Apr;33(4):878–880. doi: 10.1128/aem.33.4.878-880.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]