Abstract
Background
The heterogeneity statistic I2, interpreted as the percentage of variability due to heterogeneity between studies rather than sampling error, depends on precision, that is, the size of the studies included.
Methods
Based on a real meta-analysis, we simulate artificially 'inflating' the sample size under the random effects model. For a given inflation factor M = 1, 2, 3,... and for each trial i, we create a M-inflated trial by drawing a treatment effect estimate from the random effects model, using /M as within-trial sampling variance.
Results
As precision increases, while estimates of the heterogeneity variance τ2 remain unchanged on average, estimates of I2 increase rapidly to nearly 100%. A similar phenomenon is apparent in a sample of 157 meta-analyses.
Conclusion
When deciding whether or not to pool treatment estimates in a meta-analysis, the yard-stick should be the clinical relevance of any heterogeneity present. τ2, rather than I2, is the appropriate measure for this purpose.
Background
In meta-analysis, three principal sources of heterogeneity can be distinguished. These are (i) clinical baseline heterogeneity between patients from different studies, measured, e.g., in patient baseline characteristics and not necessarily reflected on the outcome measurement scale; (ii) statistical heterogeneity, quantified on the outcome measurement scale, that may or may not be clinically relevant and may or may not be statistically significant, and (iii) heterogeneity from other sources, e.g. design-related heterogeneity. In this article, we only deal with statistical heterogeneity. References [1-7] give an introduction to the large literature in this area. We do not discuss how to assess clinical baseline heterogeneity.
In this paper, we show that I2 increases with the number of patients included in the studies in a meta-analysis. In the light of this, we argue that I2 is in general of limited use in assessing clinically relevant heterogeneity.
The article is structured as follows. After introducing existing measures of heterogeneity in meta-analysis and discussing their properties, we illustrate the problem of interpreting the measure I2 using an example from the literature. We then present a simulation study which explores the effect of sample size inflation on I2, and finally conclude with a discussion.
Methods
Let k be the number of studies in a meta-analysis. Further, let xi be the within-study treatment effect estimate (e.g., a log odds ratio), the within-study variance of xi, and wi the weight of study i (i = 1,..., k). In this article, we always use inverse variance weights, that is, wi = 1/ if the fixed effect model is used, and wi = 1/( + τ2) if the random effects model is used (see below for definition and estimation of the heterogeneity variance τ2). Several measures of statistical heterogeneity are widely used:
1. Cochran's Q statistic, which under the null hypothesis of no heterogeneity follows a χ2 distribution with k - 1 degrees of freedom [8]. Q is given by
2. Higgins' and Thompson's I2, derived from Cochran's Q by defining [4]
3. the between-study variance, τ2, as estimated in a random effects meta-analysis. There are several proposals for estimating τ2 in a meta-analysis, such as the REML estimator or the Hedges-Olkin estimator [5-7,9]. Nevertheless, most reviewers use the moment-based estimate of τ2 [10], implemented in RevMan [11] and calculated as
4. H2, derived from Cochran's Q by defining [4]
and
5. R2, similar to H2 and calculated from τ2 and a so-called 'typical' within-study variance σ2 (which must be estimated), and defined as:
As seen here, and described elsewhere [4], some measures are directly related, and others approximately related. Table 1 shows key properties of the various measures; more details are given in [4]. In summary:
Table 1.
Properties of measures of heterogeneity.
| Measure | measured on | increasing with | ||
| scale | range | number of studies in meta-analysis | precision (size of studies) | |
| Q | absolute | [0, ∞) | yes | yes |
| I2 | percent | [0, 100%] | no | yes |
| τ, τ2 | outcome | [0, ∞) | no | no |
| H, H2 | absolute | [1, ∞) | no | yes |
| R, R2 | absolute | [1, ∞) | no | yes |
1. Q, which follows a χ2 distribution with k - 1 degrees of freedom under H0, is the weighted sum of squared differences between the study means and the fixed effect estimate. It always increases with the number of studies, k, in the meta-analysis.
2. In contrast to Q, the statistic I2 was introduced by Higgins and Thompson [4] as a measure independent of k, the number of studies in the meta-analysis. I2 is interpreted as the percentage of variability in the treatment estimates which is attributable to heterogeneity between studies rather than to sampling error.
3. τ2 describes the underlying between-study variability. Its square root, τ, is measured in the same units as the outcome. Its estimates do not systematically increase with either the number, or size, of studies in a meta-analysis.
4. H2 is a test statistic. It describes the relative difference between the observed Q and its expected value in the absence of heterogeneity. Thus it does not systematically increase with the number of studies [4]. H corresponds to the residual standard deviation in a radial (Galbraith) plot [12]. H = 1 indicates perfect homogeneity.
5. R2 is the square of a statistic R which describes the inflation of the random effects confidence interval compared to that from the fixed effect model. It does not increase with k. R2 = 1 indicates perfect homogeneity [4].
Notice that, in contrast to τ2, the measures Q, I2, H and R all depend on the precision, which is proportional to study size [13]. Thus, given an underlying model, if the study sizes are enlarged, the confidence intervals become smaller and the heterogeneity, measured (say) using I2, increases. This is reflected in the interpretation: As I2 is the percentage of variability that is due to between-study heterogeneity, 1 - I2 is the percentage of variability that is due to sampling error. When the studies become very large, the sampling error tends to 0 and I2 tends to 1. Such heterogeneity may not be clinically relevant.
We now explore this further using simulation. Note first that simply looking at the effect of scaling up all sample sizes by a common factor (leaving their treatment effects unchanged) is not appropriate. This is because if study sizes were truly to increase, estimates would approach the true value for each study and not be fixed at the original observed value. Instead, we simulate under the random effects model. Under this model, μ and τ2 are assumed constant, and the total variance in study i is + τ2, which decreases with increasing study sample size, eventually tending to τ2.
Study size inflation based on the random effects model
Suppose in a meta-analysis trial i reports treatment effect estimate xi (e.g., on the log odds scale) with observed sampling variance . Let τ2 denote the heterogeneity variance. The model is
where μ is the average treatment effect. For a given inflation factor M = 1, 2, 3,..., the model with inflated sample size (corresponding to an M-fold increase in precision) is
We generate an illustrative meta-analysis for each inflation factor. For each trial in each meta-analysis, we generate a random M-inflated trial by drawing a treatment effect estimate xM,i from this model, using /M as the within-trial sampling variance and the DerSimonian-Laird estimate for the heterogeneity parameter τ2.
Results
We use data from a large meta-analysis (of 70 trials) to estimate the effect of thrombolytic therapy in acute myocardial infarction [14]. The original analysis using the fixed effects model (Mantel-Haenszel method) gives an odds ratio of 0.747 with a 95% confidence interval (95% CI) of [0.705; 0.792]. Using the random effects model, the odds ratio is 0.732, 95% CI [0.664; 0.808]. The DerSimonian-Laird estimate of τ2 is 0.018 (H = 1.11, 95% CI [1; 1.29], I2 = 18.6%, 95% CI [0%; 40.1%]). As Q = 85, p = 0.0953, there is no evidence of heterogeneity.
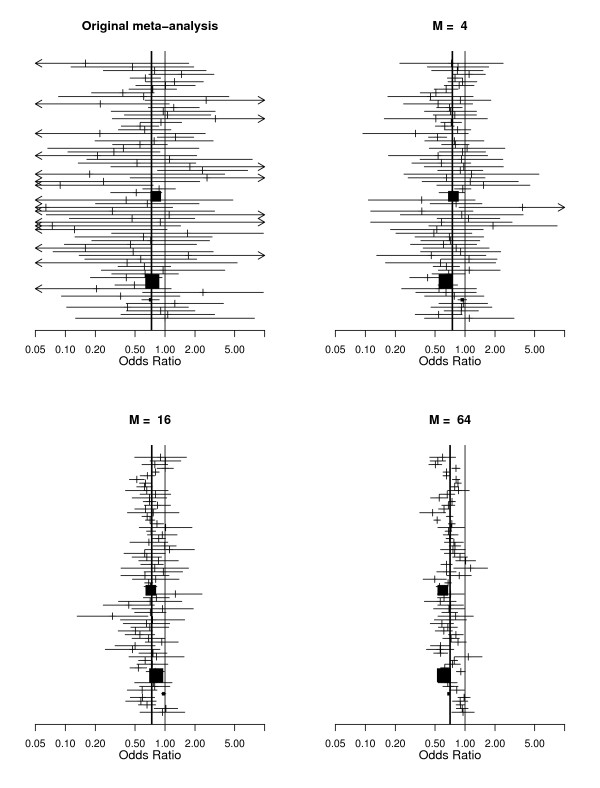
We now explore the effect of increasing M. Figure 1 shows forest plots of the original meta-analysis along with illustrative meta-analyses generated for M = 4, 16 and 64. The behavior of the heterogeneity measures is shown in Table 2. It is clear that while the variation in τ2 is essentially random, the values of Q, H and I2 increase rapidly with increasing sample size.
Figure 1.
Top left panel: Meta-analysis of thrombolytic therapy in acute myocardial infarction [14]. Other plots: illustrative randomly sampled versions of the same meta-analysis with sample-size inflation factors of M = 4, 16 and 64 (details in text).
Table 2.
Effect of increasing within trial precision (factor M) on heterogeneity measures (data in [14]).
| Factor | Measure | |||
| M | Q (P-value) | I2 | H | |
| 1 | 0.018 | 85 (0.0953) | 18.6% [0%; 40.1%] | 1.11 [1; 1.29] |
| 4 | 0.008 | 98 (0.0135) | 29.2% [4.5%; 47.6%] | 1.19 [1.02; 1.38] |
| 16 | 0.027 | 454 (<0.0001) | 84.8% [81.4%; 87.5%] | 2.56 [2.32; 2.83] |
| 64 | 0.028 | 1708 (<0.0001) | 96.0% [95.4%; 96.5%] | 4.98 [4.65; 5.32] |
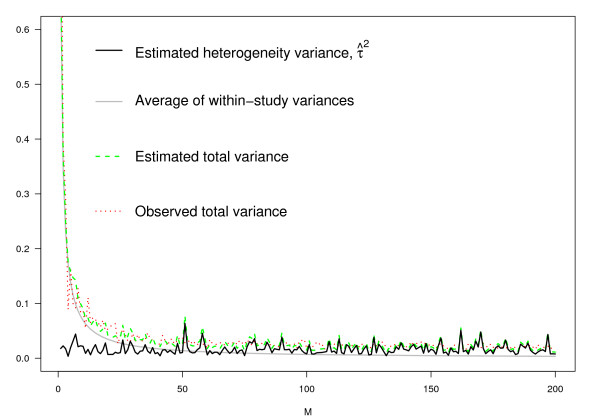
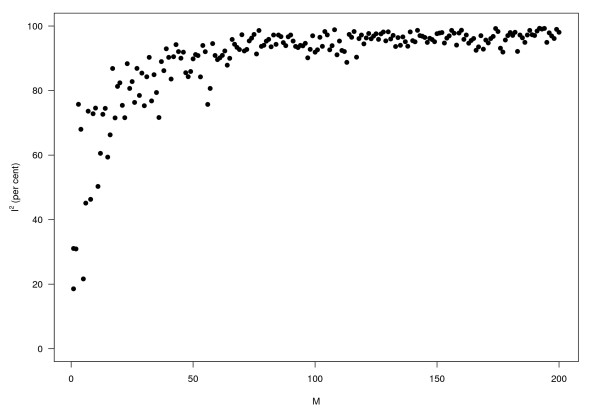
Figures 2 and 3 give two other perspectives on this. Figure 2 shows that as M increases, τ2 varies randomly, while (i) the average of the within study variances; (ii) the estimated total variance (under the model), and (iii) the observed total variance, all decrease rapidly with increasing M. Using the same data, Figure 3 shows how I2 behaves. Note how rapidly it approaches 100%.
Figure 2.
Within-study variation, decreasing with increasing sample size while heterogeneity remains constant. Details in text.
Figure 3.
Percentage I2 of variation due to heterogeneity rather than to sampling error against sample size (same simulation data as in Figure 2).
Empirical evaluation: a sample of meta-analyses
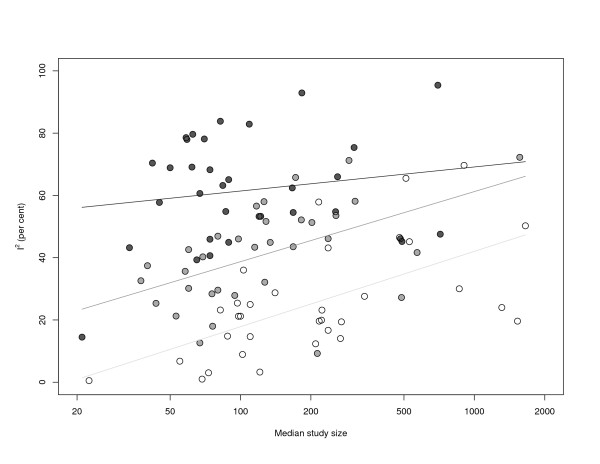
In order to examine the behavior and the order of magnitude of I2 empirically, we further looked at a sample of 157 meta-analyses with binary endpoints. This data set was kindly provided by Peter Jüni [15]. We calculated τ2 and I2 for each meta-analysis. Further, for each meta-analysis, we calculated the median study size of the contributing studies, denoted ni, i = 1,..., 157. After excluding all meta-analyses with both τ2 = I2 = 0 (n = 58), we fitted a linear model to the remaining 99 meta-analyses with I2 as outcome and and log ni as covariates (thus implicitly assuming a log-normal distribution for study size).
As expected, I2 increases with both heterogeneity (βτ = 65.873, SE = 4.788, p = 0.000) and median study size (βlog n = 8.503, SE = 1.460, p = 0.000). The residual standard error is 13.07 with an adjusted = 0.6621 (F = 97.01, df = 96, p = 0.000). That is, even after adjusting for between-study variance τ2, I2 depends strongly on study size. Figure 4 illustrates the results.
Figure 4.
I2 against median study size in a sample of 157 meta-analyses. Light, grey and black dots and regression lines correspond to the first, second and third tercile of the distribution of τ2.
Light, grey and black dots and regression lines correspond to the first, second and third tercile of the distribution of τ2. Within each class of meta-analyses, I2 is increasing with median study size.
Discussion
The main advantage of the statistic I2 is that it does not depend on the number of studies in a meta-analysis. Thus, using I2 instead of Q, it is possible to compare the statistical heterogeneity of meta-analyses with different numbers of studies [4]. Also, I2 is easily interpreted by clinicians as the percentage of variability in the treatment estimates which is attributable to heterogeneity between studies rather than to sampling error.
However, an immediate (but often overlooked) consequence of this interpretation is that I2 increases with the number of patients included in the studies in a meta-analysis. In a recent simulation using continuous outcomes, others found empirically that I2 increased with increasing numbers of patients per trial though τ2 was kept fixed [16]. Unfortunately, as demonstrated by a recent empirical study [17], reviewers seem to be unaware of this when they use I2 to decide whether to pool studies in a meta-analysis. Some authors also seem to be reluctant to call I2 a statistic, using instead words such as metric [18], index [19], or even point estimate [17,18,20]. On the other hand, the term 'statistical test' is used in connection with I2 in one of these references [20], p. 915. In another reference [18], the authors proposed an algorithm for a sensitivity analysis that successively excludes 'outlying' trials until I2 falls below a prespecified level. In response to this [21], Higgins showed that the exclusion of a large trial with its effect close to the pooled estimate can be the most efficient way to reduce I2.
Our simulation highlights the problem of interpreting heterogeneity measured by I2 as clinical heterogeneity. This is analogous to interpreting statistically significant effects (P < 0.05) as clinically relevant. In our view the decision on whether or not to pool studies in a meta-analysis should not solely be based on I2. Instead, studies with relatively large I2 may usefully be pooled when the clinically relevant heterogeneity (in efficacy and covariates) is acceptably small.
Further, as τ is measured on the same scale as the outcome, it can be directly used to quantify variability. Indeed, clinically meaningful heterogeneity on the outcome scale could be pre-specified. Thus, in advance a reviewer may decide that three studies with odds ratios of 0.8, 1 and 1.25 cannot be pooled; in other words the relative effect ratios of 0.8 = 1/1.25 are too great. This corresponds to a standard deviation τ0 = - log 0.8 = log 1.25 = 0.22 = on the log scale and thus a threshold of = 0.05 for the heterogeneity variance τ2.
While Higgins and Thompson in their papers [4,22] thoroughly described the properties of the various measures and distinguished between them, we feel current guidelines are likely to let misconceptions persist. For example, the 'Cochrane Handbook for Systematic Reviews of Interventions' (outdated Version 4.2.6, page 138) stated 'A value [of I2] greater than 50% may be considered as substantial heterogeneity'. The recent Version 5.0.1, while admitting that 'thresholds for the interpretation of I2 can be misleading, since the importance of inconsistency depends on several factors', nevertheless lists overlapping ranges of I2 which provide 'a rough guide to interpretation' (see Table 3) [23]. The result is that some reviewers conclude that studies must not be pooled if I2 > 50% [24,25]. By contrast, Section 9.5.4 of the handbook states 'The choice between a fixed-effect and a random-effects meta-analysis should never be made on the basis of a statistical test of heterogeneity'. Further some methodologists discourage reviewers from using tests for funnel plot asymmetry if I2 > 50% [26].
Table 3.
Ranges for interpretation of I2 following the Cochrane Handbook for Systematic Reviews of Interventions (Version 5.0.1) [23].
| 0% to 40% | might not be important |
| 30% to 60% | may represent moderate heterogeneity |
| 50% to 90% | may represent substantial heterogeneity |
| 75% to 100% | considerable heterogeneity |
We believe the interpretation issues stem from the concept of I2 as 'the proportion of variance (un)explained', referred to as 'widely familiar' to clinicians by Higgins and Thompson [4] (Section 4). However, there is a fundamental difference between the interpretation of the coefficient of determination in regression analysis, which is sub-consciously invoked by this phrase, and that of I2: On the one hand, (that is, the square of the correlation coefficient) is a measure of the association between the dependent and the independent variable, which homes in on the true value as the sample size increases. However, I2 tends to 100% as the number of patients increases. Although one may argue that the 'unit' corresponding to the 'observation' in a regression is the study, not the patient, this link is only strictly valid if sample size of new studies are distributed similarly to those of existing studies. This is not universally true. Often small trials are followed by larger ones. Thus I2 will tend to increase artificially as evidence accumulates.
To address this, more weight should be given to often overlooked comments by Higgins and Thompson, [4], p 1545, who state 'Note that we do not propose that our measure should be independent of the precisions of estimates observed in the studies. Thus sets of studies with identical heterogeneity τ2, but with different degrees of sampling error σ2, will produce different measures.... Describing the underlying between-study variability ... can best be achieved simply by estimating the between-study variance, τ2.'
Conclusion
When deciding whether or not to pool treatment estimates in a meta-analysis, the yard-stick should be the clinical relevance of any heterogeneity present. τ2, rather than I2 is the appropriate measure for this purpose.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
GR proposed the model for sample size inflation, did all calculations and wrote the first draft of the manuscript. GS, JC and MS contributed to the writing and approved the final version.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
GR and JC are funded by Deutsche Forschungsgemeinschaft (FOR 534 Schw 821/2-2). The authors wish to thank Peter Jüni for providing data and all reviewers and Douglas G Altman for helpful discussion.
Contributor Information
Gerta Rücker, Email: ruecker@imbi.uni-freiburg.de.
Guido Schwarzer, Email: sc@imbi.uni-freiburg.de.
James R Carpenter, Email: James.Carpenter@lshtm.ac.uk.
Martin Schumacher, Email: ms@imbi.uni-freiburg.de.
References
- Hardy RJ, Thompson SG. Detecting and describing heterogeneity in meta-analysis. Statistics in Medicine. 1998;17:841–856. doi: 10.1002/(SICI)1097-0258(19980430)17:8<841::AID-SIM781>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: A comparison of methods. Statistics in Medicine. 1999;18:2693–2708. doi: 10.1002/(SICI)1097-0258(19991030)18:20<2693::AID-SIM235>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Engels EA, Schmid CH, Terrin N, Olkin I, Lau J. Heterogeneity and statistical significance in meta-analysis: An empirical study of 125 meta-analyses. Statistics in Medicine. 2000;19:1707–1728. doi: 10.1002/1097-0258(20000715)19:13<1707::AID-SIM491>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Sidik K, Jonkman JN. Simple heterogeneity variance estimation for meta-analysis. JRSS Series C (Applied Statistics) 2005;54:367–384. doi: 10.1111/j.1467-9876.2005.00489.x. [DOI] [Google Scholar]
- Knapp G, Biggerstaff BJ, Hartung J. Assessing the amount of heterogeneity in random-effects meta-analysis. Biometrical Journal. 2006;48:271–285. doi: 10.1002/bimj.200510175. [DOI] [PubMed] [Google Scholar]
- Viechtbauer W. Confidence intervals for the amount of heterogeneity in meta-analysis. Statistics in Medicine. 2007;26:37–52. doi: 10.1002/sim.2514. [DOI] [PubMed] [Google Scholar]
- Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. doi: 10.2307/3001666. [DOI] [Google Scholar]
- Hedges LV. A random effects model for effect sizes. Psychological Bulletin. 1983;93:388–395. doi: 10.1037/0033-2909.93.2.388. [DOI] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in Clinical Trials. Controlled Clinical Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Review Manager (RevMan) [Computer program]. Version 5.0. 2008. http://www.cc-ims.net/RevMan/RevMan5/
- Galbraith RF. A note on graphical presentation of estimated odds ratios from several clinical trials. Statistics in Medicine. 1988;7:889–894. doi: 10.1002/sim.4780070807. [DOI] [PubMed] [Google Scholar]
- Mittlböck M, Heinzl H. A simulation study comparing properties of heterogeneity measures in meta-analyses. Statistics in Medicine. 2006;25:4321–4333. doi: 10.1002/sim.2692. [DOI] [PubMed] [Google Scholar]
- Olkin I. Statistical and theoretical considerations in meta-analysis. Journal of Clinical Epidemiology. 1995;48:133–146. doi: 10.1016/0895-4356(94)00136-E. [DOI] [PubMed] [Google Scholar]
- Jüni P. Department of Social and Preventive Medicine, University of Berne, Switzerland. Personal Communication. 2006.
- Friedrich JO, Adhikari N, Beyene J. The ratio of means method as an alternative to mean differences for analyzing continuous outcome variables in meta-analysis: a simulation study. BMC Medical Research Methodolology. 2008;8:32. doi: 10.1186/1471-2288-8-32. http://www.biomedcentral.com/1471-2288/8/32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis JP, Patsopoulos NA, Rothstein HR. Reasons or excuses for avoiding meta-analysis in forest plots. BMJ. 2008;336:1413–1415. doi: 10.1136/bmj.a117. http://www.bmj.com/cgi/content/full/336/7658/1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. International Journal of Epidemiology. 2008;37:1148–1157. doi: 10.1093/ije/dyn065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychological Methods. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- Ioannidis JP, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ. 2007;335:914–916. doi: 10.1136/bmj.39343.408449.80. http://www.bmj.com/cgi/content/full/335/7626/914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP. Commentary: Heterogeneity in meta-analysis should be expected and appropriately quantified. International Journal of Epidemiology. 2008;37:1158–1160. doi: 10.1093/ije/dyn204. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analysis. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. http://www.bmj.com/cgi/content/full/327/7414/557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 501 Version 501. 2008. http://www.cochrane-handbook.org
- Thomas D, Elliott E, Naughton G. Exercise for type 2 diabetes mellitus. Cochrane Database of Systematic Reviews. 2006;19:3. doi: 10.1002/14651858.CD002968.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmer A, McDonald JW, MacDonald JK. Azathioprine And 6-Mercaptopurine For Maintenance Of Remission In Ulcerative Colitis. Cochrane Database Syst Rev. 2007;24:CD000478. doi: 10.1002/14651858.CD000478.pub2. [DOI] [PubMed] [Google Scholar]
- Ioannidis JPA, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey. Canadian Medical Association Journal. 2007;176:1091–1096. doi: 10.1503/cmaj.060410. [DOI] [PMC free article] [PubMed] [Google Scholar]