As the need for extrarenal organs increases, surgeons normally responsible for the procurement and later use of kidneys will find themselves more frequently involved in the harvest of other organs, such as the liver. The procurement of kidneys in this country has become regionalized, whereas the transplantation of livers has remained geographically centralized to only a very few institutions.
Of the 30 liver transplantations done at the University of Pittsburgh in 1981, 20 livers were obtained from centers outside the geographic area normally assigned to the Transplant Organ Procurement Foundation of the University. The expansion of liver transplantation will be highly dependent upon the cultivation of this type of interinstitutional co-operation.
The method of donor hepatectomy and nephrectomy described in this report can be easily modified to conform with the kidney harvesting techniques customarily used by surgeons of the referring organ procurement center. Careful management of the various steps provides equal protection of all three potential grafts against ischemic damage, as will be shown by a review of the outcome of 56 kidneys and 30 livers obtained from 30 cadaveric donors.
METHODS
Donor nephrectomy is performed with varying techniques in different centers. It is essential that the liver transplantation team review the methods of cadaveric nephrectomy preferred at the host institution and make certain adaptations. For instance, cadaveric kidneys can be removed after thorough in situ dissection, with cooling of the organs by infusion of a cold preservation solution after they are removed from the donor. Alternatively, the infusion step can be done in situ and the organs excised thereafter, as reported by Merkel and associates (1) and Schweizer and colleagues (2). If definitive preservation by pump perfusion is desired, a segment of aorta is normally excised with each kidney and fashioned to accommodate perfusion cannulas. This is not necessary if cold storage in an ice-slush solution is preferred. Either method is compatible with the techniques of donor hepatectomy, provided that the critical step of precooling to be described is carried out.
Preliminary Steps
The abdomen is opened through a long midline incision. Extensions can be made laterally into the left side of the thorax and right flank, as is necessary. More recently, the addition of a midline sternotomy has obviated the need for these extensions and has provided excellent exposure in the upper part of the abdomen. The gross suitability of the liver and kidneys for transplantation is immediately confirmed.
If in situ perfusion of the kidneys is not the preference of the local surgeon and individual nephrectomies are desired, the left kidney can be dissected free, removed from the field and immediately preserved by the method of choice. The adjacent left anterolateral surface of the aorta is then dissected, exposing and encircling the superior mesenteric artery and celiac axis. In this adaptation, the right kidney is excised after the liver has been removed, since a segment of vena cava is usually taken with the renal vein to provide for extra length.
Should the host surgeon prefer in situ cooling of the kidneys, dissection of the upper part of the aorta is performed first. The diaphragm is incised to the aorta and the aorta encircled so that it may be clamped later. The arcuate ligament is divided, and the celiac axis and superior mesenteric artery are exposed at their origin. Special effort is not made at this time to explore the renal arteries. For adequate exposure, the left triangular ligament of the liver can be cut at this time.
With either approach, the next step is to skeletonize the structures of the portal triad. The common duct is cut to provide as much length as possible. A small incision is made in the fundus of the gallbladder, and the extrahepatic biliary tract, including the gallbladder, is washed clean to prevent later autolysis. Next, the hepatic artery is dissected from the hilus of the liver back toward the celiac axis, ligating the gastroduodenal, right gastric, splenic and left gastric arteries as the dissection proceeds proximally. In many donors, it is safer to begin at the aorta and to deal with the splenic and left gastric arteries while moving distally. Before ligating the left gastric artery, it is important to be sure that it does not have an anomalous branch to the left lobe of the liver. Such a branch can be seen and easily palpated in the superior portion of the gastrohepatic ligament and must be preserved. Anomalous branches from the superior mesenteric artery can supply the entire liver or the right lobe and, usually, can be palpated posterior to the portal vein (Fig. 1). Methods of rearterialization with these anomalies have been described previously by Starzl (3).
Fig. 1.
Anomalies of hepatic arterial supply.
The portal vein is dissected to below the junction of the splenic and superior mesenteric veins. Division of the pancreas at this level enhances the exposure to these vessels. The coronary vein and other small branches are ligated and divided, but the major vessels are left intact at this time. The vena cava is encircled below the liver and the level of entry of both renal veins identified.
If retraction of the right hepatic lobe is possible without causing undue cyanosis, the right triangular and coronary ligaments are cut, and the bare area is entered. The suprahepatic vena cava is encircled, and tributary veins—the phrenic veins are most constant—are ligated and divided. The retrohepatic vena cava is exposed, into which the only constant tributary is the right adrenal vein. If such manipulation causes color changes of the liver, it is abandoned. Hepatic artery spasm is prevented by bathing the vessel with papaverine or procaine. The final stages of vena caval dissection are deferred to the end if there is a question that the liver is being damaged.
Option of In Situ Renal Perfusion
If the preference of the host surgeons is to cool the kidneys by in situ infusion, large cannulas are introduced into the distal part of the aorta and the inferior vena cava, and a third cannula is placed into the previously dissected splenic vein as shown in Figure 2. Before proceeding to the next step, the donor is given 300 U.S.P units per kilogram of heparin.
Fig. 2.
En bloc infusion of liver and kidneys.
Principle of Precooling
Regardless of what techniques will be used for kidney harvesting and preservation, the critical step at this point for liver preservation is rapid infusion of cold lactated Ringer’s solution through the portal system. Pressures of approximately 100 centimeters of water are maintained by elevation of the infusion reservoir. The cadaver core temperature is monitored during this phase with a rectal or esophageal probe or with a thermistor-tipped pulmonary artery catheter. The liver still has an intact arterial supply during this time, but after approximately 1,000 milliliters have been infused, it can be felt to cool rapidly as the body core temperatures approach 30 degrees C. Central venous pressures are regulated by using the inferior vena cava catheter as a kind of overflow valve, thus avoiding hepatic venous hypertension.
A bolus injection of a vasolytic agent, such as phentolamine, chlorpromazine or tolazoline may be given to the donor, as is a common practice in many centers, during this phase of the procedure as reported by Belzer and collaborators (4).
Terminal Events
Precooling with portal infusion of lactated Ringer’s solution is stopped either when the core temperatures reach 30 degrees C., usually after 2,000 milliliters in an adult, or whenever hemodynamic instability occurs. The in situ renal perfusion is done by cross clamping the aorta above the celiac axis and infusing with cold, modified Collins’ solution, or the host institution’s preservation solution of choice, through the previously placed distal aortic cannula. The vena cava line is opened and allowed to drain fully. The infusion into the portal vein is changed to an electrolyte solution for kidney preservation for a final flush, amounting to 500 to 1,000 milliliters in adults.
The liver is removed, placed in slush and inspected carefully. Any defects found in the vena cava are repaired at this time. The cold kidneys are then removed, and definitive preservation is instituted by one of the conventional techniques.
If in situ perfusion of the kidney is not preferred and the left kidney is removed at the outset, aortic perfusion of the liver and the remaining kidney can be omitted, if desired, inasmuch as total body cooling to 25 to 30 degrees C. is highly protective. The right kidney is promptly removed after hepatectomy is completed and flushed with a preservation solution.
RESULTS
In the 30 donors of livers transplanted at the University of Pittsburgh, in situ kidney perfusion through the aorta was used in all. For the first four adult donors, the portal precooling stage was incomplete in two and omitted altogether in two. In two liver donors, kidneys were not harvested.
Renal Allografts
The fate of the 56 kidneys is summarized in Table I. Of the 18 kidneys obtained locally in Pittsburgh, nine, including one en bloc pair from a child, were exported and used elsewhere. Only three single kidneys and one en bloc pair of the total 38 kidneys harvested outside of Pittsburgh were transplanted at the University of Pittsburgh. Six organs were not used. Three of these six kidneys were discarded by the referring centers because of surgical errors at the conclusion of the nephrectomies. Suitable recipients were not found for two others. The sixth kidney was not used because a lower pole artery, located 8 centimeters from the main renal artery, appeared to have intimal damage. This proved not to be so upon histologic examination.
TABLE I.
OUTCOME OF KIDNEYS PROCURED IN CONCERT WITH LIVERS

|
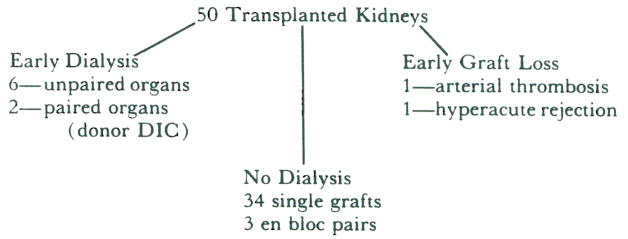
The results of transplantation of the remaining 50 kidneys are summarized in Table II. Since three kidney pairs from children were transplanted en bloc, the recipients numbered 47. One graft was lost immediately to hyperacute rejection. Another graft was lost because of an arterial occlusion resulting from a technical error in graft placement. Thirty-seven of the remaining 45 recipients achieved immediate function. The other eight required early dialysis. Prompt function of the contralateral mate to each of five of these kidneys was obtained. The remaining two kidneys were a pair taken from a donor who, in retrospect, was found to have had disseminated intravascular coagulation. The recipients of these two kidneys both required five dialysis treatments before eventual graft function was adequate. Of the other six patients requiring early dialysis, two received kidneys that were preserved initially for 25 and 36 hours in slush and then given pump perfusion for an additional 15 and 12 hours, respectively. Function was eventually adequate after three dialysis treatments in one recipient and five in the other recipient. Two other of these recipients were undergoing retransplantation and were considered high responders by virtue of their having preformed antibodies reacting with 90 per cent of panel lymphocytes. Finally, another of these patients had an early biopsy of the kidney which was interpreted as showing incomplete hyperacute rejection. All eight recipients eventually attained adequate graft function.
TABLE II.
RESULTS OF KIDNEY TRANSPLANTATION
|
DIC, Disseminated intravascular coagulation.
The number of kidneys preserved by each of the two techniques used and the mean preservation times for each group are shown in Table III. The two organs with prolonged times in each method are not included in the individual categories but are placed in the over-all group. No differences in the means which are significant were found when the data were subjected to analysis with Student’s t test.
TABLE III.
PRESERVATION TIMES FOR TRANSPLANTED KIDNEYS
| Times | No. | |
|---|---|---|
| Mean over-all pump time | 24.08±7.56 h | 32 |
| Mean pump time, dialysis group | 23.00±8.20 h | 4 |
| Mean pump time, nondialysis group | 24.23±7.61 h | 28 |
| Mean over-all slush time | 23.69±7.2 h | 13 |
| Mean slush time, dialysis group | 20.83±2.4 h | 3 |
| Mean slush time, nondialysis group | 24.55±8.0 h | 10 |
| Mean over-all preservation time | 25.16±8.4 h | 47 |
| Mean preservation time, dialysis group | 26.90±11.2 h | 9 |
| Mean preservation time, nondialysis group | 24.74±7.7 h | 38 |
h, Hours.
Hepatic Allografts
The first four hepatic homografts did not function well from the outset. The recipients died from four to 22 days later. Each of the livers had massive necrosis. The precooling by portal vein infusion had been omitted in two and had been incomplete in the other two. These problems were compounded in two of the four recipients by technical errors in biliary tract reconstruction. Beside liver failure, all four patients had infectious complications and failure of other organs.
The next 26 livers were precooled by satisfactory portal infusions in conjunction with in situ renal perfusion. After preservation from one and a half to 12 hours, all but one of these livers functioned adequately. Elevations of transaminase levels occurred occasionally but always were indicative of variable and quickly reversible ischemic injury. One of the livers had excellent early function, but a huge fungal abscess developed and the liver was replaced two weeks later. The one liver in this group that failed to function adequately early was taken from a donor found in retrospect to have disseminated intravascular coagulation. Retransplantation was done four days later, and histologic examination of the first liver revealed generalized microvascular thrombosis with coagulation necrosis. A third transplant was performed in this patient 52 days later, when severe rejection resulted in liver failure.
Of the 22 recipients of these 26 organs, all but three are alive after six weeks to six months. One of the fatalities was caused by disseminated aspergillus infection in an adult. The second was a child who died four weeks after transplant, when, at reoperation, liver abscess was found to have eroded into the intrahepatic portal vein, and exsanguination occurred. The third fatality was the recipient of three sequential liver allografts. He died of a massive intracranial hemorrhage, 46 days after the last transplant.
DISCUSSION
Previous experience with organ procurement at the University of Colorado had delineated the requirements for effective hepatic procurement to include preliminary cooling of the liver by portal infusion of a cold solution, followed by portal infusion with an electrolyte preservation solution. This was shown to eliminate hepatic warm ischemia effectively during the harvest, since the hepatic arterial supply is intact during the initial cooling phase. The ancillary benefit of this practice has been the protection of the kidneys, since effective total body cooling also occurs.
Rolles and co-workers (5) reported an incidence of early, first week, dialysis treatment of 30 per cent in 42 recipients of kidneys harvested in concert with livers. This incidence of early dialysis was strikingly better than that found when kidneys were not recovered with livers, and it was viewed as persuasive evidence that the kidneys are not jeopardized in these combination procedures.
Excluding instances of early graft loss, the incidence of first week dialysis treatment in our series was 17 per cent. This incidence also compares quite favorably with that of series in which kidneys alone were recovered, including the 17 to 19 per cent reported by Squifflet and collaborators (6), the 30 per cent reported by Feduska and co-authors (7) and the 44 per cent reported by Cho and associates (8) and 54 per cent as indicated in the report of Anderson and colleagues (9). Vaughn and co-workers (10) recently published incidences from the collected institutions of the Southeastern Organ Procurement Foundation of 40.5 per cent early dialysis for cold stored kidneys and 32.8 per cent for those that were machine perfused. The incidence of first week dialysis treatment in the present report is also no different from that seen in our own series of kidneys not harvested in combination with livers, 18 per cent. Organ loss secondary to technical errors during nephrectomy, four of 56, and the total number of kidneys not used for all reasons, six of 56, is less than the total discard incidence of 27 per cent reported by Montella (11) for the first quarter of 1981.
Finally, the cause of inadequate early graft function in six kidneys was presumed to be acute tubular necrosis. The satisfactory function of the contralateral mate, in a donor pair, to each of these six kidneys argues against indictment of the procurement procedure itself as the cause of acute tubular necrosis.
SUMMARY
In an approach to combined donor hepatectomy and nephrectomy, which is adaptable to any cadaveric kidney procurement method now in use, the importance of precooling with portal venous infusion is stressed. The over-all effectiveness of the technique in providing equal protection for the quality of all three organs is supported by the data presented. Encouragement should be given to the type of interinstitutional collaborations that are necessary for the continued progression of extrarenal organ transplantation.
Acknowledgments
Supported by grant Nos. AM-30183 and AM-29961 from the Veterans Administration and the National Institutes of Health, Bethesda, Maryland.
References
- 1.Merkel FK, Jonasson O, Bergan JJ. Procurement of cadaver donor organs; evisceration technique. Transplant Proc. 1972;4:585–589. [PubMed] [Google Scholar]
- 2.Schweizer R, Sutphin BA, Bartus SA. In situ cadaver kidney perfusion. Transplantation. 1981;32:482–487. doi: 10.1097/00007890-198112000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Starzl TE. Experience in Hepatic Transplantation. Philadelphia: W. B. Saunders Co.; 1969. [Google Scholar]
- 4.Belzer FO, Reed TW, Pryor JP, et al. Cause of renal injury in kidneys obtained from cadaver donors. Surg Gynecol Obstet. 1970;130:467–477. [PubMed] [Google Scholar]
- 5.Rolles K, Calne RY, McMaster P. Technique of organ removal and fate of kidney grafts from liver donors. Transplantation. 1979;28:44–46. doi: 10.1097/00007890-197907000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Squifflet JP, Pirson Y, Gianello P, et al. Safe preservation of human renal cadaver transplants by Euro-Collins solution up to 50 hours. Transplant Proc. 1981;13:693–696. [PubMed] [Google Scholar]
- 7.Feduska NJ, Belzer FO, Stieper KW, et al. A ten year experience with cadaver kidney preservation using cryoprecipitated plasmas. Am J Surg. 1978;135:356–361. doi: 10.1016/0002-9610(78)90065-x. [DOI] [PubMed] [Google Scholar]
- 8.Cho SI, Der Hagopian RP, Krane RS, Nasbeth DC. Regional organ preservation programs in the New England area. Surgery. 1974;75:528–534. [PubMed] [Google Scholar]
- 9.Anderson C, Sicard G, Etheredge E. Delayed primary renal function and cadaver renal allograft results. Surg Gynecol Obstet. 1979;149:697–702. [PubMed] [Google Scholar]
- 10.Vaughn WK, Mendez-Picon G, Humphries AL, Spees EK. Method of preservation is not a determinant of graft outcome in kidneys transplanted by Southeastern Organ Procurement Foundation Institutions. Transplantation. 1981;32:490–494. doi: 10.1097/00007890-198112000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Montella J. Editor. SEOPF Newsletter. 1981;2:27. [Google Scholar]




