Abstract
We report a novel 1 bp deletion (c.1834delC) in the MCT8 gene in a large Brazilian family with Allan‐Herndon‐Dudley syndrome (AHDS), an X linked condition characterised by severe mental retardation and neurological dysfunction. The c.1834delC segregates with the disease in this family and it was not present in 100 control chromosomes, further confirming its pathogenicity. This mutation causes a frameshift and the inclusion of 64 additional amino acids in the C‐terminal region of the protein. Pathogenic mutations in the MCT8 gene, which encodes a thyroid hormone transporter, results in elevated serum triiodothyronine (T3) levels, which were confirmed in four affected males of this family, while normal levels were found among obligate carriers. Through in vitro functional assays, we showed that this mutation decreases cellular T3 uptake and intracellular T3 metabolism. Therefore, the severe neurological defects present in the patients are due not only to deficiency of intracellular T3, but also to altered metabolism of T3 in central neurones. In addition, the severe muscle hypoplasia observed in most AHDS patients may be a consequence of high serum T3 levels.
Allan‐Herndon‐Dudley syndrome (AHDS; MIM 309600) is an X linked condition characterised by severe mental retardation, dysarthria, athetoid movements, muscle hypoplasia, and spastic paraplegia.1 There is large clinical interfamilial and intrafamilial variability. In severe cases, patients never gain the ability to walk or talk.2,3,4
The gene has been mapped to Xq12–Xq21.5,6 Based on the reanalysis of a large Brazilian family with AHDS, we have narrowed down the critical region for this disease gene to 3 cM between markers DXS106 and DXS986.3,4 Of the approximately 100 genes in this region, 20 have been excluded as responsible for this condition.4 Recently, it was shown that mutations in the MCT8 gene (NM_006517), coding for a thyroid hormone transporter, cause severe mental retardation associated with lack of speech, severe proximal hypotonia with poor head control and inability to keep the head upright, spastic quadriplegia, dystonic movements, and in some patients impaired vision and hearing.7,8 Patients with mutations in MCT8 also have abnormal thyroid parameters, including strongly elevated serum levels of the bioactive hormone triiodothyronine (T3) and rather low levels of the prohormone thyroxine (T4). Because of the clinical similarity of our patients and those with mutations in MCT8, we investigated if serum thyroid hormone levels were altered in those patients before analysing if MCT8 mutation causes this syndromic form of mental retardation.
PATIENTS AND METHODS
Blood samples were obtained only after informed consent by each patient's responsible guardian.
Patients
The AHDS family was re‐evaluated for clinical analysis and new blood sample collections. Genomic DNA was isolated from lymphocytes through standard methods,9 and all the six coding exons and the exon intron borders of the MCT8 gene (gi51477297) were amplified and directly sequenced on both strands using a dye terminator kit (DYEnamic; Amersham Biosciences GE) in MegaBace 1000. Primer sequences are available upon request. Mutation nomenclature was according to Den Dunnen and Antonarakis.10
Serum T3, free T3 (FT3), T4, free T4 (FT4), and thyroid stimulating hormone (TSH) levels were measured by immunofluorimetric assays in four affected patients, three obligate carriers and two non‐carriers of the pathogenic MCT8 mutation (based on segregation analysis; data not shown).
Functional analysis
Wild type and mutated human (h) MCT8‐3'UTR (2.2 kb) cDNAs were obtained joining a PCR product from hMCT8 cDNA (1842 bp) with a PCR product from genomic DNA (502 bp) by overlap extension PCR. The PCR product from the genomic DNA contained part of the coding sequence of exon 6 (which overlaps with the hMCT8 cDNA) and 227 nucleotides of the 3'UTR. Primer sequences are available on request. The most upstream primer included a HindIII restriction site and the most downstream primer a XbaI site. Wild type and mutated hMCT8‐3'UTR cDNAs were cloned into pCR Blunt II‐Topo (Invitrogen), excised with HindIII and XbaI, and subcloned in pcDNA3 (Invitrogen). The cloned fragments were sequenced on an automated ABI 3100 capillary sequencer, using the Big Dye terminator cycle sequencing method (Applied Biosystems).
JEG3 human choriocarcinoma cells were grown in DMEM‐F12 medium (Life Technologies) supplemented with 9% fetal calf serum (Life Technologies) in 6 well (uptake studies) or 24 well plates (metabolism studies). Cells were grown to 75–85% confluency, and transfected using FuGENE6 transfection reagent (Roche Applied Sciences) according to the manufacturer's protocol. Each transfection assay was performed in duplicate.
For analysis of T3 uptake, JEG3 cells were transfected with 1 µg pcDNA3 plasmid without insert or with wild type or mutated hMCT8‐3'UTR insert. After 2 days, the cells were incubated for 10 minutes at 37°C with 1 nmol/l (2×105 counts/min) [3′‐125I]T3 (Amersham) in 2 ml DMEM‐F12 medium containing 0.1% bovine serum albumin (BSA). After washing, cells were lysed and counted for radioactivity in a gamma scintillation counter (NE 1.600).
For analysis of T3 metabolism, cells were cotransfected with 0.1 µg pcDNA3 with or without hMCT8‐3'UTR insert, and 0.1 µg pCI‐neo.hD3, which encodes the human type III deiodinase (hD3) that catalyses the conversion of T3 to 3,3′‐T2. After 2 days, cells were incubated for 2 hours at 37°C with 1 nmol/l (1×106 counts/min) [3′‐125I]T3 in 0.5 ml DMEM‐F12 medium plus 0.1% BSA. After incubation, 100 µl aliquots of the medium were analysed by HPLC analysis for T3 metabolism.11
Statistical analysis
Student's t test was applied to verify if T3 uptake and metabolism differed in the presence of the mutated MCT8 compared with the normal MCT8 protein, with p<0.05 considered significant.
RESULTS
Main clinical findings
Of the six affected individuals included in our original report, two have recently died; one (case III‐11) at 25 years and the other (case II‐7) at 54 years of age. Five patients (three in this report and two in Zorick et al4) were recently re‐evaluated. Physical examination did not reveal symptoms suggestive of abnormal thyroid function. Neurological examination revealed severe mental retardation with lack of communication abilities, lack of head support, spastic quadriplegia with multiple joint contractures, reduced muscular mass, dystonic purposeless movements, and vigilant gaze. The fundus oculi was normal and patients were reactive to sounds. Clinical history and family picture analysis convincingly showed that the disability was slowly progressive, and some acquired abilities, such as sitting without support and eating independently, were lost over time.
Serum thyroid hormones and screening for mutations in the MCT8 gene
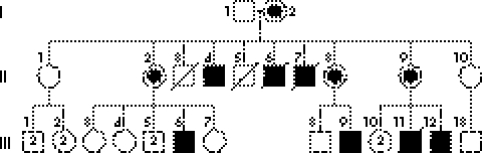
Serum thyroid hormone levels in four patients (II‐4, III‐6, III‐9 and III‐12), three obligate carriers (II‐2, II‐8 and II‐9) and two normal women (III‐3 and III‐7) are summarised in table 1. None of the family members received thyroid hormone treatment. Serum T4 and FT4 levels were in the normal range in all four tested patients, in two of the obligate carriers (II‐8, II‐9) and in both normal women (fig 1, table 1). Serum TSH was normal in all subjects except carrier II‐9, in whom it was slightly increased and carrier II‐2, in whom it was clearly elevated. In addition, II‐2 had a slightly decreased FT4 value and high serum thyroglobulin antibodies (470 U/ml), suggesting autoimmune hypothyroidism unrelated to the AHDS. Although in the three heterozygous women, the serum T3 and FT3 values were close to the upper limit of the normal range, they were similar to the values presented by the normal family members analysed. In contrast, serum T3 and FT3 concentrations were elevated in all affected men (table 1).
Table 1 Thyroid hormone levels in members of the Brazilian family with AHDS.
| No. | Genetic status | Age (years) | Free T3 (ng/dL) | Serum T3 (ng/dL) | Free T4 (ng/dL) | Serum T4 (µg/dL) | TSH (mUI/L) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| II.2 | Heterozygote | 65 | 0.39 | 173 | 0.6 | 7.1 | 26.5 | ||||||
| II.8 | Heterozygote | 52 | 0.44 | 159 | 1.0 | 8.7 | 3.0 | ||||||
| II.9 | Heterozygote | 49 | – | 152 | 0.8 | 8.6 | 4.4 | ||||||
| II.4 | Affected | 62 | 0.54 | 207 | 1.1 | 8.1 | 2.3 | ||||||
| III.6 | Affected | 34 | 0.75 | 337 | 0.8 | 8.7 | 1.6 | ||||||
| III.9 | Affected | 24 | 0.67 | 255 | 0.8 | 8.5 | 1.2 | ||||||
| III.12 | Affected | 24 | 0.62 | 253 | 0.9 | 7.1 | 4.0 | ||||||
| III.3 | Normal | 42 | 0.36 | 131 | 1.0 | 10 | 3.3 | ||||||
| III.7 | Normal | 31 | 0.40 | 159 | 1.1 | 11.8 | 1.9 | ||||||
| Normal range | 0.25–0.45 | 70–200 | 0.7–1.5 | 4.5–12.0 | 0.3–4.0 | ||||||||
Figure 1 Summarised pedigree of the Brazilian family with AHDS.
Sequence analysis of all six exons of the MCT8 gene in the propositus revealed a 1 bp deletion (c.1834delC) located five nucleotides upstream of the stop codon (fig 2). We verified that this mutation segregates with the disease in this family, and it was not detected among 100 control chromosomes, supporting its pathogenicity.
Figure 2 Partial sequence of the exon 6 of the MCT8 gene showing the deletion c.1834delC in an AHDS patient (see text for method). (A) Normal sequence; (B) corresponding sequence of the affected patient. The arrow indicates the position of deletion c.1834delC.
Functional analysis of the c.1834delC
In order to better understand the functional consequences of the c.1834delC mutation in the MCT8 gene, we evaluated the uptake of radiolabelled T3 in JEG3 cells transfected with wild type or mutated MCT8 cDNA. T3 transport by MCT8 was calculated by subtracting the uptake in JEG3 cells transfected with pcDNA3 vector without MCT8 cDNA from the measurements of the uptake in JEG3 cells transfected with wild type or mutated MCT8 cDNA. Expression of mutated MCT8 resulted in a greater than twofold reduction in T3 uptake compared with the wild type transporter (p = 0.003) (fig 3).
Figure 3 (A) T3 transport in JEG3 cells transfected with wild type or mutated hMCT8 cDNA or with vector only (see text for method). (B) T3 metabolism in JEG3 cells cotransfected with wild type or mutated MCT8 cDNA and hD3 cDNA (see text for method). Data are presented as means and ranges of duplicate observations in a typical experiment.
The effect of the MCT8 mutation on the intracellular availability of T3 was also studied by analysis of [125I]T3 metabolism in JEG3 cells cotransfected with cDNA coding for wild type or mutated MCT8 and cDNA coding for hD3. D3 inactivates T3 by inner ring deiodination to 3,3′‐T2. T3 metabolism was deduced by the difference between the quantity of radiolabelled T3 added to the cell medium and its amount in the cell medium after the incubation period. Specific T3 metabolism was calculated by subtracting the metabolism of cells transfected with empty pcDNA3 vector from that of cells expressing either mutant or wild type MCT8 construct. The mutated MCT8 construct resulted in a ninefold reduct in T3 metabolism (p = 0.017) compared with the wild type transporter (fig 3).
DISCUSSION
In the present report, we describe a novel mutation (c.1834delC) in MCT8 detected in all AHDS affected members of a large Brazilian family.3,4 Mutations in MCT8 were originally reported in young boys with severe mental retardation and very recently, Schwartz et al described mutations in this gene in six families with AHDS. There are very few reports on AHDS, but all cases reported so far are characterised by very severe mental retardation associated with various neurological dysfunctions. The inability to walk independently occurs in about 50% of patients, and speech is very compromised in all of them (Schwartz et al12 and this report). It has been suggested that the most severe forms present a phenotype that resembles cerebral palsy,2 and therefore, it seems that all pathogenic mutations found in MCT8 cause a spectrum of clinical variability of AHDS.
The mutation described by us, c.1834delC, seems not to lead to mRNA decay, as indicated both by its location in the last exon and by the results obtained with the functional analysis.13 It probably results in a frameshift, with bypassing of the wild type translation stop codon and the creation of a novel stop codon 196 nucleotides further downstream. This mRNA, translated in a protein with an abnormal C‐terminal domain with 64 additional amino acids, impairs thyroid hormone transport, the only known function of MCT8.14 Although MCT8 is capable of transporting both T3 and T4, only serum T3 and FT3, but not T4 and FT4, are increased in the AHDS patients, which has also been observed in other patients with mutations in this gene.7,8 This suggests that MCT8 is more important for T3 than for T4 metabolism.
Impaired T3 transport in AHDS patients was confirmed in this study through in vitro functional analysis of the mutated MCT8. Our findings demonstrate that the C‐terminal elongation of the MCT8 protein results in a drastic reduction of T3 transport. In addition, the strongly decreased metabolism of T3 by D3 in cells expressing mutated MCT8 compared with the wild type protein indicates that the defect in the transporter results in a large reduction in intracellular T3 availability.
MCT8 is expressed in several tissues, including liver, kidney, heart, and brain. The protein is localised in different brain areas, in particular the choroid plexus structures, neocortical and allocortical regions, striatum, and cerebellum.15 In the brain, MCT8 is localised primarily in neurones, which are the primary targets for the crucial action of T3 during fetal and neonatal brain development. This T3 is largely derived from the outer ring deiodination of T4 by the type II deiodinase (D2) in neighbouring astrocytes. MCT8 is thought to be essential for the uptake of this T3 by neurones. In addition to the nuclear receptors, which mediate the action of T3, neurones also express D3 for termination of T3 action. The defect in neuronal T3 uptake due to mutations in MCT8 will block T3 access to its intracellular receptor and degrading enzyme, with a resultant impairment of neurological development and decrease in T3 clearance.
We have observed that thyroid parameters observed in the patients with the mutation c.1834delC are less altered than in those whose disease is predicted to be due to lack of the transporter.7,8 Alternatively, considering the age of our patients (>20 years), it is possible that the abnormal thyroid parameters are more pronounced in younger patients. In this regard, it is of note that the oldest patient (II‐4) here reported, aged 62 years, had serum T3 levels close to the upper normal limit. Although the number of mutations in MCT8 is very small, different types of mutations have been described, including missense, inframe deletion, and creation of premature stop codons.7,8,12,16 It will be important to analyse the functional effects of these mutations in order to verify if there is any genotype– phenotype correlation.
All obligate carriers of the Brazilian family have normal cognitive functions. To date, there is apparently only one previous report describing a carrier female with mental retardation7 and slightly low T4 and FT4. This clinical variability possibly reflects an X inactivation deviation.
It will be interesting to investigate if in addition to an impaired CNS development, the neuromuscular abnormalities in patients with MCT8 mutations may be due to increased T3 uptake in affected muscles in the face of elevated circulating T3 levels. It is noteworthy that an excess of T3 causes muscle wasting.17,18
In conclusion, this work contributes to a better clinical and molecular characterisation of the Allan‐Herndon‐Dudley syndrome. In addition, based on our functional assays, we suggest that the neurological defect in these patients and the increased serum T3 levels are due to impaired uptake and metabolism of T3 in tissues, in particular brain. In addition, high serum T3 concentration may contribute to the dysfunction of skeletal muscle in these patients.
ACKNOWLEDGEMENTS
We gratefully acknowledge the patients and their relatives. We also thank F A A Duarte, L Armelin‐Correa, O Suzuki and C Masotti for helpful suggestions, Mrs Urbani for secretarial assistance, and W Klootwijk for technical support. This work was supported by grants from Fundação de Amparo a Pesquisa do Estado de São Paulo (CEPID/FAPESP) and Conselho Nacional de Pesquisa (CNPq), the Netherlands Organization of Scientific Research (NWO grants 903.40.204 and 916.36.139) and the Sophia Kinderziekenhuis (SKZ) Foundation.
Abbreviations
AHDS - Allan‐Herndon‐Dudley syndrome
BSA - bovine serum albumin
FT3/4 - free T3/T4
T3 - triiodothyronine
T4 - thyroxine
TSH - thyroid stimulating hormone
References
- 1.Stevenson R E, Goodman H O, Schwartz C E, Simensen R J, McLean W T, Jr, Herndon C N. llan‐Herndon syndrome. I. Clinical studies. Am J Hum Genet 199047446–453. [PMC free article] [PubMed] [Google Scholar]
- 2.Bundey S, Comley L A, Blair A. Allan‐Herndon syndrome—or X‐linked cerebral palsy? Am J Hum Genet 1991481214. [PMC free article] [PubMed] [Google Scholar]
- 3.Passos‐Bueno M R, Byth B C, Rosenberg S, Takata R I, Bakker E, Beggs A H, Pavanello R C, Vainzof M, Davies K E, Zatz M. Severe nonspecific mental retardation caused by a proximally Xp located gene: intragenic heterogeneity or a new form of X‐linked mental retardation? Am J Med Genet 199346172–175. [DOI] [PubMed] [Google Scholar]
- 4.Zorick T S, Kleimann S, Sertie A, Zatz M, Rosenberg S, Passos‐Bueno M R. Fine mapping and clinical reevaluation of a Brazilian pedigree with a severe form of X‐linked mental retardation associated with other neurological dysfunction. Am J Med Genet 2004127321–323. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz C E, Ulmer J, Brown A, Pancoast I, Goodman H O, Stevenson R E. Allan‐Herndon syndrome. II. Linkage to DNA markers in Xq21. Am J Hum Genet 199047454–458. [PMC free article] [PubMed] [Google Scholar]
- 6.Bialer M G, Lawrence L, Stevenson R E, Silverberg G, Williams M K, Arena J F, Lubs H A, Schwartz C E. Allan‐Herndon‐Dudley syndrome: clinical and linkage studies on a second family. Am J Med Genet 199243491–497. [DOI] [PubMed] [Google Scholar]
- 7.Dumitrescu A M, Liao X H, Best T B, Brockmann K, Refetoff S. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet 200474168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friesema E C, Grueters A, Biebermann H, Krude H, Von Moers A, Reeser M, Barrett T G, Mancilla E E, Svensson J, Kester M H, Kuiper G G, Balkassmi S, Uitterlinden A G, Koehrle J, Rodien P, Halestrap A P, Visser T J. Association between mutations in a thyroid hormone transporter and severe X‐linked psychomotor retardation. Lancet 20043641435–1437. [DOI] [PubMed] [Google Scholar]
- 9.Miller S A, Dykes K K, Polesky H F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 1988161215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.den Dunnen J T, Antonarakis S E. Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion. Hum Mutat 2000157–12. [DOI] [PubMed] [Google Scholar]
- 11.Kester M H A, Martinez de Mena R, Obregon M J, Marinkovic D, Howatson A, Lavado‐Autric R, Visser T J, Hume R, Morreale de Escobar G. Iodothyronine levels in the human developing brain: major regulatory roles of iodothyronine deiodinase levels in different areas. J Clin Endocrinol Metab 2004893117–3328. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz C E, May M M, Carpenter N J, Rogers R C, Martin J, Bialer M G, Ward J, Sanabria J, Marsa S, Lewis J A, Echeverri R, Lubs H A, Voeller K, Simensen R J, Stevenson R E. Allan‐Herndon‐Dudley syndrome and the monocarboxylate transporter 8 (MCT8) gene. Am J Hum Genet 20057741–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frischmeyer P A, van Hoof A, O' Donnell K, Guerrerio A L, Parker R, Dietz H C. An mRNA surveillance mechanism that eliminates transcripts lacking termination codons. Science 20022952258–2261. [DOI] [PubMed] [Google Scholar]
- 14.Friesema E C, Ganguly S, Abdalla A, Fox J E M, Halestrap A P, Visser T J. Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J Biol Chem 200327840128–40135. [DOI] [PubMed] [Google Scholar]
- 15.Heuer H, Maier M K, Iden S, Mittag J, Friesema E C, Visser T J, Bauer K. The monocarboxylate transporter 8 linked to human psychomotor retardation is highly expressed in thyroid hormone sensitive neuron populations. Endocrinology 20051461701–1706. [DOI] [PubMed] [Google Scholar]
- 16.Brockmann K, Dumitrescu A M, Best T T, Hanefeld F, Refetoff S. X‐linked paroxysmal dyskinesia and severe global retardation caused by defective MCT8 gene. J Neurol 2005252663–666. [DOI] [PubMed] [Google Scholar]
- 17.Brown J G, Millward D J. Dose response of protein turnover in rat skeletal muscle to triiodothyronine treatment. Biochim Biophys Acta 1983757182–190. [DOI] [PubMed] [Google Scholar]
- 18.Doi J, Ohtsubo A, Ohtsuka A, Hayashi K. Triiodothyronine but not thyroxine accelerates myofibrillar proteolysis via ATP production in cultured muscle cells. Biosci Biotechnol Biochem 2003672451–2524. [DOI] [PubMed] [Google Scholar]