Abstract
Spinal muscular atrophy (SMA) is the most common genetic cause of infant mortality. SMA is caused by loss of functional survival motor neuron 1 (SMN1), resulting in death of spinal motor neurons. Current therapeutic research focuses on modulating the expression of a partially functioning copy gene, SMN2, which is retained in SMA patients. However, a treatment strategy that improves the SMA phenotype by slowing or reversing the skeletal muscle atrophy may also be beneficial. Myostatin, a member of the TGF-β super-family, is a potent negative regulator of skeletal muscle mass. Follistatin is a natural antagonist of myostatin, and over-expression of follistatin in mouse muscle leads to profound increases in skeletal muscle mass. To determine whether enhanced muscle mass impacts SMA, we administered recombinant follistatin to an SMA mouse model. Treated animals exhibited increased mass in several muscle groups, elevation in the number and cross-sectional area of ventral horn cells, gross motor function improvement and mean lifespan extension by 30%, by preventing some of the early deaths, when compared with control animals. SMN protein levels in spinal cord and muscle were unchanged in follistatin-treated SMA mice, suggesting that follistatin exerts its effect in an SMN-independent manner. Reversing muscle atrophy associated with SMA may represent an unexploited therapeutic target for the treatment of SMA.
INTRODUCTION
Delivery of therapeutic agents to the central nervous system for neurodegenerative diseases faces significant obstacles. Therefore, additional targets such as skeletal muscle have been sought, which are more accessible. Enhancing muscle mass has received attention as a potential therapeutic target for the treatment of neuromuscular disorders and muscle wasting diseases (1,2). This proposed therapy does not directly target the disease-causing gene, but rather targets pathways that positively affect muscle maintenance and growth. One potentially promising target is myostatin. Myostatin is a member of the TGF-β family and is expressed almost exclusively in muscle (3). It functions as a potent negative regulator of muscle growth, and its inhibition or genetic ablation increases muscle mass and strength developmentally and in adults (4,5). Specifically, myostatin knockout animals, in comparison with wild-type, display an increased number of fast type II fibers and a decreased number of slow type I fibers (6). This change has been shown to be a result of altered development in these animals, implicating myostatin functionality in formation, proliferation or differentiation of developing muscle fibers (6). The structure and function of myostatin are highly conserved across species, and loss of functional myostatin results in increased muscle mass also in dogs, cattle and humans (7–9). Myostatin activity can be significantly reduced by neutralizing antibodies and several proteins that bind to myostatin and inhibit its activity, including follistatin, follistatin-related gene and growth and differentiation factor-associated serum protein-1 (10–12). This suggests that modulation of the myostatin signaling pathway could be exploited therapeutically. In support of this notion, the introduction of the myostatin null allele into the mdx mouse model of muscular dystrophy resulted in improved muscle regeneration (13). This proof-of-principle study was followed by attempts to block myostatin activity. The administration of the inhibitory myostatin propeptide or a stabilized anti-myostatin antibody improved the pathophysiology of the mdx mouse (14,15). Viral delivery of the inhibitory myostatin propeptide was also efficient in muscular dystrophy due to calpain deficiency, but not due to α-sarcoglycan deficiency (16). Schuelke et al. (9) reported extensively developed musculature in the absence of adverse effects in a baby with an inactivating mutation in both myostatin alleles. This suggests that myostatin blockade may become a therapeutically relevant approach to modulate muscle mass in humans. Further support comes from the recent completion of a phase I/II clinical trial of MYO-029, a neutralizing antibody, which showed adequate safety and limited efficacy (17).
Follistatin is a cystine-rich glycoprotein that binds to and inhibits several TGF-β family members (18,19), including myostatin (5,20,21). Similar to myostatin null mice, animals over-expressing follistatin also exhibit a nearly 2-fold increase in muscle mass (5). Interestingly, follistatin over-expression in a myostatin-null background leads to an even greater increase in muscle mass, to nearly 4-fold normal levels, indicating that follistatin increases muscle mass at least in part through myostatin-independent pathways (22). Follistatin has been successfully employed to enhance muscle in mouse models of disease. Follistatin delivered by recombinant adeno-associated virus increases muscle mass in both wild-type mice and mouse models of amyotrophic lateral sclerosis (ALS) and Duchenne muscular dystrophy (23,24). Importantly, not only did muscle proximal to the site of injection gain in mass, but muscles distant from the site of injection increased in weight as well (23,24).
Spinal muscular atrophy (SMA) is a neurodegenerative disease and the leading genetic cause of infant death (25). With a carrier frequency of 1:40 and an incidence of 1:6000 live births, SMA is the second most common autosomal-recessive disorder. SMA presents in a broad clinical spectrum, but all cases result from the loss the survival motor neuron 1 (SMN1) gene (26). Loss of SMN1 homologs in other animals results in embryonic lethality (27). The human genome, however, contains a second nearly identical gene called SMN2 (28). In the absence of SMN1, the SMN2 locus cannot prevent disease development, as one of the nucleotide differences disrupts a critical pre-mRNA regulatory element within exon 7, resulting in the production of a truncated and unstable SMN protein (29–31). However, SMN2 is clearly a potent disease modifier as increasing SMN2 gene copy numbers correlate with less severe forms of disease. This is possible as SMN2 does produce fully functional, full-length SMN protein, albeit at ∼10% of the levels expressed by SMN1. Recently, the first potential SMN-independent disease modifier was identified. Increased levels of plastin-3, a regulator of actin filament organization, were found to correlate with a mild SMA phenotype in humans without affecting SMN protein levels, demonstrating that the SMA phenotype might be modified by SMN-independent mechanisms (32). Here, we show that repeated administration of a potent myostatin inhibitor, follistatin, increases mean lifespan of SMA mice, improves gross motor function and increases the total number of ventral horn motor neurons. Together this points to a therapy that would benefit the quality-of-life of SMA patients. Expression levels of full-length SMN protein and plastin-3 remained essentially unchanged, suggesting a novel, SMN-independent mechanism of action.
RESULTS
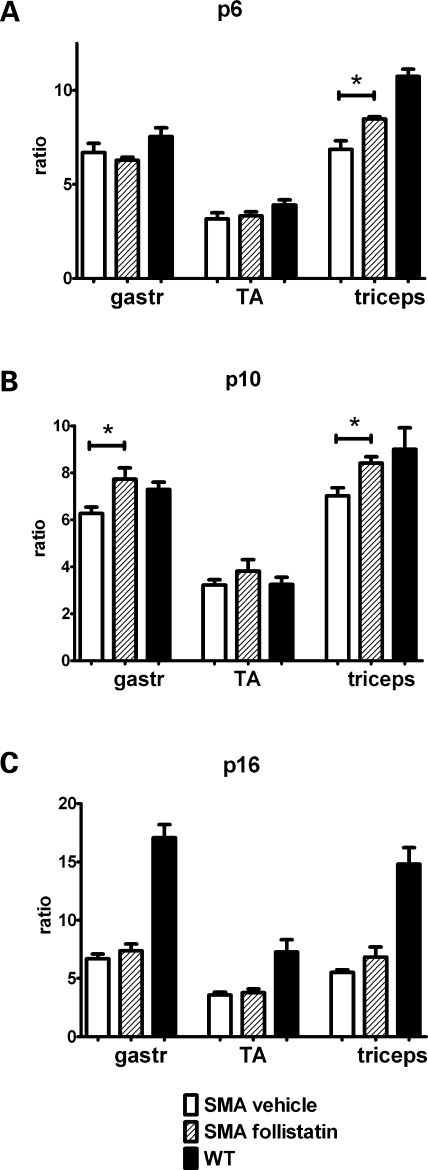
The efficacy of follistatin to increase muscle mass and modulate the SMA phenotype was tested in hSMN2+/+; hSMNΔ7+/+; Smn−/− mice, referred to as SMA mice (33). This model represents a relatively severe type of SMA with loss of lumbar motor neurons and progressive muscle weakness. SMA mice reach peak body weight at approximately post-natal day (p) 10 and die prematurely at around p16. Mice were genotyped using tail biopsy material at the day of birth (designated p1), and animals homozygous for Smn loss were randomly assigned to treatment groups. To ensure that our delivery strategy resulted in an increase in muscle mass in the neonatal context of this relatively severe SMA mouse model, the wet weight of various muscles from follistatin-treated SMA mice was compared with that of vehicle-treated animals. Animals received intraperitoneal follistatin injections 0.75 mg/kg twice daily (b.i.d.). Gastrocnemius, tibialis anterior and triceps muscles from follistatin- and vehicle-treated animals were dissected at p6, p10 and p16. Muscles from wild-type mice were included for comparison. At p6, follistatin-treated triceps muscles were larger than vehicle-treated (Fig. 1A). This difference was even more pronounced at p10, when follistatin treatment resulted in significantly larger gastrocnemius and triceps muscles, approaching the weight of muscles from wild-type littermates (Fig. 1B). As expected, as disease progression advanced and the animals approached endstage, muscle weights were similar between phosphate-buffered saline (PBS)- and follistatin-treated animals (Fig. 1C). To account for potential variation in animal size, results are shown as muscle weights indexed to brain weights. Comparison of raw muscle weights gave virtually identical results (data not shown). To more closely analyze the muscle phenotype in treated SMA mice, muscle fiber size was examined at p6, p10 and p16 in the triceps brachii. Cross-sectional areas were not significantly different between vehicle- and follistatin-treated mice (Supplementary Material, Fig. S1). This result was somewhat surprising as myostatin blockade has been shown to increase muscle fiber size (5). However, transgenic over-expression of follistatin correlated to an approximate 1.6-fold increase in fiber area, with a 327% increase in muscle weight (5). In our treatment model, we observe a more modest increase in muscle weight between 20 and 25%, and it is therefore likely that a proportional increase in fiber area would be too small to detect reliably. These data demonstrate that neonatal follistatin administration confers a temporal gain of muscle mass in SMA mice during the course of this rapidly developing neurodegenerative disease.
Figure 1.
Muscle weights of SMA mice. Gastrocnemius (gastroc), tibialis antrior (TA) and triceps brachii (triceps) muscles were dissected at p6 (A), p10 (B), or p16 (C) and weighed. Pairs of muscle from the left and right sides of the animal were combined. Muscle weight was normalized to brain weight, and the ratio of the muscle/brain weight is shown. p, post-natal day. *P<0.05 (n=3–8 average ± SE).
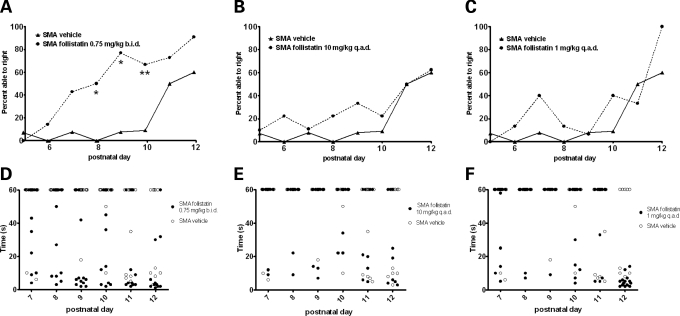
To determine whether follistatin treatment affects the severity of the SMA phenotype, we measured time-to-right (TTR) for PBS- or follistatin-treated SMA pups. TTR was previously shown to be a sensitive measurement of gross motor function for these SMA animals (34,35). To determine TTR, animals were placed on their backs, and the time required to turn upright was measured. Animals that failed to turn were recorded as 60 s. To examine a broader dosing range of recombinant follistatin, follistatin was administered at 10 mg/kg every other day (q.a.d.) and 1 mg/kg q.a.d., in addition to the initial dose of follistatin at 0.75 mg/kg b.i.d that increased muscle weight. These doses were administered q.a.d., assuming that the mice would better tolerate less frequent injections. TTR success rate was measured between p5 and p12 by adopting a turn/did-not-turn scoring system. Motor function of SMA mice was improved at all three doses tested, as a greater percentage of the follistatin-treated pups were able to right when compared with controls (Fig. 2A–C). In particular, the fraction of mice able to turn was statistically greater between p8 and p10 in the 0.75 mg/kg b.i.d. group. In addition, follistatin-treated animals tended to exhibit faster righting times (Fig. 2D–F). In Figure 2, p7 through p12 were emphasized due to the fact that about p12 all SMA animals, regardless of treatment, begin to right. Although there are clear statistical differences in the percentage of animals able to right following follistatin treatment, the severity of the disease likely impacts the ability to identify a statistically significant difference in the specific average righting times since only a small fraction of the vehicle-treated animals was able to turn at any given time point. The small sample size for the ‘vehicle-treated, but able to right’ group did not allow for a rigorous statistical analysis of the specific times to right.
Figure 2.
Follistatin improves gross motor function. Mice were placed in a prone position and allowed to turn back to their feet within a maximum of 60 s. The capacity to turn within that time was plotted against age (A–C). Significant differences are indicated by asterisk as determined by Fisher's exact test. In addition, the fastest time to turn from two consecutive trials was plotted against age (D–F). No significant differences in the mean time to turn were detected. P-values are listed in the Supplementary Material, Table S1.
Follistatin treatment improved the capacity for overall ambulation in the SMA animals (Supplementary Material, Video S1). The observed improvements in gross motor function were not accompanied by significant increases in body weight (Supplementary Material, Fig. S2), which was not unexpected given the relatively modest size of neonatal muscles. Gross motor function increase with follistatin treatment (0.75 mg/kg b.i.d.) appears to be most pronounced from p6 to p12. These data directly correlate with the time frame of increased muscle mass (Fig. 1). These findings demonstrate that administration of recombinant follistatin treatment is effective in lessening the severity of the SMA phenotype.
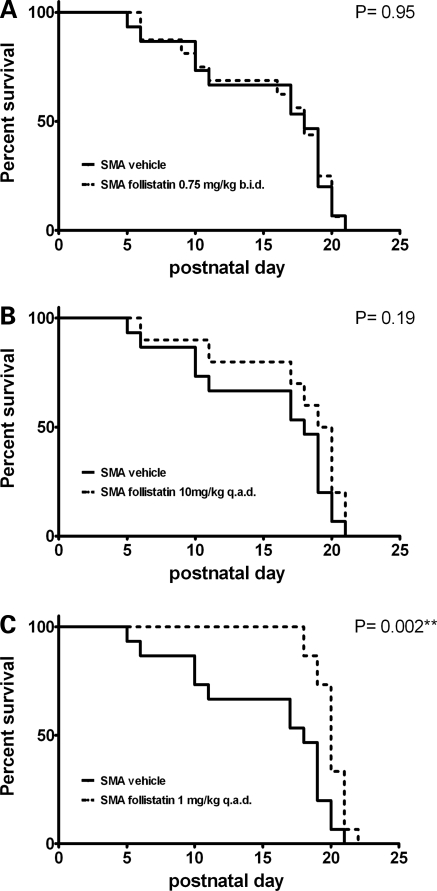
The SMA mice die prematurely following progressive muscle weakness, having an average lifespan of ∼2 weeks (33). To determine whether follistatin treatment extended survival of the SMA animals, lifespans for each of the three follistatin treatment groups were analyzed by Kaplan–Meyer survival curves. Although two groups did not exhibit significantly extended lifespans, the 1 mg/kg q.a.d. treatment increased mean lifespan by 4.6 days or ∼30% (Fig. 3A–C). It is important to note that this change is seen not by altering the maximum lifespan, but rather by preventing the earlier deaths, thus an increase in the mean survival is observed. Additionally, the early deaths occurring in the vehicle-treated group were not observed in the 1 mg/kg q.a.d. treatment group. Although the basis for the different outcomes of these dosing regimens is currently unknown, these findings demonstrate that even in a relatively severe model of disease, follistatin administration can significantly extend survival of SMA mice. The expression of follistatin in muscles of a different model of muscle wasting, the SOD-1 ALS model, was recently shown to increase muscle mass but did not affect survival (23), suggesting that the effects of follistatin on survival may be disease-specific.
Figure 3.
Survival was determined using Kaplan–Meier curves. P-values were determined by log-rank (Walton–Cox) test (n = 10 for 10 mg/kg q.a.d., n = 15 for all other groups). To correct for Bonferroni interference, a stricter significance threshold of P < 0.016 was used.
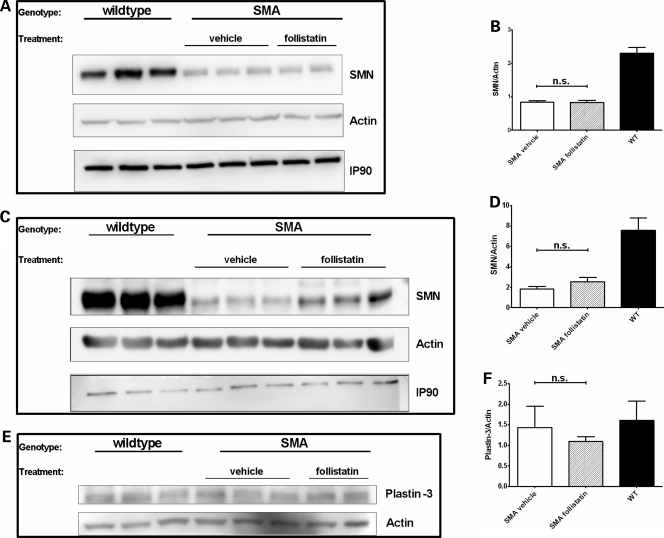
As follistatin is a known inducer of muscle mass, we hypothesized that the lessening of disease severity was SMN-independent. Therefore, we examined SMN levels in tissues from follistatin-treated SMA animals. SMN levels were determined using p10 mice of the 0.75 mg/kg b.i.d. treatment group. This experimental group was selected on the basis of the phenotypic assessment, demonstrating that timed-righting and muscle weight differences were most pronounced at this stage. Tissues from p10 animals were dissected and analyzed by western blotting for SMN protein levels. As expected, in tissues from SMA mice, SMN levels were dramatically lower than those observed in wild-type tissues, as only low levels of full-length SMN are produced by the SMN2 transgene. Consistent with our hypothesis, follistatin treatment did not elevate SMN in spinal cord (Fig. 4A and B) or triceps muscle (Fig. 4C and D), and protein levels were statistically equivalent to those of the vehicle group. Plastin-3 was recently identified as a potential SMA-modifying gene, and its expression lessens the severity of the disease (32). To determine whether the expression of this gene correlated with the lessening of the disease phenotype in follistatin-treated animals, plastin-3 levels were examined in the follistatin- and PBS-treated mice. Spinal cord protein extracts of p10 SMA pups were examined by western blot, demonstrating that plastin-3 levels were similar in follistatin-treated, PBS-treated pups and wild-type animals (Fig. 4E and F). These results demonstrate that the observed motor improvement was not correlated with changes in plastin-3 or full-length SMN levels.
Figure 4.
Follistatin treatment does not affect SMA disease modifiers. SMN protein in p10 triceps (A and B) or upper spinal cord (C and D) lysates were examined by western blot. Both actin and IP90 are shown as loading controls. Graphs were quantified and average SMN/actin ratios are shown. Plastin-3 protein levels were also examined in p10 spinal cord lysates (E and F) by western blot, using actin as a loading control. n.s., not significant.
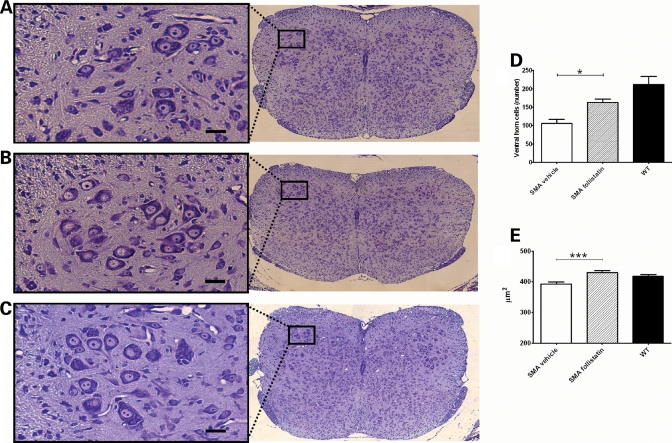
Several models of disease suggest a potentially protective interplay between both sides of the neuromuscular junction. In an ALS model, insulin-like growth factor I expression in muscle results in increased motor neuron survival (36,37), whereas enhancing the functionality of the neuromuscular junction by maintaining the expression of post-synaptic proteins ameliorates the phenotype in a mouse model of muscular dystrophy (38). Therefore, we speculated that enhanced muscle mass may lessen the severity of the SMA phenotype in part by preserving or maintaining neuronal circuitry. Spinal cord sections were analyzed to determine the number and size of ventral horn cell (VHC) numbers in follistatin-, PBS-treated and wild-type animals (Fig. 5A–C). The analysis of VHCs demonstrated that spinal cord sections from follistatin-treated animals contained approximately 180 VHCs when compared with 110 in vehicle controls (Fig. 5D). Although VHCs in follistatin-treated sections were not as abundant as in wild-type animals, they were significantly greater in numbers compared with vehicle-treated counterparts (P < 0.02; Fig. 5D). Furthermore, the average cross-sectional area of the VHCs from follistatin-treated mice was larger than that of SMA mice treated with PBS and comparable with those of wild-type animals (Fig. 5E).
Figure 5.
Analysis of VHCs of the lumbar spinal cord. Representative images of SMA vehicle (A), SMA follistatin (0.75 mg/kg b.i.d.) (B) and WT (C) spinal cords are shown of p10 animals. Lumbar VHCs were counted and the average number of VHCs per mouse (D) and the average cross-sectional area of VHCs (E) are shown (N = 3 per group).
These results suggest that follistatin treatment can delay the death of spinal motor neurons in a relatively severe mouse model of SMA and that induction of known and potential modifiers of disease, SMN2 and plastin-3, are not obligate components of this process. We provide evidence that enhanced muscle mass in SMA improves gross motor function, which is consistent with the cellular analysis of treated animals, indicating a neural protective effect for VHCs. Taken together, our results demonstrate that follistatin treatment lessens the severity of the SMA phenotype and can increase mean survival in this relatively severe model of disease. The identification of an SMN-independent therapeutic target raises the possibility that combinatorial therapy aimed at increasing both muscle mass and SMN levels may prove beneficial.
DISCUSSION
The findings presented here demonstrate that administration of follistatin and the subsequent inhibition of the myostatin pathway are beneficial in a model of a neurodegenerative disorder, SMA. Lifespan and gross motor function were improved concomitant with an increase in muscle mass and the preservation of VHCs. Although most work in the SMA field has focused on targeting the SMN2 gene, these results suggest that skeletal muscle is a viable therapeutic target that may ameliorate the severity of the SMA phenotype. Consistent with this, follistatin-treated SMA mice performed better than their vehicle-treated SMA littermates in a series of timed-righting experiments designed to monitor gross motor function. This improvement was particularly pronounced between p6 and p10 in the 0.75 mg/kg b.i.d treatment group (Fig. 2). As muscle mass was increased during this period as well (Fig. 1), it is possible that the two are causally linked. It has been shown previously that reduction of myostatin levels increases muscle mass and strength in the mouse (4,5). In particular, follistatin expression derived from a viral vector resulted in long-term functional and structural benefits in normal and diseased mice (24). It is possible that changes in the physiological composition of the follistatin-treated muscle also contributed to the observed functional improvement. The expression of muscle-derived factors is critical for the proper maturation and survival of neurons. For example, the ectopic expression of the post-synaptic protein MuSK can partially rescue lethality due to the loss of agrin, a nerve-derived factor (39). Similarly, muscle production of neurotrophin-4 induces sprouting and remodeling of motor neuron axons (40). Therefore, it is possible that follistatin treatment preserved the expression of muscle-derived neurotrophic factors in SMA mice, thus providing a neuroprotective signal.
Although it is presently unclear why optimal survival does not correlate with better motor performance (Figs 2 and 3), we suspect that sensitivity to follistatin levels may affect the outcomes in the SMA animals. This is clearly seen in the lifespan data of the 10 mg/kg q.a.d. dose. The 0.75 mg/kg b.i.d. dose may have maximal effect on myostatin blockade to aid the muscle, as seen in gross motor function improvement, but over time may have adverse effects, preventing an extension of lifespan. Along the same line, the 1 mg/kg q.a.d. dose may not have the overt effects seen in gross motor function of the 0.75 mg/kg group, but has enough of a baseline effect on the muscles to promote early survival. An alternative to systemic delivery of recombinant protein is the expression of follistatin via muscle-directed delivery of plasmid or viral expression vectors. Such an approach may be advantageous for achieving constant levels of circulating protein and has been successfully used in an ALS model (24). However, owing to size restrictions and the relatively short lifespan of the neonatal SMA mice, this was not attempted in this study. Direct injections also allowed the delivery of varying, defined amounts to assess potential differences between doses. Additionally, direct administration of recombinant follistatin to individual muscle groups is an alternative delivery paradigm; however, the requirement for repeated injections to these severely affected animals would likely have detrimental effects and complicated the interpretations.
Although it is clear that the presence of SMN2 provides an excellent target for therapeutic intervention, it was important to demonstrate in these studies that follistatin treatment did not alter the expression level of SMN protein (Fig. 4). This finding was not surprising because follistatin is not known to modulate SMN levels. Therefore, our results indicate that the lessening of the SMA phenotype occurred in an SMN-independent fashion. Motor improvements and increased lifespan were also observed after the treatment of SMA mice with TSA, a potent histone deacetylase inhibitor (34). In this study, histological and molecular analyses demonstrated increased muscle fiber diameters and improved post-natal muscle development. In parallel, SMN2-derived transcripts and SMN protein levels were increased, suggesting that the beneficial effects of TSA were at least partly mediated by the transcriptional regulation of a key SMA modifier gene, SMN2 (34). Using a different genetic model of disease, Grondard et al. (41) demonstrated improved motor behavior and extended survival in response to exercise in a relatively severe mouse model of SMA. In this study, increased levels of SMN protein were detected using immunohistochemistry of spinal cord sections. The increase in protein correlated with an increase in exon 7 inclusion in SMN2-derived transcripts (41). Collectively, the authors demonstrated a connection between the induction of SMN2 expression and exercise, although a role for SMN-independent modes of action was not examined. Further analyses of trophic factor expression and recruitment of motor units may shed light on additional influences of exercise on the SMA phenotype in this model. Interestingly, exercise decreases levels of myostatin and increases levels of follistatin in humans and rodents (42). It is therefore possible that follistatin administration elicits a subset of the more global exercise effects.
Although SMN is a ubiquitously expressed protein, its loss results in the selective death of spinal motor neurons. It is still somewhat controversial whether SMN deficiency in muscle contributes to the development of SMA or whether it is exclusively the reduction of SMN in the motor neurons that causes disease. Our report is not germane to that controversy because the beneficial effects of follistatin administration are independent of SMN protein levels. Recently, plastin-3 was identified as a potential novel modifier gene for SMA (32). Plastin-3 was shown to promote axon outgrowth by increasing F-actin levels, thereby rescuing the axonal defects due to SMN knockdown in a zebrafish model without affecting SMN levels. This finding is the first to identify an SMN-independent molecular target for the modification of the SMA phenotype. We found that follistatin treatment did not alter plastin-3 levels in the SMA mice (Fig. 4), suggesting that inhibition of the myostatin pathway offers a tractable, SMN-independent target to modulate the SMA phenotype.
A possible mechanism underlying the phenotypic improvement of follistatin-treated SMA mice is suggested by the increased number and size of VHCs in the lumbar spinal cord (Fig. 5). We hypothesize that follistatin does not regulate VHC numbers directly, but rather indirectly via its muscle-enhancing activity. This view is supported by the finding that an increase in muscle mass (e.g. by exercise) leads to changes in the gene expression profile of the spinal cord, including the upregulation of anti-apoptotic genes (43). Similarly, the positive impact of exercise (41) or histone deacetylase inhibition (34) is correlated with increased numbers and/or size of VHCs. Changes in both size and numbers of VHCs likely contribute to SMA as autopsy material from severe, type I patients demonstrated the loss of large VHCs in the spinal cord (44). It is tempting to speculate that improving muscles by various means leads to increased survival of VHCs, thereby lessening the severity of SMA. The findings presented in this report do not specifically examine the role of the muscle versus neuron in SMA, but rather demonstrate that SMN-independent targeting of muscle enhancement may be viable as an SMA therapeutic strategy. Collectively, our results demonstrate that follistatin improves the SMA phenotype independently of SMN levels. These results provide a rationale for examining combinatorial therapeutic strategies that directly address the genetic defect in SMA via an SMN-induction strategy as well as an SMN-independent strategy that enhances skeletal muscle.
MATERIALS AND METHODS
Animals and drug treatment
All animal experiments were carried out in accordance with protocols approved by the Animal Care and Use Committee of the University of Missouri. Original breeder pairs of Smn−/+; SMN2+/+; SMNΔ7+/+ mice were purchased from Jackson Laboratory. Offspring were genotyped using primer sets for the Smn gene: SMN1#5 FWD (5′-CTAGGGACTTTGAGCTACTTCC-3′) and SMN1#4 REV (5′-TGGGACACTCCAAACAGAAGGAGC-3′) and for the Smn knockout: SMN1-KO #3 FWD (5′-GCCTGCGATGTCGGTTTCTGTGAGG-3′) and SMN1-KO #3 REV (5′-CCAGCGCGGATCGGTCAGGACG-3′) in a multiplex PCR on tail biopsy material. Recombinant human follistatin (Biovision #4708) was resuspended in PBS (Invitrogen) and was administered by intraperitoneal injection at 10 µl/g of body weight, as often as indicated from p2 to p16. PBS (vehicle) was injected as a negative control. To supplement maternal feedings, the mice were hand-fed Enfamil Lipil three times per day, from p5 until death. To minimize the potential influence of litter size and SMA/wild-type ratios, pups with birth weight under 1 g and litters containing less than six pups were excluded, whereas larger litters were reduced to eight pups. In addition, the maximum ratio of SMA/wild-type pups allowed was 0.5. To assess strength, righting reflex [as described in (35)] was measured starting at p6.
Western blot analysis
Spinal cord (p10) or triceps (p9–10) tissues were harvested from wild-type or SMA pups (either follistatin or control-treated) and immediately frozen. Tissues were resuspended in buffer (50 mm Tris–HCl pH 8, 150 mm NaCl, 10% glycerol, 0.1% Roche PIC, 20 mm NaH2(PO)4, 25 mm NaF, 2 mm EDTA, 1% Triton X-100) and sonicated. Total protein was measured using the BioRad Protein Assay, according to the manufacturer's directions. Equal amounts of total protein were loaded (∼20 µg for SMN Westerns). Mouse monoclonal anti-SMN antibody (BD Biolabs) and anti-actin polyclonal rabbit antibody (Sigma) were used for SMN and actin detection, as described previously (45). The membrane was subsequently stripped with β-mercaptoethanol (0.625% β-mercaptoethanol, 0.02% SDS, 6.25% 1 M Tris pH 6.8, 45 min at 50°C) and re-probed with an anti-IP90 polyclonal rabbit antibody at a dilution of 1:2000. Anti-T Plastin C-15 (Santa Cruz) was used at 1:200 to detect plastin levels. Probes were visualized by chemiluminescence using the Pierce SuperSignal Pico reagents.
Histology and morphometry
Lumbar regions L2–L5 of spinal columns were dissected after transcardiac perfusion, post-fixed for 24 h in 4% buffered paraformaldehyde and embedded in paraffin. Thin sections at 150 µm intervals were processed for Nissl staining to highlight motor neurons. Overlapping photomicrographs from each section were joined using Photoshop, and cell body area was quantified using Multigauge software (Fujifilm). Measurements were restricted to the ventral portion of the spinal column. Motor neurons were defined by the presence of a nucleolus and having a cross-sectional area >250 µm2 (46).
Statistical analyses
Data are presented as means ± standard error, unless indicated otherwise. For pairwise comparisons of parametric data, Student's t-tests were performed. The presence or absence of the righting reflex was analyzed for each day from p6 to p12, using 2×2 contingency tables and Fisher's exact test to calculate P-values.
SUPPLEMENTARY MATERIAL
FUNDING
This work was supported by grants from FightSMA (C.L.L.); MU College of Veterinary Medicine and the National Institutes of Health (C.L.L., R01 NS41584; R01 HD054413). F.F.R. is supported by Training Grant (T32 GM008396). V.B.M. is supported by the Clinical BioDectives Program (NIH T90 DK070105).
Supplementary Material
ACKNOWLEDGEMENTS
We thank John Marston for expert technical assistance and Dr Leonard Hearne for help with the statistical analyses.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Engvall E., Wewer U.M. The new frontier in muscular dystrophy research: booster genes. FASEB J. 2003;17:1579–1584. doi: 10.1096/fj.02-1215rev. [DOI] [PubMed] [Google Scholar]
- 2.Tsuchida K., Nakatani M., Uezumi A., Murakami T., Cui X. Signal transduction pathway through activin receptors as a therapeutic target of musculoskeletal diseases and cancer. Endocr. J. 2008;55:11–21. doi: 10.1507/endocrj.kr-110. [DOI] [PubMed] [Google Scholar]
- 3.McPherron A.C., Lawler A.M., Lee S.J. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 4.Grobet L., Pirottin D., Farnir F., Poncelet D., Royo L.J., Brouwers B., Christians E., Desmecht D., Coignoul F., Kahn R., et al. Modulating skeletal muscle mass by postnatal, muscle-specific inactivation of the myostatin gene. Genesis. 2003;35:227–238. doi: 10.1002/gene.10188. [DOI] [PubMed] [Google Scholar]
- 5.Lee S.J., McPherron A.C. Regulation of myostatin activity and muscle growth. Proc. Natl Acad. Sci. USA. 2001;98:9306–9311. doi: 10.1073/pnas.151270098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Girgenrath S., Song K., Whittemore L.A. Loss of myostatin expression alters fiber-type distribution and expression of myosin heavy chain isoforms in slow- and fast-type skeletal muscle. Muscle Nerve. 2005;31:34–40. doi: 10.1002/mus.20175. [DOI] [PubMed] [Google Scholar]
- 7.Mosher D.S., Quignon P., Bustamante C.D., Sutter N.B., Mellersh C.S., Parker H.G., Ostrander E.A. A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs. PLoS Genet. 2007;3:e79. doi: 10.1371/journal.pgen.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grobet L., Martin L.J., Poncelet D., Pirottin D., Brouwers B., Riquet J., Schoeberlein A., Dunner S., Menissier F., Massabanda J., et al. A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat. Genet. 1997;17:71–74. doi: 10.1038/ng0997-71. [DOI] [PubMed] [Google Scholar]
- 9.Schuelke M., Wagner K.R., Stolz L.E., Hubner C., Riebel T., Komen W., Braun T., Tobin J.F., Lee S.J. Myostatin mutation associated with gross muscle hypertrophy in a child. N. Engl. J. Med. 2004;350:2682–2688. doi: 10.1056/NEJMoa040933. [DOI] [PubMed] [Google Scholar]
- 10.Hayette S., Gadoux M., Martel S., Bertrand S., Tigaud I., Magaud J.P., Rimokh R. FLRG (follistatin-related gene), a new target of chromosomal rearrangement in malignant blood disorders. Oncogene. 1998;16:2949–2954. doi: 10.1038/sj.onc.1201807. [DOI] [PubMed] [Google Scholar]
- 11.Tsuchida K., Arai K.Y., Kuramoto Y., Yamakawa N., Hasegawa Y., Sugino H. Identification and characterization of a novel follistatin-like protein as a binding protein for the TGF-beta family. J. Biol. Chem. 2000;275:40788–40796. doi: 10.1074/jbc.M006114200. [DOI] [PubMed] [Google Scholar]
- 12.Hill J.J., Qiu Y., Hewick R.M., Wolfman N.M. Regulation of myostatin in vivo by growth and differentiation factor-associated serum protein-1: a novel protein with protease inhibitor and follistatin domains. Mol. Endocrinol. 2003;17:1144–1154. doi: 10.1210/me.2002-0366. [DOI] [PubMed] [Google Scholar]
- 13.Wagner K.R., McPherron A.C., Winik N., Lee S.J. Loss of myostatin attenuates severity of muscular dystrophy in mdx mice. Ann. Neurol. 2002;52:832–836. doi: 10.1002/ana.10385. [DOI] [PubMed] [Google Scholar]
- 14.Bogdanovich S., Krag T.O., Barton E.R., Morris L.D., Whittemore L.A., Ahima R.S., Khurana T.S. Functional improvement of dystrophic muscle by myostatin blockade. Nature. 2002;420:418–421. doi: 10.1038/nature01154. [DOI] [PubMed] [Google Scholar]
- 15.Bogdanovich S., Perkins K.J., Krag T.O., Whittemore L.A., Khurana T.S. Myostatin propeptide-mediated amelioration of dystrophic pathophysiology. FASEB J. 2005;19:543–549. doi: 10.1096/fj.04-2796com. [DOI] [PubMed] [Google Scholar]
- 16.Bartoli M., Poupiot J., Vulin A., Fougerousse F., Arandel L., Daniele N., Roudaut C., Noulet F., Garcia L., Danos O., et al. AAV-mediated delivery of a mutated myostatin propeptide ameliorates calpain 3 but not alpha-sarcoglycan deficiency. Gene Ther. 2007;14:733–740. doi: 10.1038/sj.gt.3302928. [DOI] [PubMed] [Google Scholar]
- 17.Wagner K.R., Fleckenstein J.L., Amato A.A., Barohn R.J., Bushby K., Escolar D.M., Flanigan K.M., Pestronk A., Tawil R., Wolfe G.I., et al. A phase I/IItrial of MYO-029 in adult subjects with muscular dystrophy. Ann. Neurol. 2008;63:561–571. doi: 10.1002/ana.21338. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura T., Takio K., Eto Y., Shibai H., Titani K., Sugino H. Activin-binding protein from rat ovary is follistatin. Science. 1990;247:836–838. doi: 10.1126/science.2106159. [DOI] [PubMed] [Google Scholar]
- 19.Fainsod A., Deissler K., Yelin R., Marom K., Epstein M., Pillemer G., Steinbeisser H., Blum M. The dorsalizing and neural inducing gene follistatin is an antagonist of BMP-4. Mech. Dev. 1997;63:39–50. doi: 10.1016/s0925-4773(97)00673-4. [DOI] [PubMed] [Google Scholar]
- 20.Zimmers T.A., Davies M.V., Koniaris L.G., Haynes P., Esquela A.F., Tomkinson K.N., McPherron A.C., Wolfman N.M., Lee S.J. Induction of cachexia in mice by systemically administered myostatin. Science. 2002;296:1486–1488. doi: 10.1126/science.1069525. [DOI] [PubMed] [Google Scholar]
- 21.Amthor H., Nicholas G., McKinnell I., Kemp C.F., Sharma M., Kambadur R., Patel K. Follistatin complexes myostatin and antagonises myostatin-mediated inhibition of myogenesis. Dev. Biol. 2004;270:19–30. doi: 10.1016/j.ydbio.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 22.Lee S.J. Quadrupling muscle mass in mice by targeting TGF-beta signaling pathways. PLoS ONE. 2007;2:e789. doi: 10.1371/journal.pone.0000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller T.M., Kim S.H., Yamanaka K., Hester M., Umapathi P., Arnson H., Rizo L., Mendell J.R., Gage F.H., Cleveland D.W., et al. Gene transfer demonstrates that muscle is not a primary target for non-cell-autonomous toxicity in familial amyotrophic lateral sclerosis. Proc. Natl Acad. Sci. USA. 2006;103:19546–19551. doi: 10.1073/pnas.0609411103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haidet A.M., Rizo L., Handy C., Umapathi P., Eagle A., Shilling C., Boue D., Martin P.T., Sahenk Z., Mendell J.R., et al. Long-term enhancement of skeletal muscle mass and strength by single gene administration of myostatin inhibitors. Proc. Natl Acad. Sci. USA. 2008;105:4318–4322. doi: 10.1073/pnas.0709144105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pearn J.H., Wilson J. Acute Werdnig–Hoffmann disease: acute infantile spinal muscular atrophy. Arch. Dis. Child. 1973;48:425–430. doi: 10.1136/adc.48.6.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubowitz V. Very severe spinal muscular atrophy (SMA type 0): an expanding clinical phenotype. Eur. J. Paediatr. Neurol. 1999;3:49–51. doi: 10.1053/ejpn.1999.0181. [DOI] [PubMed] [Google Scholar]
- 27.Schrank B., Gotz R., Gunnersen J.M., Ure J.M., Toyka K.V., Smith A.G., Sendtner M. Inactivation of the survival motor neuron gene, a candidate gene for human spinal muscular atrophy, leads to massive cell death in early mouse embryos. Proc. Natl Acad. Sci. USA. 1997;94:9920–9925. doi: 10.1073/pnas.94.18.9920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lefebvre S., Burglen L., Reboullet S., Clermont O., Burlet P., Viollet L., Benichou B., Cruaud C., Millasseau P., Zeviani M., et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–165. doi: 10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]
- 29.Cartegni L., Krainer A.R. Disruption of an SF2/ASF-dependent exonic splicing enhancer in SMN2 causes spinal muscular atrophy in the absence of SMN1. Nat. Genet. 2002;30:377–384. doi: 10.1038/ng854. [DOI] [PubMed] [Google Scholar]
- 30.Lorson C.L., Hahnen E., Androphy E.J., Wirth B. A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc. Natl Acad. Sci. USA. 1999;96:6307–6311. doi: 10.1073/pnas.96.11.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monani U.R., Lorson C.L., Parsons D.W., Prior T.W., Androphy E.J., Burghes A.H., McPherson J.D. A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Hum. Mol. Genet. 1999;8:1177–1183. doi: 10.1093/hmg/8.7.1177. [DOI] [PubMed] [Google Scholar]
- 32.Oprea G.E., Krober S., McWhorter M.L., Rossoll W., Muller S., Krawczak M., Bassell G.J., Beattie C.E., Wirth B. Plastin 3 is a protective modifier of autosomal recessive spinal muscular atrophy. Science. 2008;320:524–527. doi: 10.1126/science.1155085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le T.T., Pham L.T., Butchbach M.E., Zhang H.L., Monani U.R., Coovert D.D., Gavrilina T.O., Xing L., Bassell G.J., Burghes A.H. SMNDelta7, the major product of the centromeric survival motor neuron (SMN2) gene, extends survival in mice with spinal muscular atrophy and associates with full-length SMN. Hum. Mol. Genet. 2005;14:845–857. doi: 10.1093/hmg/ddi078. [DOI] [PubMed] [Google Scholar]
- 34.Avila A.M., Burnett B.G., Taye A.A., Gabanella F., Knight M.A., Hartenstein P., Cizman Z., Di Prospero N.A., Pellizzoni L., Fischbeck K.H., et al. Trichostatin A increases SMN expression and survival in a mouse model of spinal muscular atrophy. J. Clin. Invest. 2007;117:659–671. doi: 10.1172/JCI29562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butchbach M.E., Edwards J.D., Burghes A.H. Abnormal motor phenotype in the SMNDelta7 mouse model of spinal muscular atrophy. Neurobiol. Dis. 2007;27:207–219. doi: 10.1016/j.nbd.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaspar B.K., Llado J., Sherkat N., Rothstein J.D., Gage F.H. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301:839–842. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- 37.Dobrowolny G., Giacinti C., Pelosi L., Nicoletti C., Winn N., Barberi L., Molinaro M., Rosenthal N., Musaro A. Muscle expression of a local Igf-1 isoform protects motor neurons in an ALS mouse model. J. Cell. Biol. 2005;168:193–199. doi: 10.1083/jcb.200407021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Handschin C., Kobayashi Y.M., Chin S., Seale P., Campbell K.P., Spiegelman B.M. PGC-1alpha regulates the neuromuscular junction program and ameliorates Duchenne muscular dystrophy. Genes. Dev. 2007;21:770–783. doi: 10.1101/gad.1525107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim N., Burden S.J. MuSK controls where motor axons grow and form synapses. Nat. Neurosci. 2008;11:19–27. doi: 10.1038/nn2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Funakoshi H., Belluardo N., Arenas E., Yamamoto Y., Casabona A., Persson H., Ibanez C.F. Muscle-derived neurotrophin-4 as an activity-dependent trophic signal for adult motor neurons. Science. 1995;268:1495–1499. doi: 10.1126/science.7770776. [DOI] [PubMed] [Google Scholar]
- 41.Grondard C., Biondi O., Armand A.S., Lecolle S., Della Gaspera B., Pariset C., Li H., Gallien C.L., Vidal P.P., Chanoine C., et al. Regular exercise prolongs survival in a type 2 spinal muscular atrophy model mouse. J. Neurosci. 2005;25:7615–7622. doi: 10.1523/JNEUROSCI.1245-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kollias H.D., McDermott J.C. Transforming growth factor-beta and myostatin signaling in skeletal muscle. J. Appl. Physiol. 2008;104:579–587. doi: 10.1152/japplphysiol.01091.2007. [DOI] [PubMed] [Google Scholar]
- 43.Kaspar B.K., Frost L.M., Christian L., Umapathi P., Gage F.H. Synergy of insulin-like growth factor-1 and exercise in amyotrophic lateral sclerosis. Ann. Neurol. 2005;57:649–655. doi: 10.1002/ana.20451. [DOI] [PubMed] [Google Scholar]
- 44.Wharton S.A.I. Motor Neuron Disorders. Philadelphia, PA: Butterworth Heinemann; 2003. [Google Scholar]
- 45.Mattis V.B., Rai R., Wang J., Chang C.W., Coady T., Lorson C.L. Novel aminoglycosides increase SMN levels in spinal muscular atrophy fibroblasts. Hum. Genet. 2006;120:589–601. doi: 10.1007/s00439-006-0245-7. [DOI] [PubMed] [Google Scholar]
- 46.Fischer L.R., Culver D.G., Tennant P., Davis A.A., Wang M., Castellano-Sanchez A., Khan J., Polak M.A., Glass J.D. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp. Neurol. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.