Abstract
Hypoxia sensing is the generic term for pO2-sensing in humans and other higher organisms. These cellular responses to pO2 are largely controlled by enzymes that belong to the Fe(II) α-ketoglutarate (αKG) dependent dioxygenase superfamily, including the human enzyme called the Factor Inhibiting HIF (FIH-1), which couples O2-activation to the hydroxylation of the Hypoxia Inducible Factor α (HIFα). Uncoupled O2-activation by human FIH-1 was studied by exposing the resting form of FIH-1, (αKG+Fe)FIH-1, to air in the absence of HIFα. Uncoupling lead to two distinct enzyme oxidations, one a purple chromophore (λmax = 583 nm) arising from enzyme auto-hydroxylation of Trp296, forming an Fe(III)–O–Trp296 chromophore (Y.-H. Chen, L. M. Comeaux, S. J. Eyles, M. J. Knapp, Chem. Commun. (2008) DOI:10.1039/B809099H); the other a yellow chromophore due to Fe(III) in the active site, which under some conditions also contained variable levels of an oxygenated surface residue, (oxo)Met275. The kinetics of purple FIH-1 formation were independent of Fe(II) and αKG concentrations, however product yield was saturable with increasing [αKG] and required excess Fe(II). Yellow FIH-1 was formed from (succinate+Fe)FIH-1, or by glycerol addition to (αKG+Fe)FIH-1, suggesting that glycerol could intercept the active oxidant from the FIH-1 active site and prevent hydroxylation. Both purple and yellow FIH-1 contained high-spin, rhombic Fe(III) centers, as shown by low temperature EPR. XAS indicated distorted octahedral Fe(III) geometries, with subtle differences in inner-shell ligands for yellow and purple FIH-1. EPR of Co(II)-substituted FIH-1, (αKG+Co)FIH-1, indicated a mixture of 5-coordinate and 6-coordinate enzyme forms, suggesting that resting FIH-1 can readily undergo uncoupled O2-activation by loss of an H2O ligand from the metal center.
Keywords: HIF, hypoxia, non-heme iron, α-ketoglutarate, protein oxidation, factor inhibiting HIF, asparaginyl hydroxylase
Introduction
Hypoxia sensing is crucial for tissue development and angiogenesis in most animals, as this is the signal that pO2 is below the ideal set-point. Humans and many other higher organisms utilize a complex system for sensing oxygen which relies upon the hydroxylation of specific residues on a transcription factor called the hypoxia inducible factor (HIF), a heterodimer consisting of the O2-sensitive HIFα subunit and the constitutive HIFβ subunit (also known as the Aryl Hydrocarbon Nuclear Translocator). HIF promotes the expression of genes involved in anaerobic metabolism, angiogenesis, and iron uptake in response to pO2; for reviews, see [1–4]. The key players regulating HIF are the human enzymes HIF-asparaginyl hydroxylase (previously identified as factor inhibiting HIF, FIH-1), and the HIF-prolyl hydroxylases, each of which hydroxylate residues on HIFα [5–7].
The HIF hydroxylases are members of the large and diverse superfamily of non-heme Fe(II), α––ketoglutarate dependent oxygenases. Enzymes of this class activate O2 via the oxidative decarboxylation of α–ketoglutarate (αKG), leading to an Fe(IV)=O intermediate which oxidizes or oxygenates the primary substrate (Scheme 1) [8–10]. The Fe(II) in resting enzyme is approximately octahedral, coordinated by a His2Asp/Glu facial triad, bidentate αKG, and an axial H2O ligand. A key step in the consensus mechanism is the controlled loss of the axial H2O, which ensures coupling between the formation of the Fe(IV)=O and substrate hydroxylation [8]. In this step, the axial H2O is released when prime substrate binds, forming a 5-coordinate Fe(II) center which can bind and activate O2 [11, 12]. Consequently, the controlled changes in the coordination geometry of Fe(II) is a key determinant of enzyme fidelity.
Scheme 1.
Coordination changes at Fe within the consensus mechanism of FIH-1.
Nevertheless, many enzymes from the αKG oxygenase superfamily rapidly inactivate due to metal oxidation or auto-hydroxylation. Spectroscopic methods indicate that the coordination changes described above are not absolute, and that the resting form of several enzymes contain Fe(II) in a mixture of 5C and 6C forms [13, 14], suggesting that O2 activation may occur prior to binding prime substrate. This is borne out by the need for ascorbate in turnover assays to regenerate Fe(II) from Fe(III) for maximal enzyme activity, an effect related to the dietary need of vitamin C to prevent scurvy. There are also several examples of auto-hydroxylation or auto-oxygenation in αKG oxygenases, which frequently lead to inactive enzyme [15–20], In many cases, a new charge-transfer transition appears from the coordination of hydroxylated Tyr or Trp residues to Fe(III) in the active site. In view of the examples of ready inactivation for αKG-oxygenases, it is somewhat surprising that nature would choose such enzymes as oxygen sensors.
The X-ray crystal structure of human FIH-1 indicates that the Fe(II) is 6C in resting enzyme, with a limited hydrogen bonding network [21–23]. Three aromatic residues are within 12 Å of the iron, forming ripe targets for auto-hydroxylation (Figure 1). Recently, we showed that FIH-1 forms a purple Fe(III)-O-Trp chromophore, in which Trp296 was auto-hydroxylated [24]. Herein, we test the link between auto-hydroxylation and coordination changes at the metal center, and characterize two different forms of the oxidized human FIH-1 which contain oxygenated residues.
Figure 1.
Active site of human FIH-1 (PDB 1H2K) [21].
Materials and Methods
Protein expression and purification
The gene for FIH-1 was subcloned from the IMAGE clone 4138066 [25] purchased through the American Type Culture Collection, and FIH-1 protein was expressed similar to literature precedent [26]. Briefly, PCR-amplified fragments of the FIH-1 gene were inserted into a pET28a plasmid (Novagen) via NdeI and BamHI restriction sites with an N-terminal hexa-histidine tag, and the sequence confirmed by DNA sequencing at the Yale/W.M. Keck sequencing center. E. coli BL21(DE3) cells were transformed, grown on LB plates, then grown as overnight liquid cultures at 37° C. Fresh LB media was inoculated with the overnight cultures at a 1:500 ratio, and grown at 37° C. His6-FIH-1 expression was induced by the addition of IPTG (0.5mM) at OD600 between 0.5 – 0.7. Protein was expressed for 5 hours, then the cells harvested by centrifugation and frozen.
Frozen cell paste was suspended in lysis buffer (25 mM Tris, pH 8.00, containing 0.1 mM EDTA, 0.5 mg/ml lysozyme, and 5 µM AEBSF on ice for 1 hour, followed by sonication to lyse the cells. Crude lysate was centrifuged, followed by dialysis with 50 mM Tris buffer pH 8.00 to remove EDTA. Dialysed lysate was clarified with 40 µm membrane, then loaded onto a Ni-NTA His-bind Superflow (Novagen) column (2 × 10 cm) using loading buffer (50 mM NaH2PO4, 300 mM NaCl, and 10 mM Imidazole pH 8.00). His6-FIH-1 was first washed with 15% elution buffer (50 mM NaH2PO4, 300 mM NaCl, and 250 mM imidazole pH 8.00), and then eluted with 100% elution buffer. Salt was removed by serial cycles of ultraconcentration and dilution with 50 mM Tris buffer pH 8.00. His6-FIH-1 was cleaved by thrombin at 4° C for 2 days, and then loaded onto the Ni-column again to separate FIH-1 from uncleaved His6-FIH-1. The flow-through was concentrated and loaded onto an S200 size exclusion column (1.5 × 100 cm) to purify dimeric FIH-1. Metal-free FIH-1 (Apo FIH-1) was prepared by incubation with 50 mM EDTA at 4° C for 1 day, followed by concentration and buffer exchange (50 mM HEPES pH 7.50), and frozen at −20° C. Protein concentration was determined by A280 readings, and referred to on a per monomeric subunit basis. The extinction coefficient for apo FIH-1 was determined (ε280 = 48.8 mM−1 cm−1) by the method of Gill [27].
Preparation of metal-reconstituted FIH-1
To reconstitute (αKG/succ+Fe)FIH-1, anaerobic stock solutions of apo FIH-1 (1.00 mM), FeSO4•7H2O (10 mM), and αKG (10 mM) or succinate (10 mM) in 50 mM HEPES pH 7.50 were prepared and kept in an anaerobic chamber. Stock FIH-1 (10 µl) was diluted into 100 µl by HEPES buffer with varied amounts of Fe(II) and µKG or succinate, which were added at 30 min. intervals. The mixture was incubated for 30 min. prior to measurements.
To reconstitute (αKG+Co)FIH-1, aerobic apo FIH-1 stock (1.00 mM) was diluted with 50 mM HEPES pH 7.50 to a final concentration of 100µM. Stock solutions of CoCl2 (10 mM CoCl2, 50 mM HEPES pH 7.50) and αKG (10 mM αKG/50 mM HEPES pH 7.50) were added into the protein solution at 30 min. intervals, to final concentrations of 100 µM, and 500 µM, respectively. After 30 min. incubation, the mixture was concentrated and buffer exchanged with 10 kDa cut-off microcon concentrators (Millipore), and the final FIH-1 concentration was determined by A280.
Auto-oxidation kinetics
An Agilent HP 8453 diode-array UV-Vis spectrometer was used, with masked cuvettes. Apo FIH-1 and all other reagent stocks were prepared, degassed, and then manipulated in an anaerobic chamber. Experiments were performed in 50 mM HEPES, pH 7.50 at room temperature (20 – 23°C) unless noted otherwise. Rapid oxygenation was achieved by addition of aerobic buffer to anaerobic FIH-1 samples within a sealed cuvette. Slow oxygenation was achieved by removal of the cap from a sealed cuvette containing anaerobic FIH-1 samples.
EPR Spectroscopy
EPR spectra were collected in 3 mm quartz tubes using a Bruker ELEXSYS E-500 X-band spectrometer, with a helium flow cryostat. Purple FIH-1 was prepared by slow oxygenation of (αKG+Fe)FIH-1; yellow FIH-1 was similarly prepared from an FIH-1 sample that only formed the yellow chromophore. Spectral simulations used standard spin Hamiltonians [28] within XSophe software package, version 1.1.4 [29].
X-ray spectroscopy
XAS data was collected as previously described on beamline 9-3 at Stanford Synchrotron Radiation Laboraotry (SSRL) [30]. The same FIH-1 samples used for EPR experiments (3 mM FIH-1) were syringed into polycarbonate holders closed with kapton tape, frozen in liquid nitrogen, and analyzed near 10 K using a liquid He cryostat. The ring conditions for data collection were 3 GeV and 80–100 mA. Beam line optics consisted of a Si(220) double-crystal monochromator and two rhodium coated mirrors, a flat mirror before the monochromator for harmonic rejection and vertical collimation, and a second toroidal mirror after the monochromator for focusing. X-ray fluorescence was collected using a 30-element Ge detector (Canberra). Scattering was minimized by placing a set of Soller slits with a Z-1 element filter between the sample chamber and the detector. X-ray absorption near-edge spectroscopy (XANES) was collected from ±200 eV relative to the Fe edge. Extended X-ray absorption fine structure (EXAFS) was collected to k = 16 Å−1. The X-ray energy for the K-edge of Fe was internally calibrated to 7111.2 eV using transmission data from a Fe foil. The data shown is the average of 4 – 8 scans and was analyzed using the EXAFS123 (XANES) and SIXPACK (EXAFS) software package [31] with phase and amplitude parameters obtained from prior FEFF8 calculations [32].
Mass spectrometry
Tryptic digests of FIH-1 (10 µM) were frozen in 30 µL aliquots, thawed immediately prior to analysis, and injected in 30 µL volumes for LC-MS/MS analysis. Chromatography was carried out with an Agilent 1100 HPLC connected to a 150 × 2.1 mm PLRP-S column (Polymer Laboratories, Amherst, MA) with 300 Å pore size and 3 µm particle size, using a H2O/CH3CN (0.1% formic acid) gradient at a flow rate of 200 µL/min. Preliminary work utilized a Bruker Esquire ion trap mass spectrometer, to identify tryptic peptide masses. High-resolution and MS/MS analysis was carried out using an Applied Biosystems QStar time-of-flight (TOF) mass spectrometer,
Results and Discussion
Coordination geometry of (αKG+Fe)FIH-1 is 5C/6C
The coordination geometry of the metal center in (αKG+Fe)FIH-1 was tested by EPR spectroscopy of the Co(II)-substituted enzyme, (Co)FIH-1. As the electronic environment of Co(II) changes due to coordination geometry and site symmetry, the EPR lineshape is a sensitive reporter of the local environment in Co(II)-substituted metalloenzymes [33–36]. Co(II) in biologically relevant, pseudo-octahedral environments typically has a large zero-field splitting (DSz 2 ≫ gβBS), such that only the lowest MS = |± 1/2> Kramers doublet is observable. As a consequence, the most useful information from Co(II) EPR is the rhombicity, E/D, as this reports on the electronic site symmetry. Due to the strong crystal-field preference for axial electronic symmetries in Co(II), bonding to solvent-derived ligands will adapt so that Co(II) can adopt axial electronic symmetry. On the other hand, the geometric constraints of the protein fold frequently leads to rhombic site symmetry when only protein-derived ligands are present. As a result, a reduced value of E/D for Co(II) is an indicator for the presence of solvent-derived ligands. In the case of (αKG+Co)FIH-1, a resting enzyme with three protein-derived ligands, a bidentate αKG, and a H2O would be 6C, and expected to adopt axial electronic symmetry. Should the H2O ligand be absent, the resultant 5C center would likely exhibit rhombic electronic symmetry.
(αKG+Co)FIH-1 was frozen in an EPR tube over liquid nitrogen, then inserted into a cooled (~ 20K) helium flow cryostat. X-band EPR spectra were collected over the range 4 – 20 K, with noticeably better signal intensity observed at 9.5 – 11 K. The EPR spectra of each (αKG+Co)FIH-1 sample contained at least two different signals (Figure 2). A broad axial signal near geff = 4 was apparent, as expected for Co(II) with axial electronic symmetry. Each sample also exhibited fine structure due to Co hyperfine splitting (Aobs ~ 80 G), centered near geff = 6; these hyperfine lines must arise from a Co(II) in a rhombic electronic environment, as they are were not centered on the geff = 4 feature. Sample and instrumentation conditions were varied in an attempt to isolate ‘pure’ signals from each of the Co(II) species; these included sample pH (6, 7, 10), the addition of a glassing agent, and acquisition parameters (4.2 – 20 K, 0.02 – 20 mW power). Standard conditions for optimal signal in the absence of saturation were T = 10 ± 1 K, with microwave power of 0.2 – 2.0 mW.
Figure 2.
EPR spectra of (αKG+Co)FIH-1, 10 K, 9.37 GHz, 10 G modulation; A) (αKG+Co)FIH-1 (10 mM Bis-Tris, pH 6.0), 2.0 mW; B) (αKG+Co)FIH-1 (10 mM CHES, pH 10.0), 0.2 mW; Simulation gx,y,z = 2.35 , E/D = 0.10; C) Difference spectrum (A–B); Simulation gx,y = 2.60, gx,y = 2.30, E/D = 0.23, A = 7.7 mT. * indicates cavity noise.
While both Co(II) sites were observed under all conditions attempted, the low pH sample with glassing agent (50% glycerol, 10 mM Bis-Tris, pH 6.0) exhibited the most prominent rhombic signal, whereas the high pH sample (10 mM CHES, pH 10.0) acquired at 0.2 mW power exhibited the smallest intensity for the rhombic species. This high pH sample could be simulated using giso = 2.35, E/D = 0.10† within the standard spin Hamiltonian: , where β is the Bohr magneton, B0 the applied magnetic field, S the spin operator, D the axial zero-field splitting, E/D the rhombicity of the zero-field splitting, A the hyperfine coupling constant for 59Co, and I the nuclear spin of 59Co. These electronic parameters were reasonable for axial Co(II), in which the value for |D| was much larger than the Zeeman splitting [33, 36]. This likely arose from the 6C center which contained one H2O ligand. Spectral subtraction of the high pH signal from the low pH signal produced a difference spectrum that was clearly a rhombic signal, albeit with some lineshape distortion. This difference spectrum was simulated with gx,y = 2.60, gz = 2.30, E/D = 0.23, Aiso = 7.7 mT, and while the difference spectrum is distorted, the key features expected for rhombic Co(II) are clearly present, in particular the resolved hyperfine splitting centered at geff = 6. This rhombic signal was attributed to a 5C species which likely lacks the H2O ligand.
The EPR spectra indicated that the resting form of (αKG+Co)FIH-1 was a mixture of axial and rhombic Co(II) sites, which we interpret to indicate the presence of a 5C site lacking the H2O ligand. As the Irving-Williams series predicts that Co(II) will bind a common ligand shell more tightly than Fe(II) [37–39], this suggests that the resting form of Fe-constituted FIH-1 will also exist in an equilibrium of 5C and 6C forms – in fact, the Fe(II) may favor 5C to an even greater extent than the Co(II). Such a weakened axial H2O ligand has been observed in the resting form of other αKG oxygenases, such as TauD and CytC3 [14], where the 6C resting (αKG+Fe) species exhibit significant distortions due to an elongated metal-ligand bond. Within the context of the consensus mechanism for αKG oxygenases, loss of the H2O-ligand makes the enzyme ready for O2-activation [8, 12, 40]; when H2O-loss is uncoupled from the binding of prime substrate, uncoupled oxidation can occur, as seen in select bacterial members of the αKG oxygenase superfamily [8]. This suggests that FIH-1 may uncouple O2-activation from HIFα under certain conditions, leading to enzyme oxidation at either the metal center or at the protein structure.
FIH-1 uncouples O2-activation
Reconstituting FIH-1 with Fe(II) and αKG under anaerobic conditions forms (αKG+Fe)FIH-1, which exhibits a faint chromophore with λmax = 500 nm (Figure 3). This optical feature has been assigned as a charge transfer transition between αKG and Fe(II) in other αKG oxygenases [12], indicating that resting FIH-1 in solution binds αKG in a bidentate fashion as found crystallographically [21]. This MLCT has a comparable extinction coefficient (ε500 = 0.24 mM−1 cm−1), but is slightly higher in energy than, those found for TauD [41], TfdA [17], and CS2 [42], suggesting that FIH-1 contains either a stronger Fe-αKG bond, or a more Lewis-acidic Fe(II) center, than in these other oxygenases.
Figure 3.
Difference spectra of resting FIH-1 showing the αKG-Fe(II) MLCT. A) Spectral subtraction of apo FIH-1 from (αKG+Fe)FIH-1 (1.4 mM FeSO4, 1.4 mM αKG); B) Spectral subtraction of apo FIH-1 from (αKG)FIH-1 (1.4 mM αKG); C) Spectral subtraction of apo FIH-1 from a solution of FeSO4 (1.4 mM) and αKG (1.4 mM). All samples 500 µM FIH-1 in 50 mM HEPES, pH 7.50.
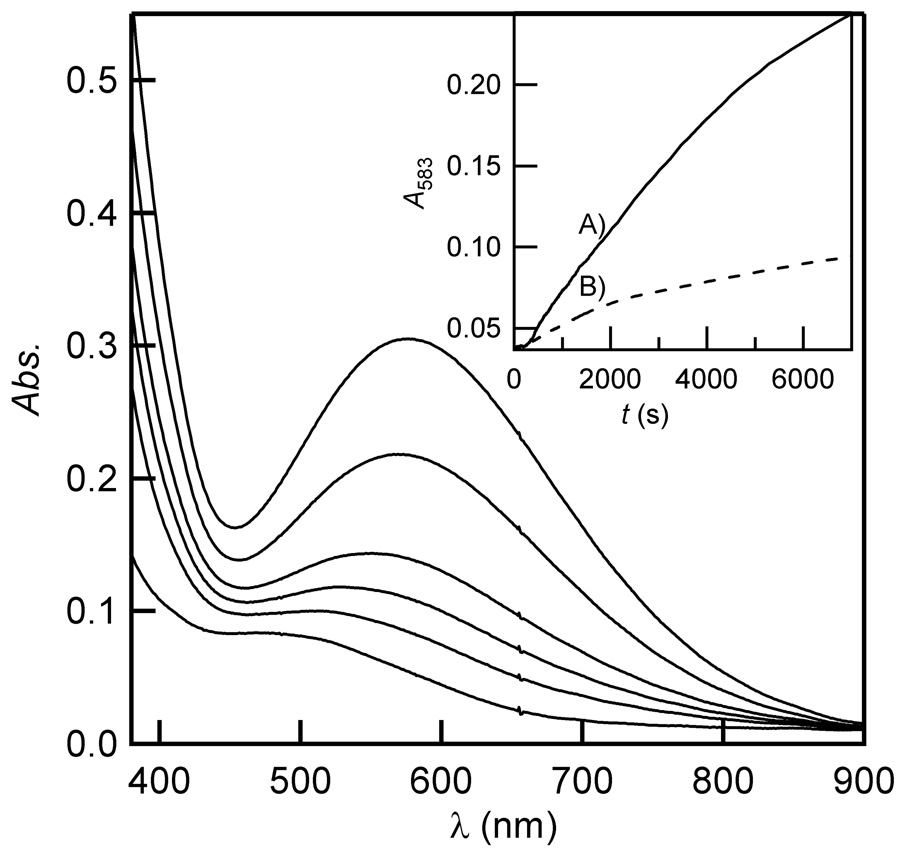
(αKG+Fe)FIH-1 auto-hydroxylated once the cuvette was opened to air, generating a purple Fe(III)-O-Trp chromophore having λmax = 583nm (ε583 = 3 × 103 M−1 cm−1, assuming full conversion of apo FIH-1) [24]. This closely resembled the charge-transfer band from Fe(III)-O-Trp centers, formed upon auto-oxidation in two bacterial members of the αKG oxygenase superfamily: TfdA (λmax = 580 nm) [17] and AlkB (λmax = 595 nm) [15], which result from O2-activation in the absence of the prime substrate. The yield of purple FIH-1 varied with different oxygenation procedures (Figure 4), however the initial rate was very slow under all tested conditions (ν0 = 10−8 M s−1),‡ as expected for uncoupled O2-activation. When 100 µL of anaerobically prepared (αKG+Fe)FIH-1 (500 µM in 50 mM HEPES, pH 7.50, 2.5 mM FeSO4, 2.5 mM αKG) was slowly oxygenated by adding 400 µL anaerobic buffer, then allowing air to diffuse into the opened cuvette, an initial lag phase was observed, followed by a large ΔA583. Conversely, when 100 µL of anaerobically prepared (αKG+Fe)FIH-1 was diluted with aerobic buffer (400 µL) then opened to air, a modest increase in A583 followed. We attribute the lag phase to air diffusing into the cuvette, as it was not observed under rapid oxygenation conditions. As the rate of oxygenation affected the yield of purple chromophore, this suggested that rapid introduction of air likely depleted the available pool of Fe(II) in solution, which both reduced yield and slowed the rate of auto-hydroxylation.
Figure 4.
Appearance of purple FIH-1 following slow exposure of (αKG+Fe)FIH-1 (100 µM apo FIH-1, 500 µM FeSO4, 500 µM αKG in 50 mM HEPES, pH 7.50) to air. Inset: Timecourses following (αKG+Fe)FIH-1 exposure to air; A) slow oxygenation; B) rapid oxygenation.
The kinetics of auto-hydroxylation were monitored for their dependence on varied concentrations of Fe(II) and αKG. Auto-oxidation reactions with varied [Fe(II)] were performed by preparing an anaerobic solution of apo FIH-1 (100 µM in 100 µL buffer), αKG (500 µM), and variable FeSO4 (50 – 500 µM), then initiating reaction by adding 100 µL aerobic buffer. Under low [Fe(II)] conditions, very little purple chromophore was produced, suggesting that air oxidation of Fe(II) depleted the pool of available metal. In contrast, at excess [Fe(II)] the rate and yield were high and indistinguishable (Figure 5), indicating that the rate-limiting step occurred after Fe(II) bound to FIH-1, provided that the Fe(II) pool was in excess.
Figure 5.
Kinetics of purple FIH-1 formation following rapid oxygenation of (αKG+Fe)FIH-1 (50 µM apo FIH-1, 25 – 250 µM FeSO4, 250 µM αKG in 50 mM HEPES, pH 7.50). The Fe:FIH-1 ratio is indicated within the figure.
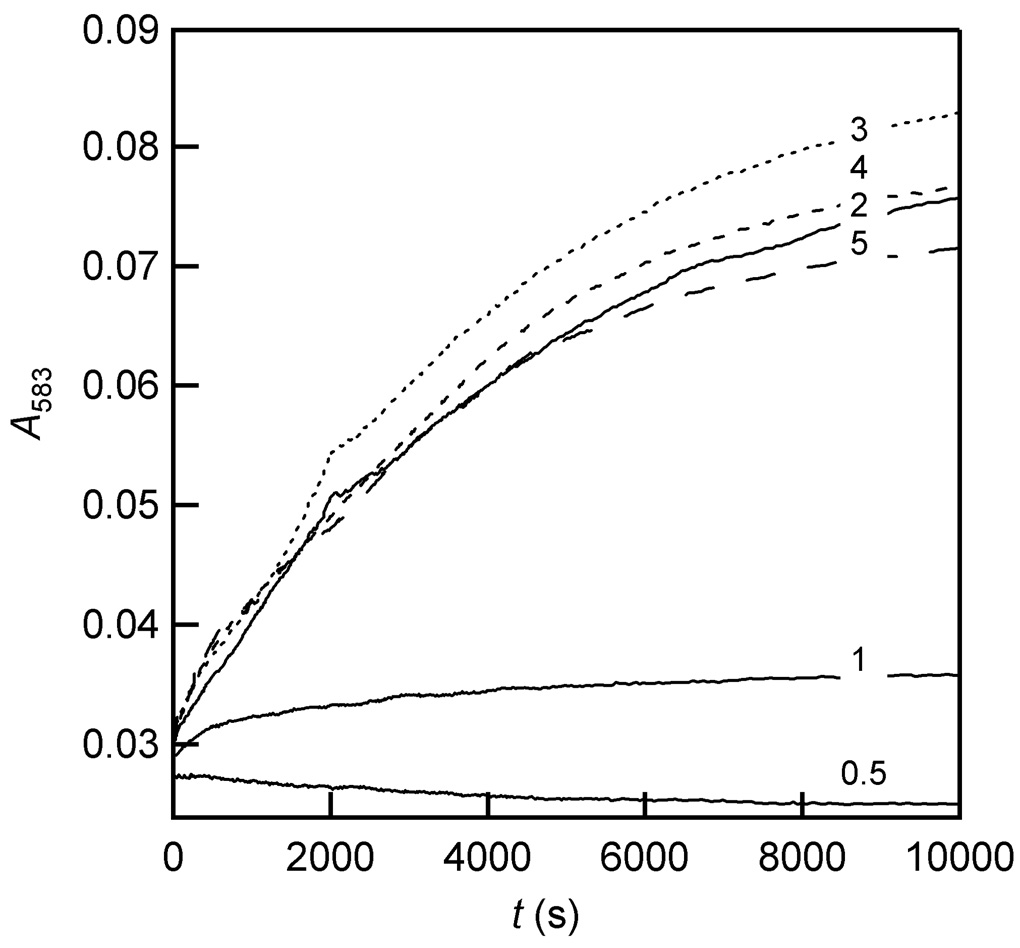
The αKG dependence of auto-hydroxylation was assessed under conditions in which FIH-1 (100 µM, 50 mM HEPES, pH 7.50) was preincubated anaerobically with saturating FeSO4 (500 µM) and variable αKG (50 – 1000 µM), then opened to air (Figure 6). The yield increased in response to [αKG] in a saturable fashion, suggesting that an αKG binding equilibrium preceded the reaction. As the rate of formation was independent of [αKG], a step after αKG binding must be rate-determining. Furthermore, as experiments in which αKG was replaced by succinate did not support purple FIH-1 formation (see below), the purple chromophore is likely formed by a high-valent oxidant within the active site, such as the canonical Fe(IV)=O.
Figure 6.
Kinetic traces (A583) for purple FIH-1 formation following air exposure of (αKG+Fe)FIH-1 (100 µM apo FIH-1, 500 µM FeSO4, 50 – 1000 µM αKG, in 50 mM HEPES, pH 7.50). The αKG:FIH-1 ratio is indicated in the figure.
Some preparations of FIH-1 formed a yellow chromophore under auto-hydroxylation conditions, with little or no yield of the Trp-O-Fe(III) species. The yellow color arose from a shoulder in the UV-Vis spectrum 300 – 400 nm, indicating metal-centered oxidation to Fe(III). As the formation of yellow FIH-1 was not uniformly observed, we suspected that trace redox agents introduced during protein purification could redirect oxidation toward iron and away from enzymatic hydroxylation.
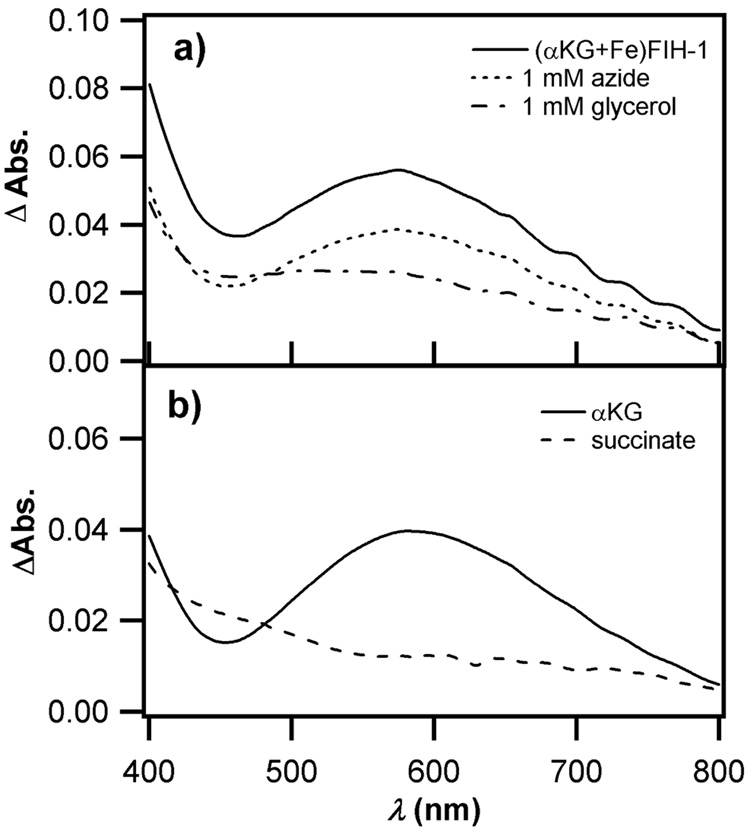
To test this model, we assayed auto-hydroxylation in the presence of 1mM NaN3 or glycerol, as these are preservatives for the dialysis membranes used in protein concentrators. Apo FIH-1 (100 µM) was incubated in 100 µL with αKG (500 µM) and FeSO4 (100 µM) under anaerobic conditions, then 100 µL of aerobic buffer containing either no additive, NaN3 (2 mM), or glycerol (2 mM) was added to initiate auto-oxidation. While the (αKG+Fe)FIH-1 sample generated purple FIH-1 in the positive control (no additive), as well as a slightly reduced yield in the presence of azide, a resolved absorption peak was not found in the presence of glycerol (Figure 7a). It appeared that 1 mM glycerol almost completely prevented the formation of the purple chromophore, suggesting that this preservative was to blame the variable yield of purple FIH-1 in different FIH-1 preparations.
Figure 7.
UV-Vis absorption changes following 10,000 sec. auto-hydroxylation. a) (αKG+Fe)FIH-1 in the presence of A) no additive; B) 1 mM NaN3; C) 1 mM glycerol. b) 27 Effect of varied organic diacid (αKG/succinate) on auto-hydroxylation, A) (αKG+Fe)FIH-1; B) (succ.+Fe)FIH-1. Conditions: 50 µM FIH-1, 50 µM FeSO4, 250 µM αKG or succinate in 50 mM HEPES, pH 7.50.
As auto-hydroxylation was specifically localized to Trp296, it likely results from a non-diffusable oxidant. In analogy with other αKG oxygenases, this implicates a metal-centered oxidant in Trp hydroxylation, such as Fe(IV)=O. The fact that glycerol quenched the auto-hydroxylation reaction suggests that glycerol can reach the active site of FIH-1 and intercept this metal-centered oxidant. Notably, the active site of FIH-1 is relatively solvent exposed in the X-ray crystal structure [21], which is consistent with this proposal.
The appearance of purple FIH-1 required αKG, Fe(II), and O2, however we wished to test whether this was due to oxidative decarboxylation chemistry. Succinate was used in place of αKG, as both succinate and αKG are anionic ligands with similar chelation modes, but succinate does not undergo oxidative decarboxylation. Apo FIH-1 (100 µM) was pre-incubated with one equivalent of FeSO4, and an excess of organic diacid (500 µM αKG or succinate), then rapidly oxygenated by dilution with an equal volume of oxygenated buffer. Quite different products were formed during the subsequent reaction, as shown by the changes in the UV-Vis spectra (Figure 7b). The HO-Trp was clearly formed in the positive control, but absent from (succ.+Fe)FIH-1. As αKG can undergo oxidative decarboxylation to form the high-valent Fe(IV)=O oxidant found in related enzymes, whereas succinate cannot, this result strongly suggests that HO-Trp formation results from the high-valent oxidant.
Metal Oxidation in Purple and Yellow FIH-1
EPR and X-ray absorption spectroscopy were used to characterize the active-site Fe in purple and yellow FIH-1. EPR spectra collected near 10 K for purple and yellow FIH-1 exhibited signals near geff = 9 and 4.27, indicating the presence of high-spin Fe(III) (Supplemental material). These signals must come from Fe(III) centers tightly associated with FIH-1, as buffer exchange did not remove these EPR signals, indicating that the cofactor of FIH-1 was oxidized upon air exposure.
The coordination geometries of purple and yellow FIH-1 were measured by Fe X-ray absorption spectroscopy. The edge energy for purple FIH-1 lies at 7124.2 eV compared to the 7125.0 eV edge energy for yellow FIH-1, indicating either that there is a small fraction of reduced iron in yellow FIH-1, or that purple FIH-1 is more Lewis-acidic than yellow FIH-1. Each sample exhibited a 1s → 3d pre-edge transition in the XANES region near 7113.5 eV, indicating that iron deviated from octahedral symmetry[43] (Figure 8).
Figure 8.
X-ray absorption spectra; A) yellow FIH-1; B) purple FIH-1. Insets show the Fourier-filtered EXAFS data, and fits using the parameters from Table 1. Samples buffer-exchanged and concentrated from FIH-1 (100 µM in 50 mM HEPES, pH 7.50) with FeSO4 (500 µM) and αKG (500 µM).
The EXAFS of each sample was fit to Fourier-filtered data from 1 to 2.3 Å−1 to obtain the best first coordination sphere fit. The best fits are shown in Table 1; Alternative fits and fits used to refine the model are shown in the supporting information. The best fit for the purple FIH-1 sample was for a six-coordinate Fe center with two different scattering distances: 2 N/O scatterers at a shorter distance (1.97 Å) and 4 N/O scatterers at a longer distance (2.11 Å). This was consistent with tighter bonds to the two protein-derived anionic ligands, Asp and Trp-O−, with longer bonds to the two His residues, and the remaining two sites occupied by a combination of H2O and organic acid (αKG or succinate). The yellow FIH-1 sample fit best to 7 N/O scatterers at the same distance (2.01 Å). It is possible that one of the anticipated carboxylate ligands, either Asp or αKG/succinate, was ordered sufficiently that the non-bonded oxygen contributed to scattering. In fact, the difference between the coordination numbers suggested by XANES (5C, or distorted 6C) and determined by EXAFS (6C or 7C) likely arises from the presence of carboxylate ligands that may adopt a bidentate binding mode. For the purposes of XANES analysis, the bidentate ligand would reduce the symmetry of the iron site, leading to an increase in the 1s → 3d peak area. The Fourier-filtered spectra of the two samples look very similar, with minor differences at 10 Å−1 and beyond (Figure 8). These differences most likely arise from subtle changes in ligand identity or geometry around the Fe center, as would be anticipated for a different pattern of protein sidechain oxygenation in yellow and purple FIH-1.
Table 1.
XAS fits to FIH-1 samples.
| Sample | n | r (Å) | σ2(×103 Å2) | E0 (eV) | r factor | 1s→3d peak area (eV) |
|---|---|---|---|---|---|---|
| Purple | 4 N/O | 2.11(3) | 6(5) | −3(3) | 0.0766 | 0.15(3) |
| 2 N/O | 1.97(4) | 3(4) | ||||
| Yellow | 7 N/O | 2.01(1) | 6.5(9) | −2(1) | 0.0337 | 0.12(1) |
Best fits reported, with number of first-shell scatterers (n), distance to iron (r), disorder parameter (σ2), phase shift (E0), and the fit r-factor. The 1s → 3d peak area was observed in the pre-edge.
Oxygenation of Sidechains
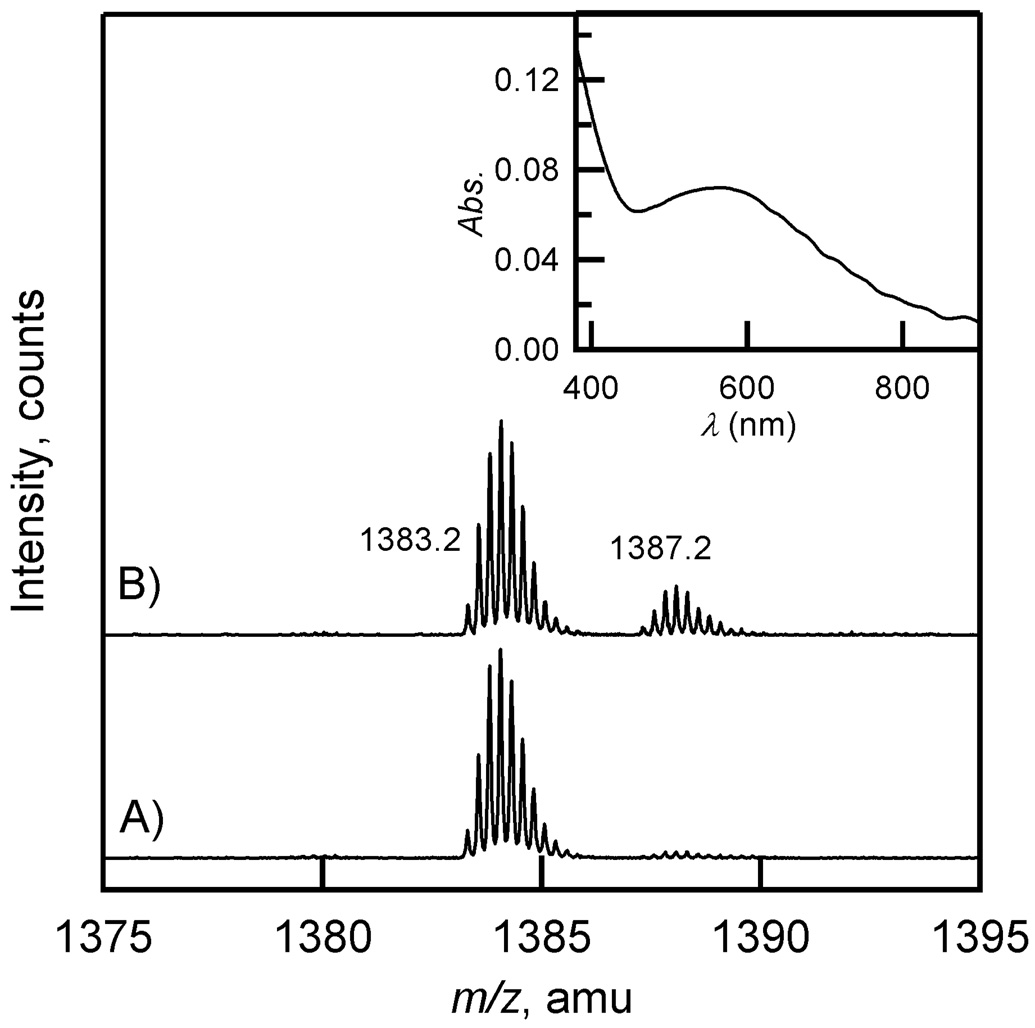
Electrospray mass spectrometry was used to identify the protein-derived oxidation products in apo, purple, and yellow FIH-1. FIH-1 samples were digested with trypsin overnight, and then analyzed and sequenced via LC-MS/MS to identify peptides containing active-site aromatics (Tyr93, Tyr102, and Trp296). The Tyr containing peptides, 76 – 99 (MH2 2+ = 1387.14 m/z) and 100 – 106 (MH+ = 977.46 m/z) were unmodified in all FIH-1 samples, and the Trp containing peptide 252 – 298 (MH4 4+ = 1379.19 m/z) was unmodified in the apo FIH-1 sample. In contrast, both purple and yellow FIH-1 samples contained the unmodified peptide intermixed with an oxygenated peptide (OMH4 4+ = 1383.19 m/z) corresponding to a single O-atom addition to peptide 252 – 298 (Figure 9).
Figure 9.
QSTAR-TOF spectra of peptide 252 – 298 from trypsin digested FIH-1. A) apo FIH-1; B) purple FIH-1; C) yellow FIH-1. The z = 4+ peak at m/z = 1379.2 is due to unmodified peptide; the z = 4+ peak at m/z = 1383.2 is due to a singly oxygenated peptide.
High quality MS/MS spectra for yellow and purple FIH-1 samples were obtained by collecting the HPLC fraction containing peptide 252 – 298 for direct injection at 5 µL/min. Scans were averaged to obtain the MS/MS profiles of the unmodified and modified peptides. The site of oxygenation in purple FIH-1 was positively identified as arising from the addition of a single O-atom to Trp296 by b- and y-ion sequencing of this peptide.
Peptide 252 – 298 from yellow FIH-1 was similarly analyzed by direct injection MS/MS using a QStar-TOF. Only limited sequencing was possible, as ions between y16 and y25 were not observable due to the dominant signal from the y25 ion. Nevertheless, oxygenation was localized to the 274 – 283 region, excluding Trp296 as the site of oxygenation. As Met oxidation was suspected, collision-induced dissociation (CID) of the infused peptide fraction was performed. The modified y25 ion with mass of 1,561.7 m/z (z = 2+) was selected for MS/MS analysis, and CID was initiated by increasing the declustering potential to 210 V. Fragmentation spectra contained a new peak at 1,529.3 m/z (z = 2+), corresponding to a total loss of 64 amu from the modified y25 (Figure 10). This is characteristic for the loss of a CH3SOH group from an oxygenated Met residue [44–46], indicating that Met275 is the site of oxygenation for yellow FIH-1. In-source CID analysis of purple FIH-1 showed that the only oxygenated residue was Trp296.
Figure 10.
Fragmentation spectra of y25 ion from peptide 252 – 298 of yellow FIH-1 via collision-induced dissociation. The z = 2+ peak at m/z = 1561.3 fragments with loss of 64 mass units to form the z = 2+ peak at m/z = 1529.3.
While the EPR and XAS data showed similar Fe(III) centers for purple and yellow FIH-1, the MS data indicated distinct peptide modifications. In the case of purple FIH-1, the UV-Vis and MS data together indicated that the oxygenated product was Fe(III)-O-Trp296. Similar Fe(III)-O-Trp chromophores are found in the bacterial enzymes TfdA and AlkB as the products of uncoupled O2-activation. The indistinct UV-Vis spectrum for yellow FIH-1, on the other hand, indicated an Fe(III) without a new (Trp/Tyr-O)− ligand; the MS data indicate that oxygenation occurred, but on a residue (Met275) located at the protein surface, outside of the active site (Figure 1).
This raised the question of whether oxo-Met275 and HO-Trp296 were mutually exclusive modifications, as for a branched reaction pathway arising from uncoupled oxidation at the active site. Apo FIH-1 (500 µM) was intentionally oxidized at Met residues for 30 min. by excess H2O2 (500 mM), followed by the addition of β-mercaptoethanol (1.5 M) [47]. The peroxide-treated apo FIH-1 was split, and one fraction analyzed by MS/MS following trypsin digestion (Figure 11A). The apo FIH-1 sample was quantitatively oxygenated on peptide 252 – 298 (z = 4+ peak at 1383.2 m/z), as the unmodified peptide (m/z = 1379.2) was not observed. Subsequent CID experiments on this sample exhibited loss of a CH3SOH group from the y25 ion, confirming that the O-atom addition formed oxo-Met275. The remaining sample of peroxide-treated FIH-1 was used for an auto-hydroxylation assay, which yielded purple FIH-1 (Figure 11B, inset). Trypsin digestion followed by MS/MS analysis indicated that a portion of peptide 252 – 298 was doubly oxygenated (z = 4+ peak at 1387.2 m/z) following auto-hydroxylation, as anticipated for the Fe(III)-O-Trp296 chromophore. Clearly, the presence of oxo-Met275 does not preclude the formation of Fe(III)-O-Trp296 on the same molecule of FIH-1, suggesting that Met275 oxidation did not eliminate further active-site chemistry.
Figure 11.
QStar-TOF spectra of peptide 252 – 298 from H2O2-treated FIH-1; A) apo FIH-1; B) (αKG+Fe)FIH-1 following auto-hydroxylation assay. The z = 4+ peaks correspond to peptides that are singly (1383.2 m/z) or doubly oxygenated (1387.2 m/z). Inset: UV-Vis spectrum of purple FIH-1 formed by rapid oxygenation of peroxide-treated (αKG+Fe)FIH-1 (50 µM apo FIH-1, 50 µM FeSO4, 250 µM αKG in 50 mM HEPES, pH 7.50).
Proposed Auto-oxidation mechanism
The consensus chemical mechanism for normal turnover in the αKG oxygenases suggests a mechanism for uncoupled O2-activation [8]. As the binding of prime substrate releases the axial H2O from Fe(II), this is the key to making enzyme ready for O2 binding. Normal turnover leads to the subsequent steps of oxidative decarboxylation, H-atom abstraction, and rebound to produce the hydroxylated product and regenerate resting enzyme. Similarly, inadvertent loss of H2O prior to prime substrate binding could lead to O2 activation in the absence of substrate oxygenation. While there are other routes for uncoupling in αKG oxygenases [16, 48], the basic theme is one of O2 entry in the absence of prime substrate.
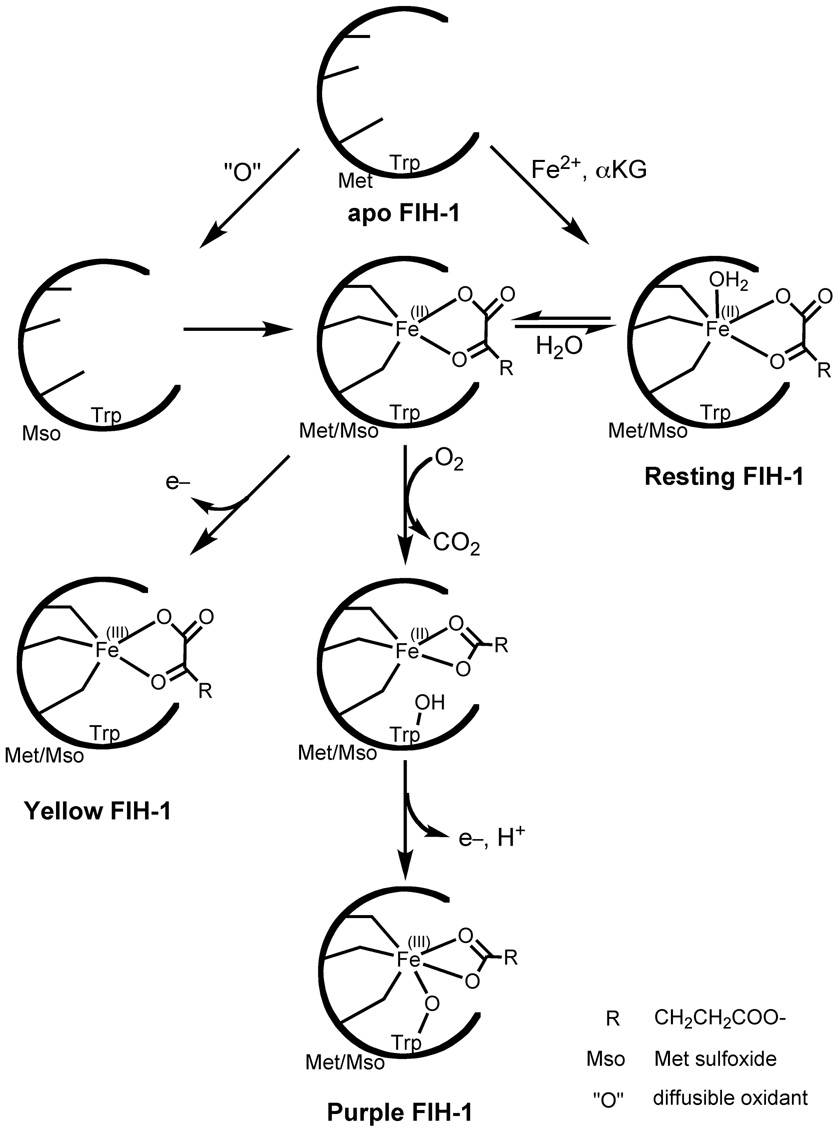
Our experimental conditions delineated the relationships between multiple enzyme forms, and suggested a role for H2O-loss in uncoupled O2-activation by FIH-1 (Scheme 2). Insofar as Co(II) could serve as a structural mimic for Fe(II), the EPR data for (αKG+Co)FIH-1 indicated that Fe(II) within the active site partitioned between a 6C form and a 5C form in which H2O was likely lost. The mechanistic data were consistent with O2 binding to the pre-formed αKG-Fe(II) center in resting (αKG+Fe)FIH-1,with a subsequent rate-determining step. Based upon what has been reported for normal turnover in other αKG oxygenases [8, 9], the step most likely to be rate-limiting is formation of the Fe(IV)=O intermediate, with subsequent steps leading to Fe(III)-O-Trp296. Yellow FIH-1 arose from a metal centered oxidation in (succ.+Fe)FIH-1 or by the action of glycerol on (αKG+Fe)FIH-1, and contained a fractional (O)Met275 modification. It is notable that (O)Met was not observed in the purple FIH-1 samples, implying that purple FIH-1 may be incapable of reacting further with O2.
Scheme 2.
Proposed mechanism for converting apo FIH-1 into purple or yellow FIH-1.
It has been proposed that auto-hydroxylation in αKG oxygenases may provide a protective function by inactivating the enzyme to minimize reactive-oxygen species (ROS) production [8], but inactivating an enzyme would also regulate its activity. The potential for auto-hydroxylation to regulate FIH-1 function is intriguing, if for no other reason than it underscores the paradox involved in O2-sensing by HIFα. As FIH-1 likely functions under physiological conditions in which [O2] ≫ [HIFα], uncoupled oxidation should be common within the cell. Although it would seem energy inefficient to regulate FIH-1 by an irreversible covalent modification, the entire hypoxia-sensing pathway relies on the irreversible hydroxylation of HIFα. Perhaps the resolution lies in kinetics: if auto-hydroxylation were kinetically sluggish, then the ratio of uncoupled turnover to normal turnover would remain small, except for conditions of elevated pO2. The normal turnover rate for FIH-1 hydroxylating HIFα is already slow (kcat/KM = 103 M−1s−1) [49], but should HIFα be depleted below 10−9 M, normal turnover may occur with an observed rate of <10−6 s−1. As purple FIH-1 formation is slightly slower than normal turnover, the relative rates of normal and uncoupled hydroxylation would prevent uncoupling from overwhelming normal turnover, while minimizing aberrant ROS production.
Supplementary Material
The EPR spectra for oxidized FIH-1, and tables of alternate X-ray absorption fits, are available through the online version of this article, at doi:10.106/j.inorgbio.2008.08-0604.
Acknowledgements
We thank the University of Massachusetts, the American Cancer Society (#IRG 93-033) and the NIH (GM077413) for funding; and the NSF for support of the EPR facility (CHE-0443180). We thank Professors S. J. Eyles and R. W. Vachet, and Dr. C. Bobst for helpful suggestions.
Abbreviations
- 5C
five-coordinate
- 6C
six-coordinate
- AEBSF
4-(2-aminoethyl)-benzenesulfonyl fluoride
- Bis-Tris
2-[Bis-(2-hydroxyethyl)-amino]-2-hydroxymethyl-propane-1,3-diol
- CHES
N-Cyclohexyl-2-aminoethanesulfonic acid
- CID
Collision-induced dissociation
- ESI-MS
electrospray ionization mass spectrometry
- EXAFS
Extended X-Ray Absorption Fine Structure
- FIH-1
the factor inhibiting HIF
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- HIF
Hypoxia Inducible Factor
- IPTG
isopropyl β-D-1-thiogalactopyranoside
- MLCT
Metal to Ligand Charge Transfer
- Mso
Methionine sulfoxide
- ROS
reactive-oxygen species
- TOF
Time-of-flight
- Tris
2-Amino-2-hydroxymethyl-propane-1,3-diol
- XANES
X-ray Absorption Near Edge Structure
- XAS
X-ray absorption spectroscopy
- αKG
alpha-ketoglutarate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
D was fixed arbitrarily at 50 cm−1 and A was fixed at zero.
Assuming ε583= 3000 M−1 cm−1 for purple FIH-1
References
- 1.Schofield CJ, Ratcliffe PJ. Nat. Rev. Mol. Cell. Bio. 2004;5:343–354. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- 2.Semenza GL. Annu. Rev. Cell. Dev. Biol. 1999;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- 3.Semenza GL. Physiol. 2004;19:176–182. doi: 10.1152/physiol.00001.2004. [DOI] [PubMed] [Google Scholar]
- 4.Metzen E, Ratcliffe PJ. J. Biol. Chem. 2004;385:223–230. doi: 10.1515/BC.2004.016. [DOI] [PubMed] [Google Scholar]
- 5.Bruick RK, McKnight SL. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 6.Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. Gene. Dev. 2002;16:1466–1471. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahon PC, Hirota K, Semenza GL. Gene. Dev. 2001;15:2675–2686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hausinger RP. Crit. Rev. Biochem. Mol. Biol. 2004;39:21–68. doi: 10.1080/10409230490440541. [DOI] [PubMed] [Google Scholar]
- 9.Price JC, Barr EW, Tirupati B, Bollinger JM, Krebs C. Biochemistry. 2003;42:7497–7508. doi: 10.1021/bi030011f. [DOI] [PubMed] [Google Scholar]
- 10.Proshlyakov DA, Henshaw TF, Monterosso GR, Ryle MJ, Hausinger RP. J. Am. Chem. Soc. 2004;126:1022–1023. doi: 10.1021/ja039113j. [DOI] [PubMed] [Google Scholar]
- 11.Zhou J, Gunsior M, Bachmann BO, Townsend CA, Solomon EI. J. Am. Chem. Soc. 1998;120:13539–13540. doi: 10.1021/ja004025+. [DOI] [PubMed] [Google Scholar]
- 12.Solomon EI, Brunold TC, Davis MI, Kemsley JN, Lee SK, Lehnert N, Neese F, Skulan AJ, Yang YS, Zhou J. Chem. Rev. 2000;100:235–349. doi: 10.1021/cr9900275. [DOI] [PubMed] [Google Scholar]
- 13.Zhou J, Kelly WL, Bachmann BO, Gunsior M, Townsend CA, Solomon EI. J. Am. Chem. Soc. 2001;123:7388–7398. doi: 10.1021/ja004025+. [DOI] [PubMed] [Google Scholar]
- 14.Neidig ML, Brown CD, Light KM, Fujimori DG, Nolan EM, Price JC, Barr EW, Bollinger JM, Krebs C, Walsh CT, Solomon EI. J. Am. Chem. Soc. 2007;129:14224–14231. doi: 10.1021/ja074557r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henshaw TF, Feig M, Hausinger RP, Inorg J. Biochem. 2004;98:856–861. doi: 10.1016/j.jinorgbio.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 16.Koehntop KD, Marimanikkuppam S, Ryle MJ, Hausinger RP, Que L. J. Biol. Inorg. Chem. 2006;11:63–72. doi: 10.1007/s00775-005-0059-4. [DOI] [PubMed] [Google Scholar]
- 17.Liu A, Ho RYN, Que LJ, Ryle MJ, Phenney BS, Hausinger RP. J. Am. Chem. Soc. 2001;123:5126–5127. doi: 10.1021/ja005879x. [DOI] [PubMed] [Google Scholar]
- 18.Saari RE, Hausinger RP. Biochemistry. 1998;37:3035–3042. doi: 10.1021/bi972388p. [DOI] [PubMed] [Google Scholar]
- 19.Liu PH, Mehn MP, Yan F, Zhao ZB, Que L, Liu HW. J. Am. Chem. Soc. 2004;126:10306–10312. doi: 10.1021/ja0475050. [DOI] [PubMed] [Google Scholar]
- 20.Sundheim O, Vågbø CB, Bjørås M, Sousa MML, Talstad V, Aas PA, Drabløs F, Krokan HE, Tainer JA, Slupphaug G. EMBO J. 2006;25:3389–3397. doi: 10.1038/sj.emboj.7601219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elkins JM, Hewitson KS, McNeill LA, Seibel JF, Schlemminger I, Pugh CW, Ratcliffe PJ, Schofield CJ. J. Biol. Chem. 2003;278:1802–1806. doi: 10.1074/jbc.C200644200. [DOI] [PubMed] [Google Scholar]
- 22.Dann CER, Bruick RK, Deisenhofer J. Proc. Natl. Acad. Sci. USA. 2002;99:15351–15356. doi: 10.1073/pnas.202614999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee C, Kim SJ, Jeong DG, S.M L, Ryu SE. J. Biol. Chem. 2003;278:7558–7563. doi: 10.1074/jbc.M210385200. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y-H, Comeaux LM, Eyles SJ, Knapp MJ. Chem. Commun. 2008 doi: 10.1039/b809099h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lennon GG, Auffray C, Polymeropoulos M, Soares MB. Genomics. 1996;33:151–152. doi: 10.1006/geno.1996.0177. [DOI] [PubMed] [Google Scholar]
- 26.Hewitson KS, McNeill LA, Riordan MV, Tian YM, Bullock AN, Welford RW, Elkins JM, Oldham NJ, Bhattacharya S, Gleadle JM, Ratcliffe PJ, Pugh CW, Schofield CJ. J. Biol. Chem. 2002;277:26351–26355. doi: 10.1074/jbc.C200273200. [DOI] [PubMed] [Google Scholar]
- 27.Gill SC, von Hippel PH. Anyl. Biochem. 1989;182:319–326. doi: 10.1016/0003-2697(89)90602-7. [DOI] [PubMed] [Google Scholar]
- 28.Abragam A, Bleaney B. Electron paramagnetic resonance of transition ions. Oxford: Clarendon Press; 1970. [Google Scholar]
- 29.Hanson GR, Gates KE, Noble CJ, Griffin M, Mitchell A, Benson S. J. Inorg. Biochem. 2004;85:903–916. doi: 10.1016/j.jinorgbio.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Leitch S, Bradley MJ, Rowe JL, Chivers PT, Maroney MJ. J. Am. Chem. Soc. 2007;129:5085–5095. doi: 10.1021/ja068505y. [DOI] [PubMed] [Google Scholar]
- 31.Padden KM, Krebs JF, MacBeth CE, Scarrow RC, Borovik AS. J. Am. Chem. Soc. 2001;123:1072–1079. doi: 10.1021/ja003282b. [DOI] [PubMed] [Google Scholar]
- 32.Ankudinov AL, Ravel B, Rehr JJ, Conradson SD. Phys. Rev. B. 1998;58:7565–7576. [Google Scholar]
- 33.Bennett B, Holz RC. J. Am. Chem. Soc. 1997;119:1923–1933. [Google Scholar]
- 34.Bennett B, Holz RC. Biochemistry. 1997;36:9837–9846. doi: 10.1021/bi970735p. [DOI] [PubMed] [Google Scholar]
- 35.Breece RM, Costello A, Bennett B, Sigdel TK, Matthews ML, Tierney DL, Crowder MW. J. Biol. Chem. 2005;280:11074–11081. doi: 10.1074/jbc.M412582200. [DOI] [PubMed] [Google Scholar]
- 36.Kumar A, Periyannan GR, Narayanan B, Kittell AW, Kim JJ, Bennett B. Biochem. J. 2007;403:527–536. doi: 10.1042/BJ20061591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huheey JE. Inorganic Chemistry: Principles of Structure and Reactivity. 3rd edn. New York, NY: Harper Collins; 1983. [Google Scholar]
- 38.Irving H, Williams RJP. Nature. 1948;162:746–747. [Google Scholar]
- 39.Irving H, Williams RJP. J. Chem. Soc. 1953:3192–3210. [Google Scholar]
- 40.Que L, Hausinger RP, Hegg EL, Ho RYN, Liu A, Mehn MP, Ryle MJ. J. Inorg. Biochem. 2001;86:388–388. [Google Scholar]
- 41.Hegg EL, Whiting AK, Saari RE, McCracken J, Hausinger RP, Que L. Biochemistry. 1999;38:16714–16726. doi: 10.1021/bi991796l. [DOI] [PubMed] [Google Scholar]
- 42.Pavel EG, Zhou J, Busby RW, Gunsior M, Townsend CA, Solomon EI. J. Am. Chem. Soc. 1998;120:743–753. [Google Scholar]
- 43.Westre TE, Kennepohl P, DeWitt JG, Hedman B, Hodgson KO, Solomon EI. J. Am. Chem. Soc. 1997;119:6297–6314. [Google Scholar]
- 44.Jiang XY, Smith JB, Abraham EC. Rapid Commun. Mass Spectrom. 1996;31:1309–1310. [Google Scholar]
- 45.Lagerwerf FM, vandeWeert M, Heerma W, Haverkamp J. Rapid Commun. Mass Spectrom. 1996;10:1905–1910. doi: 10.1002/(SICI)1097-0231(199612)10:15<1905::AID-RCM755>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 46.Reid GE, Roberts KD, Kapp EA, Simpson RJ. J. Proteome Res. 2004;3:751–759. doi: 10.1021/pr0499646. [DOI] [PubMed] [Google Scholar]
- 47.Kim YH, Berry AH, Spencer DS, Stites WE. Protein Eng. 2001;14:343–347. doi: 10.1093/protein/14.5.343. [DOI] [PubMed] [Google Scholar]
- 48.Ryle MJ, Koehntop KD, Liu AM, Que L, Hausinger RP. Proc. Natl. Acad. Sci. USA. 2003;100:3790–3795. doi: 10.1073/pnas.0636740100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ehrismann D, Flashman E, Genn DN, Mathioudakis N, Hewitson KS, Ratcliffe PJ, Schofield CJ. Biochem. J. 2007;401:227–234. doi: 10.1042/BJ20061151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The EPR spectra for oxidized FIH-1, and tables of alternate X-ray absorption fits, are available through the online version of this article, at doi:10.106/j.inorgbio.2008.08-0604.