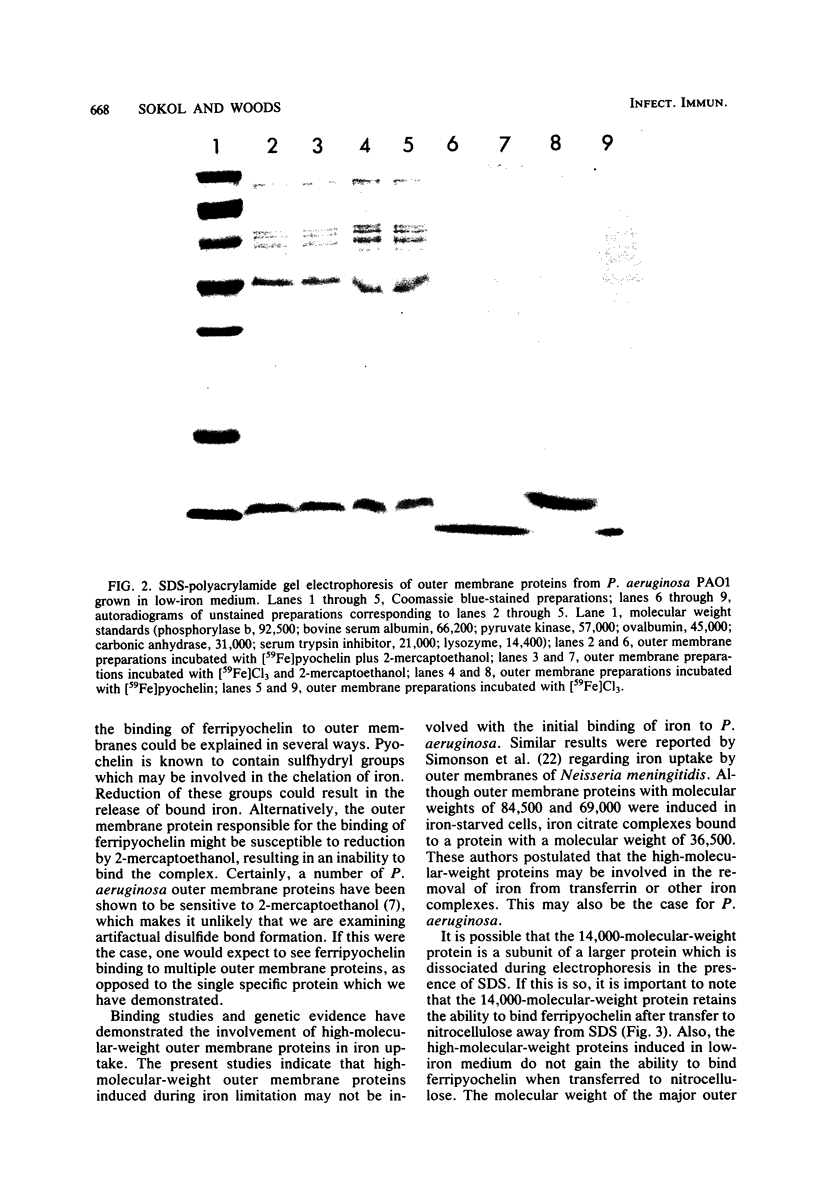
Abstract
Outer membranes prepared from Pseudomonas aeruginosa grown in low-iron medium bind three times as much [59Fe]pyochelin as do membranes from cells grown in high-iron medium. The deletion of pyochelin reduced 59Fe binding to background levels. Autoradiographic analysis of sodium dodecyl sulfate polyacrylamide gel electrophoretograms of outer membrane preparations previously incubated with [59Fe]pyochelin revealed that the iron-siderophore complex bound to a low-molecular-weight protein of approximately 14,000.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett R. L., Rothfield L. I. Genetic and physiological regulation of intrinsic proteins of the outer membrane of Salmonella typhimurium. J Bacteriol. 1976 Jul;127(1):498–504. doi: 10.1128/jb.127.1.498-504.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D., Graham R. Isolation of an iron-binding compound from Pseudomonas aeruginosa. J Bacteriol. 1979 Jan;137(1):357–364. doi: 10.1128/jb.137.1.357-364.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D. Iron uptake with ferripyochelin and ferric citrate by Pseudomonas aeruginosa. J Bacteriol. 1980 May;142(2):581–587. doi: 10.1128/jb.142.2.581-587.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiss E. H., Stanley-Samuelson P., Neilands J. B. Properties and proteolysis of ferric enterobactin outer membrane receptor in Escherichia coli K12. Biochemistry. 1982 Aug 31;21(18):4517–4522. doi: 10.1021/bi00261a050. [DOI] [PubMed] [Google Scholar]
- Futai M. Membrane D-lactate dehydrogenase from Escherichia coli. Purification and properties. Biochemistry. 1973 Jun 19;12(13):2468–2474. doi: 10.1021/bi00737a016. [DOI] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Lyle R. D. Chemical alterations in cell envelopes of polymyxin-resistant Pseudomonas aeruginosa isolates. J Bacteriol. 1979 Jun;138(3):839–845. doi: 10.1128/jb.138.3.839-845.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Carey A. M. Outer membrane of Pseudomonas aeruginosa: heat- 2-mercaptoethanol-modifiable proteins. J Bacteriol. 1979 Dec;140(3):902–910. doi: 10.1128/jb.140.3.902-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Nikaido H. Outer membranes of gram-negative bacteria. XIX. Isolation from Pseudomonas aeruginosa PAO1 and use in reconstitution and definition of the permeability barrier. J Bacteriol. 1978 Oct;136(1):381–390. doi: 10.1128/jb.136.1.381-390.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollifield W. C., Jr, Neilands J. B. Ferric enterobactin transport system in Escherichia coli K-12. Extraction, assay, and specificity of the outer membrane receptor. Biochemistry. 1978 May 16;17(10):1922–1928. doi: 10.1021/bi00603a019. [DOI] [PubMed] [Google Scholar]
- Holloway B. W., Krishnapillai V., Morgan A. F. Chromosomal genetics of Pseudomonas. Microbiol Rev. 1979 Mar;43(1):73–102. doi: 10.1128/mr.43.1.73-102.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara S., Mizushima S. Identification of an outer membrane protein responsible for the binding of the Fe-enterochelin complex to Escherichia coli cells. J Biochem. 1978 Jan;83(1):137–140. doi: 10.1093/oxfordjournals.jbchem.a131884. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McIntosh M. A., Chenault S. S., Earhart C. F. Genetic and physiological studies on the relationship between colicin B resistance and ferrienterochelin uptake in Escherichia coli K-12. J Bacteriol. 1979 Jan;137(1):653–657. doi: 10.1128/jb.137.1.653-657.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne S. M., Finkelstein R. A. The critical role of iron in host-bacterial interactions. J Clin Invest. 1978 Jun;61(6):1428–1440. doi: 10.1172/JCI109062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., Reeves P. Iron uptake in colicin B-resistant mutants of Escherichia coli K-12. J Bacteriol. 1976 Jun;126(3):1052–1062. doi: 10.1128/jb.126.3.1052-1062.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., Reeves P. The role of colicin receptors in the uptake of ferrienterochelin by Escherichia coli K-12. Biochem Biophys Res Commun. 1977 Feb 7;74(3):903–911. doi: 10.1016/0006-291x(77)91604-7. [DOI] [PubMed] [Google Scholar]
- Simonson C., Trivett T., DeVoe I. W. Energy-independent uptake of iron from citrate by isolated outer membranes of Neisseria meningitidis. Infect Immun. 1981 Feb;31(2):547–553. doi: 10.1128/iai.31.2.547-553.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and susceptibility to infectious disease. Science. 1974 May 31;184(4140):952–956. doi: 10.1126/science.184.4140.952. [DOI] [PubMed] [Google Scholar]





