Abstract
A methyl-β-cyclodextrin-induced lipid exchange technique was devised to prepare small unilamellar vesicles with stable asymmetric lipid compositions. Asymmetric vesicles that mimic biological membranes were prepared with sphingomyelin (SM) or SM mixed with 1-palmitoyl-2-oleoyl-phosphatidylcholine (POPC) as the predominant lipids in the outer leaflet and dioleoylphosphatidylcholine (DOPC), POPC, 1-palmitoyl-2-oleoyl-phosphatidyl-l-serine (POPS), or POPS mixed with 1-palmitoyl-2-oleoyl-phosphatidylethanolamine (POPE) in the inner leaflet. Fluorescence-based assays were developed to confirm lipid asymmetry. Cholesterol was introduced into these vesicles using a second methyl-β-cyclodextrin exchange step. In asymmetric vesicles composed of SM outside, DOPC inside (SMo/DOPCi) or SM outside, 2:1 mol:mol POPE:POPS inside (SMo/2:1 POPE:POPSi) the outer leaflet SM formed an ordered state with a thermal stability similar to that in pure SM vesicles and significantly greater than that in symmetric vesicles with the same overall lipid composition. Analogous behavior was observed in vesicles containing cholesterol. This shows that an asymmetric lipid distribution like that in eukaryotic plasma membranes can be conducive to ordered domain (raft) formation. Furthermore asymmetric vesicles containing ∼25 mol % cholesterol formed ordered domains more thermally stable than those in asymmetric vesicles lacking cholesterol, showing that the crucial ability of cholesterol to stabilize ordered domain formation is likely to contribute to ordered domain formation in cell membranes. Additional studies demonstrated that hydrophobic helix orientation is affected by lipid asymmetry with asymmetry favoring formation of the transmembrane configuration. The ability to form asymmetric vesicles represents an important improvement in model membrane studies and should find many applications in the future.
Over the last few decades artificial lipid bilayers of various types have been successfully used as models for biological membranes, yielding many important insights into the architecture of cell membranes. Vesicle dispersions (liposomes) have perhaps been the most useful model membrane system. However, commonly used preparation procedures do not provide control over differences in lipid composition between inner and outer leaflets (lipid asymmetry). This is a troubling limitation because biological membranes are highly asymmetric. In mammalian cells the plasma membrane outer leaflet (exofacial monolayer) is enriched in sphingolipids and phosphatidylcholine (PC),2 whereas the inner leaflet (cytofacial monolayer) is enriched in phosphatidylethanolamine (PE) and phosphatidylserine (PS) (1).
The subject of lipid asymmetry has become all the more important because of its potential role in the structure and function of lipid rafts. Lipid rafts are defined as sphingolipid and sterol-rich lipid domains that exist in the liquid-ordered (Lo) state. Rafts are thought to co-exist in many eukaryotic cell membranes with liquid-disordered (Ld) state domains rich in lipids having unsaturated acyl chains (2, 3) and have been proposed to be important for numerous cellular processes.
The physical properties of Lo domains and the lipid structure dependence of domain formation have been extensively characterized in model membrane bilayers with a symmetric lipid distribution (4–30). The ability to prepare asymmetric vesicles would allow more direct comparison of raft-forming model membranes with cell membranes. Some important progress has been made in preparing asymmetric planar bilayers (31–33). However, asymmetric lipid vesicles would be of wider utility. Ordinary vesicle preparation procedures (e.g. sonication) can yield some degree of asymmetry in some cases (34, 35), but it can be hard to control. Using pH gradients, asymmetry of small amounts of anionic lipids has been achieved (36, 37). The ability to exchange lipids in one leaflet of the bilayer can provide a method to prepare asymmetric vesicles with controlled asymmetry (38, 39). In one study, a phospholipid exchange protein was used to effectively deliver labeled phosphatidylcholines to the outer leaflet of model membranes (39). In addition, a monolayer-by-monolayer assembly method for preparation of asymmetric vesicles has been reported (40). γ-Cyclodextrins have been used to deliver small amounts of labeled phospholipids into the outer leaflet of membranes (41).
Nevertheless a facile and widely applicable method to prepare asymmetric vesicles with a wide variety of lipid compositions, including compositions that mimic cell membranes, has not been described. In this report, we introduce such a method. This procedure is based on the observation that methyl-β-cyclodextrin (MβCD) binds phospholipids at very high MβCD concentrations (42, 43). Using this method asymmetric vesicles were prepared with an external leaflet rich in sphingomyelin (SM) and an internal leaflet rich in PE and PS, similar to eukaryotic plasma membranes. Furthermore cholesterol was introduced into the asymmetric vesicles by exposure of the asymmetric vesicles to cholesterol-loaded MβCD (using lower MβCD concentrations).
The physical properties of these vesicles reveal some important differences and similarities between symmetric and asymmetric bilayers. Overall it appears that the type of asymmetry found in eukaryotic cell membranes is not a barrier to raft formation and, even more importantly, that the stabilizing effects of cholesterol upon raft formation are not restricted to symmetric membranes. Furthermore we found that lipid asymmetry influences hydrophobic helix topography. Asymmetric vesicles prepared by this method should aid many studies of the role of lipid asymmetry in membrane structure and function.
EXPERIMENTAL PROCEDURES
Materials—1,2-Dipalmitoylphosphatidylcholine (DPPC), porcine brain SM, 1,2-dioleoylphosphatidylcholine (DOPC), 1-palmitoyl-2-oleoyl-phosphatidylcholine (POPC), 1-palmitoyl-2-oleoyl-phosphatidylethanolamine (POPE), 1-palmitoyl-2-oleoyl-phosphatidyl-l-serine (POPS), 1,2-[9,10-dibromo] stearoylphosphatidylcholine (BrPC), and cholesterol (CHOL) were purchased from Avanti Polar Lipids (Alabaster, AL). 1,6-Diphenyl-1,3,5-hexatriene (DPH) and MβCD were purchased from Sigma-Aldrich. 1-(4-Trimethylammoniumphenyl)-6-phenyl-1,3,5-hexatriene p-toluenesulfonate (TMADPH), N-(Rhodamine Red-X)-1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine (rhodamine-PE), and N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine (NBD-PE) were purchased from the Molecular Probes division of Invitrogen. [3H]cholesterol was purchased from PerkinElmer Life Sciences. Lipids were dissolved in chloroform and stored at –20 °C. DPH and TMADPH were dissolved in ethanol. Concentrations were determined by dry weight or, in the case of DPH and TMADPH, by absorbance using ε = 84,800 cm–1 m–1 at 353 nm in ethanol (44). LW peptide (acetyl-K2W2L8AL8W2K2-amide) and pL4A18 peptide (acetyl-K2LA9LWLA9LK2-amide) were purchased from Anaspec (San Jose, CA). LW peptide was used without further purification, and pL4A18 was purified via reverse-phase HPLC (see below). Sephacryl S-200 and Sepharose CL-4B were purchased from Amersham Biosciences. High performance thin layer chromatography (HP-TLC) plates (Silica Gel 60) were purchased from VWR International. (Batavia, IL).
Ordinary Vesicle Preparation Procedures—All steps in this and the following procedures were carried out at room temperature except where otherwise noted. Multilamellar vesicles (MLVs) and ethanol dilution small unilamellar vesicles (SUVs) were prepared in glass tubes as described previously (6, 45). For MLVs, lipid mixtures were mixed and dried under nitrogen followed by high vacuum for at least 1 h, dispersed at 70 °C in PBS (1.8 mm KH2PO4, 10 mm Na2HPO4, 137 mm NaCl, and 2.7 mm KCl at pH 7.4), and vortexed in a multitube vortexer (VWR International) at 55 °C for 15 min. In the case of SM MLVs, to remove any small vesicles present prior to lipid exchange, the preparation was centrifuged at 11,000 × g for 5 min at room temperature. The supernatant was discarded, and the pellet obtained was resuspended to the original volume with PBS and used for further experiments. In some cases, MLVs were prepared with 0.01 mol % rhodamine-PE. To prepare sonicated SUVs, MLVs containing 8 or 16 mm unsaturated glycerophospholipids dispersed in PBS (unless otherwise noted) were sonicated in a bath sonicator (Special Ultrasonic Cleaner Model G1112SP1, Laboratory Supplies Co., Hicksville, NY) at room temperature for at least 45 min (until the solution became nearly transparent) and then diluted to the desired concentration with PBS. As judged by their elution position on Sepharose CL-4B chromatography (see below) we found that the small size of the sonicated vesicles was stable for days. For SUVs prepared by ethanol dilution, the desired lipid mixtures were dried, dissolved in 20 μl of ethanol, dispersed in 980 μl of PBS at 70 °C, and then cooled to room temperature. In some cases, the sonicated SUVs contained 0.5 mol % LW peptide and/or 0.01 mol % NBD-PE.
Cholesterol-loaded MβCD (CLC) Preparation—Generally 100 μmol of MβCD were dissolved in 600 μl of methanol and mixed with 30.8 μmol of cholesterol while vortexing at room temperature. The mixture was dried by nitrogen followed by high vacuum for at least 1 h and then dispersed in 2 ml of PBS. The resulting solution (which is turbid due to excess cholesterol) was sonicated in the bath sonicator for 3 min and then incubated in a shaker at 37 °C overnight. The CLC-containing solution was then filtered with a 0.22-μm-pore size syringe filter, and the filtrate was used in subsequent experiments.
Exchange (Asymmetric) Vesicle Preparation—First 500 μl of the resuspended pellet from a 16 mm SM MLV preparation (see above) and 95 μl of 625 mm MβCD dissolved in PBS were vortexed in the multitube vortexer at 55 °C for 2 h. Then 500 μl of 4 mm sonicated SUVs containing unsaturated glycerophospholipid were added to the MLV-MβCD mixture and vortexed at 55 °C for 30 min. After cooling for 5 min, samples were centrifuged at 11,000 × g for 5 min, and the resulting supernatant was centrifuged at 49,000 × g for another 5 min using an air-driven microultracentrifuge (Beckman Airfuge). For asymmetric SUV preparations without cholesterol, the supernatant was chromatographed on a Sepharose CL-4B column (dimensions, 25-cm length and 1-cm diameter). Fractions of 1 ml were collected with asymmetric SUVs mainly eluting in fractions 10–14. Unless otherwise noted, fraction 12 was used for further analysis. Generally the approximate lipid concentration in the peak SUV fractions was about or somewhat greater than 200 μm as estimated by the recovery of peptide using fluorescence (by comparing it with that in the vesicles prior to exchange) or the recovery of lipid using HP-TLC. In cases in which lipid concentration was not explicitly measured, 200 μm was assumed unless otherwise noted.
For asymmetric SUVs containing ∼25 mol % cholesterol, the supernatant from the centrifugation at 49,000 × g was chromatographed on a Sephacryl S-200 column (dimensions, 7-cm length and 1-cm diameter), and 1-ml fractions were collected (although we later realized 0.5 ml fractions would be better to avoid dilution of lipid). To prepare cholesterol-containing SM outside, DOPC inside SUVs (SMo/DOPCi/CHOL SUVs), 950 μl of fraction 4 from this column were transferred to a new glass tube and mixed with 50 μl of a CLC preparation at 55 °C for 30 min. To prepare SMo/2:1 POPE:POPSi/CHOL SUVs, 850 μl of fraction 4 were transferred to a new glass tube and mixed with 150 μl of the CLC preparation at 55 °C for 30 min. The samples were then chromatographed on Sepharose CL-4B as described above. Fractions 12 and 13 from the Sepharose CL-4B column were used for further analysis of cholesterol-containing asymmetric vesicles. Because of losses during this procedure, lipid concentration in the Sepharose CL-4B fractions was lower than that without cholesterol with fractions 12 and 13 each having ∼100 μm lipid as estimated by the recovery of peptide using fluorescence or lipid using HP-TLC. Although lipid recovery was occasionally even lower a 100 μm concentration in these fractions was assumed when the lipid concentration was not explicitly measured.
Fluorescence Measurements—Fluorescence was measured by a SPEX FluoroLog 3 spectrofluorometer (Jobin-Yvon, Edison, NJ) using quartz semimicrocuvettes (excitation pathlength, 10 mm; emission, 4 mm). DPH and TMADPH fluorescence was measured at an excitation wavelength of 364 nm and emission wavelength of 426 nm. Trp fluorescence was measured at an excitation wavelength of 280 nm and emission wavelength of 340 nm. Rhodamine-PE fluorescence was measured at an excitation wavelength of 560 nm and emission wavelength of 580 nm. NBD-PE fluorescence was measured at an excitation wavelength of 465 nm and emission wavelength of 534 nm. The slit bandwidths for fluorescence measurements were generally set to 4.2 nm (2-mm physical size) for excitation and 4.2 nm (2-mm physical size) for emission. Background intensities in samples lacking fluorescent probe were negligible (<1–2%) and were generally not subtracted from the reported values. An exception to this was for measurements of TMADPH fluorescence anisotropy (see below).
Steady-state Fluorescence Anisotropy Measurements—Anisotropy measurements, unless otherwise noted, were made at room temperature using a SPEX automated Glan-Thompson polarizer accessory. DPH and TMADPH anisotropy values were calculated from the fluorescence intensities with polarizing filters set at all combinations of horizontal and vertical orientations. For TMADPH experiments anisotropy was calculated after subtraction of fluorescence intensity in background samples lacking fluorophore. Anisotropy was calculated from the following equation: A = [((Ivv × Ihh)/(Ivh × Ihv)) – 1]/[((Ivv × Ihh)/(Ivh × Ihv)) + 2] where A is anisotropy and Ivv, Ihh, Ivh, and Ihv are the fluorescence intensities with the excitation and emission polarization filters, respectively, set in the vertical (v) and horizontal (h) orientations (46). For these and all the following experiments in which DPH and TMADPH were used, the fluorescence probe was added (from a ∼100 μM stock solution dissolved in ethanol) to preformed ordinary or preformed exchange vesicles to a concentration of ∼0.1 mol % of the total lipid concentration, and the samples were incubated for at least 5 min before fluorescence was measured. This was sufficient time for the fluorescence of the probe, which increases upon binding to lipid vesicles, to reach nearly maximal intensity (Ref. 47 and data not shown).
Measurement of the Temperature Dependence of Fluorescence Anisotropy—To measure the temperature dependence of DPH anisotropy, samples containing (unless otherwise noted) about 50 μm lipid and 0.1 mol % DPH added as described above were cooled to about 16 °C, and anisotropy was measured. Samples were then heated in steps of about 4 °C, measuring anisotropy at each step once temperature stabilized (as measured with a probe thermometer placed in the cuvette (Fisherbrand traceable digital thermometer with a YSI microprobe, Fisher Scientific)). This process was repeated up to 60–70 °C. The ordered domain melting temperature was defined by the midpoint of a sigmoid fit to the anisotropy versus temperature curve using SlideWrite Plus software (Advanced Graphics Software, Inc., Encinitas, CA).
Re-reconstitution Experiments—Fraction 12 of a preparation of SMo/DOPCi SUVs, SMo/2:1 POPE:POPSi SUVs, or DPPCo/DOPCi SUVs was divided into four tubes (250 μl/tube), and then 750 μl of PBS were added to each aliquot. To two aliquots, either DPH (0.5 μl of 100 μm dissolved in ethanol) or TMADPH (0.57 μl of 87.7 μm dissolved in ethanol) was added to give a final membrane composition containing ∼0.1 mol % fluorescent probe. The other two aliquots were subjected to re-reconstitution. They were dried by a nitrogen stream, dissolved in 20 μl of ethanol, and then dispersed at 70 °C in 980 μl of distilled water (which should reconstitute the PBS as well as the lipid vesicles). After cooling to room temperature, DPH or TMADPH was added as described above. Ordinary vesicles were dried and then re-reconstituted by an analogous procedure. For cholesterol-containing asymmetric SUVs fractions 12 and 13 were combined, and then the same procedure was used except that 500 μl of the combined fractions were used per tube so that the lipid concentration would be similar to that in the samples lacking cholesterol.
Extraction of TMADPH from vesicles by MβCD—To measure the MβCD concentration dependence of TMADPH extraction from the outer leaflet of vesicles at room temperature, TMADPH was added to preformed vesicles at a concentration of about 0.1 mol % of the lipid concentration. After a 5-min incubation, the initial TMADPH fluorescence of each sample was measured. Next an aliquot of MβCD from a 625 mm stock solution dissolved in PBS was added, and after incubation for 5 min TMADPH fluorescence intensity was remeasured. This was repeated for a series of aliquots of MβCD. Controls at both 0.5 and 1 mm MβCD showed that extraction by MβCD reached equilibrium within 3 min for vesicles of various lipid compositions.
High Performance Thin Layer Chromatography—HP-TLC plates were preheated at 100 °C for 30 min and cooled to room temperature, and samples were then loaded. For asymmetric SUV samples, 200–500 μl of fraction 12 from the Sepharose CL-4B column were dried by an N2 stream and dissolved in 20 μl of 1:1 chloroform:methanol (v/v) (excess salt was present as a solid). Then 5 μl of the dissolved lipid were loaded onto the plate. For each lipid standard, the desired lipid was first dried by an N2 stream, dissolved in 20 μl of ethanol, and then dispersed in the same volume of PBS as present in the asymmetric SUV sample (so that the stock solution of the standards would have the same concentration of salt as the vesicle samples). The lipid standards were redried in N2 and dissolved in 50–100 μl of 1:1 chloroform:methanol, and then desired amounts of each lipid were spotted onto the plates (generally loading a total of 8–9 μl for each spot).
For samples without cholesterol, 65:25:4 chloroform:methanol:water (v/v) was used to separate each lipid. Samples with cholesterol were generally chromatographed in two solvents. The first solvent system was 50:38:8:4 chloroform:methanol: water:acetic acid (v/v). After the solvent front migrated about halfway up the plate, the plate was air-dried for 5 min. Then the plate was rechromatographed in 1:1 hexane:ethyl acetate (v/v) until the solvent front migrated to near the top of the plate. After air drying for 10 min, the plate was evenly sprayed with a 3% (w/v) cupric acetate, 8% (v/v) phosphoric acid solution, dried for 45 min, and charred at 180 °C for 2–5 min.
Charred HP-TLC plates were scanned using an Epson 1640XL scanner (Epson America Inc., Long Beach, CA), and charred band intensity was measured by Scion Image software (Scion Corp., Frederick, MD). Lipid in samples was quantitated by comparing band intensity with that of the standards fit to an exponential intensity versus concentration curve (SlideWrite Plus software).
Sucrose Density Gradient Centrifugation—Sucrose gradient centrifugation was carried out in a Beckman L8-55M ultracentrifuge using an SW-60 rotor. Gradients for samples lacking cholesterol were prepared by freeze-thawing 3.4 ml of 25% (w/v) sucrose overnight at –20 °C in the (Beckman ultraclear) tubes used for centrifugation. Gradients for samples containing cholesterol were prepared by freeze-thawing 3.4 ml of 20% (w/v) sucrose. Next 400 μl of vesicle samples were loaded on top of the gradients, and the gradients were then centrifuged for 17 h at 38,000 rpm (average g, 148,305). After centrifugation the gradients were fractionated by pipetting into 200-μl aliquots. (The bottom, highest density fraction (fraction 18) contained 200–400 μl.) Lipids were extracted from each fraction with 2.5 ml of 2:2:1 (v/v) chloroform:methanol:water. After 5 min of low speed centrifugation the upper aqueous phase was discarded. Comparison of a control sample before and after extraction indicated that lipid was nearly fully recovered in the lower phase. The extract in the lower phase was then dried with N2 and redissolved in 15 μl of 1:1 (v/v) chloroform:methanol. Five microliters were spotted on an HP-TLC plate and chromatographed in 50:38:8:4 (v/v) chloroform:methanol:water:glacial acetic acid. The amount of SM or glycerophospholipid in the extracts was then quantified by HP-TLC as described above. For cholesterol-containing samples, cholesterol with trace [3H]cholesterol was used, and the amount of cholesterol in one-fifth of each fraction was measured by scintillation counting. The amount of SM or phospholipid in the remainder of the fractions was quantified by analysis of lipid extracts using HP-TLC and charring as described above. (For cholesterol-containing samples the silica gel on the upper portion of the plate, which contained radiolabeled cholesterol, was scraped off and discarded prior to charring!) Sucrose concentrations in the fractions were estimated using a refractometer (Abbe Precision Refractometer, Bausch & Lomb, Rochester, NY).
Peptide Topography Experiments—pL4A18 peptide was purified via reverse-phase HPLC using a C18 column as described previously (48). Purified peptide was dried under a nitrogen stream, redissolved in 1:1 (v/v) water:2-propanol, and stored at 4 °C. For pL4A18-containing samples, the peptide was added to the lipid mixtures (at 1 mol % of total lipid concentration) and dried under nitrogen. The lipid films were re-resuspended in chloroform and dried under nitrogen followed by high vacuum for at least 1 h, dispersed at 70 °C in PBS, and then sonicated to SUVs as described above. In this experiment, exchange vesicles were prepared using mixed MLVs. First 300 μl of 16 mm SM MLVs were mixed with 57 μl of 625 mm MβCD and 200 μl of 16 mm POPC MLVs were mixed with 38 μl of 625 mm MβCD. Each was vortexed using a multitube vortexer at 55 °C for 2 h, and then the two MLV-MβCD mixtures were combined in one glass tube. Next 500 μl of 4 mm 1:1 POPE: POPS SUVs containing 1 mol % pL4A18 peptide were added to the tube with the MLV-MβCD mixtures and vortexed at 55 °C for 30 min. The exchange SUVs were then isolated as described above. Trp fluorescence emission spectra measurements were taken by a SPEX 2 FluoroLog spectrofluorometer (Jobin-Yvon) using quartz semimicrocuvettes (excitation pathlength, 10 mm; emission, 4 mm) at room temperature as described previously (48). The slit bandwidths for this measurement were set to 4.5 nm (2.5-mm physical size) for excitation and 9 nm (5 mm) for emission. Trp fluorescence emission spectra were measured at an excitation wavelength of 280 nm and emission wavelength over the range of 300–400 nm and subjected to 21-point Savitsky-Golay smoothing (49). Fluorescence from background samples (containing lipids but lacking peptide) was subtracted from reported values.
RESULTS
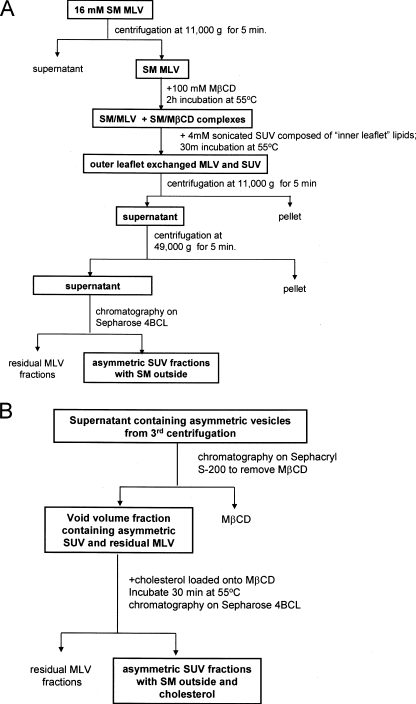
Exchange (Asymmetric) Vesicle Preparation—Our aim was to prepare asymmetric vesicles with SM in the outer leaflet and glycerophospholipids containing at least one unsaturated acyl chain in the inner leaflet. To accomplish this, SM MLVs were incubated with 100 mm MβCD to allow the formation of MβCD-SM complexes (Fig. 1A). This high concentration of MβCD was necessary to load the SM onto the MβCD and dissolved a significant fraction of the SM MLVs (about 25% solubilization as judged by the decrease in optical density at 450 nm; not shown). An elevated temperature was used (55 °C) so that the SM would be in a disordered fluid state, and a high SM concentration was used (16 mm prior to centrifugation) to saturate the MβCD so that it would not dissolve the SUVs added in the following step and so that exchange would result in an SUV population with an outer leaflet predominantly composed of SM. SUVs composed of unsaturated glycerophospholipids (4 mm DOPC, POPC, POPS, or a 1:1 or 2:1 POPS:POPE (mol:mol) mixture) were then added, and the MLVs were removed by centrifugation. To separate the exchanged SUVs from both MβCD and residual MLVs the supernatant was then chromatographed on Sepharose CL-4B.
FIGURE 1.
Flow chart summary of methods for producing exchange (asymmetric) phospholipid vesicles. Shown are procedures for preparing exchange vesicles without cholesterol (A) and with cholesterol (B).
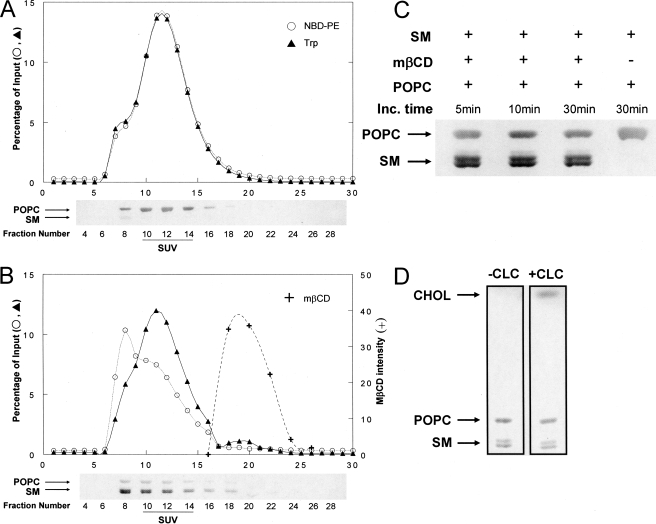
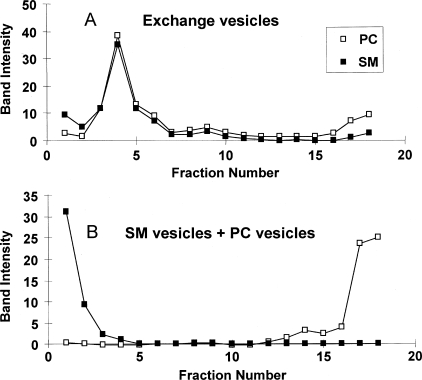
Fig. 2A shows the Sepharose CL-4B chromatographic profile for a control sample in which POPC SUVs (labeled with NBD-PE and a transmembrane peptide, LW peptide) and SM MLVs were mixed in the absence of MβCD. NBD and peptide fluorescence exhibited nearly identical profiles and recoveries (∼85%), mainly eluting in fractions containing SUVs, with a chromatography profile of the SUVs characteristic of that for SUVs having a 250 ± 30-Å diameter (50). As judged by the fluorescence of rhodamine-PE incorporated into the MLVs, a small amount (<1%) of residual MLVs was present and mainly eluted in void volume fractions, which contain vesicles too large to enter the beads (not shown). HP-TLC analysis detected only a trace of SM (SM:POPC ratio, 0.02) in SUV-containing fractions.
FIGURE 2.
Preparation of exchange vesicles. A, Sepharose CL-4B chromatographic profile of control sample without MβCD. B, Sepharose CL-4B chromatographic profile of exchange vesicle preparation. The x axis gives fraction numbers. Percentage of input, percentage of amount in the sample after exchange (i.e. prior to centrifugation and loading on the column). ○, fluorescence of NBD-PE; ▴, Trp fluorescence of LW peptide; +, MβCD (intensity of MβCD bands after charring HP-TLC plates). Charred HP-TLC plates containing selected fractions are shown below the column profiles. Upper band, POPC; lower doublet, SM. (Natural SM migrates as a doublet (90).) C, HP-TLC profile of SUV fraction 12 from Sepharose CL-4B chromatography after (from left to right) incubation (Inc.) of 4 mm POPC SUVs for 5, 10, and 30 min with the resuspended pellet from 16 mm SM MLVs (after preincubation at 55 °C for 2 h with 100 mm MβCD) or for 30 min with the resuspended pellet from 16 mm SM MLVs (preincubated at 55 °C for 2 h with PBS). D, HP-TLC analysis of Sepharose CL-4B SUV fraction from SMo/POPCi exchange vesicles incubated with PBS (left lane) or CLC (5 mm MβCD final concentration) (right lane). (This CLC concentration results in higher cholesterol content (∼45 mol %) than in the samples used for all of the fluorescence studies (∼25 mol %).)
In contrast, when an SM MLV-MβCD mixture was co-incubated with POPC SUVs, HP-TLC analysis showed considerable SM transfer into SUV fractions within minutes (Fig. 2, B and C). The Sepharose CL-4B elution profile for fluorescence markers (Fig. 2B) showed that some of the NBD-PE was transferred to the fractions containing large vesicles, presumably indicative of partial exchange of NBD-PE into (residual) MLVs present, whereas the elution profile of LW peptide, which should not be extracted from membranes by MβCD, was largely unaltered from that in the absence of MβCD, i.e. the peptide remained SUV-associated. Consistent with these observations ∼68% of the NBD-PE was recovered in the eluted fractions (the decrease in recovery was likely due to NBD-PE transfer to MLVs, which were removed by pelleting), whereas ∼80% of the non-exchangeable LW peptide was recovered in the eluted fractions, an amount similar to that recovered in the absence of MβCD. Thus, it appears that the SUVs remained intact during exchange. In the presence of MβCD there was also an increase in rhodamine-PE fluorescence in void volume fractions to almost 5% of the original amount in the MLVs (not shown). This may be due to the presence of some SM vesicles slightly too small to pellet and that form either when MβCD is added to SM MLVs or when the SM MLV-MβCD mixture is diluted with SUVs. Vesicles in these fractions were also observed when the SM MLV-MβCD mixtures by themselves (i.e. without incubation with SUVs) were applied to the Sepharose CL-4B column. Column profiles similar to that in Fig. 2A were observed in preparations in which SM was exchanged into DOPC SUVs or 2:1 (mol:mol) POPE:POPS SUVs.
We refer to the SUVs present after the lipid exchange step as exchange vesicles. Based on HP-TLC the exchange of lipids resulted in SM:POPC ratios of 1–1.2:1 mol:mol. If 66% of the total lipid resides in the outer leaflet of SUVs, this is equivalent to exchange of 75–82% of the outer leaflet.
Preparation of Cholesterol-containing Exchange Vesicles—To more closely imitate eukaryotic membranes, cholesterol was introduced into the exchange vesicles. As illustrated in Fig. 1B, in the first step SM was exchanged into the SUVs. Then samples were subjected to centrifugation to remove MLVs followed by chromatography on a Sephacryl S-200 column to remove MβCD with minimal dilution of the vesicles. Void volume fractions contained the lipid vesicles (SUVs and residual MLVs) (not shown). To incorporate cholesterol the exchange vesicles were then incubated with CLC using a lower MβCD concentration (generally 2.5 mm for PC SUVs and 7.5 mm for POPE:POPS SUVs) so that the MβCD would not bind phospholipid (43). In the final step, the cholesterol-containing asymmetric SUVs were separated from other components on a Sepharose CL-4B column. HP-TLC analysis of the lipid composition of SUV fractions showed incorporation of ∼25% cholesterol (not shown) and that introduction of cholesterol did not appreciably alter vesicle phospholipid composition (Fig. 2D).
Comparison of Ordinary and Exchange SUVs Using Fluorescence Anisotropy—To test for asymmetry, ordered domain formation by ordinary and exchange vesicles was compared by measuring the steady-state fluorescence anisotropy of DPH and TMADPH added to the vesicles. DPH dissolves throughout the lipid bilayer, whereas TMADPH, which anchors to the polar interface by a charged quaternary amino group and does not flip rapidly between inner and outer leaflets, was restricted to the outer leaflet (see below) (51, 52). High anisotropy was observed for these probes in gel (Lβ) and Lo state bilayers (referred to collectively as ordered states), whereas low anisotropy was observed in the Ld (or Lα) state. Thus, at room temperature both DPH and TMADPH exhibited much higher anisotropy in gel phase SM vesicles than in Ld phase vesicles containing unsaturated glycerophospholipids (DOPC, POPC, or POPE:POPS) (Table 1) (53, 54). However, the anchoring of TMADPH restricts its motional range so its anisotropy was higher than that of DPH in Ld state vesicles.
TABLE 1.
Fluorescence anisotropy in ordinary and exchange (asymmetric) vesicles at room temperature
Average anisotropy and S.D. are shown. Sample number is shown in parentheses. Ordinary vesicles with SM and/or CHOL were formed by ethanol dilution; those without SM and/or CHOL were formed by sonication. Ex, exchange vesicles. Ratios are mol:mol. Samples contained ∼50 μm lipid dispersed in PBS, except for POPC-containing samples that had ∼100 μm lipid. Fluorescence probe was 0.1 mol % of total lipid. The percent ordered state bilayer (from DPH A) or outer leaflet (from TMADPH A) was estimated from the following equation: Percent ordered = (A – A100% Ld)/(A100% ordered – A100% Ld). Without CHOL, A is that in an SM/unsaturated lipid mixture, A100% ordered is that in SM, and A100% Ld is that in the appropriate unsaturated lipid. In CHOL-containing samples CHOL was also present. This formula assumes that gel, Lo, and Ld domains have A values similar to that in pure gel, Lo, and Ld bilayers, respectively. Notice that if ordinary POPE:POPS:CHOL vesicles partly form ordered domains at 23 °C (61) this formula underestimates inner leaflet percent ordered in exchange vesicles with these lipids. LW, LW peptide.
|
Sample composition
|
Anisotropy (A)
|
Percent ordered
|
||
|---|---|---|---|---|
| DPH | TMADPH | DPH | TMADPH | |
| SM | 0.309 ± 0.012 (10) | 0.350 ± 0.013 (7) | ≡100 | ≡100 |
| DOPC | 0.115 ± 0.005 (6) | 0.261 ± 0.010 (3) | ≡0 | ≡0 |
| POPC + LW | 0.128 ± 0.005 (4) | 0.274 ± 0.011 (4) | ≡0 | ≡0 |
| POPC | 0.120 ± 0.004 (5) | 0.272 ± 0.012 (5) | ≡0 | ≡0 |
| 2:1 POPE:POPS + LW | 0.152 ± 0.002 (4) | 0.264 ± 0.005 (4) | ≡0 | ≡0 |
| 2:1 POPE:POPS | 0.143 ± 0.003 (7) | 0.259 ± 0.009 (4) | ≡0 | ≡0 |
| 2:1 SM:DOPC | 0.209 ± 0.016 (7) | 0.293 ± 0.013 (4) | 49 | 36 |
| 2:1 SM:POPC + LW | 0.212 ± 0.012 (3) | 0.287 ± 0.005 (3) | 46 | 17 |
| 2:1 SM:POPC | 0.202 ± 0.011 (3) | 0.285 ± 0.010 (3) | 43 | 17 |
| 6:2:1 SM:POPE:POPS + LW | 0.237 ± 0.013 (3) | 0.292 ± 0.008 (3) | 54 | 33 |
| 6:2:1 SM:POPE:POPS | 0.233 ± 0.018 (6) | 0.290 ± 0.015 (6) | 54 | 34 |
| Ex SMo/DOPCi | 0.255 ± 0.011 (6) | 0.350 ± 0.007 (3) | 72 | 100 |
| Ex SMo/POPCi + LW | 0.248 ± 0.014 (3) | 0.337 ± 0.007 (3) | 66 | 83 |
| Ex SMo/POPCi | 0.263 ± 0.003 (3) | 0.349 ± 0.006 (3) | 76 | 99 |
| Ex SMo/POPE:POPSi + LW | 0.270 ± 0.011 (3) | 0.339 ± 0.016 (3) | 75 | 87 |
| Ex SMo/POPE:POPSi | 0.271 ± 0.004 (4) | 0.352 ± 0.013 (3) | 77 | 102 |
| 3:1 SM:CHOL | 0.320 ± 0.011 (10) | 0.345 ± 0.015 (8) | ≡100 | ≡100 |
| 3:1 DOPC:CHOL | 0.140 ± 0.006 (6) | 0.259 ± 0.009 (4) | ≡0 | ≡0 |
| 2:1:1 POPE:POPS:CHOL | 0.200 ± 0.011 (4) | 0.267 ± 0.005 (3) | ≡0 | ≡0 |
| 2:1:1 SM:DOPC:CHOL | 0.267 ± 0.013 (6) | 0.310 ± 0.010 (3) | 71 | 59 |
| 6:2:1:3 SM:POPE:POPS:CHOL | 0.277 ± 0.010 (5) | 0.314 ± 0.005 (3) | 64 | 60 |
| Ex SMo/DOPCi/CHOL | 0.290 ± 0.004 (3) | 0.352 ± 0.003 (3) | 83 | 108 |
| Ex SMo/POPE:POPSi/CHOL | 0.296 ± 0.004 (4) | 0.346 ± 0.004 (5) | 80 | 101 |
Table 1 also shows that intermediate anisotropy values were observed in ordinary SUVs containing a 2:1 mol:mol ratio of SM to total unsaturated glycerophospholipids. These values reflect the presence of co-existing SM-rich gel domains and glycerophospholipid-rich Ld domains in such vesicles at room temperature (55, 56). From DPH anisotropy, which should report the average of inner and outer leaflet fluidity, 43–54% of the DPH fluorescence originated from the (SM-rich) ordered state domains for SM mixtures with DOPC, POPC, or 2:1 mol: mol POPE:POPS. Because DPH usually partitions equally between ordered and disordered domains (57, 58), this should be close to the percentage of the bilayer that is in the ordered (here gel) state. (Differences in DPH intensity when it is located within an ordered and disordered state environment have little influence upon this conclusion. For the lipids used in this study, both in the absence and presence of cholesterol, we found that the intensity of DPH in ordered state vesicles was only 5–20% larger than in vesicles in the Ld state.) TMADPH anisotropy indicated a smaller fraction of TMADPH fluorescence originating from the ordered domains (17–36% depending on the identity of the unsaturated glycerophospholipid) consistent with the tendency of TMADPH to partition somewhat more favorably into disordered domains (59).
The anisotropy of exchange SUVs containing SM and either DOPC, POPC, or 2:1 POPE:POPS exhibited a striking contrast with that of ordinary vesicles of similar overall lipid composition. The anisotropy and calculated percentage of DPH fluorescence originating from ordered domains (66–77% depending on the identity of the unsaturated glycerophospholipid) was higher than for ordinary vesicles. Furthermore no matter what unsaturated glycerophospholipid was used, high anisotropy values were observed for TMADPH, indicating that the fluorescence of TMADPH incorporated into the outer leaflet arose almost totally from ordered domains. This means that the outer leaflet of these vesicles was more ordered than the bilayer as a whole and thus much more ordered than the inner leaflet. This is the expectation if there is an asymmetric distribution of lipids such that the outer leaflet is predominantly composed of SM in the ordered gel state.
The difference between the percent ordered domains sensed in the entire vesicle by DPH and in the outer leaflet in TMADPH can be used to estimate the percent ordered domains in the inner leaflet. Assuming that the DPH anisotropy reflects a combination of that in the inner and outer leaflet; ⅔ of the total lipid (and DPH) in the exchange SUVs is in the outer leaflet (60); ∼17–31% of the inner leaflet lipid in exchange vesicles would be in an ordered state (in samples lacking peptide). This is consistent with an inner leaflet composed predominantly of the unsaturated lipids, which tend to form disordered domains. In other words, the fluorescence data indicated that the exchange vesicles had a highly asymmetric lipid distribution with an SM-rich outer leaflet and unsaturated glycerophospholipid-rich inner leaflet. It also indicated that ordered domain formation in the outer leaflet did not require ordered domain formation in the inner leaflet.
Anisotropy in exchange vesicles containing LW peptide was similar to that in its absence, suggesting that asymmetry of the exchange vesicles was maintained. However, there was a small decrease in TMADPH anisotropy hinting that peptide may alter domain organization (see “Discussion”).
The results above imply that the vesicles made by the exchange procedure have an asymmetric lipid composition, as confirmed by the experiments described in the following sections. To describe the lipid composition and asymmetry of such vesicles we propose the designations “o” = outer leaflet lipid and “i” = inner leaflet lipid. We use “/” to separate the names of lipids in different leaflets, “:” to separate lipid names when the lipids are in the same leaflet or in ordinary vesicles, and “-” to separate lipid names when referring to both asymmetric and ordinary vesicles. Thus, a vesicle composed of SM, PE, and PS would be designated SMo/PE:PSi when the SM is in the outer leaflet and PE and PS are in the inner leaflet, designated SM:PE:PS in ordinary symmetric vesicles, and designated SM-PE-PS when asymmetry is undefined when talking about symmetric and asymmetric vesicles at the same time.
Cholesterol-containing vesicles were also studied. Ordinary vesicles containing 2:1 SM:unsaturated glycerophospholipids plus 25 mol % cholesterol gave DPH and TMADPH anisotropy values consistent with a mixture of ordered (Lo) and disordered fluid (Ld) domains (Table 1). Indeed ordered/disordered domain co-existence in these mixtures has been found previously using fluorescence quenching (61). The apparent percent ordered domains forming in the presence of cholesterol was higher than in its absence as noted previously (4, 6, 13).
Exchange vesicles containing cholesterol showed a significant degree of ordered domain formation as judged by DPH anisotropy and fully ordered outer leaflets as judged by TMADPH anisotropy. Again this indicates an asymmetric lipid distribution with an SM-rich outer leaflet and unsaturated glycerophospholipid-rich inner leaflet. As in the case of ordinary vesicles, exchange vesicles containing cholesterol showed a higher overall level of ordered domain formation than exchange vesicles lacking cholesterol (80–83%). Based on comparison of DPH and TMADPH anisotropy, we estimate that ∼40–50% of the inner leaflet was in an ordered state in cholesterol-containing exchange vesicles. (In SMo/2:1 POPE:POPSi/CHOL vesicle inner leaflet order may be higher if ordinary POPE:POPS:CHOL vesicles are already partly in an ordered state (Table 1 and Ref. 61).) This suggests that the SM-rich outer leaflet induces a greater level of ordering within the inner leaflet in the presence of cholesterol than in its absence.
Asymmetry was relatively stable. Anisotropy of both DPH and TMADPH (added to exchange vesicles right before measuring anisotropy) did not change significantly for at least 1–2 days after sample preparation both for SMo/POPCi or for SMo/2:1 POPE: POPS/CHOL vesicles (in each case the vesicles also contained LW peptide) (data not shown). Additional controls confirmed that TMADPH flips slowly from the outer to the inner leaflet. In exchange vesicles movement of TMADPH to the inner leaflet should have been accompanied by a decrease in anisotropy, but we found only a very small drop in anisotropy over a period of a few hours. In addition, accessibility of TMADPH to extraction by externally added MβCD (see below) only decreased slightly over several hours (not shown).
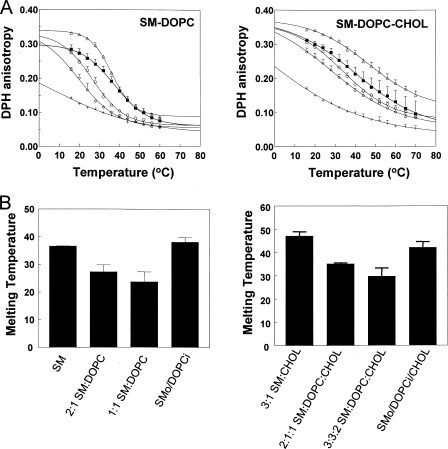
Comparison of the Thermal Stability of Ordered Domains in Ordinary and Exchange SUVs—To further characterize differences between ordinary and exchange vesicles the thermal stability of ordered domains was determined via the temperature dependence of DPH fluorescence anisotropy. As temperature is increased ordered state lipids melt and anisotropy shows a distinct transition from high to low values. The melting temperature (Tm) can be defined as the midpoint of this transition, the temperature at which the decrease in anisotropy per °C is a maximum (57, 62).
Fig. 3 shows the temperature dependence of DPH anisotropy and Tm values derived from these curves. SUVs composed of SM showed high DPH anisotropy at low temperature (∼16 °C) (Fig. 3A) and a Tm slightly above 35 °C (Fig. 3B) as expected (63, 64). DOPC vesicles exhibited a low anisotropy that decreased slowly as temperature increased, characteristic of the Ld state. Ordinary vesicles composed of SM:DOPC mixtures exhibited intermediate anisotropy at low temperature due to the co-existence of Ld and gel domains (Fig. 3A, left panel) as noted above. As expected, they exhibited a lower Tm than that of pure SM vesicles, 27 °C for 2:1 SM:DOPC and 24 °C for 1:1 SM:DOPC (Fig. 3B, left panel). This behavior contrasts with that of SMo/DOPCi exchange vesicles with an overall lipid composition similar to that of the ordinary vesicles. As in the case of ordinary vesicles, these vesicles had an intermediate anisotropy at low temperature (Fig. 3A), but their melting transition occurred at about as high a temperature as that of pure SM vesicles (Fig. 3B), indicating that the unsaturated glycerophospholipid-rich, largely Ld state inner leaflet did not have a deleterious effect on the stability of ordered domain formation by the SM-rich outer leaflet.
FIGURE 3.
Measurement of ordered domain thermal stability. A, temperature dependence of DPH anisotropy in ordinary and exchange vesicles. Left panel, SM vesicles (▵), ordinary 2:1 mol:mol SM:DOPC vesicles (○), ordinary 1:1 SM:DOPC vesicles (⋄), SMo/DOPCi vesicles (▪), and DOPC vesicles (+). Right panel, 3:1 SM:CHOL vesicles (▵), ordinary 2:1:1 SM:DOPC:CHOL vesicles (○), ordinary 3:3:2 SM:DOPC:CHOL vesicles (⋄), SMo/DOPCi/CHOL vesicles with about 25 mol % CHOL (▪), and 3:1 DOPC:CHOL vesicles (+). PBS-dispersed samples contained ∼50 μm total lipid with 0.1% DPH. Average anisotropy values from triplicate experiments and S.D. are shown. Error bars are defined as standard deviation (S.D.). B, Tm values for curves shown in A. The average derived from averaging Tm values from different samples and S.D. in Tm values is shown. The x axis labels give vesicle lipid compositions.
Ordered domain thermal stability was also measured for vesicles containing ∼25 mol % cholesterol (Fig. 3, A and B, right panels). The pattern of Tm values versus vesicle composition mirrored that in vesicles without cholesterol with higher and nearly equivalent Tm observed for SM:CHOL and exchange vesicles and lower Tm observed for ordinary vesicles with an overall lipid composition similar to that of the exchange vesicles.
A very similar anisotropy and Tm pattern was observed for SMo/POPE:POPSi, SMo/POPE:POPSi/CHOL exchange vesicles, and the corresponding ordinary vesicles (see supplemental Fig. S1). The Tm of DPPCo/DOPCi exchange vesicles was also higher (by ∼5 °C) than that of ordinary 2:1 DPPC:DOPC vesicles but not as high as in pure DPPC vesicles (supplemental Fig. S1). This may reflect a lower level of DPPC exchange into SUVs (relative to that of SM). Nevertheless the observation that ordered domains in DPPC-containing exchange vesicles had a higher Tm than in ordinary vesicles suggests that the higher stability of ordered domains in asymmetric vesicles is not restricted to ordered domains composed of SM.
It should be noted that both ordinary and exchange vesicles exhibited significantly higher Tm values (by 5–10 °C) in the presence of cholesterol. This thermal stabilization of ordered domains by cholesterol has been observed previously in the case of ordinary vesicles and is one of the key in vitro observations suggesting that Lo-like ordered domains might form in cell membranes under physiological temperatures (4, 9, 14). The fact that stabilization of ordered domains by cholesterol was observed in exchange vesicles indicates that this crucial property of cholesterol can be retained in asymmetric bilayers (see “Discussion”).
Controls confirmed that the difference between the Tm values in ordinary and exchange vesicles was not due to residual ethanol in the ordinary vesicles. Addition of ethanol to exchange vesicles (or any vesicles containing ordered domains) only decreased Tm by 1–2 °C (not shown). Also the asymmetric arrangement of lipids in the exchange vesicles was largely maintained after heating to above Tm. Tm values remeasured after heating exchange vesicles to 60–70 °C and cooling to 16 °C were only generally 1–2 °C lower than those of initial samples (not shown).
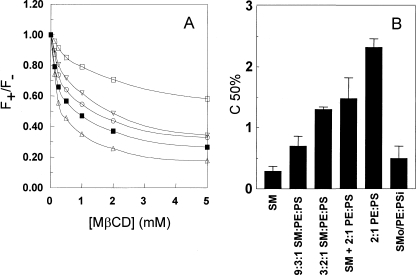
Confirmation of Asymmetry Using TMADPH Binding to Vesicles—An alternate explanation of the high Tm values in exchange vesicles is that they contain separate populations of SM vesicles and unexchanged unsaturated glycerophospholipid vesicles. To rule this out and further confirm asymmetry, several methods were used. The first asymmetry test was based on the observation that POPS (an anionic lipid) renders MβCD-induced extraction of outer leaflet TMADPH (a cationic molecule) from vesicles more difficult. Fig. 4 shows that extraction of TMADPH from bilayers by MβCD could be detected by a decrease in TMADPH fluorescence upon addition of MβCD (Fig. 4 A). That this fluorescence decrease was due to extraction was confirmed by centrifugation experiments measuring the amount of TMADPH bound to the outermost leaflet of SM MLVs in the absence and presence of MβCD. Most of the TMADPH appeared in the MLV-containing pellet without MβCD but in the supernatant after addition of MβCD (supplemental Fig. S2).
FIGURE 4.
MβCD-induced extraction of TMADPH from ordinary and exchange vesicles. A, dependence of TMADPH fluorescence upon MβCD concentration for various vesicle compositions. Vesicles were composed of 200 μm 2:1 mol:mol POPE:POPS (□), a 1:1 mixture of 100 μm SM vesicles and 100 μm 2:1 POPE:POPS vesicles (▿), 200 μm 9:2:1 SM:POPE:POPS (○), ∼200 μm SMo/2:1 POPE:POPSi (▪), and 200 μm SM (▵). F+ is TMADPH fluorescence intensity in the presence of MβCD, and F– is TMADPH fluorescence intensity in the absence of MβCD. Representative curves for single samples are shown. B, comparison of C50% values for various vesicle compositions shown in A plus for ordinary vesicles with a composition of 3:2:1 SM:PE:PS and total lipid concentration of 200 μm. C50% is the concentration of MβCD at which the decrease of F+/F– is half as large as the decrease in F+/F– in the presence of 20 mm MβCD. Lipid concentrations and composition are as in A. The x axis labels give lipid compositions. The average of triplicate experiments and S.D. are shown. Error bars are defined as standard deviation (S.D.).
Fig. 4B quantifies the lipid composition dependence of the ability of MβCD to extract TMADPH from the outer leaflet of vesicles via the parameter C50% defined as the MβCD concentration resulting in half as much extraction as at 20 mm MβCD. Extraction of the cationic TMADPH molecules from 2:1 mol: mol POPS:POPE vesicles, which are anionic, was much more difficult (C50% ∼ 1.9 mm) than extraction from zwitterionic SM vesicles (C50% ∼ 0.29 mm). Consistent with an asymmetric structure with little POPS in the outer leaflet, SMo/POPE: POPSi exchange vesicles exhibited a low C50% value (∼0.5 mm) close to that of pure SM vesicles and less than that of ordinary vesicles containing 75% SM (C50%∼ 0.7 mm) or of TMADPH bound to a 1:1 mixture of SM vesicles and 2:1 POPE:POPS vesicles (C50% ∼ 1.3 mm). It should be noted that the C50% values were affected by lipid concentration such that the lower the concentration of lipid, the lower the C50% value (not shown). We confirmed that for the exchange vesicle preparations the low C50% value observed was not due to a lower lipid concentration than in the ordinary vesicle preparations (data not shown).
C50% values also showed that it was more difficult to extract TMADPH from DOPC vesicles (C50% ∼ 0.64 mm) than from SM vesicles as predicted by the slightly higher affinity of TMADPH for disordered domains than ordered domains (59). However, this difference was too small to use in evaluating lipid asymmetry in SMo/DOPCi vesicles. Complications arising from extraction of cholesterol by MβCD precluded meaningful TMADPH extraction experiments on cholesterol-containing preparations. We also confirmed asymmetry based on the dependence of Ca2+-induced vesicle aggregation upon the amount of anionic lipid exposed on the outer surface of vesicles (see supplemental Fig. S3).
Distinguishing Asymmetric Vesicles from Mixtures of Two Types of Ordinary Vesicles Using Sucrose Gradient Centrifugation—The methods above were suitable for assaying asymmetry for vesicles containing anionic lipid. To confirm that separate SM and unsaturated lipid vesicles were not being formed by the exchange procedure in vesicles lacking anionic lipid, sucrose density gradient centrifugation was used. SMo/PCi exchange vesicles were prepared from SUVs containing a 2:3 mol:mol mixture of DOPC with a tetrabrominated derivative of DOPC (BrPC). The latter lipid has a high density, allowing BrPC-containing SUVs to be distinguished from vesicles formed from unlabeled lipids on density gradients (65). Fig. 5 shows the density profile for SMo/2:3DOPC:BrPCi exchange vesicles. As assayed by HP-TLC analysis SM and PC primarily both located in fraction 4. (In a separate experiment both SM and BrPC in ordinary SUVs composed of 5:2:3 SM:DOPC:BrPC were found to predominantly locate in fraction 5 (not shown).) In contrast, in the case of a mixture of SM vesicles and 3:2 DOPC:BrPC vesicles, the SM vesicles mainly located in the lowest density fraction, whereas the PC vesicles were mainly in the highest density fractions. These results confirm that the exchange procedure does not result in the formation of separate SM and PC vesicles.
FIGURE 5.
Sucrose density gradient centrifugation of SMo/PCi vesicles. A, sucrose gradient profile of exchange SUVs containing ∼200 μm lipid composed of SMo/6:4 (mol:mol) BrPC:DOPCi. B, sucrose gradient profile of a mixture of 100 μm SUVs composed of SM and 100 μm SUVs composed of 6:4 BrPC:DOPC. TLC band intensity is shown for SM (▪) and total PC (□). Fractions from left to right are from low to high density. Band intensities for SM and PC should not be directly compared because the intensity of charred bands is dependent on lipid type. Sucrose concentrations, estimated from the index of refraction, in fractions with peak lipid concentrations were: fraction 1 (SM), 1%, fraction 4 (exchange SUVs), 16%, and fraction 17–18 (PC), 26–28%.
When centrifuged under conditions with a shallower sucrose gradient, SMo/∼2:3 DOPC:BrPCi exchange vesicles containing ∼25 mol % cholesterol located at a similar average sucrose density as vesicles without cholesterol but with the lipids spread into a wider number of fractions (data not shown). There was a constant phospholipid:cholesterol ratio over these fractions but some heterogeneity in the SM:PC ratio such that the estimated SM content varied over the range 50 ± 15% (data not shown).
Re-reconstitution Confirms That Exchange Induces the Formation of Asymmetric Vesicles—The exact lipid composition of asymmetric vesicles was difficult to measure with very high accuracy. To compare the physical properties of ordinary and exchanged vesicles with identical lipid compositions, a re-reconstitution procedure was developed. Ordinary and exchange vesicle samples were dried, destroyed by solubilization in ethanol, and then re-reconstituted in vesicles by dispersion into aqueous buffer.
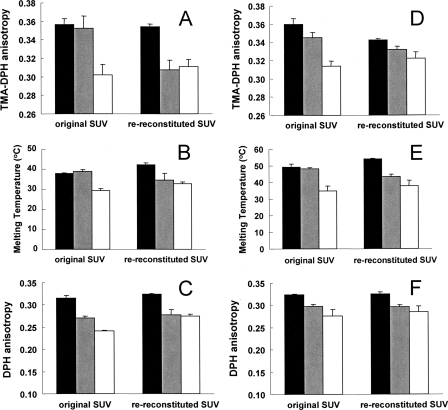
Ideally ordinary and exchange vesicles with identical lipid compositions should have different properties before re-reconstitution but identical properties after re-reconstitution. Fig. 6 shows that this was to a large degree true. Before re-reconstitution, SMo/POPE-POPSi vesicles (gray bars) showed an outer leaflet TMADPH anisotropy (A) and thermal melting temperature (B) similar to that of SM vesicles (black bars) and significantly higher than that of ordinary 6:2:1 SM:POPE:POPS vesicles (open bars). Overall ordered domain formation in the SMo/POPE-POPSi vesicles, as judged by DPH anisotropy (C), was intermediate between that of SM vesicles and 6:2:1 SM:POPE: POPS vesicles. After re-reconstitution the anisotropy and ordered domain melting temperatures of the exchange vesicles decreased and reached levels almost identical to those of ordinary mixed lipid vesicles. (Notice that there is a much smaller change in these properties for the ordinary vesicles and that it is generally in the opposite direction of that in the exchange vesicles, which is inconsistent with the hypothesis that some property other than asymmetry explains the decrease in anisotropy and melting temperatures in the exchange vesicles.) Clearly very little, if any, of the physical differences between the ordinary and exchange vesicles was due to a difference in lipid composition. Similar behavior upon re-reconstitution was observed for analogous vesicles containing cholesterol (Fig. 6, D–F). However, there was a small residual difference between ordinary and exchange vesicles after re-reconstitution, perhaps reflecting a small difference between their lipid composition.
FIGURE 6.
Effect of re-reconstitution upon the level and thermal stability of ordered domains. A–C, SM-POPE-POPS vesicles. D–F, SM-POPE-POPS-CHOL vesicles. A and D, TMADPH anisotropy at room temperature. B and E, ordered domain Tm. C and F, DPH anisotropy at room temperature. Black bars, SM vesicles (A–C) or 3:1 SM:CHOL vesicles (D–F). Gray bars, exchange SMo/2:1 POPE:POPSi vesicles (A–C) or SMo/2:1 POPE:POPSi vesicles with ∼25 mol % cholesterol (D–F). Unfilled bars, ordinary 6:2:1 SM:POPE:POPS vesicles (A–C) or ordinary 6:2:1:3 SM:POPE:POPS:CHOL vesicles (D–F). Original SUV, SUVs before re-reconstitution. Re-reconstituted SUV, SUVs after re-reconstitution. Average values from triplicate experiments and S.D. are shown. Error bars are defined as standard deviation (S.D.).
Analogous decreases in the level of outer leaflet ordered domains and ordered domain thermal stability upon re-reconstitution were also observed for SMo/DOPCi, SMo/DOPCi/CHOL, and DPPCo/DOPCi exchange vesicles and the corresponding ordinary vesicles (supplemental Fig. S4). (Notice that for SMo/DOPCi and SMo/DOPCi/CHOL SUVs the ordinary vesicles were 1:1 SM:DOPC rather than 2:1 and thus still have lower anisotropy and Tm values than the exchange vesicles after re-reconstitution.)
To summarize, all of the methods described above show that the behavior and properties of the exchange vesicles are inconsistent with the hypothesis that they have a symmetric lipid distribution or the hypothesis that they are composed of separate SM-containing and unsaturated glycerophospholipid-containing populations. Instead the data all point to their having an asymmetric SMoutside/unsaturated glycerophospholipidinside lipid distribution.
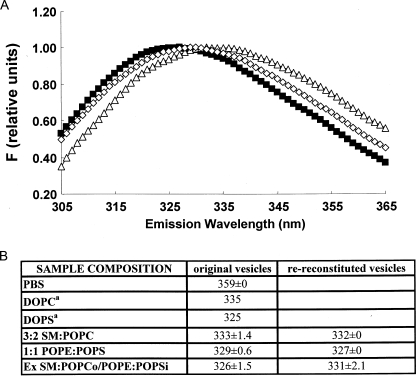
Lipid Asymmetry Affects the Extent of Transmembrane Insertion by Hydrophobic Helices—A long standing question in membrane structure is whether lipid asymmetry affects the topography of the hydrophobic helices found in membrane proteins. To test the hypothesis that asymmetry can affect topography, a hydrophobic helix (pL4A18) with a topography that is sensitive to lipid composition was used (48). Previous studies have shown that pL4A18 forms a transmembrane (TM) helix in PS vesicles but can only form a non-TM helix associated with the surface of the bilayer in zwitterionic vesicles composed of PC (48). pL4A18 has a Trp residue in the center of its hydrophobic sequence, and these helix configurations can be distinguished by the wavelength of maximum Trp fluorescence emission (λmax) (Fig. 7A). In the TM state, our previous studies show that pL4A18 Trp emission is significantly more blue shifted (λmax 325 nm) in PS vesicles than in the non-TM, surface-bound state formed in PC vesicles (λmax 335 nm) (Fig. 7). (We have demonstrated a similar difference in Trp emission in TM and non-TM configurations for hydrophobic helices with many other sequences (48, 66–68).) Therefore, fluorescence was used to define pL4A18 topography in symmetric and asymmetric vesicles composed of anionic and zwitterionic lipids. We found peptide behavior consistent with these results in ordinary vesicles (Fig. 7B). In ordinary zwitterionic SM:POPC vesicles, λmax was 333 nm, close to that in DOPC, whereas in ordinary 1:1 POPE: POPS vesicles (which have 50% anionic lipid), λmax was 329 nm, intermediate between that in fully zwitterionic (PC or SM:PC) and fully anionic (PS) vesicles. This indicates that in SM:POPC pL4A18 is mainly non-TM, whereas it is in a mixture of TM and non-TM configurations in 1:1 POPE:POPS. It should be noted that pL4A18 is membrane-bound in both of these cases. When dissolved in aqueous solution pL4A18 had a much more red shifted fluorescence (λmax 359 nm) (Fig. 7B).
FIGURE 7.
Effect of lipid asymmetry on the topography of membrane-associating hydrophobic helix pL4A18. A, Trp fluorescence emission spectra of pL4A18 in ordinary 3:2 SM:POPC vesicles (▵), ordinary 1:1 POPE:POPS vesicles (⋄), and SM:POPCo/POPE:POPSi vesicles (▪). Fluorescence intensities have been normalized to 1 at λmax. B, λmax values for pL4A18 peptide in ordinary and exchange (Ex) vesicles. Original vesicles, before re-reconstitution. Average λmax and S.D. is shown for duplicate or, in the case of exchange vesicles and the original 1:1 POPE:POPS vesicles prior to exchange, triplicate preparations. DOPS, dioleoylphosphatidylserine. a, data from Ref. 48.
The behavior of pL4A18 inserted into asymmetric ∼3:2 SM:POPCo/1:1POPE:POPSi vesicles was distinctly different from that in ordinary vesicles. As shown in Fig. 7, the Trp fluorescence of pL4A18 was about as highly blue shifted (λmax 326 nm) in asymmetric vesicles as in fully anionic PS vesicles. This blue shifted fluorescence indicates the formation of a fully TM topography in the asymmetric vesicles even though they contain only a low percentage of PS (16 mol % based on HP-TLC). It is especially noteworthy that this λmax is more blue shifted than in vesicles composed entirely of either the outer leaflet (3:2 SM:POPC) or inner leaflet (1:1 POPE:POPS) lipids rather than an average of these values. That this blue shift is due to lipid asymmetry was confirmed by remeasuring λmax after destroying asymmetry by reconstitution. After re-reconstitution of the vesicles, pL4A18 fluorescence red shifted significantly (λmax 331 nm). This value is between that of the 3:2 SM:POPC and 1:1 POPE:POPS vesicles as predicted if the vesicles become symmetric after re-reconstitution. (It should be noted that in control TLC experiments we confirmed that there had been about the expected total amount of lipid exchanged into the asymmetric vesicles, although the amount of SM was slightly lower than expected, and that, as in the case of the other asymmetric vesicles studied, after exchange the ordered domains had a thermal stability higher than that measured after re-reconstitution, confirming lipid asymmetry. Furthermore sucrose gradient experiments confirmed that the PC and SM exchanged into the same set of vesicles (data not shown).)
DISCUSSION
Preparation of Asymmetric Vesicles—This report introduces an MβCD-induced exchange method to prepare small unilamellar vesicles with a stable asymmetry in which the outer leaflets are composed largely of sphingomyelin or other lipids containing saturated acyl chains and the inner leaflets contain various glycerophospholipids with unsaturated acyl chains. Cholesterol can be incorporated into these vesicles with maintenance of phospholipid asymmetry. In addition, mixtures of lipids can be introduced by exchange, which results in great flexibility in the lipid compositions that can be prepared. The procedure is relatively rapid, requires little special equipment or materials, and produces an ample amount of exchange vesicles. It works with many different lipids and can be used at elevated temperatures that might aid exchange for lipids with high Tm values. Introduction of cholesterol without disruption of the vesicles is possible because cholesterol-MβCD complexes can be prepared at MβCD concentrations too low to bind phospholipids (43) and is no doubt aided by rapid cholesterol equilibration between the inner and outer leaflets (69). Although the final cholesterol concentration in the inner and outer leaflets is unknown, the need to maintain mass balance between the leaflets requires a similar final mole fraction of cholesterol in the two leaflets.
The experimental conditions that give maximal lipid change are empirical and are likely affected by the concentration of MβCD, MLVs, and SUVs and the identity of the lipids used. As noted under “Results,” the first step in the process involves solubilization of some of the MLVs by the MβCD. We chose an excess of MLVs, i.e. an amount that is not totally dissolved by MβCD, so that the MβCD will be saturated with lipid and not dissolve the SUVs. Because the amount of MLVs that dissolves is not well defined, it is hard to say what exact excess is present, but even if that were known it might not help define the best exchange conditions because the difference in the relative affinity of MβCD for different types of lipids in MLVs and SUVs could also affect exchange. MβCD affinity for lipids is complex to determine because of the cooperative nature of the MβCD-lipid interaction, which only becomes strong enough to allow lipid binding at high MβCD concentration (43). Both the affinity of MβCD for lipid and its binding stoichiometry may be dependent upon its concentration. However, it is important to note that the exchange method is practical without a full study of all of these variables. We found that we can define optimal conditions for exchange by varying these parameters slightly when different lipid compositions are used.
The lipid concentration of asymmetric vesicles obtained after chromatography can be variable. The final lipid concentration was determined explicitly when desired, but it is important to note that in many cases the exact vesicle concentration is not important. It is the composition of the membrane that controls vesicle properties, not the number of vesicles in solution.
It should be noted that the small decrease in anisotropy that was detected in exchange vesicles in the presence of the LW peptide might be significant. The LW peptide used has a structure that strongly disfavors its insertion into ordered domains in ordinary vesicles, and it appears to nucleate disordered domains in ordinary vesicles (70). Thus, the decrease in TMADPH anisotropy in the presence of LW peptide may suggest that it induces formation of a small amount of disordered domains in the outer leaflet of the exchange vesicles, and this property might be shared by transmembrane proteins in cell membranes.
Further studies will be necessary to define conditions suitable to prepare additional types of exchange vesicles. Preliminary studies indicate that by changing experimental conditions unsaturated lipids can be exchanged into small unilamellar vesicles composed largely of SM.3 Other preliminary studies indicate that the exchange procedure can be used to prepare asymmetric large unilamellar vesicles. Such vesicles would lack the curvature of SUVs and are more appropriate model membranes for many types of experiments. However, SUVs themselves are likely to be very useful. It should be noted in this regard that studies of membrane-inserted hydrophobic helices have found little difference between SUVs and large unilamellar vesicles (71).
Insights into the Lipid Behavior in Eukaryotic Plasma Membranes from the Physical Properties of Plasma Membrane-mimicking Asymmetric Vesicles—The asymmetric vesicles we prepared mimic plasma membranes in having a sphingolipid-rich outer leaflet and unsaturated glycerophospholipid-rich inner leaflet and thus provide important insights into the relationship between lipid structure and biological membrane organization. Previous studies in supported planar bilayers showing that ordered domains inner and outer leaflets are not always in close register suggested that the formation of ordered domains in both leaflets is not an absolute requirement for ordered domain formation (72, 73). The experiments in this report show that the thermal stability of SM-rich ordered state domains in the outer leaflet is not adversely affected by interactions with an inner leaflet composed of unsaturated lipids that (by themselves) should tend to form disordered domains at least in small vesicles. It was also found that the significant increase in the stability of ordered domains in the presence of cholesterol, a crucial cholesterol property observed in symmetric model membrane vesicles, is maintained in asymmetric membranes and thus should occur in cell membranes. It has been assumed that cholesterol stabilizes ordered domains in cells based on detergent insolubility data, but complications can make it difficult to properly interpret detergent insolubility (74). Combined these biophysical properties indicate that in the sphingolipid- and cholesterol-rich plasma membrane the sphingolipid-rich outer leaflet is very likely to exist in the Lo state.
Furthermore there seems to be some degree of ordering of the inner leaflet by an ordered outer leaflet. The effect of the outer leaflet upon the inner leaflet may partly explain why the overall level of membrane order is higher when the ordered state-favoring and disordered state-favoring lipids are asymmetrically disposed than when they are symmetrically disposed. The induction of an ordered state in the inner leaflet by ordered domains in the outer leaflet is consistent with the coupling between ordered formation in inner and outer leaflets in SM vesicles that was detected in an early study (75) and with the very recent observations of Kiessling et al. (32) that were made in planar bilayers insulated from the substrate and in which it was found that when cholesterol was present an SM-rich outer leaflet could induce order in an inner leaflet composed of a physiological mixture of mammalian PC, PE, and PS. It is also consistent with observations in unsupported planar bilayers and giant unilamellar vesicles indicating that ordered domains in opposite leaflets tend to exist in register with one another, indicating that there is some degree of coupling between ordered domains in opposite leaflets (15, 17). However, it is difficult to evaluate the behavior of the inner leaflet with the assays available at present. For example, some of the order we estimate to exist in the inner leaflet of asymmetric vesicles may reflect a change in the dynamics of inner leaflet DPH molecules when they are trapped in a disordered environment that is only one leaflet wide. It should also be noted that we have not yet determined whether the inner leaflet undergoes segregation into separate ordered and disordered domains under the influence of the ordered domains in the outer leaflet. This will require additional methods for specifically evaluating the domain structure in the inner leaflet at the nanodomain level. In any case, further studies of interleaflet coupling will clearly be an important goal.
The Effect of Lipid Asymmetry upon the Orientation of Membrane-inserted Hydrophobic Helices—The observation that transmembrane polypeptides can be incorporated in exchange vesicles without disruption of asymmetry should allow the use of asymmetric vesicles to carry out more detailed studies of protein-lipid interaction than in symmetric vesicles. An important finding of this report in this regard is that lipid asymmetry, in which one membrane leaflet has more anionic lipids than the other, can stabilize the formation of transmembrane topography by hydrophobic helices. This could have important implications for membrane protein topography in vivo. A very recent investigation of the behavior of hydrophobic helices during co-translational membrane insertion in natural membranes by Lerch-Bader et al. (76) has shown that the extent of translocon-dependent formation of a transmembrane state (relative to formation of a non-transmembrane state) can be enhanced by cationic residues flanking the hydrophobic sequence. Our results show that this might be explained by an asymmetric distribution of anionic lipid as is believed to occur in natural membranes. Lipid asymmetry may also be important in controlling the extent of transmembrane insertion in cases of post-translational membrane insertion (77–79). Whether this stabilization of transmembrane topography is exclusively electrostatic in origin or involves other specific lipid-peptide interactions will be investigated in future studies.
Other Applications of Asymmetric Vesicles—Asymmetric vesicles should also be crucial for other applications. One is refining the interpretation and applications of detergent insolubility in cell membranes. Detergent insolubility (especially using Triton X-100) has been widely used to identify the presence of sphingolipid- and cholesterol-rich domains in cells and to isolate detergent-insoluble sphingolipid- and cholesterol-rich membranes (74, 80, 81). The latter property allows studies of the lipid and protein composition of these membranes and how composition changes under different physiological conditions (3, 82–84). Nevertheless there are many cases in which detergent insolubility results are ambiguous (18, 74, 85–89), and comparison of the detergent insolubility properties of asymmetric model membrane vesicles with those of cell membranes should clarify the relationship between detergent insolubility and domain formation and aid the development of improved detergent insolubility methods for detection of ordered domains in cells.
Finally asymmetric vesicles may find applications in drug encapsulation. The availability of asymmetric vesicles (especially large vesicles) would allow the design of vesicles in which the lipid composition of the inner leaflet is compatible with the encapsulated drug, whereas the composition of the outer leaflet is compatible with the surrounding biological milieu.
Supplementary Material
Acknowledgments
We acknowledge MiJin Son for measurements of lipid solubilization by MβCD and SUV stability.
This work was supported, in whole or in part, by National Institutes of Health Grant GM48596. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental methods and Figs. S1–S4.
Footnotes
The abbreviations used are: PC, phosphatidylcholine; PE, phosphatidylethanolamine; PS, phosphatidylserine; Lo, liquid-ordered; Ld, liquid-disordered; MβCD, methyl-β-cyclodextrin; SM, sphingomyelin; POPC, 1-palmitoyl-2-oleoyl-phosphatidylcholine; DOPC, 1,2-dioleoylphosphatidylcholine; POPS, 1-palmitoyl-2-oleoyl-phosphatidyl-l-serine; POPE, 1-palmitoyl-2-oleoyl-phosphatidylethanolamine; o, outside; i, inside; DPPC, 1,2-dipalmitoylphosphatidylcholine; BrPC, 1,2-[9,10-dibromo]stearoylphosphatidylcholine; CHOL, cholesterol; DPH, 1,6-diphenyl-1,3,5-hexatriene; TMADPH, 1-(4-trimethylammoniumphenyl)-6-phenyl-1,3,5-hexatriene p-toluenesulfonate; rhodamine-PE, N-(Rhodamine Red-X)-1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine; NBD-PE, N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine; HPLC, high pressure liquid chromatography; HP-TLC, high performance thin layer chromatography; MLV, multilamellar vesicle; SUV, small unilamellar vesicle; CLC, cholesterol-loaded MβCD; TM, transmembrane.
M. Son and E. London, unpublished observations.
References
- 1.Verkleij, A. J., Zwaal, R. F., Roelofsen, B., Comfurius, P., Kastelijn, D., and van Deenen, L. L. (1973) Biochim. Biophys. Acta 323 178–193 [DOI] [PubMed] [Google Scholar]
- 2.Brown, D. A., and London, E. (1997) Biochem. Biophys. Res. Commun. 240 1–7 [DOI] [PubMed] [Google Scholar]
- 3.Brown, D. A., and London, E. (1998) Annu. Rev. Cell Dev. Biol. 14 111–136 [DOI] [PubMed] [Google Scholar]
- 4.Ahmed, S. N., Brown, D. A., and London, E. (1997) Biochemistry 36 10944–10953 [DOI] [PubMed] [Google Scholar]
- 5.Feigenson, G. W., and Buboltz, J. T. (2001) Biophys. J. 80 2775–2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Megha Bakht, O., and London, E. (2006) J. Biol. Chem. 281 21903–21913 [DOI] [PubMed] [Google Scholar]
- 7.Megha, and London, E. (2004) J. Biol. Chem. 279 9997–10004 [DOI] [PubMed] [Google Scholar]
- 8.Nybond, S., Bjorkqvist, Y. J., Ramstedt, B., and Slotte, J. P. (2005) Biochim. Biophys. Acta 1718 61–66 [DOI] [PubMed] [Google Scholar]
- 9.Schroeder, R., London, E., and Brown, D. (1994) Proc. Natl. Acad. Sci. U. S. A. 91 12130–12134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veatch, S. L., Gawrisch, K., and Keller, S. L. (2006) Biophys. J. 90 4428–4436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang, J., Megha, and London, E. (2004) Biochemistry 43 1010–1018 [DOI] [PubMed] [Google Scholar]
- 12.Wang, T. Y., and Silvius, J. R. (2001) Biophys. J. 81 2762–2773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu, X., Bittman, R., Duportail, G., Heissler, D., Vilcheze, C., and London, E. (2001) J. Biol. Chem. 276 33540–33546 [DOI] [PubMed] [Google Scholar]
- 14.Xu, X., and London, E. (2000) Biochemistry 39 843–849 [DOI] [PubMed] [Google Scholar]
- 15.Korlach, J., Schwille, P., Webb, W. W., and Feigenson, G. W. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 8461–8466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dietrich, C., Bagatolli, L. A., Volovyk, Z. N., Thompson, N. L., Levi, M., Jacobson, K., and Gratton, E. (2001) Biophys. J. 80 1417–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samsonov, A. V., Mihalyov, I., and Cohen, F. S. (2001) Biophys. J. 81 1486–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang, T. Y., Leventis, R., and Silvius, J. R. (2000) Biophys. J. 79 919–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang, T. Y., Leventis, R., and Silvius, J. R. (2001) Biochemistry 40 13031–13040 [DOI] [PubMed] [Google Scholar]
- 20.Bjorkqvist, Y. J., Nyholm, T. K., Slotte, J. P., and Ramstedt, B. (2005) Biophys. J. 88 4054–4063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramstedt, B., and Slotte, J. P. (2006) Biochim. Biophys. Acta 1758 1945–1956 [DOI] [PubMed] [Google Scholar]
- 22.Chiantia, S., Ries, J., Kahya, N., and Schwille, P. (2006) Chemphyschem 7 2409–2418 [DOI] [PubMed] [Google Scholar]
- 23.Kahya, N., and Schwille, P. (2006) Mol. Membr. Biol. 23 29–39 [DOI] [PubMed] [Google Scholar]
- 24.Chiantia, S., Kahya, N., Ries, J., and Schwille, P. (2006) Biophys. J. 90 4500–4508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fidorra, M., Duelund, L., Leidy, C., Simonsen, A. C., and Bagatolli, L. A. (2006) Biophys. J. 90 4437–4451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sot, J., Bagatolli, L. A., Goni, F. M., and Alonso, A. (2006) Biophys. J. 90 903–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Veatch, S. L., and Keller, S. L. (2003) Biophys. J. 85 3074–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veatch, S. L., Soubias, O., Keller, S. L., and Gawrisch, K. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 17650–17655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ayuyan, A. G., and Cohen, F. S. (2008) Biophys. J. 94 2654–2666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ira, and Johnston, L. J. (2008) Biochim. Biophys. Acta 1778 185–197 [DOI] [PubMed] [Google Scholar]
- 31.Collins, M. D., and Keller, S. L. (2008) Proc. Natl. Acad. Sci. U. S. A. 105 124–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiessling, V., Crane, J. M., and Tamm, L. K. (2006) Biophys. J. 91 3313–3326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crane, J. M., Kiessling, V., and Tamm, L. K. (2005) Langmuir 21 1377–1388 [DOI] [PubMed] [Google Scholar]
- 34.Malewicz, B., Valiyaveettil, J. T., Jacob, K., Byun, H. S., Mattjus, P., Baumann, W. J., Bittman, R., and Brown, R. E. (2005) Biophys. J. 88 2670–2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barsukov, L. I., Victorov, A. V., Vasilenko, I. A., Evstigneeva, R. P., and Bergelson, L. D. (1980) Biochim. Biophys. Acta 598 153–168 [DOI] [PubMed] [Google Scholar]
- 36.Eastman, S. J., Hope, M. J., and Cullis, P. R. (1991) Biochemistry 30 1740–1745 [DOI] [PubMed] [Google Scholar]
- 37.Hope, M. J., Redelmeier, T. E., Wong, K. F., Rodrigueza, W., and Cullis, P. R. (1989) Biochemistry 28 4181–4187 [DOI] [PubMed] [Google Scholar]
- 38.Bloj, B., and Zilversmit, D. B. (1981) Mol. Cell. Biochem. 40 163–172 [DOI] [PubMed] [Google Scholar]
- 39.Everett, J., Zlotnick, A., Tennyson, J., and Holloway, P. W. (1986) J. Biol. Chem. 261 6725–6729 [PubMed] [Google Scholar]
- 40.Pautot, S., Frisken, B. J., and Weitz, D. A. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 10718–10721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanhuanpaa, K., and Somerharju, P. (1999) J. Biol. Chem. 274 35359–35366 [DOI] [PubMed] [Google Scholar]
- 42.Ottico, E., Prinetti, A., Prioni, S., Giannotta, C., Basso, L., Chigorno, V., and Sonnino, S. (2003) J. Lipid Res. 44 2142–2151 [DOI] [PubMed] [Google Scholar]
- 43.Anderson, T. G., Tan, A., Ganz, P., and Seelig, J. (2004) Biochemistry 43 2251–2261 [DOI] [PubMed] [Google Scholar]
- 44.Du, H., Fuh, R. A., Li, J., Corkan, A., and Lindsey, J. S. (1998) Photochem. Photobiol. 68 141–142 [Google Scholar]
- 45.Bakht, O., and London, E. (2007) in Lipid Rafts (McIntosh, T.J., ed) pp. 29–40, Humana Press, Totowa, NJ
- 46.Lacowicz, J. (1999) Principles of Fluorescence Spectroscopy, 2nd Ed., pp. 298–299, Kluwer Academic/Plenum Publishers, New York
- 47.London, E., and Feligenson, G. W. (1978) Anal. Biochem. 88 203–211 [DOI] [PubMed] [Google Scholar]
- 48.Shahidullah, K., and London, E. (2008) J. Mol. Biol. 379 704–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Savitzky, A., and Golay, M. J. E. (1964) Anal. Chem. 36 1627–1639 [Google Scholar]
- 50.Huang, C. (1969) Biochemistry 8 344–352 [DOI] [PubMed] [Google Scholar]
- 51.Prendergast, F. G., Haugland, R. P., and Callahan, P. J. (1981) Biochemistry 20 7333–7338 [DOI] [PubMed] [Google Scholar]
- 52.Kaiser, R. D., and London, E. (1998) Biochemistry 37 8180–8190 [DOI] [PubMed] [Google Scholar]
- 53.Koynova, R., and Caffrey, M. (1994) Chem. Phys. Lipids 69 1–34 [DOI] [PubMed] [Google Scholar]
- 54.Koynova, R., and Caffrey, M. (1998) Biochim. Biophys. Acta 1376 91–145 [DOI] [PubMed] [Google Scholar]
- 55.Shaikh, S. R., Dumaual, A. C., Castillo, A., LoCascio, D., Siddiqui, R. A., Stillwell, W., and Wassall, S. R. (2004) Biophys. J. 87 1752–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Untrach, S. H., and Shipley, G. G. (1977) J. Biol. Chem. 252 4449–4457 [PubMed] [Google Scholar]
- 57.Lentz, B. R., Barenholz, Y., and Thompson, T. E. (1976) Biochemistry 15 4521–4528 [DOI] [PubMed] [Google Scholar]
- 58.London, E., and Feigenson, G. W. (1981) Biochim. Biophys. Acta 649 89–97 [Google Scholar]
- 59.Beck, A., Heissler, D., and Duportail, G. (1993) Chem. Phys. Lipids 66 135–142 [DOI] [PubMed] [Google Scholar]
- 60.Huang, C., and Mason, J. T. (1978) Proc. Natl. Acad. Sci. U. S. A. 75 308–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bakht, O., Pathak, P., and London, E. (2007) Biophys. J. 93 4307–4318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wenz, J. J., and Barrantes, F. J. (2003) Biochemistry 42 14267–14276 [DOI] [PubMed] [Google Scholar]
- 63.Koynova, R., and Caffrey, M. (1995) Biochim. Biophys. Acta 1255 213–236 [DOI] [PubMed] [Google Scholar]
- 64.Sripada, P. K., Maulik, P. R., Hamilton, J. A., and Shipley, G. G. (1987) J. Lipid Res. 28 710–718 [PubMed] [Google Scholar]
- 65.Dawidowicz, E. A., and Rothman, J. E. (1976) Biochim. Biophys. Acta 455 621–630 [DOI] [PubMed] [Google Scholar]
- 66.Caputo, G. A., and London, E. (2003) Biochemistry 42 3275–3285 [DOI] [PubMed] [Google Scholar]
- 67.Krishnakumar, S. S., and London, E. (2007) J. Mol. Biol. 374 1251–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krishnakumar, S. S., and London, E. (2007) J. Mol. Biol. 374 671–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leventis, R., and Silvius, J. R. (2001) Biophys. J. 81 2257–2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fastenberg, M. E., Shogomori, H., Xu, X., Brown, D. A., and London, E. (2003) Biochemistry 42 12376–12390 [DOI] [PubMed] [Google Scholar]
- 71.Ren, J., Lew, S., Wang, Z., and London, E. (1997) Biochemistry 36 10213–10220 [DOI] [PubMed] [Google Scholar]
- 72.Rinia, H. A., Snel, M. M., van der Eerden, J. P., and de Kruijff, B. (2001) FEBS Lett. 501 92–96 [DOI] [PubMed] [Google Scholar]
- 73.Crane, J. M., and Tamm, L. K. (2004) Biophys. J. 86 2965–2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.London, E., and Brown, D. A. (2000) Biochim. Biophys. Acta 1508 182–195 [DOI] [PubMed] [Google Scholar]
- 75.Schmidt, C. F., Barenholz, Y., Huang, C., and Thompson, T. E. (1978) Nature 271 775–777 [DOI] [PubMed] [Google Scholar]
- 76.Lerch-Bader, M., Lundin, C., Kim, H., Nilsson, I., and von Heijne, G. (2008) Proc. Natl. Acad. Sci. U. S. A. 105 4127–4132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Antignani, A., and Youle, R. J. (2006) Curr. Opin. Cell Biol. 18 685–689 [DOI] [PubMed] [Google Scholar]
- 78.Fujita, K., Krishnakumar, S. S., Franco, D., Paul, A. V., London, E., and Wimmer, E. (2007) Biochemistry 46 5185–5199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shepard, L. A., Heuck, A. P., Hamman, B. D., Rossjohn, J., Parker, M. W., Ryan, K. R., Johnson, A. E., and Tweten, R. K. (1998) Biochemistry 37 14563–14574 [DOI] [PubMed] [Google Scholar]
- 80.Brown, D. A., and Rose, J. K. (1992) Cell 68 533–544 [DOI] [PubMed] [Google Scholar]
- 81.Brown, D. A., and London, E. (2000) J. Biol. Chem. 275 17221–17224 [DOI] [PubMed] [Google Scholar]
- 82.Young, R. M., Holowka, D., and Baird, B. (2003) J. Biol. Chem. 278 20746–20752 [DOI] [PubMed] [Google Scholar]
- 83.Sohn, H. W., Tolar, P., Jin, T., and Pierce, S. K. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 8143–8148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sohn, H. W., Pierce, S. K., and Tzeng, S. J. (2008) J. Immunol. 180 793–799 [DOI] [PubMed] [Google Scholar]
- 85.Shogomori, H., and Brown, D. A. (2003) Biol. Chem. 384 1259–1263 [DOI] [PubMed] [Google Scholar]
- 86.Heerklotz, H. (2002) Biophys. J. 83 2693–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Heerklotz, H., Szadkowska, H., Anderson, T., and Seelig, J. (2003) J. Mol. Biol. 329 793–799 [DOI] [PubMed] [Google Scholar]
- 88.London, E. (2005) Biochim. Biophys. Acta 1746 203–220 [DOI] [PubMed] [Google Scholar]
- 89.Lichtenberg, D., Goni, F. M., and Heerklotz, H. (2005) Trends Biochem. Sci. 30 430–436 [DOI] [PubMed] [Google Scholar]
- 90.Ramstedt, B., Leppimaki, P., Axberg, M., and Slotte, J. P. (1999) Eur. J. Biochem. 266 997–1002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.