Abstract
Oct4 is a known master regulator of stem cell renewal and differentiation. Expression of Oct4 during differentiation is regulated by promoter methylation by the nucleosome remodeling and histone deacetylation (NuRD) complex. Here, we show that Cdk2ap1, a negative regulator of Cdk2 function and cell cycle, promotes Oct4 promoter methylation during murine embryonic stem cell differentiation to down-regulate Oct4 expression. We further show that this repressor function of Cdk2ap1 is dependent on its physical interaction with the methyl DNA-binding protein, Mbd3. Our data support a potential molecular link between the known differentiation promoters, including bone morphogenetic proteins and transforming growth factor signaling, and embryonic stem cell differentiation.
Embryonic stem cells (ESC)5 maintain pluripotency and indefinite self-renewal through yet-to-be defined molecular mechanisms. Pluripotent murine ES cells (mESC) are grown and maintained either on feeder cells or in the presence of leukemia inhibitory factor (LIF) to inhibit spontaneous differentiation (1–4). Bone morphogenetic protein (BMP) signaling also plays a role in the maintenance of pluripotency, especially in the absence of serum and feeder cells (5–8). Pluripotency of mESC is dictated by the expression of three transcriptional master regulators, Oct3/4, Nanog, and Sox2, and their expression levels relative to each other (5, 9).
Octamer 4 (Oct4; aka POU domain, class 5, transcription factor 1, or Pou5f1) is a homeodomain-containing transcription factor that has been shown to be absolutely essential for formation and maintenance of pluripotent stem cells (10). Induction of ESC differentiation, either by retinoic acid or by removal of LIF, has been shown to result in an increase in promoter methylation and silencing of Oct4 expression (11, 12). Failure of mESC to down-regulate Oct4 expression has been shown to result in an inability of the mESC to differentiate. In addition, ESC can also be induced to differentiate by exposure to various signaling molecules, including various BMPs and TGFβ (5, 13–16). As ES cells progress through the differentiation process members of the Nucleosome Remodeling and histone Deacetylation (NuRD) complex, including germ cell nuclear factor (GCNF), MBD2, and MBD3, are recruited at the promoter regions of the negative regulators of differentiation resulting in epigenetic modification and silencing of gene expression (17–21). Although factors and mechanisms regulating expression of Oct4 are being better understood, the molecular mediators of signaling pathways inducing ESC differentiation remain to be identified.
We had previously identified Cdk2ap1 in a screen for negative regulators of oral carcinogenesis (22, 23). CDK2AP1 was shown to negatively regulate the S-phase kinase, CDK2, and is a downstream target of the TGFβ pathway (24, 25). To understand the normal physiological function, we have generated mice with targeted deletion of the Cdk2ap1 gene. Animals with homozygous deletion of Cdk2ap1 display peri-implantation lethality between 3.5 and 5.5 days after coitus. We have also generated Cdk2ap1–/– mESC by targeted deletion of the Cdk2ap1 locus in LW1 cells (26). Analyses of the ability of these cells to differentiate following removal of LIF or exposure to retinoic acid showed a loss of differentiation potential and maintained expression of markers of pluripotency, including Oct4 and Nanog, supporting an essential function for Cdk2ap1 in stem cell differentiation and early embryonic development.6 Interestingly, Cdk2ap1 (aka DOC-1) was also identified as a core component of the MBD2-NuRD and MBD3-NuRD complexes in a tandem affinity purification and mass spectrometry identification study to identify components of the NuRD complex (27). Comparison of Cdk2ap1–/– and Mbd3–/– murine ESC revealed an overlapping phenotype. Deletion of both genes results in similar phenotypes, viz. early embryonic lethality and sustained Oct4 and Nanog expression, following LIF withdrawal (17). Based on these observations, we hypothesized that the Cdk2ap1-Mbd3-NuRD complex is required for proper silencing of the Oct4 expression following induction of differentiation of murine ES cells.
In this study, we present data supporting a role for Cdk2ap1 in negative regulation of Oct4 expression during differentiation of mESC into embryoid bodies (EB) following withdrawal of LIF. Comparison of the methylation status of the Oct4 promoter in Cdk2ap1+/+ and Cdk2ap1–/– mESC showed significant reduction in the EB derived from Cdk2ap1–/– ES cells. We further show that the hypomethylation of Oct4 promoter is reversible and dependent on the physical interaction of Cdk2ap1 with MBD3. These data support a necessary role for Cdk2ap1, and specifically, the Cdk2ap1-MBD3-NuRD complex in proper orchestration of gene expression patterns during the differentiation of murine ES cells.
EXPERIMENTAL PROCEDURES
Cell Culture and Nucleic Acid Isolation—WT and three Cdk2ap1–/– mESC clones, 272-9, 272-10, and 272-13 (26), were used. mESC were cultured on gelatinized tissue culture dishes in mESC growth medium (mESGM): Dulbecco's modified Eagle's medium containing 15% fetal bovine serum (Bio-West), nonessential amino acids, 2 mm l-glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin, 0.12 mm β-mercaptoethanol, and ∼1000 units/ml LIF. All reagents were purchased from Invitrogen unless otherwise specified. MBD3–/– mESC were generously provided by Dr. Brian Hendrich.
Embryoid bodies were harvested on day 0 and day 8 of differentiation and genomic DNA was isolated using the DNeasy kit (Qiagen). Total RNA was isolated with TRI Reagent (Molecular Research Center) according to the manufacturer's protocol.
cDNA Constructs—Mouse Cdk2ap1 open reading frame was cloned into pMSCV-IRES-EGFP obtained by cloning an IRES-EGFP fragment into the retroviral vector, pMSCV (Clontech). Cdk2ap1Δ deletion mutant (deletion of amino acids 8–15) was generated by PCR amplification and cloned into the pMSCV-IRES-EGFP vector. C-terminal Myc-tagged MBD3 was generated by PCR amplification using mouse MBD3 cDNA (Open Biosystems) and subcloned into the pMSCV-IRES-EGFP.
Gene Transfer and EB Formation—Retroviral transduction of mESC was carried out in mESGM supplemented with 5 μg/ml Polybrene (Sigma) for 4 h. Medium was replaced after 4 h with fresh mESGM, and cells were grown for 24 h before use in further experiments. For EB formation, cells from confluent cultures were collected by trypsinization, washed, and resuspended in mESGM without LIF in Petri dishes (BD Falcon). Cells were fed every other day by allowing the EB to settle and replacing the supernatant. EB were collected on day 8 for analyses.
ChIP Analysis—ChIP analysis was performed using the ChIP-IT™ kit (Active Motif) according to the manufacturer's protocol. Primer sequences will be provided upon request.
Co-immunoprecipitation Analysis—2.5 mg of cell lysates was used for co-IP analysis, which was performed according to standard protocols.
Quantitative PCR—Quantitative analysis was performed on an ABI 7700 real-time PCR machine with SYBR Green PCR master mix. Primer pairs were designed from previously published sequences (28).
Statistical Analysis—Statistical significance was determined using the two-tailed Student's t test.
RESULTS
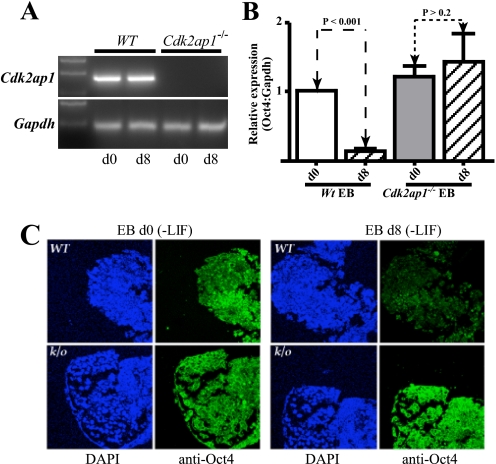
Loss of Cdk2ap1 Results in Deregulation of Oct4 Silencing during mES Differentiation—Initial analyses of Cdk2ap1–/– mESC revealed a failure of these cells to differentiate upon LIF withdrawal and down-regulate expression of the pluripotency markers, Oct4 and Nanog. Analysis of Oct4 expression by quantitative RT-PCR showed sustained expression in EB derived from Cdk2ap1–/– mESC when compared with those derived from WT mESC (Fig. 1B). This was further validated by immunofluorescence staining of EB (Fig. 1C).
FIGURE 1.
Retention of Oct4 expression in differentiating Cdk2ap1–/– mESC. A, expression of Cdk2ap1 in WT and Cdk2ap1–/– mESC at d0 and d8 of differentiation. Gapdh, glyceraldehyde-3-phosphate dehydrogenase. B, quantitative RT-PCR analysis of Oct4 expression in day 0 and day 8 embryoid bodies showed sustained expression of Oct4 at day 8 of differentiation. Data were obtained in triplicate from three individual Cdk2ap1–/– mESC lines. C, immunofluorescence analysis of Oct4 expression in day 0 EB and day 8 EB confirmed the RT-PCR data. Intense Oct4 staining was detected in day 8 Cdk2ap1–/– EB.
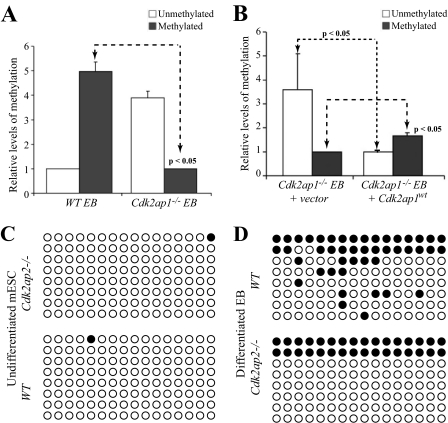
Loss of Oct4 Promoter Methylation during Differentiation of Cdk2ap1–/– mESC—Expression of Oct4 in embryonic stem cells is regulated both by transcription factors and by epigenetic regulation. Although transcription factors play an important role in maintaining Oct4 expression in undifferentiated stem cells, epigenetic regulation is the main mechanism of down-regulation of Oct4 expression during differentiation (29–31). To understand the cause of deregulated Oct4 expression in differentiating Cdk2ap1–/– EB, we performed methylation-specific PCR (MSP) and bisulfite sequencing of the Oct4 promoter. In Cdk2ap1–/– EB, we observed a significant reduction in the levels of Oct4 promoter methylation when compared with those in the WT EB, both by MSP (Fig. 2A, p < 0.05) and by bisulfite sequencing (Fig. 2D). No significant difference was observed in promoter methylation in undifferentiated ES cells (Fig. 2C). To determine whether this difference in methylation of the Oct4 promoter in differentiating EB was related to the Cdk2ap1 status, we re-expressed Cdk2ap1WT into Cdk2ap1–/– ESCs by retroviral transduction. Rescue of Cdk2ap1–/– mESC with Cdk2ap1WT re-established the methylation patterns in differentiated EB to WT levels (Fig. 2B), although not to the same extent as the WT EB. This is probably due to the transduction efficiency and/or the expression levels in the transduced mESC cells.
FIGURE 2.
Cdk2ap1 alters methylation status of the Oct4 promoter. A, Oct4 promoter methylation, as determined by quantitative MSP, showed higher levels of hypomethylated DNA in the Cdk2ap1–/– EB when compared with the WT EB. B, increased methylation of the Oct4 promoter was observed in Cdk2ap1–/– EB expressing Cdk2ap1WT. C, bisulfite sequencing analyses of the Oct4 promoter showed no methylation in undifferentiated wild type and the Cdk2ap1–/– mESC. D, induction of differentiation by LIF withdrawal results in an increase in DNA methylation (filled circles) in EB obtained from wild type mESC. This was not seen in a majority of EB derived from the Cdk2ap1–/– mESC.
Cdk2ap1 Interacts with Mbd3 via Eight Amino Acid Residues in the N Terminus—MBD3, a methyl CpG-binding protein, has been shown to be required for the ability of mESC to differentiate into all four embryonic lineages, ectoderm, mesoderm, endoderm, and trophectoderm (17, 32). Kaji et al. (17, 32) showed that loss of Mbd3 expression resulted in improper silencing of the stem cell pluripotency factors, including Oct4 and Nanog, leading to a loss of differentiation potential of the mESC in the absence of LIF. Interestingly, the Cdk2ap1–/– ESC showed a phenotype very similar to that of the Mbd3–/– mESC in that both cells are resistant to differentiation upon removal of LIF and both cells maintain expression of markers of ES pluripotency following induction of differentiation (Figs. 1 and 3B, lane 3). Additionally, Cdk2ap1 was recently identified as a core component of the MBD2-NurD and MBD3-NuRD complexes that are involved in establishing the early epigenetic modification signatures in various cell lineages derived from ES cells (27). We thus hypothesized that Cdk2ap1 regulates the coalescence of the MBD-NuRD complex by interacting with MBD3. To test this hypothesis, we performed co-immunoprecipitation analyses using bacterially expressed deletion mutants of CDK2AP1 and MBD3. We identified a stretch of 8 amino acids in the N terminus of CDK2AP1 (amino acids 8–15; Cdk2ap1Δ) that were required for direct interaction of CDK2AP1 with MBD3 (supplemental Fig. 1). We tested this interaction in situ using Cdk2ap1–/– mESC. Because the levels of Mbd3 are relatively low in mouse ES cells (28) and due to the lack of clean anti-Mbd3 antibodies, we transduced mESC with a retrovirus expressing myc-Mbd3 and performed reciprocal IP-Western analyses using Cdk2ap1 or Cdk2ap1Δ and Myc-MBD3. As seen in supplemental Fig. 1B, Cdk2ap1WT, but not Cdk2ap1Δ, was able to pull down Myc-MBD3. Reciprocal IP using anti-Myc antibody to pull down MBD3-interacting proteins successfully pulled down Cdk2ap1 but not Cdk2ap1Δ (supplemental Fig. 1C). These data show that Cdk2ap1 and Mbd3 physically interact via an N-terminal domain. Interaction of endogenous Cdk2ap1 and Mbd3 was confirmed by co-IP analyses of mESC and 293T cell lysates (supplemental Fig. 1, D and E).
FIGURE 3.
Cdk2ap1-Mbd3-complex associates with the Oct4 promoter and regulates Oct4 expression. A, ChIP analysis using anti-Cdk2ap1 antibody showed a positive association of Cdk2ap1 protein with the Oct4 promoter (lane 2). Specificity of this association was confirmed by the absence of a PCR product in Cdk2ap1–/– EB (lane 3) and presence of a PCR product in the EB rescued with Cdk2ap1WT (lane 5). Cdk2ap1 association with the Oct4 promoter requires its interaction with Mbd3. This is evidenced by the absence of a PCR product in the Mbd3–/– EB (lanes 4 and 7) or in EB derived from the Cdk2ap1–/– ESC expressing the Mbd3-interaction mutant, Cdk2ap1Δ (lane 6). –Ab control, antibody control. B, the positive association of Cdk2ap1-MBD3 complex correlated with down-regulation of Oct4 expression in day 8 embryoid bodies. Gapdh, glyceraldehyde-3-phosphate dehydrogenase.
Cdk2ap1-Mbd3 Interaction Is Required for the Differentiation-specific Methylation and Silencing of the Oct4 Promoter—As seen in Fig. 1, loss of Cdk2ap1 expression in mESC resulted in a loss of differentiation-induced silencing of Oct4 expression. Analysis of methylation status of the Oct4 promoter revealed reduced methylation, potentially resulting in deregulation of Oct4 expression (Figs. 1 and 2). To test whether the Cdk2ap1-containing complex associates with the Oct4 promoter, we performed ChIP using anti-Cdk2ap1 antibody. As seen in Fig. 3A, lanes 2 and 3, we were able to successfully IP and amplify the Oct4 promoter in EB derived from WT but not Cdk2ap1–/– mESC. This lack of binding correlated with the sustained expression of Oct4 in the Cdk2ap1–/– EB (Fig. 3B, lanes 1 and 2). Re-expression of CDK2AP1WT cDNA into Cdk2ap1–/– ESC resulted in CDK2AP1 association with the Oct4 promoter and restoration of proper silencing of Oct4 expression in day 8 EB derived from the rescued cells (Fig. 3A, lane 5, and B, lane 4). This supports the hypothesis that Cdk2ap1 associates with the Oct4 promoter during differentiation of ES cells.
Because Cdk2ap1 lacks a defined DNA-binding domain, we hypothesized that it associates with the Oct4 promoter via its interaction with the MBD3-NuRD. To test whether Mbd3, and by extension the NuRD complex, is required for Cdk2ap1 binding at the Oct4 promoter, we expressed an Mbd3-interaction mutant, Cdk2ap1Δ, into Cdk2ap1–/– ES cells. As seen in Fig. 3A (lane 6), we see a significantly reduced association of the mutant Cdk2ap1Δ with the Oct4 promoter when compared with either the wild type EB or those derived from Cdk2ap1–/– mESC rescued with Cdk2ap1WT. To further confirm whether the Cdk2ap1-Mbd3 interaction is required for association of Cdk2ap1 with the Oct4 promoter, we performed ChIP analysis in EB derived from Mbd3–/– mESC. Mbd3–/– ES cells (wild type for Cdk2ap1 expression) are incapable of differentiation in the absence of LIF and maintain Oct4 expression following LIF withdrawal (17). ChIP analysis using anti-Cdk2ap1 antibody in the Mbd3–/– EB did not show any association of the Cdk2ap1 protein with the Oct4 promoter (Fig. 3A, lanes 4 and 7). These data together strongly support our hypothesis that the interaction of Cdk2ap1-Mbd3, as components of the NuRD complex, is required for association of Cdk2ap1 with the Oct4 promoter and proper silencing of gene expression during differentiation (Fig. 3).
DISCUSSION
Embryonic development is a well orchestrated phenomenon wherein genes required to maintain pluripotency are silenced or down-regulated upon exposure to differentiation cues. The master regulator of pluripotency, Oct4, is constitutively expressed in undifferentiated stem cells. Upon withdrawal of LIF and/or exposure to differentiation-inducing agents, including retinoic acid, BMPs, fibroblast growth factors, etc., Oct4 expression is down-regulated in all of the resulting embryonic lineages except the germ cell lineage. Levels of Oct4 protein are down-regulated as the stem cells progressively commit to various lineages and differentiate. Various studies have demonstrated the importance Oct4 expression in the ability of stem cell to self-renew (30). Loss of Oct4 expression early in embryogenesis results in peri-implantation lethality during early embryogenesis (30, 31, 33). Conversely, increase in Oct4 expression has also been shown to result in increased expression of markers of primitive endoderm and mesoderm lineages and reduced pluripotency (34).
During embryonic stem cell differentiation, in addition to transcriptional control, gene expression is regulated by epigenetic events, including DNA methylation and histone deacetylation. These modifications result in conversion of the euchromatin to heterochromatin, and thus, silencing of the target loci. CpG islands in promoter regions of various genes have been shown to be targets of DNA methylation, an epigenetic modification that has been shown to be a major mechanism of gene silencing (35). DNA methylation has been shown to be the predominant mechanism responsible for the down-regulation of Oct4 expression during ESC differentiation (11, 12). In undifferentiated ES and embryonal carcinoma cells, the two regulator enhancer elements, present in the proximal and distal regions of the Oct4 promoter, are occupied by transcription factors, and thus, protected from DNA methylation (11, 36). Upon induction of differentiation, various members of the NuRD complex are sequentially recruited to and methylate the Oct4 promoter, resulting in silencing of gene expression (17, 28, 37). Loss of DNA methylation and its effect on ESC differentiation was demonstrated in a couple of elegant knock-out studies involving DNMT3a/3b and MBD3 (17, 38). Analysis of Dnmt-1, -3a, and -3b triple knock-out and MBD3–/– murine ESCs showed that although these cells were viable and expressed all markers of stem cell pluripotency, including Oct4, Nanog, and Sox2, they failed to down-regulate the expression of these genes following exposure to differentiation cues (17, 32), demonstrating that the NuRD complex is required for proper regulation of gene expression during stem cell differentiation.
To understand the biological role of Cdk2ap1, we generated homozygous knock-out ES cells. These cells lack the ability to differentiate in the absence of LIF. Our data showed that Cdk2ap1 negatively regulates the expression of Oct4 expression during ESC differentiation. This function of Cdk2ap1 requires a direct interaction with Mbd3. The lack of the Mbd3-interaction results in hypomethylation of the Oct4 promoter and deregulation of Oct4 expression in differentiating EB. We hypothesize that Cdk2ap1, through its interaction with Mbd3, functions as a potential “Velcro” factor to hold the NuRD complex together as a functional unit (Fig. 4).
FIGURE 4.
Schematic for the role of Cdk2ap1 as a component of the NuRD complex and in mediation of extracellular signals. Up-regulation of Cdk2ap1 in mESC by TGFβ-Smad pathway, accompanied by an initiating event that brings together the NuRD complex, results in hypermethylation of the Oct4 promoter. Cdk2ap1 potentially functions as a Velcro factor to keep the members of the NuRD complex together. Increase in methylation (Me) of the Oct4 promoter then results in the down-regulation of Oct4 expression and loss of self-renewal potential.
Cdk2ap1 expression is regulated by the TGFβ signaling pathway. This regulation is mediated by the binding of the SMAD3 and SMAD4 proteins to the Smad regulatory elements in the promoter region of the CDK2AP1 gene (24). Although upstream activators of the expression or activity of the NuRD complex remain to be identified, our data presented support a potential molecular link between the extracellular signals dictating the differentiation processes and the negative regulation of expression of stem cell pluripotency factors. Thus, we could speculate that the TGFβ pathway, through regulation of Cdk2ap1 expression, could regulate gene expression via epigenetic silencing and hence differentiation of mESC (Fig. 4). Validation of this hypothesis will contribute to an identification of and/or understanding of the regulators of Oct4 expression downstream of various signaling pathways.
Supplementary Material
Acknowledgments
We thank V. Palanisamy and B. Henson for critical reading of this manuscript and M. Stovall and L. Dysim for administrative support.
This work was supported, in whole or in part, by National Institutes of Health Grant DE014857 and by a National Institutes of Health T32 grant (Grant DE07296 to Y. K.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental “Materials and Methods” and a supplemental figure.
Footnotes
The abbreviations used are: ESC, embryonic stem cells; ES, embryonic stem; mESC, murine ESC; mESGM, mESC growth medium; Cdk2ap1, Cdk2-associated protein, member 1; TGFβ, transforming growth factor β; BMP, bone morphogenetic protein; LIF, leukemia inhibitory factor; NuRD, nucleosome remodeling and histone deacetylation; EB, embryoid bodies; IRES, internal ribosomal entry site; EGFP, enhanced green fluorescent protein; MBD, methyl DNA-binding protein; IP, immunoprecipitation; ChIP, chromatin IP; RT-PCR, reverse transcription-PCR; MSP, methylation-specific PCR; WT, wild type.
Y. Kim, A. Deshpande, Y.-S. Dai, J. Kim, A. Conway, A. Lindgren, A. Clark, and D. T. Wong, manuscript in preparation.
References
- 1.Gough, N. M., and Williams, R. L. (1989) Cancer Cells 1 77–80 [PubMed] [Google Scholar]
- 2.Gough, N. M., Williams, R. L., Hilton, D. J., Pease, S., Willson, T. A., Stahl, J., Gearing, D. P., Nicola, N. A., and Metcalf, D. (1989) Reprod. Fertil. Dev. 1 281–288 [DOI] [PubMed] [Google Scholar]
- 3.Williams, R. L., Hilton, D. J., Pease, S., Willson, T. A., Stewart, C. L., Gearing, D. P., Wagner, E. F., Metcalf, D., Nicola, N. A., and Gough, N. M. (1988) Nature 336 684–687 [DOI] [PubMed] [Google Scholar]
- 4.Smith, A. G., Heath, J. K., Donaldson, D. D., Wong, G. G., Moreau, J., Stahl, M., and Rogers, D. (1988) Nature 336 688–690 [DOI] [PubMed] [Google Scholar]
- 5.Chambers, I. (2004) Cloning Stem Cells 6 386–391 [DOI] [PubMed] [Google Scholar]
- 6.Di-Gregorio, A., Sancho, M., Stuckey, D. W., Crompton, L. A., Godwin, J., Mishina, Y., and Rodriguez, T. A. (2007) Development (Camb.) 134 3359–3369 [DOI] [PubMed] [Google Scholar]
- 7.Temple, S. (2003) Cell 115 247–248 [DOI] [PubMed] [Google Scholar]
- 8.Ying, Q. L., Nichols, J., Chambers, I., and Smith, A. (2003) Cell 115 281–292 [DOI] [PubMed] [Google Scholar]
- 9.Rao, S., and Orkin, S. H. (2006) Genome Biol. 7 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nichols, J., Zevnik, B., Anastassiadis, K., Niwa, H., Klewe-Nebenius, D., Chambers, I., Scholer, H., and Smith, A. (1998) Cell 95 379–391 [DOI] [PubMed] [Google Scholar]
- 11.Deb-Rinker, P., Ly, D., Jezierski, A., Sikorska, M., and Walker, P. R. (2005) J. Biol. Chem. 280 6257–6260 [DOI] [PubMed] [Google Scholar]
- 12.Hattori, N., Nishino, K., Ko, Y. G., Hattori, N., Ohgane, J., Tanaka, S., and Shiota, K. (2004) J. Biol. Chem. 279 17063–17069 [DOI] [PubMed] [Google Scholar]
- 13.Zhang, J., and Li, L. (2005) Dev. Biol. 284 1–11 [DOI] [PubMed] [Google Scholar]
- 14.Rao, M. (2004) Dev. Biol. 275 269–286 [DOI] [PubMed] [Google Scholar]
- 15.Ogawa, K., Saito, A., Matsui, H., Suzuki, H., Ohtsuka, S., Shimosato, D., Morishita, Y., Watabe, T., Niwa, H., and Miyazono, K. (2007) J. Cell Sci. 120 55–65 [DOI] [PubMed] [Google Scholar]
- 16.Watabe, T., Yamashita, J. K., Mishima, K., and Miyazono, K. (2006) Methods Mol. Biol. 330 341–351 [DOI] [PubMed] [Google Scholar]
- 17.Kaji, K., Caballero, I. M., MacLeod, R., Nichols, J., Wilson, V. A., and Hendrich, B. (2006) Nat. Cell Biol. 8 285–292 [DOI] [PubMed] [Google Scholar]
- 18.Crook, J. M., Dunn, N. R., and Colman, A. (2006) Nat. Cell Biol. 8 212–214 [DOI] [PubMed] [Google Scholar]
- 19.Wang, J., Rao, S., Chu, J., Shen, X., Levasseur, D. N., Theunissen, T. W., and Orkin, S. H. (2006) Nature 444 364–368 [DOI] [PubMed] [Google Scholar]
- 20.Lan, Z. J., Chung, A. C., Xu, X., DeMayo, F. J., and Cooney, A. J. (2002) J. Biol. Chem. 277 50660–50667 [DOI] [PubMed] [Google Scholar]
- 21.Mullen, E. M., Gu, P., and Cooney, A. J. (2007) PPAR Res. 2007 61563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Todd, R., McBride, J., Tsuji, T., Donoff, R. B., Nagai, M., Chou, M. Y., Chiang, T., and Wong, D. T. (1995) FASEB J. 9 1362–1370 [DOI] [PubMed] [Google Scholar]
- 23.Tsuji, T., Duh, F. M., Latif, F., Popescu, N. C., Zimonjic, D. B., McBride, J., Matsuo, K., Ohyama, H., Todd, R., Nagata, E., Terakado, N., Sasaki, A., Matsumura, T., Lerman, M. I., and Wong, D. T. (1998) J. Biol. Chem. 273 6704–6709 [DOI] [PubMed] [Google Scholar]
- 24.Hu, M. G., Hu, G. F., Kim, Y., Tsuji, T., McBride, J., Hinds, P., and Wong, D. T. (2004) Cancer Res. 64 490–499 [DOI] [PubMed] [Google Scholar]
- 25.Shintani, S., Ohyama, H., Zhang, X., McBride, J., Matsuo, K., Tsuji, T., Hu, M. G., Hu, G., Kohno, Y., Lerman, M., Todd, R., and Wong, D. T. (2000) Mol. Cell. Biol. 20 6300–6307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim, Y., McBride, J., Zhang, R., Zhou, X., and Wong, D. T. (2005) Oncogene 24 407–418 [DOI] [PubMed] [Google Scholar]
- 27.Le Guezennec, X., Vermeulen, M., Brinkman, A. B., Hoeijmakers, W. A., Cohen, A., Lasonder, E., and Stunnenberg, H. G. (2006) Mol. Cell. Biol. 26 843–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gu, P., Le Menuet, D., Chung, A. C., and Cooney, A. J. (2006) Mol. Cell. Biol. 26 9471–9483 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Buitrago, W., and Roop, D. R. (2007) J. Investig. Dermatol. 127 260–262 [DOI] [PubMed] [Google Scholar]
- 30.Pesce, M., and Scholer, H. R. (2001) Stem Cells (Dayton) 19 271–278 [DOI] [PubMed] [Google Scholar]
- 31.Watson, C. M., and Tam, P. P. (2001) Cell Struct. Funct. 26 123–129 [DOI] [PubMed] [Google Scholar]
- 32.Kaji, K., Nichols, J., and Hendrich, B. (2007) Development (Camb.) 134 1123–1132 [DOI] [PubMed] [Google Scholar]
- 33.Ovitt, C. E., and Scholer, H. R. (1998) Mol. Hum. Reprod. 4 1021–1031 [DOI] [PubMed] [Google Scholar]
- 34.Niwa, H., Miyazaki, J., and Smith, A. G. (2000) Nat. Genet. 24 372–376 [DOI] [PubMed] [Google Scholar]
- 35.Siegfried, Z., Eden, S., Mendelsohn, M., Feng, X., Tsuberi, B. Z., and Cedar, H. (1999) Nat. Genet. 22 203–206 [DOI] [PubMed] [Google Scholar]
- 36.Chew, J. L., Loh, Y. H., Zhang, W., Chen, X., Tam, W. L., Yeap, L. S., Li, P., Ang, Y. S., Lim, B., Robson, P., and Ng, H. H. (2005) Mol. Cell. Biol. 25 6031–6046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feldman, N., Gerson, A., Fang, J., Li, E., Zhang, Y., Shinkai, Y., Cedar, H., and Bergman, Y. (2006) Nat. Cell Biol. 8 188–194 [DOI] [PubMed] [Google Scholar]
- 38.Tsumura, A., Hayakawa, T., Kumaki, Y., Takebayashi, S., Sakaue, M., Matsuoka, C., Shimotohno, K., Ishikawa, F., Li, E., Ueda, H. R., Nakayama, J., and Okano, M. (2006) Genes Cells 11 805–814 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.