Abstract
Growth hormone (GH) pretreatment of 3T3-L1 adipocytes resulted in a concentration- and time-dependent inhibition of insulin-stimulated glucose uptake. Surprisingly, this occurred without significant effect on insulin-stimulated glucose transporter (GLUT) 4 translocation or fusion with the plasma membrane. In parallel, the inhibitory actions of chronic GH pretreatment also impaired insulin-dependent activation of phosphatidylinositol (PI) 3-kinase bound to insulin receptor substrate (IRS)-2 but not to IRS-1. In addition, insulin-stimulated Akt phosphorylation was inhibited by GH pretreatment. In contrast, overexpression of IRS-2 or expression of a constitutively active Akt mutant prevented the GH-induced insulin resistance of glucose uptake. Moreover, small interfering RNA-mediated IRS-2 knockdown also inhibited insulin-stimulated Akt activation and glucose uptake without affecting GLUT4 translocation and plasma membrane fusion. Together, these data support a model in which chronic GH stimulation inhibits insulin-dependent activation of phosphatidylinositol 3-kinase through a specific interaction of phosphatidylinositol 3-kinase bound to IRS-2. This inhibition leads to suppression of Akt activation coupled to glucose transport activity but not translocation or plasma membrane fusion of GLUT4.
Insulin is the major anabolic hormone whose action plays pivotal roles in tissue development, growth, and the maintenance of glucose homeostasis. Insulin regulates glucose metabolism at several levels, reducing hepatic glucose production and increasing the rate of glucose uptake into skeletal muscle and adipose tissue. Insulin-responsive glucose transporter isoform GLUT45 is expressed in skeletal muscle and adipose tissue and is known to be responsible for the glucose uptake into these tissues (1–3). Thus, glucose uptake through GLUT4 plays a central role in the regulation of postprandial glucose clearance from the plasma. Dysfunction in the ability of insulin to stimulate glucose uptake results in states of insulin resistance that plays a major role in the development of type 2 diabetes mellitus.
Insulin binding to the extracellular domain of the insulin receptor on the plasma membrane of target cells activates its intrinsic cytoplasmic tyrosine kinase activity (4, 5). The activated insulin receptor tyrosine kinase phosphorylates a variety of substrates, including insulin receptor substrates (IRSs) and Shc (6, 7). Tyrosine phosphorylation of these substrates leads to their binding to several Src homology 2 domain-containing signaling molecules, including p85 PI 3-kinase regulatory subunit and Grb2 (6, 8). This binding resulted in activation of distinct signaling cascades, for example Rasmitogen-activated protein kinase (MAPK) and PI 3-kinase cascades (7, 9). It is well known that these two pathways are directly linked to specific downstream biological responses that account for several of the known actions of insulin. In particular, the PI 3-kinase-dependent pathway is a well established requirement for insulin-induced glucose uptake in adipose and muscle tissue (10–16).
Activated PI 3-kinase phosphorylates phosphatidylinositol and generates phosphoinositide 3,4,5-triphosphate (PIP3). PIP3 production recruits Akt kinase to the plasma membrane, where Akt is phosphorylated by phosphoinositide-dependent kinases, resulting in activation of the Akt kinase (17). Activated Akt phosphorylates various Akt substrates, including AS160, GTPase-activating protein for Rab10 (18). Recently, it was reported that phosphorylation and inhibition of AS160 and subsequent activation of small GTP-binding protein Rab10 are sufficient for GLUT4 translocation to plasma membrane (19).
Growth hormone (GH) is well known to possess bioactivities regulating both growth and metabolism. In various tissues, GH is known to mediate anti-insulin effects on glucose and lipid metabolism. Insulin resistance is often observed in acromegalic patients, and GH administration to GH-deficient patients has been observed to increase the incidence of diabetes mellitus (20–22). Recently, we have reported that the GH transgenic rat, in which human GH was highly expressed, showed an insulin-resistant phenotype in both muscle and adipose tissues (23). Similarly, chronic GH treatment in cultured 3T3-L1 adipocytes was also found to impair insulin-induced glucose uptake (24). Together, these studies indicate that chronic GH treatment can result in insulin-resistant glucose uptake in vitro as well as in vivo.
Thus, this study was undertaken to evaluate the molecular mechanism of GH-induced impairment of insulin-dependent glucose uptake in 3T3-L1 adipocytes. Here we showed that chronic GH pretreatment inhibits insulin-induced glucose uptake without affecting GLUT4 translocation, through the reduction of IRS-2-associated PI 3-kinase activity.
EXPERIMENTAL PROCEDURES
Materials—Dulbecco's modified Eagle's medium (DMEM), phosphate-buffered saline (PBS), and Hanks' buffered salt solution were purchased from Nissui (Tokyo, Japan). Calf serum and fetal bovine serum (FBS) were obtained from JRH Bioscience (Tokyo, Japan). Penicillin and streptomycin were obtained from Ban'yu Pharmaceutical Co. (Tokyo, Japan). Recombinant human GH was kindly donated from Dainippon Sumitomo Pharmaceutical Co., Ltd. (Osaka, Japan), and CR Pharmaceuticals Co., Ltd. (Kobe, Japan). Bovine insulin was obtained from Sigma. Anti-IRS-1 antibody and anti-IRS-2 antibody were raised in rabbits as described previously (25). Anti-JAK2 antibody, anti-GLUT1 antibody, anti-GLUT4 antibody, and anti-IRβ antibody were obtained from Santa Cruz Biotechnology. Anti-PI 3-kinase p85 antibody and anti-c-Myc antibody were obtained from Upstate Biotechnology, Inc. (Lake Placid, NY). Anti-phospho-Akt (Ser-473) antibody and anti-Akt antibody were obtained from Cell Signaling Technology, Inc. (Beverly, MA). Anti-phospho-Akt (Thr-308) antibody, anti-β-actin antibody, and anti-phosphotyrosine antibody were obtained from Sigma. Texas Red dye-conjugated anti-mouse IgG was obtained from Jackson ImmunoResearch (West Grove, PA). Protein A-Sepharose and 2-deoxy-d-[2,6-3H]glucose (1 mCi/ml) were purchased from Amersham Biosciences. Wheat germ agglutinin-agarose was obtained from Seikagaku Co. (Tokyo, Japan). Random control, IRS-1-, and IRS-2-specific siRNAs were purchased from RNAi Corp. (Tokyo, Japan). The sequence of the IRS-1 siRNA used was CUC GAG AGC UGU UUC AAC AUC. The sequence of the IRS-2 siRNA used was GCC CGA ACC UCA AUA ACA ACA. The control siRNA sequence was GUA CCG CAC GUC AUU CGU AUC. Other chemicals were of reagent grade available commercially.
Cell Culture of 3T3-L1 Adipocytes—Murine 3T3-L1 preadipocytes were purchased from the American Type Tissue Culture repository. 3T3-L1 preadipocytes were maintained at 37 °C in a humidified 5% CO2 controlled atmosphere in DMEM supplemented with 10% calf serum, 50 IU/ml penicillin, 50 μg/ml streptomycin, 0.5 μg/ml amphotericin B (Sankyo, Tokyo, Japan). 3T3-L1 preadipocytes were induced to differentiate into adipocytes as described previously (27). Briefly, confluent cultures were incubated with DMEM containing 10% FBS, 1 μg/ml insulin, 1 mm dexamethasone, and 0.5 mm isobutyl-1-methylxanthine. After 4 days, the medium was changed to DMEM containing 10% FBS and 1 μg/ml insulin for an additional 2 days. The medium was then changed to DMEM containing 10% FBS. Cells were used for experiments at 6–10 days after inducing differentiation, when more than 90% of cells displayed an adipocyte phenotype.
Electroporation of 3T3-L1 Adipocytes—Transient transfection of 3T3-L1 adipocytes was described previously (26). Briefly, fully differentiated 3T3-L1 adipocytes were detached from the tissue culture plates by trypsin buffer (0.25% trypsin, 0.02% EDTA in PBS) and electroporated with a total of 100 μg of plasmid or 1 nmol of siRNA using the Gene Pulser II (Bio-Rad) at 0.15 kV and 0.95 microfarad.
Measurement of Glucose Uptake—3T3-L1 adipocytes were incubated with the indicated concentrations of insulin in Krebs-Ringer phosphate buffer for 15 min at 37 °C. Then 0.1 mm 2-deoxy-d-glucose containing 10 μCi/ml 2-deoxy-d-[2,6-3H]glucose was added, and cells were incubated for 10 min at 37 °C. The reaction was terminated by addition of ice-cold PBS containing 10 mm d-glucose. Cells were lysed with 0.05 n NaOH containing 0.1% SDS, and radioactivity taken up by the cells was measured by liquid scintillation counter.
Subcellular Fractionation—3T3-L1 adipocytes were scraped and homogenized in HES buffer (20 mm Tris-HCl, pH 7.4, 255 mm sucrose, 1 mm EDTA) with 20 strokes using a Dounce homogenizer. Homogenates were centrifuged at 16,000 × g for 15 min. The resulting pellet obtained from this spin was layered onto 1.12 m sucrose in HES buffer and centrifuged at 101,000 × g for 70 min. This yielded a white fluffy band at the interface, plasma membrane fraction. The plasma membrane fraction was resuspended in HES buffer and pelleted at 48,000 × g for 20 min. The resulting pellets were resuspended in Tris/Triton lysis buffer (50 mm Tris-HCl, pH 8.0, 150 mm NaCl, 1 mm NaF, 1 mm EDTA, 1 mm EGTA, 1.5 mm MgCl2, 10% glycerol, 1% Triton X-100, 10 μg/ml leupeptin, 5 μg/ml pepstatin, 20 μg/ml phenylmethylsulfonyl fluoride, 100 kallikrein-inactivating units/ml aprotinin, 0.5 mm Na3VO4, and 10 mg/ml p-nitrophenyl phosphate).
Biotinylation of Cell Surface Protein and Isolation of Biotinylated Protein—3T3-L1 adipocytes pretreated with or without GH were stimulated with insulin. Cells were washed with icecold PBS and incubated with 0.5 mg/ml NHS-LC (succinimidyl-6-[biotinamido]hexanoate) biotin (Pierce) in PBS for 30 min at 4 °C. The reaction was stopped by rinsing the plates three times with 15 mm glycine in ice-cold PBS. The cells were then collected and solubilized with Tris/Triton lysis buffer. For isolating biotinylated protein, whole cell lysates were mixed with streptavidin-agarose beads (Sigma), and the suspension was incubated at 4 °C overnight. The streptavidin-agarose beads were spun down and washed three times with lysis buffer, once with NaCl buffer (50 mm Tris-HCl, pH 8.0, 1 m NaCl, 1 mm NaF, 1 mm EDTA, 1 mm EGTA, 1.5 mm MgCl2, 10% glycerol, 1% Triton X-100), once with 10% Tris/Triton lysis buffer in distilled water, and once with lysis buffer containing 0.1% in SDS. The pellet as biotinylated protein and supernatant as nonbiotinylated protein were collected (28–30).
Immunofluorescence and GLUT4-myc-GFP Translocation Assay—3T3-L1 adipocytes were electroporated with or without siRNA along with pGlut4-myc-green fluorescent protein (GFP). Two days after electroporation, cells were serum-starved for 2 h and treated with or without 100 nm GH followed by insulin treatment. Cells were fixed without permeabilization and immunostained with anti-Myc antibody. Cells were imaged using confocal fluorescence microscopy (OLYMPUS, Tokyo, Japan). To detect GLUT4 translocation to the cell surface, we quantified the ratio of Myc-rimed cells to GFP-transfected cells.
Analyses of Insulin Signals—3T3-L1 adipocytes were serum-starved for 2 h in DMEM containing 0.1% bovine serum albumin. Cells were treated with or without 100 nm GH followed by treatment with 0.1 nm insulin. Cells were harvested by Tris/Triton lysis buffer. After centrifugation of the homogenates at 12,000 × g for 10 min at 4 °C, supernatant was collected as whole cell lysates. The whole cell lysates were subjected to protein assay using protein assay kit (Bio-Rad). One mg of whole cell lysates was used for immunoprecipitation with indicated antibodies. Precipitants were separated by SDS-PAGE and then immunoblotted with the indicated antibodies.
PI 3-Kinase Activity Assay—PI 3-kinase activity assay was carried out as described before (31). Briefly, 3T3-L1 adipocytes were serum-starved for 2 h in DMEM containing 0.1% bovine serum albumin followed by pretreatment with or without 100 nm GH for 24 h. Cells were treated with or without 0.1 nm insulin for 10 min. Cells were lysed by Tris/Nonidet P-40 lysis buffer (50 mm Tris-HCl, pH 8.0, 150 mm NaCl, 1 mm NaF, 1 mm EDTA, 1 mm EGTA, 1.5 mm MgCl2, 10% glycerol, 1% Nonidet P-40, 10 μg/ml leupeptin, 5 μg/ml pepstatin, 20 μg/ml phenylmethylsulfonyl fluoride, 100 kallikrein-inactivating units/ml aprotinin, 0.5 mm Na3VO4, and 10 mg/ml p-nitrophenyl phosphate). One mg of whole cell lysates was immunoprecipitated with anti-IRS-1 antibody or anti-IRS-2 antibody. Immunoprecipitates were washed once with Tris/Nonidet P-40 buffer, LiCl buffer (100 mm Tris-HCl, pH 7.5, and 500 mm LiCl), distilled water, and TNE buffer (10 mm Tris-HCl, pH 7.5, 150 mm NaCl, and 1 mm EDTA) and finally resuspended in 40 μl of reaction buffer (20 mm Tris-HCl, pH 7.5, 100 mm NaCl, and 0.5 mm EGTA). Kinase reaction was initiated by incubation of immunocomplex in reaction buffer with 20 mm [γ-32P]ATP (4 μCi/mmol), 20 mm MgCl2, and 0.5 μg/μl phosphatidylinositol at 25 °C for 25 min. Reaction was stopped by adding chloroform/methanol/HCl (10:20:1). A lipid product was extracted, spotted onto silica gel plate, and developed with chloroform/methanol/NH4OH/water (43:38:6:6). 32P radioactivity incorporated into phosphatidylinositol was measured by autoradiography as PI 3-kinase activity.
Adenovirus Preparation and Infection to 3T3-L1 Cells—The recombinant adenoviruses, Ade-myr-Akt (constitutive active form of Akt) and Ade-LacZ (LacZ) were prepared described before (32). The recombinant adenovirus to express IRS-2 was constructed as described before (33). Adenoviruses were amplified in human embryonic kidney 293 cells. 3T3-L1 adipocytes were infected with adenovirus by incubating cells with 100 multiplicities of infection of adenovirus. Cells were used for experiments after 48 h of infection.
Statistical Analysis—Statistical analyses of data were performed by two-way or three-way analysis of variance followed by Fisher's PSLD post-hoc test using StatView software (Abacus Concepts, Inc., Berkeley, CA). The results shown are the mean ± S.E. p < 0.05 was considered statistically significant.
RESULTS
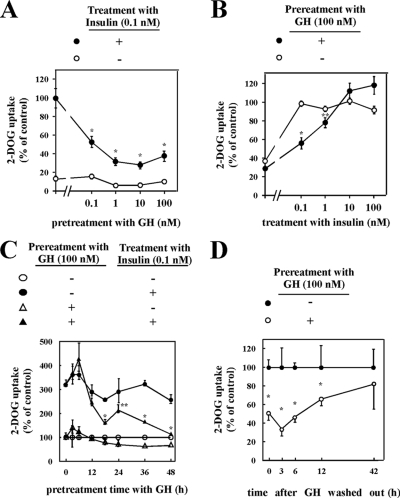
Chronic GH Pretreatment Inhibits Insulin-stimulated Glucose Uptake in 3T3-L1 Adipocytes—It has been reported that chronic GH pretreatment inhibits insulin-dependent glucose uptake in cultured adipocytes (24). To confirm these data, fully differentiated 3T3-L1 adipocytes were pretreated with various concentrations of GH for 24 h and subsequently subjected to an acute (15 min) stimulation with 0.1 nm insulin. In the absence of GH pretreatment, insulin stimulation resulted in a robust increase in glucose uptake (Fig. 1A). However, pretreatment with GH inhibited insulin-stimulated glucose uptake in a GH concentration-dependent manner. As expected, insulin-stimulated glucose uptake occurred in an insulin concentration-dependent manner in the absence of GH pretreatment (Fig. 1B). GH inhibited insulin-stimulated glucose uptake at low insulin concentrations, but at higher concentrations (10–100 nm insulin) the full extent of glucose uptake was achieved. These data demonstrate that GH pretreatment results in an insulin-dependent decrease in sensitivity (concentration) but not responsiveness (maximum effect).
FIGURE 1.
Effects of chronic GH pretreatment on insulin-induced glucose uptake. A, 3T3-L1 adipocytes were serum-starved for 2 h, pretreated with the indicated concentrations of GH for 24 h, and then stimulated without (○) or with (•) 0.1 nm insulin for 15 min. Cells were assayed for glucose uptake as described under “Experimental Procedures.” Glucose uptake by cells without GH pretreatment and with insulin stimulation was used as control. B, 3T3-L1 adipocytes were serum-starved for 2 h, pretreated without (○) or with (•) 100 nm GH for 24 h, and then treated with the indicated concentrations of insulin for 15 min. Cells were assayed for glucose uptake as described under “Experimental Procedures.” Glucose uptake by cells without GH pretreatment and with 0.1 nm insulin stimulation was used as control. C, 3T3-L1 adipocytes were pretreated without (•, ○) or with (▴, ▵) 100 nm GH for indicated periods, and then treated without (○, ▵) or with (•, ▴) 0.1 nm insulin for 15 min. Next, cells were assayed for glucose uptake as described under “Experimental Procedures.” Glucose uptake by cells without GH treatment and without insulin stimulation was used as control. The results are presented as the means ± S.E. of five wells. The difference between insulin-stimulated cells with and without GH pretreatment is significant with p < 0.01 (*) or p < 0.05 (**). D, cells were pretreated with 100 nm GH for 24 h, and GH was washed out by changing medium without GH, followed by incubation for the indicated time. Cells were treated with 0.1 nm insulin for 15 min, and glucose uptake was measured. Glucose uptake by cells without GH pretreatment was used as control. The results are presented as the means ± S.E. of five wells. The difference between cells with and without insulin stimulation is significant with p < 0.05 (*). 2-DOG, 2-deoxy-d-glucose.
We next examined the time dependence of GH pretreatment on insulin-stimulated glucose uptake (Fig. 1C). GH pretreatment for up to 48 h had no significant effect on basal glucose uptake. However, after 18 h of GH pretreatment, the extent of insulin-stimulated glucose uptake was significantly reduced. The GH reduction of insulin-stimulated glucose uptake continued to decrease with longer GH pretreatment, and by 48 h glucose uptake was completely refractory to insulin stimulation. In comparison, the effect of GH was also slowly reversible in that following 24 h of GH pretreatment, wash out of GH resulted in a time-dependent recovery of insulin-stimulated glucose uptake (Fig. 1D). Taken together, these data demonstrate that GH pretreatment of 3T3-L1 adipocytes results in a reversible, time-, and concentration-dependent inhibition of insulin-stimulated glucose uptake.
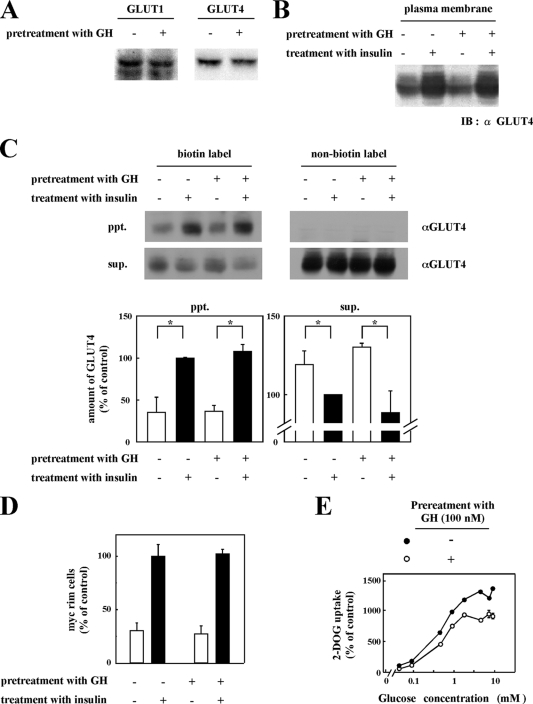
Chronic GH Pretreatment Does Not Affect Insulin-stimulated GLUT4 Translocation to Plasma Membrane—Two facilitative glucose transporters are expressed in 3T3-L1 adipocytes with the GLUT1 isoform primarily contributing to basal glucose uptake and GLUT4 to insulin-stimulated glucose uptake (2, 34). The cellular expression levels of GLUT1 and GLUT4 were unaffected by chronic GH treatment (Fig. 2A). Insulin stimulation increases glucose uptake by the translocation of the GLUT4 isoform from intracellular storage sites to the plasma membrane (35). As expected, biochemical isolation of a plasma membrane-enriched fraction demonstrated the increased appearance of the GLUT4 protein following insulin stimulation (Fig. 2B, 1st and 2nd lanes). Surprisingly however, chronic GH pretreatment had no significant effect on the extent of insulin-stimulated GLUT4 appearance in the plasma membrane fraction (Fig. 2B, 3rd and 4th lanes).
FIGURE 2.
Effects of chronic GH pretreatment on insulin-induced translocation of GLUT4 to plasma membrane. A, 3T3-L1 adipocytes pretreated with or without 100 nm GH for 24 h were lysed by lysis buffer. Lysates were carried out for immunoblotting with anti-GLUT1 antibody or anti-GLUT4 antibody. B, 3T3-L1 adipocytes were pretreated with or without 100 nm GH for 24 h and then treated with or without 0.1 nm insulin for 20 min. Subcellular fractions were prepared as described under “Experimental Procedures.” Proteins in plasma membrane fraction were separated by SDS-PAGE and immunoblotted (IB) with anti-GLUT4 antibody. C, 3T3-L1 adipocytes pretreated with or without 100 nm GH followed by treatment with 0.1 nm insulin were biotinylated by incubating with NHS-LC biotin. For isolating biotinylated protein, whole cell lysates were incubated with streptavidin-agarose beads. The pellet (ppt) as biotinylated protein and supernatant (sup.) as nonbiotinylated protein were collected. Biotinylated proteins and nonbiotinylated proteins were separated by SDS-PAGE and immunoblotted with anti-GLUT4 antibody. Under the blotting images, the quantitative data are shown. The results are presented as the means ± S.E. of three independent experiments. The difference between cells with and without insulin stimulation is significant with p < 0.05 (*). D, 3T3-L1 adipocytes were electroporated with pGLUT4-myc-GFP as described under “Experimental Procedures.” Electroporated cells pretreated with or without 100 nm GH for 24 h were treated with or without 0.1 nm insulin for 15 min. Cells were then fixed without permeabilization and immunostained with anti-Myc antibody to detect cells with GLUT4 fused to plasma membrane. Average of percentage of cells showing GLUT4-myc-GFP rim on the cell surface was calculated. The ratio of GLUT4-myc on the cell surface in insulin-stimulated cells without GH pretreatment was used as control. E, 3T3-L1 adipocytes pretreated with or without 100 nm GH for 24 h were treated with 0.1 nm insulin for 15 min. Then various concentrations (0.045, 0.091, 0.45, 0.91, 1.82, 4.5, 7.28, and 9.1 mm) of 2-deoxy-d-glucose (2-DOG) containing 10 mCi/ml 2-deoxy-d-[2,6-3H]glucose were added, and radioactivity taken up by the cells was measured. Glucose uptake by the cells with insulin stimulation without GH pretreatment and treated with 0.045 mm 2-deoxyglucose was used as control.
To determine whether GH pretreatment altered the fusion (exofacial exposure) of the GLUT4 protein with the plasma membrane, intact adipocytes were incubated with NHS-LC biotin to label plasma membrane proteins with biotin. Precipitation of cell lysates with streptavidin-agarose beads demonstrated an insulin-stimulated increase in the amount of exofacial labeled GLUT4 (Fig. 2C, 1st and 2nd lanes). Similar to the translocation assay, insulin was fully capable of inducing the plasma membrane fusion (exofacial exposure) of GLUT4 in cells chronically pretreated with GH (Fig. 2C, 3rd and 4th lanes). As controls, there was a reciprocal decrease in the amount of GLUT4 presence in the supernatant following streptavidin-agarose precipitation (Fig. 2C, bottom panel). Moreover, there was no precipitation of the GLUT4 protein by streptavidin-agarose beads without NHS-LC biotin labeling (Fig. 2C, 5th to 8th lanes). These data from three experiments are quantified in Fig. 2C, bar graphs of the lower panel. To morphologically confirm these biochemical findings, we next expressed the GLUT4 protein harboring an exofacial Myc epitope tag. 3T3-L1 adipocytes were electroporated with myc-GLUT4-GFP-expressing plasmids, and the cell exofacial exposure of the Myc epitope was determined following various treatments by confocal fluorescent microscopy (Fig. 2D). Insulin stimulation increased the amount of the exofacial exposure of the Myc epitope, and this was again unaffected by chronic GH pretreatment.
The inhibition of insulin-stimulated glucose uptake by GH pretreatment with normal GLUT4 translocation and plasma membrane fusion could have arisen from a reduction in hexokinase activity that phosphorylates 2-deoxyglucose to generate the product 2-deoxyglucose 6-phosphate. To exclude this possibility, insulin-induced glucose uptake was measured in the cells with various concentrations of glucose. Fig. 2E shows that the GH-induced inhibition of glucose uptake was independent of glucose concentration, suggesting that glucose metabolism was not inhibited by GH pretreatment. Taken together, these data indicate that chronic GH pretreatment did not affect insulin-stimulated GLUT4 translocation or exofacial exposure (fusion) with the plasma membrane but that the reduction in glucose uptake primarily reflects changes in glucose transport activity.
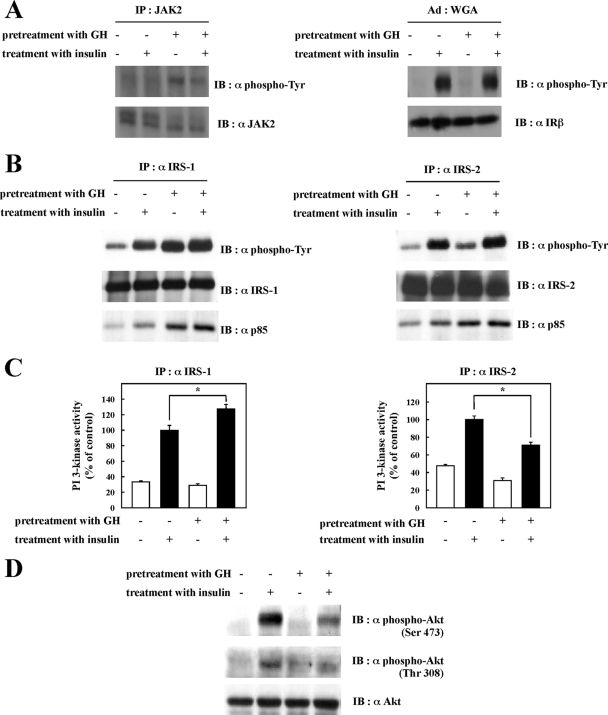
Chronic GH Pretreatment Inhibits Insulin-dependent Activation of PI 3-Kinase Bound to IRS-2 and Akt Phosphorylation—GH binding to the GH receptor activates the JAK tyrosine kinase pathway that can also signal through IRS tyrosine phosphorylation. To investigate the effect of chronic GH pretreatment on insulin signaling pathways, fully differentiated 3T3-L1 adipocytes were pretreated with 100 nm GH for 24 h followed by stimulation with 0.1 nm insulin, and the activation of signal target molecules in response to insulin stimulation was assessed. We confirmed that GH pretreatment induced JAK2 tyrosine phosphorylation, and insulin receptor tyrosine phosphorylation, which is known to reflect tyrosine kinase activation, was not changed by GH pretreatment (Fig. 3A). Insulin-induced IRS-1 and IRS-2 tyrosine phosphorylation was slightly enhanced by GH pretreatment (Fig. 3B). However, GH stimulation itself increased both IRS-1 and IRS-2 tyrosine phosphorylation. The amount of the p85 PI 3-kinase regulatory subunit associated with IRS-1 and IRS-2 was also enhanced by GH treatment, which reflected enhancement of IRS tyrosine phosphorylation (Fig. 3B). IRS-1-associated PI 3-kinase activity was also enhanced by GH pretreatment (Fig. 3C, left panel). Surprisingly, IRS-2-associated PI 3-kinase activity was suppressed by GH treatment even though the amount of PI 3-kinase associated with IRS-2 was enhanced (Fig. 3C, right panel).
FIGURE 3.
Effects of chronic GH pretreatment on insulin signal activation. After being serum-starved for 2 h, 3T3-L1 adipocytes were pretreated with or without 100 nm GH for 24 h and then treated with or without 0.1 nm insulin for 5 min. Cells were solubilized with Tris/Triton lysis buffer. A, whole cell lysates were immunoprecipitated (IP) with anti-JAK2 antibody, and the immunoprecipitates were subjected to immunoblotting (IB) with anti-phosphotyrosine or anti-JAK2 antibody (left panel). Insulin receptor was semi-purified with wheat germ agglutinin (WGA)-agarose from whole cell lysates. Semi-purified insulin receptor was separated by SDS-PAGE and immunoblotted with anti-phosphotyrosine antibody or anti-insulin receptor-β antibody (right panel). B, whole cell lysates were immunoprecipitated with anti-IRS-1 antibody or anti-IRS-2 antibody. Immunoprecipitates were separated by SDS-PAGE and immunoblotted with anti-phosphotyrosine antibody, anti-IRS-1 antibody, anti-IRS-2 antibody, or anti-p85 PI 3-kinase antibody. C, whole cell lysates were immunoprecipitated with anti-IRS-1 antibody or anti-IRS-2 antibody. PI 3-kinase activity in the immunocomplexes was measured as described under “Experimental Procedures.” PI 3-kinase activities were quantified, and the results are presented as the means ± S.E. of three independent experiments. The difference between insulin-stimulated cells with and without GH pretreatment is significant with p < 0.05 (*). D, whole cell lysates were separated by SDS-PAGE and immunoblotted with anti-phospho-Akt (Ser-473) antibody, anti-phospho-Akt (Thr-308) antibody, or anti-Akt antibody.
IRS-dependent activation of the PI 3-kinase generates the production of PIP3 that is necessary for the phosphorylation of Akt on Ser-473 and Thr-308, resulting in the activation of Akt kinase activity. As shown in Fig. 3D, Akt phosphorylation was also inhibited by chronic GH pretreatment and that inhibition correlated with the suppression of IRS-2-associated PI 3-kinase activity.
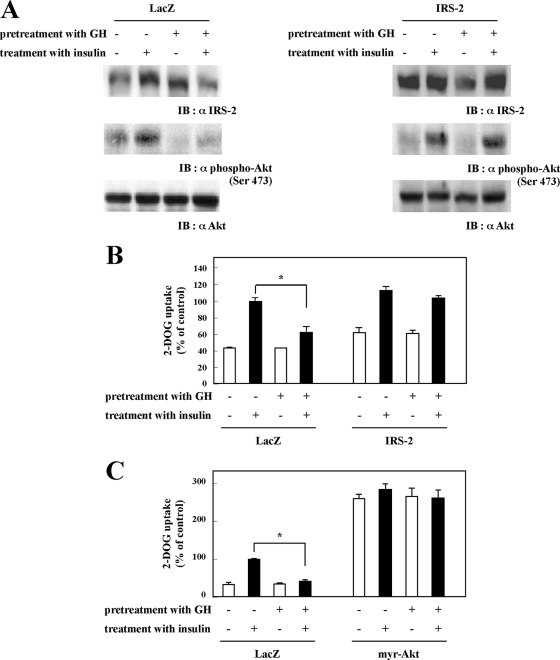
IRS-2 Mediates the GH Inhibition of Insulin-stimulated Glucose Uptake—Data presented in Fig. 3 suggest that IRS-2 coupling to Akt activation may be the target of GH that is responsible for the inhibition of insulin-stimulated glucose uptake. As observed previously, GH pretreatment suppressed insulin-stimulated Akt phosphorylation in LacZ adenovirus-infected cells (Fig. 4A, left panel). In contrast, overexpression of IRS-2 by adenovirus infection completely protected against the inhibition of insulin-stimulated Akt phosphorylation by GH pretreatment (Fig. 4A, right panel). The restoration of insulin-stimulated Akt phosphorylation by IRS-2 overexpression occurred concomitant with the recovery of insulin-stimulated glucose uptake in the GH-pretreated cells (Fig. 4B). In parallel, expression of a constitutively active Akt mutant (myr-Akt) resulted in the basal increase in glucose uptake that was no longer insulin-sensitive (Fig. 4C). Importantly, myr-Akt expression completely protected the cells from the GH inhibition of insulin-stimulated glucose uptake.
FIGURE 4.
Effects of IRS-2 or myr-Akt overexpression on glucose uptake. 3T3-L1 adipocytes were infected with adenovirus containing cDNA encoding LacZ or IRS-2. Forty eight hours after infection, cells were serum-starved for 2 h and then pretreated with or without 100 nm GH for 24 h, followed by stimulation with or without 0.1 nm insulin for 20 min. A, cells were solubilized with Tris/Triton lysis buffer. Whole cell lysates were separated by SDS-PAGE and immunoblotted (IB) with anti-phospho-Akt (Ser-478) antibody and anti-Akt antibody. B, cells were assayed for glucose uptake as described under “Experimental Procedures.” The results are presented as the means ± S.E. of five wells. The difference between insulin-stimulated cells with and without GH pretreatment is significant with p < 0.01 (*). C, 3T3-L1 adipocytes were infected with adenovirus containing cDNA encoding LacZ or myristoylated Akt (myr-Akt). Forty eight hours after infection, cells were serum-starved for 2 h, then pretreated with or without 100 nm GH for 24 h followed by stimulation with or without 0.1 nm insulin for 20 min, and assayed for glucose uptake as described under “Experimental Procedures.” The results are presented as the means ± S.E. of five wells. The difference between insulin-stimulated cells with and without GH pretreatment is significant with p < 0.01 (*).
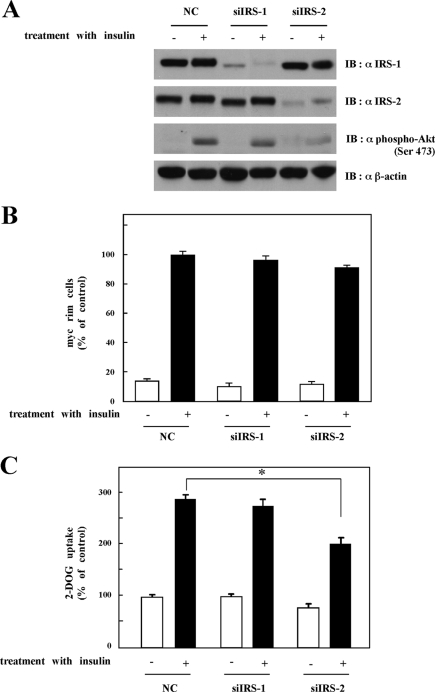
If IRS-2-dependent regulation of Akt activation is the site of GH-induced insulin resistance, then reduction of IRS-2 levels should mimic the effect of GH pretreatment. To test this hypothesis, we reduced IRS-1 or IRS-2 protein levels by RNA interference-mediated gene silencing (Fig. 5). 3T3-L1 adipocytes were electroporated with an IRS-1- or IRS-2-specific siRNA that resulted in a marked reduction in targeted IRS protein levels without any significant effect on the other IRS protein level (Fig. 5A). IRS-1 knockdown had no significant effect on insulin-stimulated Akt activation, GLUT4 translocation, or glucose uptake (Fig. 5, A–C). On the other hand, in IRS-2 knockdown cells, there was a reduction in insulin-stimulated Akt phosphorylation (Fig. 5A). Although GLUT4 translocation to plasma membrane in response to insulin was not inhibited by IRS-2 knockdown (Fig. 5B), insulin-induced glucose uptake was significantly inhibited by the reduction in IRS-2 protein levels similar to that observed for chronic GH pretreatment (Fig. 5C).
FIGURE 5.
Effects of IRS-2 knockdown on Akt activation and glucose uptake. A, 3T3-L1 adipocytes were electroporated with siRNA against nonrelevant control (NC), against IRS-1 (siIRS-1), or against IRS-2 (siIRS-2) as described under “Experimental Procedures.” Twenty four hours after electroporation, cells were serum-starved for 24 h. After washing, cells were treated with or without 100 nm insulin for 20 min. Cells were solubilized with Tris/Triton lysis buffer. Whole cell lysates were separated by SDS-PAGE and immunoblotted (IB) with anti-IRS-1 antibody, anti-IRS-2 antibody, anti-phospho-Akt (Ser-473), or anti-β-actin antibody. B, 3T3-L1 adipocytes were electroporated with siRNA against nonrelevant control (NC), against IRS-1 (siIRS-1), or against IRS-2 (siIRS-2) along with pGLUT4-myc-GFP. Twenty four hours after electroporation, cells were serum-starved for 2 h and then treated with or without 100 nm insulin for 20 min. Cells were then fixed without permeabilization and immunostained with anti-Myc antibody to detect cells with GLUT4 fused to plasma membrane. Average of percentage of cells showing GLUT4-myc-GFP rim on the cell surface was calculated. The ratio of GLUT4-myc on the cell surface in insulin-stimulated nonrelevant control cells was used as control. C, 3T3-L1 adipocytes were electroporated with siRNA against nonrelevant control (NC), against IRS-1 (siIRS-1), or against IRS-2 (siIRS-2). Cells were assayed for glucose uptake as described under “Experimental Procedures.” The results are presented as the means ± S.E. of five wells. Glucose uptake by the nonrelevant control cells without insulin was used as control. The difference between insulin-stimulated cells electroporated with siRNA against nonrelevant control and IRS-2 is significant with p < 0.001 (*).
DISCUSSION
In this study, we have observed that chronic GH pretreatment does not impair insulin-stimulated GLUT4 translocation to plasma membrane, yet results in a reduction in insulin-stimulated glucose uptake. Recently, several studies have also observed an apparent uncoupling of GLUT4 translocation and glucose uptake. For example, in L6 rat skeletal muscle cells, high leptin levels reduced insulin-stimulated glucose uptake despite normal GLUT4 translocation (36). Nelson et al. (37) suggested that, in 3T3-L1 adipocytes, high glucose levels impaired GLUT4 intrinsic activity. Smith et al. (38) reported that genistein, an inhibitor of tyrosine kinase, inhibited insulin-stimulated glucose transport without affecting translocation of GLUT4 in isolated rat adipocytes. PBP10, a rhodamine B-labeled 10-amino-acid peptide, which binds to phosphoinositides, itself induced GLUT4 translocation to the plasma membrane, without any increase in glucose uptake (39). Taken together with these reports, our data indicate that GLUT4 translocation and fusion with the plasma membrane are not sufficient to enhance glucose uptake per se but that additional activation steps are required. Thus, by comparing glucose uptake with GLUT4 translocation, we have successfully separated GLUT4-mediated glucose uptake into GLUT4 translocation step and activation step.
Previously, we have reported that adipose and muscle tissue isolated from human GH transgenic rat display insulin resistance and proposed that this resulted from an impairment of GLUT4 activation in vivo (23). To examine the potential mechanism(s), we determined the effect of GH pretreatment in cultured 3T3-L1 adipocytes. The current data demonstrate that chronic GH pretreatment impaired insulin-induced activation of PI 3-kinase bound to IRS-2, but not to IRS-1, leading to inhibition of insulin-induced glucose uptake without affecting GLUT4 translocation. IRS-2 overexpression or myr-Akt expression restored GH-induced impairment of insulin-dependent glucose uptake. In addition, IRS-2 knockdown showed similar phenotype to chronic GH pretreatment. These data revealed that chronic GH pretreatment reduced IRS-2-PI 3-kinase-Akt pathway, and this reduction was the reason why glucose uptake was impaired in GH-pretreated cells. Consistent with this interpretation, IRS-1 knockdown did not affect insulin-induced glucose uptake, suggesting that it is possible that PI 3-kinase associated with IRS-1 or IRS-2 facilitates different roles in glucose uptake. Similarly, studies using siRNA-mediated gene silencing revealed different functions for IRS-1 and IRS-2 in L6 myotube cells (40). We have reported that IRS-1 and IRS-2 showed different localization in COS-7 cells (41), suggesting that IRS-1 and IRS-2 are not functionally identical and therefore could result in differential spatial localization of PI 3-kinase.
Analyses of insulin signal activation revealed that chronic GH treatment impaired IRS-2-associated PI 3-kinase activity even though the amount of PI 3-kinase p85 regulatory subunit bound to IRS-2 was enhanced. There are several alternative hypotheses that might account for the suppression of IRS-2-associated PI 3-kinase activity despite a physical increase in the amount of PI 3-kinase binding. First, some proteins, which bind to IRS-2, but not to IRS-1, in a GH-dependent manner, inhibited PI 3-kinase activation. It is well known that several proteins, including PTEN, that have phosphatidylinositol phosphatase activity antagonize the PI 3-kinase activity. These proteins could be candidates for GH-induced IRS-2-associated protein. Alternatively, chronic GH treatment induced post-translational modification of IRS-2 and not IRS-1 leading to the inability of IRS-2 to activate PI 3-kinase. Recently, we reported that IRS-associated protein, 53BP2S, impaired GLUT4 translocation to the plasma membrane (26). Thus proteins that bind to IRS might modulate the insulin signals through inhibition of IRS-2-associated PI 3-kinase activity.
Surprisingly, chronic GH pretreatment or IRS-2 knockdown impaired insulin-induced activation of Akt but did not affect GLUT4 translocation to plasma membrane. It is well established that insulin results in a robust activation of Akt that is in larger excess than required for GLUT4 translocation (42–44). Thus it is likely that a low level of Akt activation is sufficient for GLUT4 translocation, whereas greater Akt activity is required for activation of glucose uptake. In addition, it is also possible that appropriate subcellular localization of Akt activation is required to engage substrate required for GLUT4 activation. Previously, we reported that IRS-1 and IRS-2 are localized differently and thus altering the balance of Akt association with these docking proteins by chronic GH treatment could result in different substrate accessibilities. This disturbance would then account for the inability of activated Akt to phosphorylate Akt substrates essential for GLUT4 activation. Preliminary data suggest that chronic GH pretreatment suppressed insulin-stimulated Akt activation and substrate phosphorylation in the plasma membrane fraction with relatively normal Akt activation in the low density microsome fraction, in which GLUT4 vesicle is enriched.6 Identification of plasma membrane Akt substrates, whose phosphorylation is suppressed by chronic GH pretreatment, is one approach that may resolve the potential differential signaling pathways of IRS-1- and IRS-2-mediated Akt activation.
Although additional studies will be required to resolve these issues, our data clearly demonstrate that chronic GH pretreatment of adipocytes impairs GLUT4 glucose transport activity without affecting translocation to the plasma membrane. In addition, chronic GH pretreatment inhibits IRS-2-associated PI 3-kinase leading to an impairment of GLUT4 transport activity on the plasma membrane.
Acknowledgments
We thank Dr. Takaaki Aoyagi (Institute of Microbial Chemistry, Tokyo, Japan) for leupeptin and pepstatin. We acknowledge the helpful discussion with Dr. Asako Takenaka (Meiji University, Kanagawa, Japan), and we thank Dr. Susan H. Hall (School of Medicine, University of North Carolina, Chapel Hill) for the help in preparing this manuscript.
This work was supported, in whole or in part, by a National Institutes of Health grant (to J. E. P.). This work was also supported by a grant-in-aid for international joint research (to S.-I. T.) from Japan Society for the Promotion of Science, Grants-in-aid for Scientific Research (B)(2) 11460126, Exploratory Research 15658080, and Scientific Research (A)(2) 16208028) from the Ministry of Education, Science and Culture of Japan (to S.-I. T.), and by the Program for Promotion of Basic Research Activities for Innovative Biosciences (to F. H.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: GLUT, glucose transporter; GH, growth hormone; PI, phosphatidylinositol; IRS, insulin receptor substrate; PIP3, phosphoinositide 3,4,5-triphosphate; PDK, phosphoinositide-dependent kinase; DMEM, Dulbecco's modified Eagle's medium; PBS, phosphate-buffered saline; FBS, fetal bovine serum; NC, nonrelevant control; GFP, green fluorescent protein; PI, phosphatidylinositol; siRNA, small interfering RNA.
T. Ogata, K. Kasahara, F. Hakuno, and S.-I. Takahashi, unpublished data.
References
- 1.Bryant, N. J., Govers, R., and James, D. E. (2002) Nat. Rev. Mol. Cell Biol. 3 267–277 [DOI] [PubMed] [Google Scholar]
- 2.Ducluzeau, P. H., Fletcher, L. M., Vidal, H., Laville, M., and Tavare, J. M. (2002) Diabetes Metab. 28 85–92 [PubMed] [Google Scholar]
- 3.Watson, R. T., Kanzaki, M., and Pessin, J. E. (2004) Endocr. Rev. 25 177–204 [DOI] [PubMed] [Google Scholar]
- 4.Gronborg, M., Wulff, B. S., Rasmussen, J. S., Kjeldsen, T., and Gammeltoft, S. (1993) J. Biol. Chem. 268 23435–23440 [PubMed] [Google Scholar]
- 5.Ullrich, A., and Schlessinger, J. (1990) Cell 61 203–212 [DOI] [PubMed] [Google Scholar]
- 6.Skolnik, E. Y., Lee, C. H., Batzer, A., Vicentini, L. M., Zhou, M., Daly, R., Myers, M. J., Jr., Backer, J. M., Ullrich, A., White, M. F., and Schlessinger, J. (1993) EMBO J. 12 1929–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White, M. F. (1997) Diabetologia 40 Suppl. 2, 2–17 [DOI] [PubMed] [Google Scholar]
- 8.Backer, J. M., Myers, M. G., Jr., Shoelson, S. E., Chin, D. J., Sun, X. J., Miralpeix, M., Hu, P., Margolis, B., Skolnik, E. Y., Schlessinger, J., and White, M. F. (1992) EMBO J. 11 3469–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White, M. F. (2002) Am. J. Physiol. 283 E413–E422 [DOI] [PubMed] [Google Scholar]
- 10.Cheatham, B., Vlahos, C. J., Cheatham, L., Wang, L., Blenis, J., and Kahn, C. R. (1994) Mol. Cell. Biol. 14 4902–4911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clarke, J. F., Young, P. W., Yonezawa, K., Kasuga, M., and Holman, G. D. (1994) Biochem. J. 300 631–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang, T., Sweeney, G., Rudolf, M. T., Klip, A., Traynor-Kaplan, A., and Tsien, R. Y. (1998) J. Biol. Chem. 273 11017–11024 [DOI] [PubMed] [Google Scholar]
- 13.Kotani, K., Carozzi, A. J., Sakaue, H., Hara, K., Robinson, L. J., Clark, S. F., Yonezawa, K., James, D. E., and Kasuga, M. (1995) Biochem. Biophys. Res. Commun. 209 343–348 [DOI] [PubMed] [Google Scholar]
- 14.Martin, S. S., Haruta, T., Morris, A. J., Klippel, A., Williams, L. T., and Olefsky, J. M. (1996) J. Biol. Chem. 271 17605–17608 [DOI] [PubMed] [Google Scholar]
- 15.Okada, T., Kawano, Y., Sakakibara, T., Hazeki, O., and Ui, M. (1994) J. Biol. Chem. 269 3568–3573 [PubMed] [Google Scholar]
- 16.Sharma, P. M., Egawa, K., Huang, Y., Martin, J. L., Huvar, I., Boss, G. R., and Olefsky, J. M. (1998) J. Biol. Chem. 273 18528–18537 [DOI] [PubMed] [Google Scholar]
- 17.Galetic, I., Andjelkovic, M., Meier, R., Brodbeck, D., Park, J., and Hemmings, B. A. (1999) Pharmacol. Ther. 82 409–425 [DOI] [PubMed] [Google Scholar]
- 18.Sano, H., Kane, S., Sano, E., Miinea, C. P., Asara, J. M., Lane, W. S., Garner, C. W., and Lienhard, G. E. (2003) J. Biol. Chem. 278 14599–14602 [DOI] [PubMed] [Google Scholar]
- 19.Sano, H., Eguez, L., Teruel, M. N., Fukuda, M., Chuang, T. D., Chavez, J. A., Lienhard, G. E., and McGraw, T. E. (2007) Cell Metab. 5 293–303 [DOI] [PubMed] [Google Scholar]
- 20.Beck, P., Schalch, D. S., Parker, M. L., Kipnis, D. M., and Daughaday, W. H. (1965) J. Lab. Clin. Med. 66 366–379 [PubMed] [Google Scholar]
- 21.Cerasi, E., and Luft, R. (1964) Lancet 2 769–771 [DOI] [PubMed] [Google Scholar]
- 22.Yuen, K., Cook, D., Ong, K., Chatelain, P., Fryklund, L., Gluckman, P., Ranke, M. B., Rosenfeld, R., and Dunger, D. (2002) Clin. Endocrinol. 57 333–341 [DOI] [PubMed] [Google Scholar]
- 23.Cho, Y., Ariga, M., Uchijima, Y., Kimura, K., Rho, J. Y., Furuhata, Y., Hakuno, F., Yamanouchi, K., Nishihara, M., and Takahashi, S. (2006) Endocrinology 147 5374–5384 [DOI] [PubMed] [Google Scholar]
- 24.Takano, A., Haruta, T., Iwata, M., Usui, I., Uno, T., Kawahara, J., Ueno, E., Sasaoka, T., and Kobayashi, M. (2001) Diabetes 50 1891–1900 [DOI] [PubMed] [Google Scholar]
- 25.Ariga, M., Nedachi, T., Akahori, M., Sakamoto, H., Ito, Y., Hakuno, F., and Takahashi, S. (2000) Biochem. J. 348 409–416 [PMC free article] [PubMed] [Google Scholar]
- 26.Hakuno, F., Kurihara, S., Watson, R. T., Pessin, J. E., and Takahashi, S. (2007) J. Biol. Chem. 282 37747–37758 [DOI] [PubMed] [Google Scholar]
- 27.Elmendorf, J. S., Chen, D., and Pessin, J. E. (1998) J. Biol. Chem. 273 13289–13296 [DOI] [PubMed] [Google Scholar]
- 28.Hare, J. F., and Lee, E. (1989) Biochemistry 28 574–580 [DOI] [PubMed] [Google Scholar]
- 29.Ross, S. A., Scott, H. M., Morris, N. J., Leung, W. Y., Mao, F., Lienhard, G. E., and Keller, S. R. (1996) J. Biol. Chem. 271 3328–3332 [DOI] [PubMed] [Google Scholar]
- 30.Sasson, S., Kaiser, N., Dan-Goor, M., Oron, R., Koren, S., Wertheimer, E., Unluhizarci, K., and Cerasi, E. (1997) Diabetologia 40 30–39 [DOI] [PubMed] [Google Scholar]
- 31.Nedachi, T., Akahori, M., Ariga, M., Sakamoto, H., Suzuki, N., Umesaki, K., Hakuno, F., and Takahashi, S. I. (2000) Endocrinology 141 2429–2438 [DOI] [PubMed] [Google Scholar]
- 32.Sakoda, H., Ogihara, T., Anai, M., Funaki, M., Inukai, K., Katagiri, H., Fukushima, Y., Onishi, Y., Ono, H., Fujishiro, M., Kikuchi, M., Oka, Y., and Asano, T. (2000) Diabetes 49 1700–1708 [DOI] [PubMed] [Google Scholar]
- 33.Miyake, S., Makimura, M., Kanegae, Y., Harada, S., Sato, Y., Takamori, K., Tokuda, C., and Saito, I. (1996) Proc. Natl. Acad. Sci. U. S. A. 93 1320–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scheepers, A., Joost, H. G., and Schurmann, A. (2004) JPEN J. Parenter. Enteral. Nutr. 28 364–371 [DOI] [PubMed] [Google Scholar]
- 35.Watson, R. T., and Pessin, J. E. (2001) Recent Prog. Horm. Res. 56 175–193 [DOI] [PubMed] [Google Scholar]
- 36.Sweeney, G., Keen, J., Somwar, R., Konrad, D., Garg, R., and Klip, A. (2001) Endocrinology 142 4806–4812 [DOI] [PubMed] [Google Scholar]
- 37.Nelson, B. A., Robinson, K. A., and Buse, M. G. (2000) Diabetes 49 981–991 [DOI] [PubMed] [Google Scholar]
- 38.Smith, R. M., Tiesinga, J. J., Shah, N., Smith, J. A., and Jarett, L. (1993) Arch. Biochem. Biophys. 300 238–246 [DOI] [PubMed] [Google Scholar]
- 39.Funaki, M., Randhawa, P., and Janmey, P. A. (2004) Mol. Cell. Biol. 24 7567–7577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang, C., Thirone, A. C., Huang, X., and Klip, A. (2005) J. Biol. Chem. 280 19426–19435 [DOI] [PubMed] [Google Scholar]
- 41.Kabuta, T., Hakuno, F., Asano, T., and Takahashi, S. (2002) J. Biol. Chem. 277 6846–6851 [DOI] [PubMed] [Google Scholar]
- 42.Guilherme, A., and Czech, M. P. (1998) J. Biol. Chem. 273 33119–33122 [DOI] [PubMed] [Google Scholar]
- 43.Kim, Y. B., Nikoulina, S. E., Ciaraldi, T. P., Henry, R. R., and Kahn, B. B. (1999) J. Clin. Investig. 104 733–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Venable, C. L., Frevert, E. U., Kim, Y. B., Fischer, B. M., Kamatkar, S., Neel, B. G., and Kahn, B. B. (2000) J. Biol. Chem. 275 18318–18326 [DOI] [PubMed] [Google Scholar]