Abstract
Vascular endothelial growth factor (VEGF) is a potent mitogen and permeability factor for endothelial cells that plays a central role in angiogenesis, vascular maintenance, inflammation, and cancer. VEGF also mediates the homeostatic adaptation to hypoxic conditions by promoting an increase in vascular density to compensate for decreased oxygenation. This process is triggered by an oxygen-sensitive transcription factor, hypoxia-inducible factor-1 (HIF1α), which becomes active in hypoxic tissues, leading to the synthesis and secretion of VEGF. The role of HIF1α in other processes that involve angiogenesis such as in inflammation is less clear. Of interest, endothelial cells not only respond to but also store and secrete VEGF, which is required for the maintenance of the integrity of the vascular system. How this intracellular pool of VEGF is regulated is still not understood. Here, we found that CXCL8/IL8, a potent proangiogenic and inflammatory chemokine, up-regulates VEGF mRNA and protein levels in endothelial cells by acting on its cognate receptor, CXCR2, and that this results in the autocrine activation of VEGFR2. Surprisingly, this process does not involve HIF1α but instead requires the activation of the transcription factor NFκB. Furthermore, we identified the components of the CBM complex, Carma3, Bcl10, and Malt1, as key mediators of the CXCL8/IL8-induced NFκB activation and VEGF up-regulation. Together, these findings support the existence of an NFκB-mediated pathway by which the proinflammatory chemokine CXCL8/IL8 controls the expression of VEGF in endothelial cells, thereby promoting the activation of VEGF receptors in an autocrine fashion.
The members of the vascular endothelial growth factor (VEGF)2 family of proteins are members of the platelet-derived growth factor superfamily of polypeptide growth factors, which bind and activate their cognate tyrosine kinase receptors, VEGF receptors 1, 2, and 3 (VEGFR1, VEGFR2, and VEGFR3), thereby promoting the proliferation, survival, and migration of endothelial cells (1). These molecules are essential for vasculogenesis and angiogenesis during development and are required for vascular maintenance in adults (2). VEGF-A, referred herein as VEGF, is the best studied member of the family and has been extensively implicated in a multitude of physiological and pathological conditions (3). The production and secretion of VEGF is regulated by a now well understood mechanism by which cells deprived of oxygen induce the expression of this potent angiogenic factor through the activation of the hypoxia-inducible factor-1 (HIF1), which in turn increases the formation of blood vessels and thus restores the supply of nutrients and oxygen (4). The HIF1 transcription factor is composed of two subunits, HIF1α and HIF1β, the former being synthesized in most cells but rapidly degraded by a mechanism that involves its oxygen-dependent ubiquitination and degradation in the proteasome (4). Under hypoxic conditions, HIF1α accumulates and translocates to the nucleus where it binds together with HIF1β to specific hypoxia-responsive elements (HREs), thereby activating the transcription of target genes. The crucial role of HIF1 in VEGF regulation is underscored by the observation that homozygous deletion of HIF1α results in embryonic lethality at midgestation (embryonic day 10.5) due to severely impaired vascular formation among other abnormalities (5).
Recent elegant studies in which the VEGFA gene was conditionally deleted in endothelial cells in adult mice revealed that these cells not only respond to VEGF but also express VEGF and that the latter is required for the maintenance of the integrity of the vascular system (2). In addition, accumulating evidence suggests that HIF1-independent mechanisms regulating VEGF expression may also exist (6). For instance, the proinflammatory chemokine CXCL8/IL8 can rescue the proangiogenic phenotype in HIF1α-deficient cancer cells, probably by preserving VEGF expression and secretion by a HIF1α-independent mechanism (7). As how the endogenous pool of VEGF in endothelial cells is regulated is still unknown, these findings prompted us to examine whether CXCL8/IL8 may control VEGF expression in endothelial cells. Indeed, we show here that CXCL8/IL8 promotes the NFκB-dependent autocrine activation of VEGFR2 receptors in endothelial cells by modulating their intracellular VEGF pool, which in turn may contribute to the proangiogenic response to inflammatory processes.
EXPERIMENTAL PROCEDURES
Cell Lines, Tissue Culture, and Transfections—SV40-immortalized murine endothelial cells (SVEC4–10) where purchased from the ATCC (Manassas, VA). Cell culture, DNA transfections using ExGen 500 (Fermentas, Hanover, MD) and transfection of siRNAS (Qiagen) at a final concentration of 50 nm using HiPerFect (Qiagen) were performed as described (8). At least two target sequences were used for each knockdown experiment: Carma3, Mm_Card10_1 and_2; Bcl10, Mm_Bcl10_1 and _2; Malt1, Mm_Malt1_1 and _2; TAK1, Mm_Map3k7_1 and _2; HIF1α, Mm_HIF1α_1 and _2. pGIPZ shRNA lentivectors targeting murine CXCR2 were from Open Biosystems (Huntsville, AL) (RMM4431-99211322). Lentiviral production was performed using standard protocols.
Reporter Activity, Nuclear NFκB Assays, and VEGF Levels—SVEC cells were transfected with different expression vectors in combination with 100 ng of a 5× NFκB luciferase reporter (Stratagene, La Jolla, CA) or a human VEGFA promoter, pGL3 VEGFA(–2274 + 379) (9) and 50 ng of pCEFLmyc humanized Renilla luciferase (hRL) enhanced GFP, an EF-1α-driven Renilla reniformis for normalization. Luciferase activity and NFκB binding assays using nuclear extracts (TransAM NFκB p65, Active Motif, Carlsbad, CA) were performed as described (10). VEGF levels in serum-free cell supernatants were determined using the mouse VEGF Quantikine immunoassay (R&D Systems, Minneapolis, MN).
Immunoblot Assays—Western blotting was performed as described previously (10). Rabbit polyclonal anti-Carma3 was purchased from Imgenex (San Diego, CA). Goat polyclonal anti TAK1 (M-17), rabbit polyclonal anti-Bcl 10 (H-197), and Malt1 (H-300) and mouse monoclonal antibodies anti-VEGF (C-1) and anti-tubulin (TU-02) were from Santa Cruz Biotechnology (Santa Cruz, CA), and mouse monoclonal p-IκBα (Ser-32/36), rabbit polyclonal anti-phosphorylated IKK (α/β), anti-IKKβ, and anti IκBα were from Cell Signaling Technology, Inc (Danvers, MA). Goat anti-rabbit and anti-mouse secondary antibodies were from Southern Biotech (Birmingham, AL). Representative Western blots of experiments that were repeated 3–4 times are shown in every case.
Quantitative PCR—Total RNA was isolated from cultures and processed as described (10). One μg of cDNA was used as template for quantitative PCR using iQ SYBR Green Supermix (Bio-Rad). Samples were analyzed using a Bio-Rad iCycler iQ multicolor real-time PCR detection system. Oligonucleotides used for amplification were: murine Vegfa, forward, 5′-atgaactttctgctctcttgggtg-3′, and reverse, 5′-gacttctgctctccttctgtcgtg-3′; murine Gapdh, forward, 5′-gaaggtgaaggtcggagtc-3′, and reverse, 5′-gaagatggtgatgggatttc-3′.
Statistical Analysis—ANOVA followed by the Tukey t test was used to analyze the differences between experimental groups. Data analysis was performed using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA).
RESULTS AND DISCUSSION
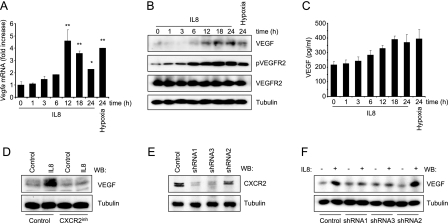
As CXCL8/IL8 is a proangiogenic chemokine (11), we asked whether this inflammatory mediator affects the endogenous levels of VEGF in endothelial cells. Using immortalized murine endothelial cells in monolayer cultures, we observed that CXCL8/IL8 promotes the accumulation of Vegfa mRNA and protein in endothelial cells under normoxic conditions, reaching levels comparable with those provoked by the exposure to hypoxia (1% O2) (Fig. 1, A and B). This effect was reflected by the enhanced secretion of VEGF to the culture media (Fig. 1C). However, the release of VEGF was more limited, in response to both CXCL8/IL8 and hypoxia, in line with the existence of an intracellular pool of VEGF in endothelial cells (2). Increased expression of VEGF in response to CXCL8/IL8 was paralleled by the accumulation of the active, phosphorylated form of VEGFR2, to an extent similar to that achieved under hypoxic conditions (Fig. 1B). To confirm that the effect of CXCL8/IL8 was dependent on the activation of its cognate receptors, we examined whether the induction of VEGF expression was sensitive to the blockade by a specific CXCR2 inhibitor, SB-225002. As shown in Fig. 1D, SB-225002 prevented the increase of VEGF levels in response to IL8. We further confirmed these results by knocking down CXCR2 using specific shRNAs. In cells where CXCR2 was effectively knocked down, CXCL8/IL8-mediated up-regulation of VEGF was impaired (Fig. 1E).
FIGURE 1.
CXCL8/IL8 induces the up-regulation of VEGF in endothelial cells through CXCR2. A, serum-starved SVEC cells were treated for the indicated times with 100 ng/ml CXCL8/IL8 or exposed to 1% O2. Vegfa mRNA levels were determined by quantitative PCR, and cellular (B) and secreted (C) VEGF levels were determined in lysates and culture media, respectively. pVEGFR2, phosphorylated VEGFR2. D, serum-starved SVEC cells were pretreated (30 min) with vehicle (Control) or 50 nm SB-225002 (CXCR2inh), stimulated for 12 h with phosphate-buffered saline (Control) or 100 ng/ml CXCL8/IL8, and analyzed by Western blot (WB). E, SVEC cells were infected with lentiviruses encoding for GFP and murine CXCR2 shRNA or control shRNA. Cells were selected by fluorescence-activated cell sorter. Two groups showed diminished CXCR2 levels (shRNA1 and shRNA3) by Western blot analysis, whereas another displayed inefficient knockdown (shRNA2) and served as an additional control. F, analysis of VEGF up-regulation in response to 12 h of treatment with 100 ng/ml CXCL8/IL8 in SVECs with different CXCR2 expression. ANOVA test, *, p < 0.01, **, p < 0.001.
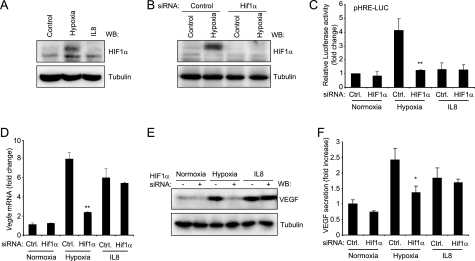
In most cell types, including tumor cells, VEGF secretion is up-regulated in response to hypoxia through the HIF1α/HIF1β transcription factor. As hypoxic conditions are unlikely to occur in capillary endothelial cells, which are exposed to the general circulation and have a similar PO2 as arterial blood (12), we wanted to address the contribution of HIF1 to CXCL8/IL8 induction of VEGF expression and secretion in endothelial cells. First, as shown in Fig. 2A, hypoxia treatment induced HIF1α accumulation, likely reflecting its stabilization, but CXCL8/IL8-treated cells did not. Furthermore, using HIF1α siRNA that led to the selective knockdown of HIF1α (Fig. 2B), we observed that hypoxia, but not CXCL8/IL8, induced an HRE reporter system (9) (Fig. 2C). However, under conditions in which HIF1α function is inhibited, CXCL8/IL8 still retained the ability to induce a large increase in VEGFA promoter-driven transcription (Fig. 2D), as well as Vegfa mRNA and protein levels (Fig. 2, E and F), thus strongly suggesting that the mechanism by which CXCL8/IL8 induces VEGF in endothelial cells is HIF1-independent.
FIGURE 2.
CXCL8/IL8 induces VEGF expression independently of HIF1α. A, SVEC cells were subjected to hypoxia or treated with 100 ng/ml for 12 h as in Fig. 1. Total cell lysates were analyzed by Western blot (WB) against HIF1α. B, SVEC cells were transfected with control (Control) or HIF1α siRNA, and 5 days later, they were subjected to hypoxia for 12 h and analyzed by Western blotting against HIF1α. C, parallel cells were transfected on day 4 with an HRE reporter plasmid (pHRE-Luc) and a Renilla luciferase expression vector. Cells were exposed to hypoxia or treated with 100 ng/ml CXCL8/IL8 for 12 h. Firefly luciferase activity was determined and normalized against Renilla luciferase counts for each sample. D, Vegfa mRNA was analyzed as in Fig. 1 in cells transfected with control or HIF1α siRNA and exposed to hypoxia or 100 ng/ml CXCL8/IL8 for 12 h. Results are shown as-fold change when compared with control siRNA-transfected cells maintained in normoxia. E, Western blot against VEGF from cells treated as in panel D is shown. F, the levels of VEGF in supernatants from cells treated as in panel D are expressed as -fold increase respect to control siRNA-transfected cells maintained in normoxia. ANOVA test, *, p < 0.01.
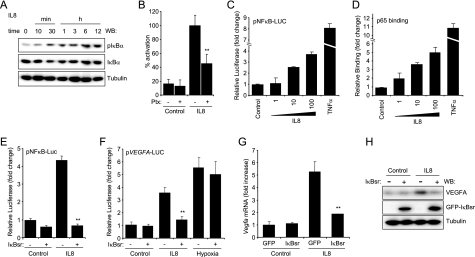
The murine Vegfa promoter contains, in addition to the hypoxia-responsive elements, two putative proximal NFκB-response elements (13), which prompted us to examine whether this transcription factor contributes to VEGF expression in response to CXCL8/IL8. Activation of the NFκB family of transcription factors involves two main molecular routes described as canonical and non-canonical pathways. Ultimately these mechanisms converge on the activation of the IKK complex, formed by the kinases IKKα and IKKβ and the regulatory subunit IKKγ (14), leading to the phosphorylation of the IκB inhibitory proteins and nuclear translocation of the NFκB dimers, which regulate the expression of multiple genes (14). Of interest, CXCL8/IL8 is a prototypical target of NFκB, but whether this proinflammatory cytokine induces NFκB remains largely unexplored. Thus, we first examined whether CXCL8/IL8 induces NFκB activation. As shown in Fig. 3A, CXCL8/IL8 rapidly increased the phosphorylation of IKBα with the concomitant decrease on the levels of IκB followed by the recovery of IκB at later time points due to the NFκB transcriptional response of the IκB promoter (15). The increased phosphorylation of IκB was significantly inhibited by pertussis toxin treatment (Fig. 3B), suggesting the contribution of a G protein of the Gi family in this response. The activation of NFκB in response to CXCL8/IL8 was reflected by increased NFκB-driven promoter expression (Fig. 3C) and NFκB nuclear binding activity to its response elements (Fig. 3D). These responses were also significantly inhibited by pertussis toxin treatment (not shown). In all cases, the potent NFκB inducer TNFα was used as a control.
FIGURE 3.
CXCL8/IL8 induces VEGF expression through an NFκB-dependent mechanism. A, time course experiment from SVEC cells stimulated with 100 ng/ml CXCL8/IL8. Representative Western blots (WB) against pIκBα (Ser-32/36), IκBα, and tubulin. B, SVEC cells pretreated 16 h with pertussis toxin (Ptx, 20 ng/ml) were stimulated for 10 min with 100 ng/ml CXCL8/IL8, and Western blot analyses of pIκBα and tubulin were performed. Bars represent densitometric analysis of the resulting pIκBα bands normalized to tubulin bands for each triplicate sample as the percentage of the level of pIκBα (Ser-32/36) achieved after IL8 stimulation, which was considered 100%. C, SVEC cells were transfected with NFκB-luciferase reporter plasmid (pNFκB-LUC) and a Renilla luciferase expression vector. Serum-starved cells were stimulated for 12 h with varying concentrations of CXCL8/IL8 (ng/ml) or 10 ng/ml TNFα, and results were expressed as -fold change with respect to untreated control cells. D, nuclear extracts of parallel cultures were assayed for NFκB binding activity, and results were expressed as -fold change with respect to untreated control cells. E, reporter assay on SVEC cells cotransfected with an NFκB reporter plasmid, a Renilla luciferase expression vector, and either a GFP vector control (–) or a vector encoding GFP-IκBsr. Serum-starved cells were stimulated with 100 ng/ml CXCL8/IL8 for 12 h or left untreated. Reporter activity in quadruplicate samples was represented as -fold change with respect to GFP control untreated cells. F, SVEC cells were cotransfected with a VEGFA-luc reporter vector (pVEGFA-LUC), a Renilla luciferase expression vector, and either GFP control vector (–) or GFP-IκBsr encoding vector (+). Cells were serum-starved, subjected to hypoxia (1% O2), stimulated with 100 ng/ml CXCL8/IL8 for 12 h, or left untreated (Control) as depicted in panel E. Firefly luciferase determinations were normalized by Renilla counts, and results from quadruplicate samples were represented as -fold change with respect to GFP control untreated cells. G, Vegfa mRNA levels were determined in total RNA from parallel cells. H, Western blots against VEGF and IκBα in cell lysates from SVEC cultures transfected with GFP (–) or GFP-IκBsr. ANOVA test, **, p < 0.001.
To explore the role of NFκB in CXCL8/IL8 stimulation in VEGF expression, we used an IκB mutant (IκBα S32A/S36A, IκB superrepressor, IκBsr) protein resistant to phosphorylation and degradation, acting as a constitutive repressor (16). As show in Fig. 3E, CXCL8/IL8 induced a 4-fold activation of an NFκB-Luc reporter plasmid, and this activation was completely inhibited by IκBsr. Furthermore, IκBsr prevented the stimulation of the VEGFA promoter reporter by CXCL8/IL8 but had no effect on the activation induced by hypoxia (Fig. 3F). In addition, IκBsr effectively reduced the levels of CXCL8/IL8-induced Vegfa mRNA and the production and secretion of VEGF (Fig. 3, G and H). These results provided evidence that an NFκB-dependent mechanism drives the expression of VEGF induced by CXCL8/IL8.
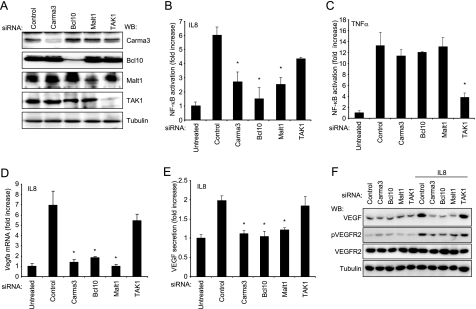
Although recent efforts addressing how cytokine receptors, such as TNFα and IL1, activate NFκB have provided a detailed molecular understanding of this process (14), the molecular events by which the large family of G protein-coupled receptors activate NFκB is still much less understood. Recent evidence suggests that lysophosphatidic acid receptors stimulate NFκB through a molecular complex known as CBM, which is formed by Carma3, Bcl10, and Malt1 (17). Thus, we used an siRNA-mediated knockdown approach to address whether this complex is also implicated in the activation of NFκB by CXCR2-CXCL8/IL8. We identified siRNA duplexes that effectively knock down the expression of Carma3, Bcl10, Malt1, and also the kinase TAK1, which is essential in mediating the activation of NFκB induced by TNFα (14) (Fig. 4A). Interestingly, knockdown of any of the components of the CBM complex significantly reduced the activation of NFκB in response to CXCL8/IL8, whereas TAK1 siRNA had only a marginal effect (Fig. 4B). Conversely, Carma3, Bcl10, and Malt1 had no effect on NFκB activation induced by TNFα, whereas knockdown of TAK1 strongly impaired TNFα induced activation of NFκB (Fig. 4C). Moreover, the levels of Vegfa mRNA and VEGF expression and secretion were drastically reduced by the knockdown of Carma3, Bcl10, and Malt1, whereas diminishing TAK1 levels had minimum or no effect (Fig. 4, E and F). Consistently with these findings, inhibition of NFκB activation by interfering with the function of the CBM complex prevented the increased activation of VEGFR2 in endothelial cells in response to CXCL8/IL8. Together, these findings support the emerging role of the CXCL8/IL8-CXCR2-initiated signaling axis controlling NFκB activation in stimulating the expression of VEGF, thereby promoting the autocrine activation of VEGFR2.
FIGURE 4.
Requirement of the CBM complex for CXCL8/IL8-induced up-regulation of VEGF. A, siRNA-mediated knockdown of Carma3, Bcl10, Malt1, and Tak1 in SVECs, as judged by Western blot (WB) analysis for each protein, using tubulin as a loading control. B and C, luciferase assays of cells transfected with the indicated siRNAs and NFκB luciferase and Renilla luciferase encoding plasmids at day 4. Cells were serum-starved and stimulated with CXCL8/IL8 (100 ng/ml) or TNFα (10 ng/ml) for 12 h as indicated. D and E, their Vegfa mRNA (D) and secreted VEGF levels (E) are represented. F, Western blots for VEGF, phospho-VEGFR2 (pVEGFR2) and total VEGFR2 after 12 h treatment with 100 ng/ml CXCL8/IL8 in cells transfected with siRNAs. ANOVA test, *, p < 0.01.
VEGF and CXCL8/IL8 play a key function in acute and chronic inflammation, which is characterized by enhanced vascular permeability and angiogenesis (18). The activated endothelium secretes CXCL8/IL8 in areas of injury (19), thus inducing the rapid recruitment neutrophils and macrophages that provide the first line of defense against bacterial and viral infection. These leukocytes in turn secrete prestored VEGF and CXCL8/IL8 to facilitate their extravasation to the adjacent tissue through a hypoxia-independent mechanism (20). Our results may provide a mechanism by which CXCL8/IL8 and likely other inflammatory mediators may in turn promote the expression of VEGF in endothelial cells under non-hypoxic conditions. Although hypoxia can still occur during inflammation, this is often the result of ischemic conditions or due to secondary edema in severe cases (21). During the first stages of the inflammatory process, however, there is normal blood flow, and tissue oxygenation is presumably normal, in particular for endothelial cells. The NFκB-mediated VEGF accumulation may also explain why HIF1α-conditional endothelial knockout animals only display severe phenotype under hypoxic conditions (22) and how HIF1α-depleted cancer cells can maintain their angiogenic phenotype (7).
Although the murine Vegfa gene has two NFκB-response elements (23), no consensus NFκB sites have been described in the human VEGFA promoter. Instead, cryptic NFκB binding sites or the existence of activating interactions between NFκB and other well characterized transcription factors acting on the VEGFA promoter, such as AP1 or Sp1, could explain why stimuli typically activating NFκB can increase VEGFA transcription (24), whereas inhibition of NFκB signaling results in down-regulation of VEGF levels (25), an area that warrants further investigation. On the other hand, endothelial cells not only respond to, but also express, VEGF (2). This endogenous pool of VEGF may be strictly required for endothelial cell survival, thereby preserving the vascular integrity (2). Thus, our findings suggest that in addition to the VEGF released by leukocytes at the site of inflammation, a myriad of chemokines and cytokines, including TNFα and IL1-β, that accumulate in the inflammatory microenvironment may also enhance the autocrine or intracrine activation of VEGF receptors in endothelial cells by stimulating VEGF expression through NFκB. This may in turn enable endothelial cells to survive the harsh conditions found in the inflammatory milieu, an interesting possibility that warrants further investigation. Overall, our present results suggest that the activation of NFκB in endothelial cells by locally produced CXCL8/IL8 and other inflammatory mediators may initiate an autocrine/intracrine mechanism, resulting in the activation of VEGFR2, which may in turn contribute to the angiogenic response that characterizes the inflammatory process.
This work was supported, in whole or in part, by a National Institutes of Health Intramural AIDS Targeted Antiviral Program and by the a grant from the NIDCR (National Institutes of Health). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor; IL, interleukin; CBM, Carma3, Bcl10, and Malt1; HIF1, hypoxia-inducible factor-1; HRE, hypoxia-responsive elements; siRNA, small interfering RNA; shRNA, short hairpin RNA; SVEC, SV40-immortalized murine endothelial cells; IKK, IκB kinase; IκBsr, IκB superrepressor; pIκBα, phosphorylated IκBα; TNF, tumor necrosis factor; ANOVA, analysis of variance; GFP, green fluorescent protein.
References
- 1.Ferrara, N., Gerber, H. P., and LeCouter, J. (2003) Nat. Med. 9 669–676 [DOI] [PubMed] [Google Scholar]
- 2.Lee, S., Chen, T. T., Barber, C. L., Jordan, M. C., Murdock, J., Desai, S., Ferrara, N., Nagy, A., Roos, K. P., and Iruela-Arispe, M. L. (2007) Cell 130 691–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carmeliet, P., and Jain, R. K. (2000) Nature 407 249–257 [DOI] [PubMed] [Google Scholar]
- 4.Pugh, C. W., and Ratcliffe, P. J. (2003) Nat. Med. 9 677–684 [DOI] [PubMed] [Google Scholar]
- 5.Ryan, H. E., Lo, J., and Johnson, R. S. (1998) EMBO J. 17 3005–3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arany, Z., Foo, S. Y., Ma, Y., Ruas, J. L., Bommi-Reddy, A., Girnun, G., Cooper, M., Laznik, D., Chinsomboon, J., Rangwala, S. M., Baek, K. H., Rosenzweig, A., and Spiegelman, B. M. (2008) Nature 451 1008–1012 [DOI] [PubMed] [Google Scholar]
- 7.Mizukami, Y., Jo, W. S., Duerr, E. M., Gala, M., Li, J., Zhang, X., Zimmer, M. A., Iliopoulos, O., Zukerberg, L. R., Kohgo, Y., Lynch, M. P., Rueda, B. R., and Chung, D. C. (2005) Nat. Med. 11 992–997 [DOI] [PubMed] [Google Scholar]
- 8.Gavard, J., and Gutkind, J. S. (2006) Nat. Cell Biol. 8 1223–1234 [DOI] [PubMed] [Google Scholar]
- 9.Sodhi, A., Montaner, S., Patel, V., Zohar, M., Bais, C., Mesri, E. A., and Gutkind, J. S. (2000) Cancer Res. 60 4873–4880 [PubMed] [Google Scholar]
- 10.Martin, D., Galisteo, R., Ji, Y., Montaner, S., and Gutkind, J. S. (2008) Oncogene 27 1844–1852 [DOI] [PubMed] [Google Scholar]
- 11.Koch, A. E., Polverini, P. J., Kunkel, S. L., Harlow, L. A., DiPietro, L. A., Elner, V. M., Elner, S. G., and Strieter, R. M. (1992) Science 258 1798–1801 [DOI] [PubMed] [Google Scholar]
- 12.Nathan, A. T., and Singer, M. (1999) Br. Med. Bull. 55 96–108 [DOI] [PubMed] [Google Scholar]
- 13.Ramanathan, M., Pinhal-Enfield, G., Hao, I., and Leibovich, S. J. (2007) Mol. Biol. Cell 18 14–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayden, M. S., and Ghosh, S. (2008) Cell 132 344–362 [DOI] [PubMed] [Google Scholar]
- 15.Sun, S. C., Ganchi, P. A., Ballard, D. W., and Greene, W. C. (1993) Science 259 1912–1915 [DOI] [PubMed] [Google Scholar]
- 16.Squarize, C. H., Castilho, R. M., Sriuranpong, V., Pinto, D. S., Jr., and Gutkind, J. S. (2006) Neoplasia (N.Y.) 8 733–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klemm, S., Zimmermann, S., Peschel, C., Mak, T. W., and Ruland, J. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 134–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagy, J. A., Vasile, E., Feng, D., Sundberg, C., Brown, L. F., Detmar, M. J., Lawitts, J. A., Benjamin, L., Tan, X., Manseau, E. J., Dvorak, A. M., and Dvorak, H. F. (2002) J. Exp. Med. 196 1497–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Utgaard, J. O., Jahnsen, F. L., Bakka, A., Brandtzaeg, P., and Haraldsen, G. (1998) J. Exp. Med. 188 1751–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koehne, P., Willam, C., Strauss, E., Schindler, R., Eckardt, K. U., and Buhrer, C. (2000) Am. J. Physiol. 279 H817–H824 [DOI] [PubMed] [Google Scholar]
- 21.Weibel, E. R. (1984) The Pathway for Oxygen: Structure and Function in the Mammalian Respiratory System, Harvard University Press, Cambridge, MA
- 22.Tang, N., Wang, L., Esko, J., Giordano, F. J., Huang, Y., Gerber, H. P., Ferrara, N., and Johnson, R. S. (2004) Cancer Cell 6 485–495 [DOI] [PubMed] [Google Scholar]
- 23.Shima, D. T., Kuroki, M., Deutsch, U., Ng, Y. S., Adamis, A. P., and D'Amore, P. A. (1996) J. Biol. Chem. 271 3877–3883 [DOI] [PubMed] [Google Scholar]
- 24.Huang, S., Robinson, J. B., Deguzman, A., Bucana, C. D., and Fidler, I. J. (2000) Cancer Res. 60 5334–5339 [PubMed] [Google Scholar]
- 25.Shibata, A., Nagaya, T., Imai, T., Funahashi, H., Nakao, A., and Seo, H. (2002) Breast Cancer Res. Treat. 73 237–243 [DOI] [PubMed] [Google Scholar]