Abstract
Despite intense interest, the molecular mechanisms underlying the association of apoE4 with Alzheimer disease are not clear. Because the function (or dysfunction) of a protein is based on its structure, this review focuses on the effects of the structural differences among the isoforms on neurodegeneration. Understanding how apoE4 structure impacts neurodegeneration is likely to provide mechanistic insight as well as potential therapeutic approaches to blunt or reduce its effects.
ApoE4 is the major genetic risk factor for AD2 (1–3). ApoE is well known as a lipid transport protein in the plasma and central nervous system and plays a key role in the maintenance and repair of neurons (4–6). Of the three common isoforms, apoE4 is associated with the greatest risk of AD and the lowest age of onset, apoE2 with the lowest risk and highest age of onset, and apoE3 with intermediate risk and age of onset. Several hypotheses have been proposed to explain this association (for review, see Ref. 7). Nevertheless, despite concerted efforts, the mechanism remains unknown.
The variable effect of apoE isoforms in AD provides an opportunity to correlate their structural and biophysical differences with their respective impact. This approach is based on the principle that the structure and biophysical properties of a protein determine its normal function or (in the case of mutations) its dysfunction. In this review, we focus on the mechanistic insights gained from comparisons of the apoE isoforms. Because apoE may contribute to AD through both Aβ-dependent and Aβ-independent pathways (8), we will consider those pathways as they relate to structural correlations.
Common Structural Features of ApoE Isoforms
The three common apoE isoforms are coded at a single gene locus on chromosome 19; each contains 299 amino acids. ApoE has two structural domains, an N-terminal domain and a C-terminal domain, which fold independently (Fig. 1) (9, 10). In the lipid-free state, the two domains are connected by a flexible “hinge region” (10). As shown by x-ray crystallography, the N-terminal domain forms an extended four-helix bundle (11). The region of apoE that interacts with members of the LDL receptor family is enriched in basic residues, is located in the N-terminal domain, and includes residues 136–150 and Arg-172 (Fig. 1) (12). The structure of the C-terminal domain is not known. It is modeled as a series of α-helices based on secondary structure prediction. This domain contains the major lipoprotein-binding elements of apoE, residues 244–272 (13).
FIGURE 1.
Model of the domain structure of apoE. The N-terminal domain consists of a four-helix bundle. Red, helix 1; blue, helix 2; green, helix 3; yellow, helix 4. The C-terminal domain is modeled as a series of α-helices. The N-terminal domain contains the LDL receptor-binding region on helix 4 and Arg-172 in the hinge region. The C-terminal domain contains the major lipoprotein-binding elements.
Structural and Biophysical Differences in ApoE Isoforms
Structural Differences—The three isoforms are distinguished by arginyl/cysteinyl differences at positions 112 and 158: apoE2 contains cysteine at both positions, apoE3 contains cysteine at position 112 and arginine at position 158, and apoE4 contains arginine at both positions (Fig. 1) (14). These differences exert profound effects on the structure and properties of the isoforms and thus hold the key to understanding their different effects in AD. Substitution of cysteine for arginine at position 158 in apoE2 markedly reduces its LDL receptor-binding activity, whereas the same substitution at position 112 has no effect on the binding activity of apoE3 compared with apoE4 (14). It is not known whether the low risk of AD associated with apoE2 is related to its lower receptor-binding activity.
The presence of cysteine in apoE3 and apoE2 results in formation of disulfide-linked homo- and heterodimers, which cannot occur with apoE4 because it lacks cysteine (15). In plasma, ∼50% of apoE3 and apoE2 molecules are disulfide-linked (15); in CSF, a major portion of apoE3 exists as dimers (16). ApoE3 homodimers affect lipid binding and reduce LDL receptor-binding activity (15). Although AD does not affect the level of apoE dimers in CSF (16), the influence of these forms on AD has not been examined.
Stability Differences—With denaturation, apoE exhibits two independent unfolding transitions, with the N-terminal domain being more stable than the C-terminal domain (17). Although the C-terminal domains of the isoforms are equally stable, the N-terminal domains have different unfolding curves and stabilities. This is not surprising, as the substitutions that distinguish the isoforms lie within the N-terminal domain. The N-terminal domain is the least stable in apoE4, the most stable in apoE2, and of intermediate stability in apoE3. In addition, the apoE4 denaturation curve displays a prominent shoulder, evidence of stable folding intermediates with features of a molten globule state: an ensemble of partially unfolded structures (18). In the apoE4 molten globule, the four-helix bundle is partially unfolded and displays both a loss of helical content, which exposes proteolytic cleavage sites, and an increase in β-structure (18).
ApoE4 has a much greater tendency to form a molten globule state than apoE3, which in turn has a greater tendency than apoE2. Thus, apoE4 is distinguished by a lower stability and a greater tendency to form a molten globule state. Moreover, these differences in the isoforms correlate with the magnitude of their effects in AD (apoE4 ≫ apoE3 > apoE2). Because of its instability, apoE4 also has an increased propensity to aggregate. When incubated at 37 °C, apoE4 displays the greatest tendency to form high molecular weight aggregates (19).
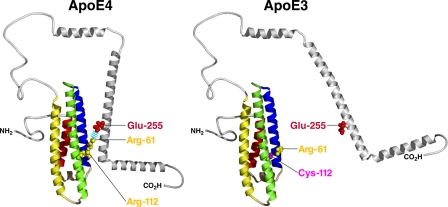
Domain Interaction—One of the first isoform-specific differences identified was referred to as domain interaction. This concept was introduced to explain the preferential binding of apoE4 to lower density lipoproteins and of apoE3 to higher density lipoproteins (20). Over the years, this model has been refined based on x-ray crystallography, site-directed mutagenesis, fluorescent resonance energy transfer, and EPR studies (13, 21, 22). The key to domain interaction is the difference in the conformation of Arg-61. In apoE4, Arg-112 causes the Arg-61 side chain to extend away from the four-helix bundle, allowing an interaction with Glu-255 and resulting in a compact structure (Fig. 2). In contrast, Cys-112 in apoE3 causes the Arg-61 side chain to be tucked between two helices, making it less accessible for interaction with Glu-255 and resulting in a more extended structure. The apoE4 model in Fig. 2 is based on three distance constraints between the two domains determined by EPR (22).
FIGURE 2.
Influence of domain interaction on the structure of apoE.
Potential Effects of Structural Differences in AD
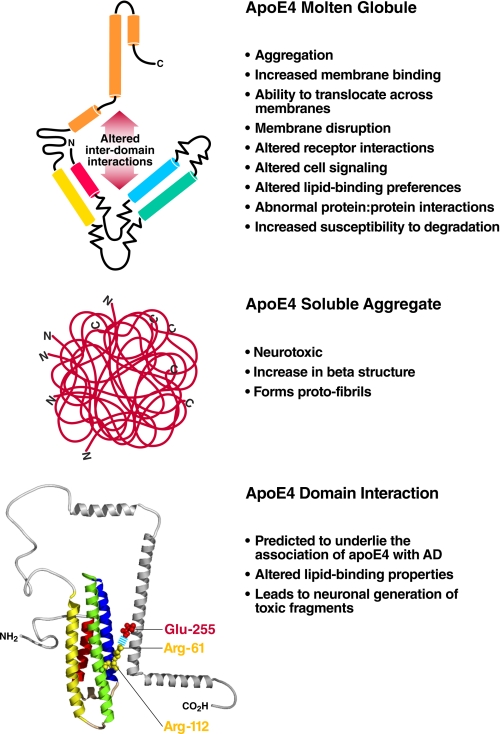
Stability/Molten Globule Formation—Molten globules have been implicated in a wide variety of physiological processes (Fig. 3, upper) (for review, see Ref. 23). An apoE4 molten globule could exert a number of effects that might contribute to a disease phenotype. For example, the instability of the N-terminal domain of apoE4 may explain its increased ability to bind and remodel phospholipid micelles (17). As a result, lipoprotein particles containing apoE4 could differ from apoE3 particles in their composition. In AD, the lipid-binding properties of apoE4 could also influence cellular function, resulting in pathological effects. For instance, apoE4 increases Aβ-induced lysosomal leakage in cultured neuronal cells to a greater extent than apoE3, suggesting that apoE4 acts cooperatively with Aβ to destabilize lysosomal membranes, potentially leading to neurodegeneration (24).
FIGURE 3.
Potential effects of the structural properties of apoE4 on AD. Upper, molten globule formation; middle, aggregation; lower, domain interaction.
Aggregation—Many mutations can destabilize native conformations and promote formation of aggregates with properties of amyloid fibrils (25). Thus, the destabilization of compactly folded apoE could result in misfolding to form amyloid fibrils. This misfolding could provide the basis for amyloid formation. In AD patients, apoE is co-localized in amyloid plaques, one of the pathological features of the disease (26). In addition, the apoE isoforms differ in their ability to promote Aβ amyloid formation. Incubation of purified apoE with Aβ promotes Aβ fibrillization as shown by electron microscopy (27) and thioflavin T reactivity (28).
In AD patients, amyloid deposition correlates with the gene dosage of apoE4 (29). In mouse models of AD expressing mutant forms of the human APP and apoE isoforms, apoE4 is more effective than apoE3 in promoting Aβ deposition (30). The apoE isoforms differ in their ability to co-aggregate with Aβ. ApoE4 forms high molecular weight aggregates characterized by a significant increase in β-structure and fibril protofilaments (Fig. 3, middle), which could promote Aβ nucleation, polymerization, and plaque formation. ApoE may also contribute to amyloid deposition by serving as a pathological chaperon (31). Furthermore, apoE4 aggregates are neurotoxic, another potential mechanism for neurodegeneration in AD (19). The propensity of apoE4 to aggregate is related to its molten globule state rather than domain interaction (19).
Domain Interaction—We hypothesized that domain interaction contributes to the association of apoE4 with AD (Fig. 3, lower) (21). Domain interaction alters lipid binding and explains the binding preference of apoE4 for large, triglyceride-rich, lower density lipoproteins (21). These lipoproteins are not present in CSF, where the major lipoproteins are small, cholesterolrich, high density lipoproteins. The lower avidity of apoE4 for these small lipoproteins could result in less effective transport of lipids that are required for neuronal synaptogenesis, maintenance, and repair.
Normally, apoE is expressed in astrocytes and other glial cells. However, under stressful conditions, neurons also express apoE (32). Because of domain interaction, apoE4 produced within neurons is subject to cleavage of the C terminus by a neuronal protease. The resulting fragments escape the secretory pathway and enter the cytosol, disrupting the cytoskeleton, impairing mitochondrial energy production, and ultimately leading to neuronal death (33, 34). These C-terminally truncated fragments of apoE4 are observed in AD patients and in transgenic mice expressing apoE4 in neurons (8). In mice, these toxic fragments cause neurodegeneration and cognitive deficits (34). Notably, domain interaction has been demonstrated in neuronal cells (35).
Mouse Models of ApoE4 Structural Properties
One difficulty in linking a given structural property of apoE4 to a pathogenic mechanism or pathway in AD is that apoE4 differs from apoE3 and apoE2 in at least three ways: it lacks cysteine, has lower stability and a greater tendency to form a molten globule state, and exhibits domain interaction. From a mechanistic and therapeutic prospective, it is essential to ascertain the relative contributions of these structural features to neurodegeneration. Until recently, this was not possible, as all apoE4 structural features were simultaneously present in the available human apoE knock-in and transgenic mouse models.
To circumvent these problems, we took advantage of the fact that that mouse WT apoE does not exhibit apoE4 instability/molten globule formation or domain interaction and does not contain cysteine (36, 37). Our approach was to identify the amino acid differences in human apoE4 responsible for domain interaction and instability/molten globule formation and then “humanize” mouse apoE by introducing those residues into mice by gene targeting. Using this approach, we engineered domain interaction into mouse apoE (37). Although it contains the equivalents of Arg-112 and Glu-255, mouse apoE lacks the equivalent of Arg-61, required for domain interaction. Instead, it contains threonine at this position, and therefore, its functional behavior resembles that of apoE3. Interestingly, only human apoE contains Arg-61; all other species contain threonine. In this gene-targeted mouse model, mouse Arg-61 apoE does not display the instability of apoE4 (36), making it a specific model of domain interaction. Thus, any phenotype in this model is associated with a direct or indirect effect of domain interaction.
Expression of Arg-61 apoE is governed by the normal murine control and regulatory elements, and Arg-61 apoE is expressed at normal physiological levels. Also, any species difference effects are minimized: there is only one amino acid difference between Arg-61 apoE and WT apoE versus a 28% sequence difference between human and mouse apoE. Species effects are an important consideration in mouse models. For example, in transgenic mice expressing human APP and human apoE isoforms on a mouse apoE knock-out background, amyloid deposition is more extensive in apoE4 mice than in apoE3 mice; but in both cases, the levels are lower than in human APP WT apoE mice, indicating a species difference in amyloid deposition between human and mouse apoE (38).
Arg-61 ApoE Mouse Model—Arg-61 apoE mice show no evidence of gliosis or neuronal loss, but they nevertheless display a significant phenotype. In the brain, the level of Arg-61 apoE is ∼40% lower than that of WT apoE, and this difference is independent of sex, age, or brain region (39). The lower level of Arg-61 apoE reflects decreased secretion by astrocytes, the main producer of apoE in a noninjured or nonstressed brain, despite identical levels of Arg-61 and WT apoE mRNAs. A similar reduction in apoE4 was observed in apoE4 knock-in mice compared with apoE3 mice (39). These results demonstrate that domain interaction is the basis for the reduction in both models.
The lower brain levels of apoE are associated with an age-dependent deficit in synaptophysin, a marker of vesicle proteins in presynaptic terminals. One-year-old mice also exhibit reductions in Bassoon, a marker of active zone proteins in presynaptic terminals, and in neuroligin-1, a postsynaptic marker (40). These results demonstrate that neurodegeneration in this model is associated with domain interaction but not with neuronal loss. Moreover, the synaptic deficits caused functional impairments that were revealed when mice were exposed to a novel environment by moving them from their home cage to a larger uncovered cage with different bedding and five novel objects in a different room. Fos and Arc expression was determined in the dentate gyrus as a measure of synaptic activity. Before exposure, there were no differences in the levels of Fos or Arc in WT and Arg-61 mice. After exposure, however, the WT mice had higher levels of both markers, demonstrating that a functional deficit is also associated with apoE domain interaction. In addition, the Arg-61 mice had a spatial memory deficit in a water maze paradigm (40).
An interesting picture is emerging to explain this phenotype based on two potential mechanisms. First, the reduced secretion of apoE by Arg-61 apoE astrocytes is accompanied by a corresponding decrease in cholesterol secretion (40). A reduction in the availability of cholesterol could contribute to inefficient maintenance and synapse formation. Second, Arg-61 apoE does not accumulate intracellularly in astrocytes, indicating that it is targeted for degradation (41). This finding raised the interesting possibility that Arg-61 apoE is recognized as an abnormally folded protein in the ER, eliciting a UPR. In fact, all three UPR pathways are activated to a greater extent in Arg-61 apoE astrocytes than in WT astrocytes (41). Potentially, a chronic ER stress response could globally impair the ability of astrocytes to support and maintain neurons by sequestration of chaperons or reduced protein translation. In support of a global astrocyte dysfunction, Arg-61 mice had lower levels of astrocyte-specific GLT1 (glutamate transporter-1) than WT mice (40). Inefficient glutamate clearance from synapses could result in excitotoxicity, causing neurodegeneration. Altered glutamate uptake and excitotoxicity have been suggested to contribute to AD (42).
These findings raise the possibility that astrocytes contribute more directly to the association of apoE4 with AD than was previously appreciated. A potential scenario is that apoE4 domain interaction impairs the ability of astrocytes to support neuronal function over most of a lifetime. Although this support is compromised, it is effective enough in the absence of brain stress. However, with the addition and accumulation of the effects of stressors later in life (i.e. ischemia, oxidative stress, or Aβ toxicity), astrocyte support becomes more ineffective as the need to increase apoE4 secretion to support neurons results in a further increase in the ER stress (Fig. 4). At this point, without effective support, neurons begin to express apoE. With apoE4, this leads to the generation of C-terminally truncated fragments that are neurotoxic, leading to more extensive neurodegeneration and AD. This scenario represents a potential mechanism to explain the greater risk and lower age of onset of AD in apoE4 subjects.
FIGURE 4.
Ability of WT and Arg-61 apoE astrocytes to repair and maintain neurons. Arg-61 apoE astrocytes are less effective than WT astrocytes in supporting neurons. Potential mechanisms include decreased secretion of apoE and cholesterol, decreased GLT1 transporter levels, and a UPR stress response.
In summary, the Arg-61 apoE model of domain interaction provides compelling evidence that apoE4 can act independently of Aβ to promote neurodegeneration and features of AD, as suggested previously (8). In addition, the model suggests a role for astrocytes in the association of apoE4 with AD. More important, these findings indicate that domain interaction is a viable apoE4 therapeutic target. Indeed, disrupting domain interaction with small molecules abolishes apoE4-enhanced Aβ production in cultured cells (43).
Mouse ApoE Molten Globule Model—The amino acids in human apoE4 required to humanize mouse WT apoE with respect to instability of the N-terminal domain have been identified; the substitutions involved are T61R, G83T, and Q113G (36). Because Arg-61 is required to introduce the instability, it was necessary to replace Glu-255 with alanine in the gene-targeting vector so as to abolish domain interaction (21). This mouse model has been generated and is currently under evaluation to determine the specific effect of apoE4 molten globule formation in the absence of domain interaction.
Conclusions and Future Perspectives
Although the association of apoE4 with AD is not completely resolved, comparisons of the structural and biophysical differences among the apoE isoforms are providing insights into the mechanisms involved. These studies provide additional evidence that apoE contributes to AD pathology by acting through both Aβ-dependent and Aβ-independent pathways and suggest a role for astrocytes. The challenge for the future will be to directly link apoE-related mechanisms to pathogenic processes and disease progression in studies of in vivo models. Mouse models specific for apoE4 structural properties will be invaluable in establishing such links.
Supplementary Material
Acknowledgments
We thank John Carroll and Chris Goodfellow for graphics, Stephen Ordway and Gary Howard for editorial assistance, and Linda Turney for manuscript preparation.
This work was supported, in whole or in part, by National Institutes of Health Grant PO1 AG022074 (Project 2, to K. H. W.) and Grant CO6 RR0118928 from the National Center for Research Resources. This work was also supported by American Health Assistance Foundation Pilot Grant for Alzheimer's Disease Research A2006-231 (to N. Z.), the Alzheimer's Association Zenith Award (to K. H. W.), and the Gladstone Institutes. This is the tenth article of eleven in the Thematic Minireview Series on the Molecular Basis of Alzheimer Disease. This minireview will be reprinted in the 2009 Minireview Compendium, which will be available in January, 2010.
Footnotes
The abbreviations used are: AD, Alzheimer disease; Aβ, amyloid β-protein; LDL, low density lipoprotein; CSF, cerebrospinal fluid; APP, amyloid precursor protein; WT, wild-type; ER, endoplasmic reticulum; UPR, unfolded protein response.
References
- 1.Corder, E. H., Saunders, A. M., Strittmatter, W. J., Schmechel, D. E., Gaskell, P. C., Small, G. W., Roses, A. D., Haines, J. L., and Pericak-Vance, M. A. (1993) Science 261 921–923 [DOI] [PubMed] [Google Scholar]
- 2.Saunders, A. M., Strittmatter, W. J., Schmechel, D., St George-Hyslop, P. H., Pericak-Vance, M. A., Joo, S. H., Rosi, B. L., Gusella, J. F., Crapper-MacLachlan, D. R., Alberts, M. J., Hulette, C., Crain, B., Goldgaber, D., and Roses, A. D. (1993) Neurology 43 1467–1472 [DOI] [PubMed] [Google Scholar]
- 3.Farrer, L. A., Cupples, L. A., Haines, J. L., Hyman, B., Kukull, W. A., Mayeux, R., Myers, R. H., Pericak-Vance, M. A., Risch, N., and Van Duijn, C. M. (1997) J. Am. Med. Assoc. 278 1349–1356 [PubMed] [Google Scholar]
- 4.Nathan, B. P., Bellosta, S., Sanan, D. A., Weisgraber, K. H., Mahley, R. W., and Pitas, R. E. (1994) Science 264 850–852 [DOI] [PubMed] [Google Scholar]
- 5.Mahley, R. W. (1988) Science 240 622–630 [DOI] [PubMed] [Google Scholar]
- 6.Weisgraber, K. H., and Mahley, R. W. (1996) FASEB J. 10 1485–1494 [DOI] [PubMed] [Google Scholar]
- 7.Huang, Y., Weisgraber, K. H., Mucke, L., and Mahley, R. W. (2004) J. Mol. Neurosci. 23 189–204 [DOI] [PubMed] [Google Scholar]
- 8.Mahley, R. W., Weisgraber, K. H., and Huang, Y. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 5644–5651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aggerbeck, L. P., Wetterau, J. R., Weisgraber, K. H., Wu, C.-S. C., and Lindgren, F. T. (1988) J. Biol. Chem. 263 6249–6258 [PubMed] [Google Scholar]
- 10.Wetterau, J. R., Aggerbeck, L. P., Rall, S. C., Jr., and Weisgraber, K. H. (1988) J. Biol. Chem. 263 6240–6248 [PubMed] [Google Scholar]
- 11.Wilson, C., Wardell, M. R., Weisgraber, K. H., Mahley, R. W., and Agard, D. A. (1991) Science 252 1817–1822 [DOI] [PubMed] [Google Scholar]
- 12.Hatters, D. M., Peters-Libeu, C. A., and Weisgraber, K. H. (2006) Trends Biochem. Sci. 31 445–454 [DOI] [PubMed] [Google Scholar]
- 13.Dong, L.-M., Wilson, C., Wardell, M. R., Simmons, T., Mahley, R. W., Weisgraber, K. H., and Agard, D. A. (1994) J. Biol. Chem. 269 22358–22365 [PubMed] [Google Scholar]
- 14.Weisgraber, K. H. (1994) Adv. Protein Chem. 45 249–302 [DOI] [PubMed] [Google Scholar]
- 15.Weisgraber, K. H., and Shinto, L. H. (1991) J. Biol. Chem. 266 12029–12034 [PubMed] [Google Scholar]
- 16.Montine, K. S., Bassett, C. N., Ou, J. J., Markesbery, W. R., Swift, L. L., and Montine, T. J. (1998) J. Lipid Res. 39 2443–2451 [PubMed] [Google Scholar]
- 17.Morrow, J. A., Segall, M. L., Lund-Katz, S., Phillips, M. C., Knapp, M., Rupp, B., and Weisgraber, K. H. (2000) Biochemistry 39 11657–11666 [DOI] [PubMed] [Google Scholar]
- 18.Morrow, J. A., Hatters, D. M., Lu, B., Höchtl, P., Oberg, K. A., Rupp, B., and Weisgraber, K. H. (2002) J. Biol. Chem. 277 50380–50385 [DOI] [PubMed] [Google Scholar]
- 19.Hatters, D. M., Zhong, N., Rutenber, E., and Weisgraber, K. H. (2006) J. Mol. Biol. 361 932–944 [DOI] [PubMed] [Google Scholar]
- 20.Weisgraber, K. H. (1990) J. Lipid Res. 31 1503–1511 [PubMed] [Google Scholar]
- 21.Dong, L.-M., and Weisgraber, K. H. (1996) J. Biol. Chem. 271 19053–19057 [DOI] [PubMed] [Google Scholar]
- 22.Hatters, D. M., Budamagunta, M. S., Voss, J. C., and Weisgraber, K. H. (2005) J. Biol. Chem. 280 34288–34295 [DOI] [PubMed] [Google Scholar]
- 23.Ptitsyn, O. B. (1995) Adv. Protein Chem. 47 83–229 [DOI] [PubMed] [Google Scholar]
- 24.Ji, Z.-S., Miranda, R. D., Newhouse, Y. M., Weisgraber, K. H., Huang, Y., and Mahley, R. W. (2002) J. Biol. Chem. 277 21821–21828 [DOI] [PubMed] [Google Scholar]
- 25.Fändrich, M., Fletcher, M. A., and Dobson, C. M. (2001) Nature 410 165–166 [DOI] [PubMed] [Google Scholar]
- 26.Wisniewski, T., Lalowski, M., Golabek, A., Vogel, T., and Frangione, B. (1995) Lancet 345 956–958 [DOI] [PubMed] [Google Scholar]
- 27.Sanan, D. A., Weisgraber, K. H., Russell, S. J., Mahley, R. W., Huang, D., Saunders, A., Schmechel, D., Wisniewski, T., Frangione, B., Roses, A. D., and Strittmatter, W. J. (1994) J. Clin. Investig. 94 860–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wisniewski, T., Castaño, E. M., Golabek, A., Vogel, T., and Frangione, B. (1994) Am. J. Pathol. 145 1030–1035 [PMC free article] [PubMed] [Google Scholar]
- 29.Schmechel, D. E., Saunders, A. M., Strittmatter, W. J., Crain, B. J., Hulette, C. M., Joo, S. H., Pericak-Vance, M. A., Goldgaber, D., and Roses, A. D. (1993) Proc. Natl. Acad. Sci. U. S. A. 90 9649–9653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holtzman, D. M., Bales, K. R., Tenkova, T., Fagan, A. M., Parsadanian, M., Sartorius, L. J., Mackey, B., Olney, J., McKeel, D., Wozniak, D., and Paul, S. M. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 2892–2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wisniewski, T., and Frangione, B. (1992) Neurosci. Lett. 135 235–238 [DOI] [PubMed] [Google Scholar]
- 32.Xu, Q., Bernardo, A., Walker, D., Kanegawa, T., Mahley, R. W., and Huang, Y. (2006) J. Neurosci. 26 4985–4994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brecht, W. J., Harris, F. M., Chang, S., Tesseur, I., Yu, G.-Q., Xu, Q., Fish, J. D., Wyss-Coray, T., Buttini, M., Mucke, L., Mahley, R. W., and Huang, Y. (2004) J. Neurosci. 24 2527–2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris, F. M., Brecht, W. J., Xu, Q., Tesseur, I., Kekonius, L., Wyss-Coray, T., Fish, J. D., Masliah, E., Hopkins, P. C., Scearce-Levie, K., Weisgraber, K. H., Mucke, L., Mahley, R. W., and Huang, Y. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 10966–10971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu, Q., Brecht, W. J., Weisgraber, K. H., Mahley, R. W., and Huang, Y. (2004) J. Biol. Chem. 279 25511–25516 [DOI] [PubMed] [Google Scholar]
- 36.Hatters, D. M., Peters-Libeu, C. A., and Weisgraber, K. H. (2005) J. Biol. Chem. 280 26477–26482 [DOI] [PubMed] [Google Scholar]
- 37.Raffaï, R. L., Dong, L.-M., Farese, R. V., Jr., and Weisgraber, K. H. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 11587–11591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fryer, J. D., Simmons, K., Parsadanian, M., Bales, K. R., Paul, S. M., Sullivan, P. M., and Holtzman, D. M. (2005) J. Neurosci. 25 2803–2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramaswamy, G., Xu, Q., Huang, Y., and Weisgraber, K. H. (2005) J. Neurosci. 25 10658–10663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhong, N., Scearce-Levie, K., Ramaswamy, G., and Weisgraber, K. H. (2008) Alzheimer's Dement. 4 179–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramaswamy, G., and Weisgraber, K. (2007) Society for Neuroscience: Neuroscience Meeting Planner, Program No. 154.1/R22
- 42.Walton, H. S., and Dodd, P. R. (2007) Neurochem. Int. 50 1052–1066 [DOI] [PubMed] [Google Scholar]
- 43.Ye, S., Huang, Y., Müllendorff, K., Dong, L., Giedt, G., Meng, E. C., Cohen, F. E., Kuntz, I. D., Weisgraber, K. H., and Mahley, R. W. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 18700–18705 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.