Abstract
Glucocorticoids (GCs) exert profound influences on many physiologic functions by virtue of their diverse roles in growth, development, and maintenance of homeostasis. We previously created a novel gain of function in the human glucocorticoid receptor (hGR), hGRM604L, which is active at GC concentrations 5–10-fold lower than wild-type GR. To gain a greater insight into GC physiology in vivo, we inserted this mutant GR (GRM610L in mice) into mice via homologous recombination. Mice expressing the allele are phenotypically normal with respect to GC function. However, corticosterone levels, ACTH levels, and adrenocortical size are markedly reduced, suggesting they are phenotypically normal because the mutant GR alters the basal regulation of the hypothalamic-pituitary-adrenal axis. We demonstrate via physiologic and immunologic studies that GRM610L mice have increased sensitivity to GCs in vivo. Sensitivity to the actions of endogenous GCs may be an important factor underlying the development of many human diseases including hypertension, obesity, and diabetes. Our model may provide a new and powerful tool for the study of GC physiological and pathological processes in vivo.
Glucocorticoids (GCs)2 are extraordinary hormones, important for maintenance of basal and stress-related homeostasis and essential for life. They modulate the expression of ∼10% of human genes and function in every organ and in a wide variety of physiologic, cellular, and molecular networks (1). The biological action of glucocorticoids is mediated primarily through the activation of the cytoplasmic glucocorticoid receptor (GR), a member of the nuclear receptor family of ligand-dependent transcription factors. Like other members of the family, GR possesses a modular structure consisting of three major domains (2): an N-terminal, a central DNA binding domain, and a C-terminal ligand binding domain. There are many aspects of GC action that we do not yet know or are just beginning to appreciate.
Glucocorticoid secretion is determined through a complex series of feedback regulation. Endogenous cortisol binds to GR in the hypothalamus, acting as a potent negative regulator of the hypothalamic-pituitary-adrenal (HPA) axis. Alterations in the number or sensitivity of GR to glucocorticoids can significantly influence HPA axis activity and, in particular, can regulate hormone levels by mediating the strength of cortisol feedback inhibition (3, 4). Abnormalities in glucocorticoid sensitivity can be divided into two major groups: resistance (CR) and hypersensitivity (CH). To our knowledge, only one case of CH has been reported, in a patient with Cushingoid manifestations despite persistent hypocortisolemia (5); the molecular basis of cortisol hypersensitivity was not elucidated. Polymorphisms in GR may also change glucocorticoid sensitivity, as has been suggested for the N363S polymorphism, which has been linked with excess adiposity in European populations (6). CR was first described as an inherited disorder, characterized by hypercortisolism without Cushingoid features (7). Diminished GC sensitivity in the hypothalamus results in higher cortisol secretion by the adrenal glands, thus maintaining relative glucocorticoid balance in tissues also bearing diminished GC sensitivity. CR is caused by loss-of-function mutations in the GC receptor (GR) (8–11). Patients homozygous for such mutations exhibit severe hypertension and hypokalemia due to hypercortisolemia and secondary activation of the mineralocorticoid receptor. Heterozygotes, on the other hand, generally exhibit relatively mild symptomatology, although virilization and hirsuitism has been reported in female patients due to shunting of cortisol precursors into the androgen biosynthetic pathway (12).
We previously described the construction of a mutant human glucocorticoid receptor with increased activity in the presence of GCs (13). The mutation, a substitution of leucine for methionine at residue 604 in the hormone-binding domain of the receptor, increases the affinity of the receptor for GCs by creating a novel helix 3-helix 5 contact. The receptor has increased sensitivity, being activated by 5–10-fold lower corticosterone concentration that GRWT in vitro without any alteration in specificity. This is one of a small group of gain of function mutations that have been described in the steroid hormone receptor family (14, 15). Whether such point mutations can be responsible for in vivo glucocorticoid hypersensitivity remains to be shown. The availability of a GR with increased activity suggests an intriguing reagent for the study of tissue-specific glucocorticoid effect. To gain new insights into this gain of function of GR function and more directly examine glucocorticoid sensitivity in vivo, we aimed to take advantage of this mutation receptor with increased steroid affinity to study physiological aspects of glucocorticoid biology relevant to the genetics of endocrine activity via creation of a knock-in mouse bearing this gain of function receptor.
MATERIALS AND METHODS
Animal Care—All experiments were performed according to an Institutional Animal Care and Use Committee-approved protocol at Yale School of Medicine, which is consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
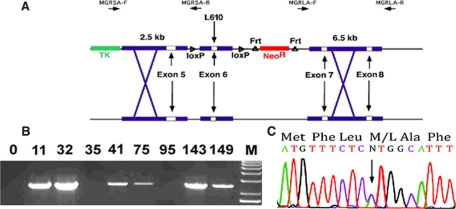
Targeting Vector Construction and Homologous Recombination to Create GRM610L Mice—The targeting vector utilized is described in Fig. 1A. A single base pair mutation converting nucleotide 1828 in the mouse GR sequence from A to C, thereby creating an M610L substitution, was produced in exon 6 using the QuikChange kit (Stratagene); the resulting product was sequenced in full prior to insertion into the cloning vector. Exon 6 lies on a 1.4-kb EcoRV fragment that was placed between two flanking arms of ∼2.5 and 6.5 kb in length, which span exons 5–8. The targeting vector includes a neomycin phosphoreductase cassette flanked by frt sites for positive selection and the herpes simplex virus tk gene for negative selection in ES cells. Details of the exact cloning strategy used are available upon request. The targeting construct was inserted into ES cells derived from SV129/REJ mice, and homologous recombinants were identified via a combination of long range PCR and Southern blotting.
FIGURE 1.
GRM610L knock-in strategy. A, the structure of the 14-kb mouse GR exon 6 region is shown. Exon 6, bearing the M610L mutation, is inserted between flanking loxP sites, which in turn is flanked by a 2.5-kb short arm containing exon 5 and a 6.5-kb long arm containing exons 7 and 8. The neomycin phosphor-reductase cassette is flanked by frt sites as indicated. Gene targeting was assayed via long-range PCR. B, long-range PCR of short arm DNA using the MGRSA-F and MGRSA-R primers. Clones 11, 32, 41, 75, 143, and 149 showed evidence of homologous recombination. This was subsequently confirmed via long-range PCR on the long arm and Southern blotting of EcoRV-digested DNA (data not shown). C, robust expression of GRM610L RNA in vivo. Total RNA was prepared from kidney from a mouse heterozygous for the GRM610L allele, GR exons 4–9 were amplified via reverse transcriptase-PCR and then sequenced using the exon 4 forward primer. These primers were chosen as they lie outside sequences utilized in our targeting vector. The wild-type and L610 alleles are identical except at the (indicated) site of the M610L substitution. At this nucleotide, there is evidence for approximately equivalent expression of the two alleles.
Mice were of a mixed C57BL/6X129 background. They were housed and bred according to international standard conditions with a 12-h dark, 12-h light cycle (lights on from 6:30 a.m. to 6:30 p.m.). We mated GRM610L heterozygous (GR +/L) mice such that each pregnant female produces +/+ (wild-type), +/L, and L/L (GRM610L homozygous) offspring. Six-generation littermate knock-in and control mice, which were 3–6 months old, were used for all experiments. Genotyping was performed by PCR using these primers: GRSHF1 (forward), ACAGCAGGTTTTGTTGACTTTG; GRSHR1 (reverse), GTGATGGCAGCTTAGGTGTATG. All mouse protocols were in accordance with National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee of Yale University.
Plasma ACTH, Corticosterone, and Aldosterone Measurement—Plasma for measurement of corticosterone and ACTH was obtained by rapid retro-orbital phlebotomy into heparinized capillary tubes with a total time from first handling the animal to completion of bleeding not exceeding 30 s. Blood was collected at circadian nadirs (9:30 a.m.) and peaks (9:30 p.m.) from adult male mice (3–6 months old) on ice, and plasma was separated by centrifugation and stored at -80 °C until assay. Plasma corticosterone and ACTH concentration were determined using commercially available radioimmunoassay kits with 125I-corticosterone and 125I-ACTH (ICN Pharmaceuticals Inc., Costa Mesa, CA). Aldosterone was measured on collected plasma via a commercially available kit as well (DSL-8600, Diagnostic Systems Laboratories, Inc.). For evaluation of the stress release of corticosterone and ACTH, blood samples were taken in the morning (9:30–10 a.m.) immediately after 30 min of restraint stress for which animals were placed in 50-ml conical tubes (plastic conical tube with the bottom removed) (n = 10–12 per genotype and gender). For the fasting test, blood samples were collected after food was removed for 36 h.
ACTH Stimulation Testing—Wild type or GRM610L mice were bled by retro-orbital phlebotomy 20 min after intraperitoneal injection of vehicle (phosphate-buffered saline with 0.3% bovine serum albumin) or ACTH (10 mg/kg of body weight, ACTH-24 from Sigma, freshly dissolved in phosphate-buffered saline). We performed synthetic ACTH-24 stimulation testing at 10:00 a.m. Plasma corticosterone concentrations were determined by radioimmunoassay as described above.
Adrenal Weight—For analysis of adrenals, adult male and female mice of each genotype (n = 10–16) were evaluated at 3–4 months of age. Whole body weight was measured and bilateral adrenals were isolated and cleaned of fat. The weight of the combined left and right adrenals were determined and corrected by body weight to generate corresponding adrenal weight index values. Adrenals were fixed by overnight immersion in a solution of 4% paraformaldehyde in phosphate-buffered saline and then processed for paraffin embedding. Sections were cut at 4 μm, and stained with hematoxylin and eosin. Sections were blinded to the genotype.
Blood Pressure Measurement—Arterial pressure in littermate controls (n = 6) and GRM610L mice on each treatment was measured by radiotelemetry. Briefly, the mice were anesthetized with a mixture of ketamine/xylazine (100 and 10 mg/kg, respectively). A Data Sciences catheter was inserted into the left carotid artery, and a transmitter was placed to a subcutaneous pocket on the right flank (16). Baseline values were continuously recorded every minute for 4 days beginning 1 week after surgery. Mice from each group (+/+, +/L, and L/L) were then given DEX (1 mg, 10 mg/liter in drinking water) for 4 days. After treatment, mice were allowed to recover until blood pressure reached baseline. Values were analyzed as 12 h means reflecting the day (6:30AM to 6:30 p.m.) and night periods (6:30 p.m. to 6:30 a.m.).
Bone Marrow Transfer—Bone marrow was flushed from tibias and femurs of donor mice as described (17). Wild type B6 recipients received 2 500-centrigray fractions of γ-irradiation and were reconstituted with T cell-depleted bone marrow from GRM610L homozygous, heterozygous, or wild-type littermate controls.
Flow Cytometry—Bone marrow chimeric mice were sacrificed, and bone marrow, spleen, thymus, and lymph node cells were harvested. Single cell suspensions were made and cells were stained with antibodies against CD4, CD8, B220, and CD11b (clones, colors, and sources) and analyzed on a FACS Calibur (BD Biosciences, Franklin Lakes, NJ).
Physiologic Assessment of GRM610L Mice—Physiologic assessment of the mice was done in conjunction with the Yale Mouse Molecular Phenotyping Center. Blood samples were taken by retro-orbital phlebotomy in GRM610L and wild-type littermates, collected in a heparinized capillary tube, and immediately centrifuged. Serum levels of glucose (fasting for 15 h), triglyceride, cholesterol, and high density lipoprotein were measured using a COBAS Mira Plus blood chemistry analyzer. Plasma concentrations of Na+, K+, Cl-, BUN, Ca2+, and Mg2+ were measured by standard atomic absorption flame photometry. Dual energy x-ray absorptiometry was used for measurement of bone density. GRM610L and wild-type littermates were anesthetized and body weights recorded, and a small x-ray source was used to expose the entire animal to a cone shaped beam of both high and low energy x-rays. A high-resolution picture (0.18 × 0.18 mm pixel resolution) was taken of an image of the x-rays hitting a luminescent panel, and accuracy was determined by using phantoms of known values. Correlation of lean tissue mass of the total body was excellent (r2 = 0.99), whereas the smaller components of fat mass and bone mass were very good (r2 = 0.86 and r2 = 0.92, respectively). Software provided by the manufacturer was used to analyze the data and generate values for BMC, BMD, and lean body mass.
Human Mutation Screening—Genomic DNA was extracted from peripheral blood of 200 control subjects using standard techniques. Mutation detection was sought in hGRM604L and performed by PCR, the Reveal Mutation Discovery System, and direct sequencing, as described previously (18).
Animal Treatment and Serum Testosterone Analysis—11 to 15 wild-type, heterozygous, and homozygous animals in each group were injected with 0.1 mg/kg DEX in 0.25 ml of saline or vehicle intraperitoneally, housed separately for 3 h, then sacrificed for blood and liver collection. Serum testosterone concentrations were measured by radioimmunoassay as described previously (19). Interassay and intraassay variations were within 15%.
Liver Microsome Preparation and 11β-HSD1 Assay—Liver microsomes were prepared from DEX or vehicle-treated mice according to a previously described method. Briefly, 100 mg of liver was homogenized in 0.01 m phosphate-buffered saline containing 0.25 mm sucrose and centrifuged at 750 × g for 30 min to collect the supernatant. The supernatant was centrifuged at 12,000 × g for 30 min twice to remove mitochondria, and then ultracentrifuged at 105,000 × g for 60 min twice to collect the microsomes. 11β-HSD1 activity was assayed as described previously (20). Briefly, 25 nm [3H]dehydrocorticosterone (11-DHC) was incubated with 2 μg of liver microsome in the presence of 200 μm NADPH and 10 mm glucose 6-phosphate reactions for 10 min, at which time linear reaction rates were confirmed. The reaction was stopped by adding 2 ml of ice-cold ether. Steroids were extracted, and the organic layer dried under nitrogen. Steroids were separated chromatographically on thin layer plates in chloroform and methanol (90:10), and the radioactivity was measured using a scanning radiometer (System AR2000, Bioscan Inc., Washington, DC). The percentage conversion of 11-DHC to corticosterone was calculated by dividing the radioactive counts identified as 11-DHC (or corticosterone, respectively) by the total counts associated with corticosterone plus 11-DHC.
Statistical Methods—Data are presented as the mean ± S.E. Statistical analysis was by one-way analysis of variance or unpaired t test (two tailed) with significance accepted for p < 0.05. The PRISM software package (GraphPad, San Diego, CA) was used for all analyses.
RESULTS
Generation of a Gain of Function GR Knock-in Mouse—The targeting construct we used is described in Fig. 1A. The construct was linearized and electroporated into mouse strain 129 SV/REJ embryonic stem (ES) cells by the Animal Genomics Service at Yale University School of Medicine and G418, ganciclovir-resistant clones were selected. A total of 190 clones were screened via long-range PCR. Six constructs were identified that showed evidence of homologous recombination of the targeting construct into the mouse genome (Fig. 1B). Sequencing of the long-range PCR products confirmed their identity and Southern blotting confirmed homologous recombination as well (data not shown). To assess expression of the mutant allele, we amplified GR mRNA from these ES cells via reverse transcriptase-PCR, using mouse GR exon 4 and exon 9 primers that lie outside the targeting construct. On sequencing, we found evidence of robust expression of both the wild-type and mutant alleles (data not shown), confirming that the construct had inserted into the mouse genome at the appropriate site.
ES cells from two distinct clones, lines 11 and 32, carrying the mutant GRM610L allele were inserted into mouse blastocysts by the Gene Targeting Service at Yale University, and chimeric mice were generated by standard methods. Chimeric mice were bred with C57/BL6J mice to yield the F0 generation. The F0 mouse was crossed with a mouse constitutively expressing Flp recombinase (21) to remove the neomycin resistance cassette from the mouse germline (Fig. 1A). All subsequent experiments were performed on mice lacking the neomycin cassette. The phenotype of mice from lines 11 and 32 were identical, and so further characterization was performed on line 32.
GRM610L Mice Are Phenotypically Normal—We first assessed GRM610L mRNA expression in heterozygous mice via amplification of kidney GR mRNA. We found evidence of robust expression of both the wild-type and mutant alleles (Fig. 1C). We therefore moved on to assess phenotypic characteristics of these mice.
Mating of heterozygotes (GR +/L) results in production of homozygous (GR L/L), GR +/L, and wild-type mice (+/+). GRM610L mice were born in the expected Mendelian ratio (26.2% L/L, 48.4% +/L, and 25.4% +/+ of the first 252 mice genotyped at 3 weeks of age). Birth weight, postnatal growth, and development were indistinguishable from wild-type littermates (Table 1), and no visible abnormalities were found in GRM610L mice. GRM610L mice, the oldest of which are now 1.5 year of age, are equal in size to their littermates and appear healthy. Homozygous and heterozygous males and females were fertile, delivering litters of 6–12 pups at 20–21 days gestation. There were no significant differences in serum electrolytes, glucose, or lipid levels, bone mineral density, or percent body fat between wild-type, heterozygous, and homozygous mice (Table 1).
TABLE 1.
Body composition and serum measurements in GRM610L and wild-type littermates All data are from three- to four-month-old wild-type, GR +/L, GR L/L, and littermates and are expressed as the mean ± S.E. The number of animals is indicated in parentheses. Serum was isolated following a 15-h fast for lipid and glucose measurements. Total body bone density and whole body fat content were measured by dual energy x-ray absorptiometry in 10 pairs of gender matched littermates with or without the GRM610L allele. There were no significant differences between the groups for any parameter measured.
| WT (GR +/+) | Heterozygotes (GR +/L) | Homozygotes (GR L/L) | |
|---|---|---|---|
| Birth weight | 1.35 ± 0.03 (32) | 1.36 ± 0.02 (38) | 1.348 ± 0.03 (25) |
| Adult weight | 29.64 ± 1.05 (14) | 30.32 ± 1.647 (14) | 29.28 ± 1.34 (14) |
| Glucose | 120 ± 8.52 (10) | 123.5 ± 8.08 (10) | 123.6 ± 5.66 (10) |
| Cholesterol | 89.39 ± 3.81 (10) | 87.31 ± 3.19 (10) | 89.46 ± 3.12 (10) |
| Triglycerides | 78.88 ± 5.2 (10) | 73.94 ± 5.9 (10) | 72.73 ± 5.98 (10) |
| HDL | 70.84 ± 3.76 (10) | 69.04 ± 2.49 (10) | 70.62 ± 2.26 (10) |
| Na+ | 148.8 ± 0.72 (11) | 148.6 ± 0.99 (20) | 149.1 ± 0.52 (16) |
| K+ | 4.273 ± 0.16 (11) | 4.288 ± 0.12 (16) | 4.54 ± 0.14 (15) |
| Cl | 115.1 ± 0.76 (11) | 115 ± 0.89 (20) | 115.4 ± 0.7 (16) |
| Ca | 8.545 ± 0.11 (9) | 8.622 ± 0.09 (9) | 8.772 ± 0.13 (13) |
| Mg | 1.847 ± 0.06 (9) | 1.79 ± 0.06 (9) | 1.672 ± 0.04 (13) |
| BUN | 21.45 ± 1.05 (25) | 21.48 ± 1.21 (26) | 23.04 ± 0.97 (33) |
| Bone density | 0.054 ± 0.002 (23) | 0.053 ± 0.004 (14) | 0.054 ± 0.003 (10) |
| % of Fat | 19.96 ± 7.22 (23) | 20.42 ± 4.54 (14) | 19.66 ± 5.73 (10) |
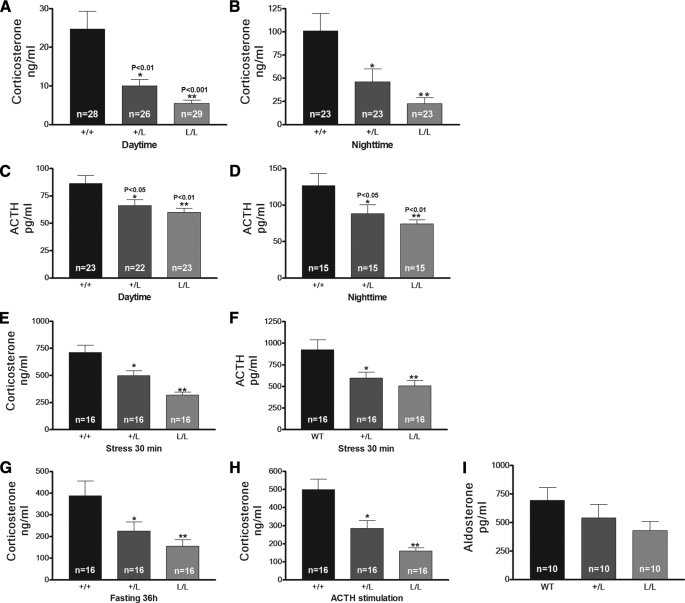
HPA Axis Suppression in GRM610L Mice—To assess basal function of the HPA axis in GRM610L mice, we collected plasma at 9:30 a.m. (morning) and 9:30 p.m. (night) from GRM610L mice and control littermates at 3–4 months of age. Homozygous and heterozygous mutant mice had very low plasma corticosterone levels when compared with wild-type littermates, both at the morning nadir at 9:30 a.m. (5.50 ± 0.82 ng/ml (L/L) and 9.99 ± 1.68 ng/ml (+/L) versus 24.79 ± 4.51 ng/ml (WT); p < 0.001; Fig. 2A) and at the evening diurnal peak at 9:00 p.m. (22.50 ± 6.44 (L/L) and 46.18 ± 13.96 ng/ml (+/L) versus 101.10 ± 18.63 ng/ml (WT) p < 0.001; Fig. 2B). Additionally, we found that heterozygous and homozygous GRM610L mice showed significantly decreased basal (9:30 a.m.) and peak (9:30 p.m.) ACTH levels compared with wild-type littermates, but that the circadian rhythm was maintained (Fig. 2, C and D). These data indicate that the HPA axis differs markedly between the mice, and suggest that the HPA axis feedback is appropriately down-regulating corticosterone production in GRM610L mice via suppression of ACTH. Nevertheless, the finding that GRM610L mice are phenotypically normal, save for a reset HPA axis and markedly reduced plasma corticosterone levels, confirms that our mutant allele is functional in vivo with activity that mimics our in vitro observations.
FIGURE 2.
Circulating corticosterone and ACTH levels are reduced in GRM610L mice. A and B, daytime (A) and nighttime (B) plasma corticosterone levels in GRM610L mice and WT littermates. C and D, daytime (C) and nighttime (D) plasma ACTH levels in GRM610L mice and WT littermates. GRM610L heterozygous and homozygous mice have a highly significant decrease in plasma corticosterone and ACTH levels compared with wild-type animals. E and F, corticosterone (E) and ACTH (F) levels in control and GRM610L littermates after 30-min restraint stress. G, corticosterone levels in control and GRM610L littermates after 36 h fasting. H, corticosterone levels in control and GRM610L mice littermates 20 min after ACTH injection (10 μg/kg, intraperitoneal). I, aldosterone levels in control and GRM610L mice littermates. *, p < 0.05; **, p < 0.01.
Given the reduction in basal corticosterone seen in GRM610L mice, we undertook dynamic testing of the HPA axis in these mice and compared the corticosterone and ACTH responses with those of WT littermates. Because a major function of GR in the control of the HPA axis is its regulation after challenges such as stress, we analyzed the response of GRM610L mice to acute restraint and food deprivation stress. Restraint stress for 30 min led to a strong elevation of plasma corticosterone and ACTH levels in mice of both genotypes (Fig. 2, E and F). Despite the qualitatively similar response in GRM610L mice, the elevation of the corticosterone and ACTH levels during restraint was significantly smaller in GRM610L mice. Similarly, food deprivation stress led to significant increases in serum corticosterone levels, but the level of corticosterone was significantly lower in GRM610L mice compared with WT littermates (Fig. 2G, p < 0.01). To clarify the role of ACTH in the regulation of corticosterone levels in GRM610L mice, we assessed the corticosterone response to ACTH. ACTH injection (10 μg/kg, intraperitoneal) led to a 5-fold induction of corticosterone levels in both genotypes. Because basal corticosterone levels in mutant animals (+/L 58.01 ± 15.67 ng/ml and L/L 32.33 ± 5.77 ng/ml) are lower than in controls (90.62 ± 15.11 ng/ml), the 5-fold induction resulted in lower absolute GC levels in mutant (+/L 283.90 ± 43.87 and L/L 159.60 ± 18.05 ng/ml) mice compared with control animals (500.2 ± 56.54 ng/ml, Fig. 2H). This indicates that even conditions of ACTH evoke a lower secretion of GCs in mutant animals. These dynamic tests of the HPA axis suggest that the GRM610L allele is functional in vivo and that the HPA axis of the mouse is intact.
Given the potential contribution of plasma corticosterone to the renin-angiotensin-aldosterone system, we assessed plasma aldosterone levels as well (Fig. 2I). Interestingly, we found a trend toward decreased circulating aldosterone levels in both homozygous (429.0 ± 81.4 pg/ml) and heterozygous mice (539.8 ± 118.7 pg/ml) compared with WT littermates (693.2 ± 132.5 pg/ml, p = 0.069 versus L/L).
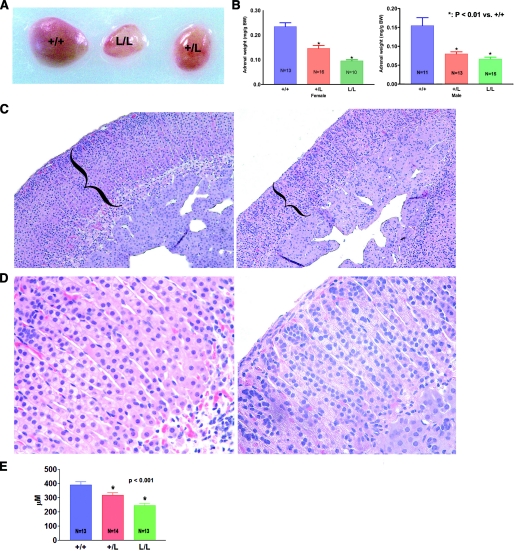
Reduced Adrenal Gland Size in GR L/L and GR +/L Mice— Because corticosterone levels are very low in GR L/L mice, this led us to check adrenal size and morphology in these mice. The adrenal glands from GR L/L and +/L mice were significantly smaller than those found in wild-type littermates; the adrenal weight/body weight ratio in GRM610L mice was ∼41% (L/L) and 56% (+/L) of that in WT female littermates, with similar reductions in adrenal size in male mice as well (Fig. 3, A and B). This is consistent with the decreased corticosterone levels observed in these mice. Histologic examination of the adrenal glands from GRM610L mice confirmed these findings. The adrenal cortex is composed of three zones: the outer zona glomerulosa synthesizes mineralocorticoids, the zona fasciculata is the area primarily responsible for corticosterone production in the mouse, and the inner zona reticularis primarily synthesizes androgens (22, 23). On hematoxylin and eosin staining, the adrenal architecture of GRM610L mice was markedly altered compared with +/+ mice, with marked cortical thinning (Fig. 3, C–F). In particular, the zona fasciculata of the adrenal cortex was markedly reduced in size, whereas the zona glomerulosa and medullary cells appeared relatively unaffected (Fig. 3F). To quantify this difference, we assessed adrenal cortical thickness in these mice. We found that the mean adrenal cortical thickness was 390.6 ± 21.6 μm(+/+), 318.4 ± 16.2 μm (+/L), and 246.1 ± 14.5 μm (L/L), indicating a highly significant reduction in adrenocortical size in GRM610L mice (Fig. 3G, p < 0.001).
FIGURE 3.
GRM610L mice have markedly reduced adrenal weight compared with control animals. A, gross appearance of adrenal glands taken from +/+, +/L, and L/L littermates. B, mean adrenal weight of 3–4-month-old female and male GRM610L mice and wild-type littermates. Adrenal weight is expressed as milligrams/g body weight. GR +/L and L/L mouse adrenals are significantly smaller than those from wild-type littermates in both male and female mice (*, p < 0.001 versus +/+). C, low (×10) and high (×40) power views of hematoxylin and eosin-stained adrenal tissue sections derived from a +/+ mouse (left panel) and an L/L mouse (right panel). There is marked thinning of the adrenal cortex (bracketed) in GRM610L mice. D, mean adrenal cortical thickness in +/+ (n = 13), +/L (n = 14), and L/L (n = 13) mice. There was a marked and highly significant reduction in adrenal cortical thickness in GRM610L mice.
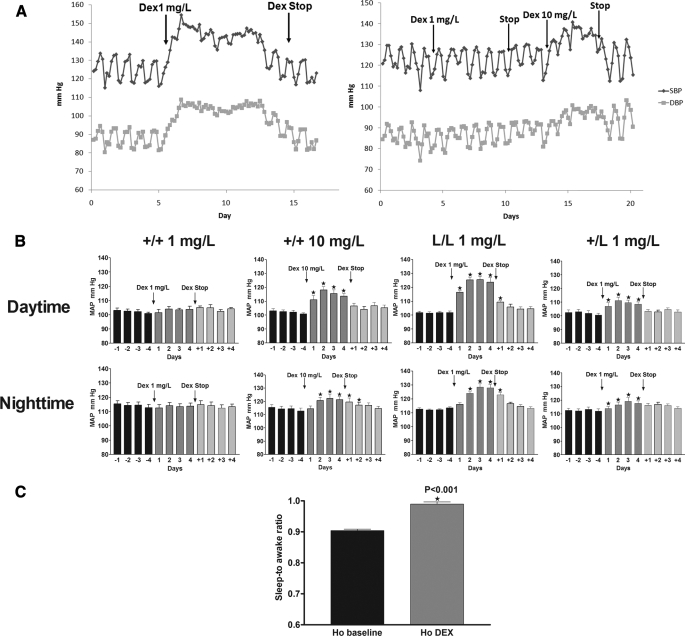
Low Dose Dexamethasone Increases Blood Pressure in GRM610L Mice, but Not in WT Mice—We hypothesized that GRM610L mice would have increased response to exogenously administered GC, and we therefore assessed the blood pressure response to dexamethasone administered in the drinking water. Blood pressure was measured directly in 3-month-old conscious male mice using radiotelemetry and was recorded for 12 days (4 days for baseline; 4 days DEX treatment; 4 days for following discontinuation of DEX). Mean arterial pressure (MAP) at baseline did not differ between the mutant and WT mice (p = 0.9262). Mice exhibited normal diurnal variation, with an elevated MAP at night (awake) and reduced MAP during the day (sleep) in both genotypes. When we treated GRM610L homozygous mice and WT littermates with the synthetic glucocorticoid DEX in their drinking water (1 mg/liter, about 0.1 mg/kg/day) for 4 days, blood pressure increased within hours of GC therapy and remained elevated for as long as steroid therapy was continued (Fig. 4A). Daytime MAP increased by 21 mm Hg and nighttime MAP by 12 mm Hg in DEX-treated GR L/L mice compared with baseline (p < 0.0001 for each point, Fig. 4B). We saw a similar but less pronounced rise in MAP in GR +/L treated with 1 mg/ml DEX (Fig. 4B). Notably, in each of these mice, the rise in BP was most pronounced during the daytime phase; the sleep-to-awake BP ratio was higher in DEX-treated GR L/L mice by 8.5% compared with their baseline MAP (Fig. 4C, p < 0.001), indicating that the dexamethasone-induced MAP elevation in GRM610L mice is exaggerated in the sleep phase. These data suggest the dexamethasone-induced rise in blood pressure mimics that which is observed in Cushing syndrome, with the characteristic loss of diurnal variation (Fig. 4A). Interestingly, when GC therapy was discontinued (after 4 days of therapy), there was a rapid reversal of GC-induced hypertension, blood pressure dropped within hours to baseline levels with an almost immediate resumption of normal diurnal variation.
FIGURE 4.
The GRM610L allele increases sensitivity to exogenous glucocorticoids in vivo. A, baseline blood pressure was recorded for 4 days in an L/L mouse (left panel) or in a +/+ (right panel) littermate. Subsequently, dexamethasone was provided in the drinking water (1 mg/liter) and blood pressure was followed radiotelemetrically. The blood pressure tracing of a representative mouse is shown. The initiation and termination of dexamethasone are indicated. Each data point represents the average of all measurements obtained within a 4-h window. DEX (1 mg/liter) induces a rapid rise in BP with loss of normal diurnal variation in L/L mice, but not in +/+ littermates; and that this rise in BP rapidly reverses following discontinuation of dexamethasone. Subsequently, 10 mg/liter DEX was provided to the +/+ mouse, and a rapid rise in BP was observed. B, 12-h means of MAP in male GR +/+ (n = 6), L/L (n = 6), or +/L (n = 6) littermates before (baseline) and after treatment with DEX (1–10 mg/liter) in the drinking water. MAP values were measured by telemetry. Values were analyzed as 12 h means reflecting the day and night periods and expressed as mean ± S.E.; C, mean ± S.E. values for sleep-to awake BP ratio of GR L/L mice before and after 1 mg/liter DEX treatment.
Although provision of 1 mg/liter DEX had a marked effect on blood pressure in GRM610L mice, the same dose had no effect on blood pressure in wild-type littermates (Fig. 4, A and B). However, when we increased the dexamethasone dose 10-fold, blood pressure rose to a similar degree as to what we observed in GR L/L mouse at 1 mg/liter.
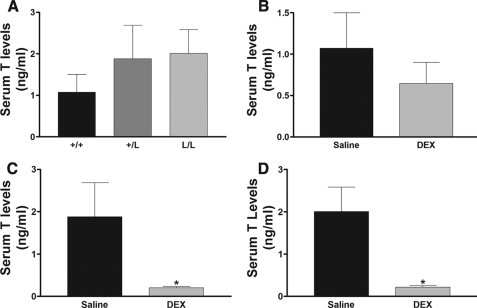
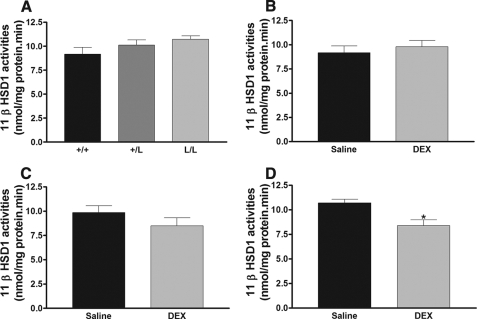
To measure other effects of the GRL604 allele in vivo, we measured serum testosterone and liver 11β-HSD1 activities in wild-type, heterozygous, and homozygous mice treated with either saline or DEX. Previous work has shown that serum testosterone levels are down-regulated by glucocorticoid. There was no significant difference in baseline serum testosterone levels in wild-type, heterozygous, and homozygous mice (Fig. 5A). However, provision of low dose DEX (0.1 mg/kg intraperitoneally) had no significant effect on serum testosterone levels in wild-type mice (Fig. 5B), but caused a highly significant reduction in serum testosterone levels in mice heterozygous and homozygous for the GRL604 allele (Fig. 5, C and D). Similarly, glucocorticoids have been shown to cause a down-regulation in 11β-HSD1 activity in the liver. We found no significant difference in 11β-HSD1 activity in wild-type, heterozygous, or homozygous mice (Fig. 6A). Low dose DEX had no effect on hepatic 11β-HSD1 activity in wild-type mice (Fig. 6B), but caused a nonsignificant reduction (p = 0.26) in 11β-HSD1 activity in heterozygous mice (Fig. 6C) and a significant reduction in hepatic 11β-HSD1 activity in homozygous mice (Fig. 6D). These data again support that the GRM610L allele has increased activity in vivo compared with the wild-type allele.
FIGURE 5.
A, serum testosterone (T) levels in wild-type (+/+, n = 11), heterozygous (+/L, n = 8), and homozygous (L/L, n = 8) mice. B–D, the effect of low-dose dexamethasone (DEX, 0.1 mg/kg intraperitoneally) treatment on serum testosterone levels in wild-type (B, n = 12), heterozygous (C, n = 9), and homozygous (D, n = 6) mice. Data are expressed as mean ± S.E. *, p < 0.05 compared with saline treatment.
FIGURE 6.
A, 11β-HSD1 activities in livers of wild-type (+/+, n = 5), heterozygous (+/L, n = 5), and homozygous (L/L, n = 4) mice. B–D, the effects of low-dose dexamethasone (DEX, 0.1 mg/kg intraperitoneally) treatment on 11β-HSD1 activity in wild-type (panel B, n = 7), heterozygous (panel C, n = 7), and homozygous (panel D, n = 8) mice. Data are expressed as mean ± S.E. *, p < 0.05 versus saline treatment.
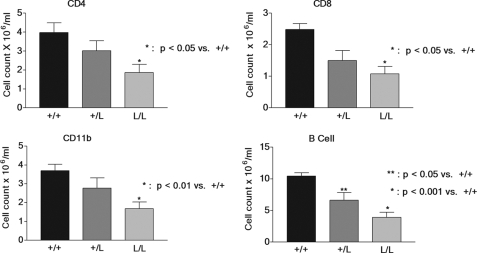
Decreased Immune Cell Counts in Bone Marrow Chimeras Derived from GRM610L Mice—Our findings suggested that phenotypic effects of the GRM610L mutation are limited by the low corticosterone levels induced by the HPA axis. To test the activity of the mutant receptor in a normal corticosterone setting and to determine whether the presence of GRM610L allele in an individual tissue would cause a specific glucocorticoid-induced effect in these cells, we transferred bone marrow from wild-type and mutant mice into irradiated C57/BL6J mice, and measured immune cell counts in these bone marrow chimera. We reasoned that the normal corticosterone levels in the recipient mice would suppress the GRM610L immune cells more than wild-type immune cells. We found a highly significant reduction in peripheral CD4, CD8, macrophage (CD11b), and B cell (CD220) counts in mice that received bone marrow from homozygous mice compared with mice that received wild-type bone marrow (Fig. 7). There was evidence of a dose-response relationship as well, for mice receiving bone marrow from GRM610L heterozygotes had significant reductions in CD220 cell counts and a strong trend toward lower CD4, CD8, and macrophage counts. These data confirm that the mutant allele has increased activity compared with the wild-type allele when placed in a mouse with normal corticosterone levels and suggest that tissue-specific expression of GRM610L results in a tissue-specific glucocorticoid effect in our model system.
FIGURE 7.
Decreased immune cell counts in wild-type recipients of GRM610L bone marrow. Irradiated C57/BL6 mice were transplanted with T-cell depleted bone marrow from mice carrying 0, 1, or 2 copies of the GRM610L allele. Ten mice were transplanted in each group. Ten weeks later (after engraftment), blood was removed via standard retroorbital bleeding techniques, and prepared for fluorescence-activated cell sorter analysis. Cell counts for the indicated cell types were assessed. One-way analysis of variance and Tukey Cramer analysis was used to compare the experimental groups with the wild-type control.
Our finding that GRM610L mice have no obvious clinical phenotype at baseline led us to question whether such a polymorphism might be present in humans. We screened 200 human control samples for the presence of the mutation, however, but found no evidence of this mutation. Whether this mutation might be present in a population of individuals selected for steroid sensitivity remains an open question.
DISCUSSION
We previously demonstrated that substitution of leucine for methionine at residue 604 in the human GR leads to a gain in sensitivity of the mutant receptor in vitro (13). Here, we extend this finding to establish a knock-in mouse carrying this point mutation (residue 610 in the mouse) of the GR and demonstrate that the homologous mutation in mice leads to a similar gain of function in vivo. Mice carrying the mutant allele do not display any obvious phenotypic abnormalities in terms of serum electrolyte, glucose or lipid levels, bone mineral density, serum testosterone levels, or percent body fat when compared with wild-type littermates. We believe the absence of a clinical manifestation of glucocorticoid hypersensitivity in these mice is due to a “resetting” of the HPA axis, presumably mediated by the hypothalamic GR, which brings about a compensatory decrease in basal corticosterone secretion to keep balance between need and production. GRM610L mice markedly decrease corticosterone levels, both basally and in response to stress or ACTH stimulation. This phenotype is similar to what has been observed in mice overexpressing a GR transgene (24), confirming that GR activity can be regulated by changes in ligand affinity or receptor numbers. Coupled with the significant decrease in ACTH levels we observed in heterozygotes and homozygotes, our data suggests enhanced glucocorticoid feedback inhibition in GRM610L mice, which is likely due to hypersensitivity on the part of hypothalamic GRs.
Glucocorticoids act via intracellular receptors of 2 types: GR and mineralocorticoid (MR) receptors (25). MR binds corticosterone with 10-fold higher affinity than GR and, in addition, binds the mineralocorticoid aldosterone with equal affinity (26, 27). Until recently, it had been commonly believed that hippocampal MR serves as the primary regulator of basal HPA activity by virtue of its high affinity, and that GR provides feedback regulation during stress, when endogenous levels of glucocorticoids are high (28–30). However, more recent evidence argues against this hypothesis. Mice lacking GR in the brain (31) or forebrain (32) have elevated basal GC plasma levels, indicating that MR is not sufficient to compensate for the lack of GR in the brain. Our finding that GRM610L mice have marked suppression of the HPA axis both at baseline and during stress is consistent with this idea, and suggests that GR is a primary mediator of both basal and stress regulation of the HPA axis. In contrast, the contribution of brain MR to corticosteroid feedback regulation has been assessed in mice lacking MR in the forebrain; these mice showed normal basal corticosterone levels as well as normal CRH mRNA and GR protein expression in the paraventricular nucleus (33). These results indicate that limbic MR is dispensable for the maintenance of basal circadian HPA axis activity. Based on our GRM610L mice, in which a single amino acid change in GR markedly alters HPA axis activity, and genetically engineered GR or MR mouse studies, GR and not MR seems to function as the primary regulator of the HPA system activity both at baseline and following stress.
GCs are necessary for maintenance of BP in clinical and experimental models of adrenalectomy. Glucocorticoid deficiency can result in hypotension (Addison disease), weight loss, hypoglycemia, and death, especially in the setting of stress (34). Conversely, exogenous or endogenous glucocorticoid excess can contribute to the development of hypertension, insulin resistance, hyperglycemia, weight gain, hyperhomocysteinemia, and atherosclerosis. Hypertension occurs in ∼75% of patients with Cushing syndrome (35, 36) and 20% of patients treated with oral glucorticoids (37). Glucocorticoid-induced hypertension has traditionally been ascribed to the promiscuous activation of renal MR by cortisol or other GCs. Our finding that markedly reduced doses of dexamethasone were sufficient to induce sustained hypertension in GRM610L mice, but not in GRWT mice, suggests that, at least in this model system, DEX-mediated hypertension is mediated primarily if not exclusively by GR and that mineralocorticoid effects are negligible. These, together with some clinical findings that cortisol increases blood pressure in essential hypertension because of a change in tissue sensitivity rather than a change in circulating concentration (38), suggest that GR hypersensitivity may contribute to hypertension. Further studies will be required to determine the contribution of GR to the hypertension induced by corticosterone, a steroid with both glucocorticoid and mineralocorticoid effects, and also to determine the mechanism by which GR mediates this hypertension. Nevertheless, these data suggest that this model system can provide a useful method to determine the relative contribution of GR and MR to glucocorticoid dependent effects.
In rodent models, increased GC levels disrupt and suppress endocrine signaling in the male reproductive axis (39, 40). Elevations in circulating corticosteroid in response to stress are associated with testicular involution and a significant drop in testosterone secretion. These changes are accompanied by diminished libido and fertility (41). A similar pattern is observed in rats subjected to immobilization stress (42). A number of in vitro studies have shown that increased glucocorticoid directly inhibits testosterone production by Leydig cells (43, 44). Leydig cells contain glucocorticoid receptors (45). Moreover, it is now known that glucocorticoid directly inhibits the transcription of genes encoding testosterone biosynthetic enzymes (43, 46–49) and these same genes are positively induced by luteinizing hormone (50). The results here confirm that suppression of testosterone synthesis is mediated directly by the glucocorticoid receptor.
For GCs sensitivity and healthy individuals, GCs are important therapeutic agents used for the treatment of various inflammatory and autoimmune diseases and variability in the sensitivity to GCs is observed in patients treated with GCs (51). In clinical observations, a considerable variability among subjects is seen in their sensitivity to GC therapy, both with regard to efficacy and side effects (51, 52). In the normal population, some individuals develop severe adverse effects on low dose glucocorticoid therapy, whereas others do not develop side effects even during long-term therapy with much higher doses. Clinical and laboratory evidence suggest that patients can be divided into “steroid-sensitive” and “resistant” groups (53). Generalized glucocorticoid resistance and hypersensitivity states represent diametrically opposite examples of this process. This implies that each subject, when treated with GCs, needs an individually optimized dose to maintain a balance between beneficial and adverse effects associated with GC treatment. Mutations in the hormone-binding domain of the GR gene have been described as the cause of familial glucocorticoid resistance (53, 54); the abnormal receptor does not properly regulate the HPA axis, thereby leading to elevated plasma ACTH levels and increased circulating cortisol concentrations. Clinical syndromes of glucocorticoid hypersensitivity have been less well studied. Iida et al. (5) described a patient affected with Cushingoid symptoms despite low plasma glucocorticoid levels; an underlying etiology was never identified. Recent evidence suggests that human carriers of a common GR polymorphism (N363S) have increased sensitivity to GCs in vivo, but in vitro studies on the N363S polymorphism found no effects on its ability to bind hormone or activate transcription (55). Our findings show that the M610L substitution in the mouse GR ligand binding domain in vivo is associated with an increased sensitivity to GCs, which is consistent with human GRM604L that causes glucocorticoid hypersensitivity in vitro. These data reinforce the idea that the pathophysiological contribution of glucocorticoids are not always inadequately assessed by measurement of circulating GC concentrations and raise the possibility that gain of function mutations in GR could underlie GC sensitivity in healthy humans. We failed to identify GRM604L in 200 healthy controls, suggesting that this is not a common clinical polymorphism. Further screening of patients identified with GC hypersensitivity will be required to determine whether, indeed, this clinical entity exists. However, our unique mouse model to approach the molecular mechanism of glucocorticoid hypertension, for the first time, establishes that glucocorticoid hypersensitivity can be linked to genetic alteration of the GR.
In conclusion, the GRM610L mice increase the sensitivity to GCs 10-fold in vivo, as evidenced by alterations in HPA axis, decreased adrenal size, altered immune cell count/function in bone marrow chimeras, and the altered hypertensive response to exogenous GCs. This work confirms the importance of helix 3-helix 5 interactions in the anchoring of steroidal GR agonists (13, 56). Our present study demonstrated that the GRM610L mouse serves as a unique model to approach the molecular metabolism of glucocorticoid hypersensitivity. This model should prove useful in distinguishing the relative contribution of GR versus other receptors to glucocorticoid-mediated events, such as hypertension. Furthermore, we believe that an animal model permitting tissue-specific expression of the GRM610L allele will provide a valuable tool for the study of glucocorticoid effects in vivo.
Acknowledgments
We thank Chantal M. Sottas, Han Lin, Lei Dong, and Guo-xin Hu for technical assistance of measurement of testosterone and 11β-HSD1.
This work was supported, in whole or in part, by National Institutes of Health Grant R03-DK064907 (to D. S. G.) from the NIDDK. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: GC, glucocorticoids; GR, glucocorticoid receptor; HPA, hypothalamic-pituitary-adrenal; ES, embryonic stem; MAP, mean arterial pressure; ACTH, adrenocorticotropic hormone; DEX, dexamethasone; DHC, dehydrocorticosterone; WT, wild type; BP, blood pressure; MR, mineralocorticoid receptor.
References
- 1.Galon, J., Franchimont, D., Hiroi, N., Frey, G., Boettner, A., Ehrhart-Bornstein, M., O'Shea, J. J., Chrousos, G. P., and Bornstein, S. R. (2002) FASEB J. 16 61-71 [DOI] [PubMed] [Google Scholar]
- 2.Beato, M., Herrlich, P., and Schutz, G. (1995) Cell 83 851-857 [DOI] [PubMed] [Google Scholar]
- 3.Svec, F. (1985) Life Sci. 36 2359-2366 [DOI] [PubMed] [Google Scholar]
- 4.Gomez, F., De Kloet, E. R., and Armario, A. (1998) Am. J. Physiol. 274 R420-R427 [DOI] [PubMed] [Google Scholar]
- 5.Iida, S., Nakamura, Y., Fujii, H., Nishimura, J., Tsugawa, M., Gomi, M., Fukata, J., Tarui, S., Moriwaki, K., and Kitani, T. (1990) J. Clin. Endocrinol. Metab. 70 729-737 [DOI] [PubMed] [Google Scholar]
- 6.Buemann, B., Black, E., Holst, C., Toubro, S., Echwald, S., Pedersen, O., Astrup, A., and Sorensen, T. (2005) Obes. Res. 13 862-867 [DOI] [PubMed] [Google Scholar]
- 7.Vingerhoeds, A. C., Thijssen, J. H., and Schwarz, F. (1976) J. Clin. Endocrinol. Metab. 43 1128-1133 [DOI] [PubMed] [Google Scholar]
- 8.Karl, M., Lamberts, S. W., Detera-Wadleigh, S. D., Encio, I. J., Stratakis, C. A., Hurley, D. M., Accili, D., and Chrousos, G. P. (1993) J. Clin. Endocrinol. Metab. 76 683-689 [DOI] [PubMed] [Google Scholar]
- 9.Charmandari, E., Raji, A., Kino, T., Ichijo, T., Tiulpakov, A., Zachman, K., and Chrousos, G. P. (2005) J. Clin. Endocrinol. Metab. 90 3696-3705 [DOI] [PubMed] [Google Scholar]
- 10.Irving, J. A., Minto, L., Bailey, S., and Hall, A. G. (2005) Cancer Res. 65 9712-9718 [DOI] [PubMed] [Google Scholar]
- 11.Feng, J., Zheng, J., Bennett, W. P., Heston, L. L., Jones, I. R., Craddock, N., and Sommer, S. S. (2000) Am. J. Med. Genet. 96 412-417 [DOI] [PubMed] [Google Scholar]
- 12.Lamberts, S. W., Poldermans, D., Zweens, M., and de Jong, F. H. (1986) J. Clin. Endocrinol. Metab. 63 1328-1333 [DOI] [PubMed] [Google Scholar]
- 13.Zhang, J., Simisky, J., Tsai, F. T., and Geller, D. S. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 2707-2712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu, C., Warriar, N., and Govindan, M. V. (1995) Biochemistry 34 14163-14173 [DOI] [PubMed] [Google Scholar]
- 15.Warriar, N., Yu, C., and Govindan, M. V. (1994) J. Biol. Chem. 269 29010-29015 [PubMed] [Google Scholar]
- 16.Butz, G. M., and Davisson, R. L. (2001) Physiol. Genomics 5 89-97 [DOI] [PubMed] [Google Scholar]
- 17.Matte, C. C., Liu, J., Cormier, J., Anderson, B. E., Athanasiadis, I., Jain, D., McNiff, J., and Shlomchik, W. D. (2004) Nat. Med. 10 987-992 [DOI] [PubMed] [Google Scholar]
- 18.Abelson, J. F., Kwan, K. Y., O'Roak, B. J., Baek, D. Y., Stillman, A. A., Morgan, T. M., Mathews, C. A., Pauls, D. L., Rasin, M. R., Gunel, M., Davis, N. R., Ercan-Sencicek, A. G., Guez, D. H., Spertus, J. A., Leckman, J. F., Dure, L. S., Kurlan, R., Singer, H. S., Gilbert, D. L., Farhi, A., Louvi, A., Lifton, R. P., Sestan, N., and State, M. W. (2005) Science 310 317-320 [DOI] [PubMed] [Google Scholar]
- 19.Cochran, R. C., Ewing, L. L., and Niswender, G. D. (1981) Investig. Urol. 19 142-147 [PubMed] [Google Scholar]
- 20.Lakshmi, V., and Monder, C. (1985) J. Steroid Biochem. 22 331-340 [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez, C. I., Buchholz, F., Galloway, J., Sequerra, R., Kasper, J., Ayala, R., Stewart, A. F., and Dymecki, S. M. (2000) Nat. Genet. 25 139-140 [DOI] [PubMed] [Google Scholar]
- 22.Paust, H. J., Loeper, S., Else, T., Bamberger, A. M., Papadopoulos, G., Pankoke, D., Saeger, W., and Bamberger, C. M. (2006) Exp. Clin. Endocrinol. Diabetes 114 6-10 [DOI] [PubMed] [Google Scholar]
- 23.Deschepper, C. F., Olson, J. L., Otis, M., and Gallo-Payet, N. (2004) J. Appl. Physiol. 97 369-376 [DOI] [PubMed] [Google Scholar]
- 24.Reichardt, H. M., Umland, T., Bauer, A., Kretz, O., and Schutz, G. (2000) Mol. Cell. Biol. 20 9009-9017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Kloet, E. R., Van Acker, S. A., Sibug, R. M., Oitzl, M. S., Meijer, O. C., Rahmouni, K., and de Jong, W. (2000) Kidney Int. 57 1329-1336 [DOI] [PubMed] [Google Scholar]
- 26.Jacobson, L., and Sapolsky, R. (1991) Endocr. Rev. 12 118-134 [DOI] [PubMed] [Google Scholar]
- 27.Funder, J. W. (1997) Annu. Rev. Med. 48 231-240 [DOI] [PubMed] [Google Scholar]
- 28.Juruena, M. F., Cleare, A. J., Papadopoulos, A. S., Poon, L., Lightman, S., and Pariante, C. M. (2006) Psychopharmacology 189 225-235 [DOI] [PubMed] [Google Scholar]
- 29.Giordano, R., Bo, M., Pellegrino, M., Vezzari, M., Baldi, M., Picu, A., Balbo, M., Bonelli, L., Migliaretti, G., Ghigo, E., and Arvat, E. (2005) J. Clin. Endocrinol. Metab. 90 5656-5662 [DOI] [PubMed] [Google Scholar]
- 30.Howell, M. P., and Muglia, L. J. (2006) Front. Neuroendocrinol. 27 275-284 [DOI] [PubMed] [Google Scholar]
- 31.Tronche, F., Kellendonk, C., Kretz, O., Gass, P., Anlag, K., Orban, P. C., Bock, R., Klein, R., and Schutz, G. (1999) Nat. Genet. 23 99-103 [DOI] [PubMed] [Google Scholar]
- 32.Boyle, M. P., Brewer, J. A., Funatsu, M., Wozniak, D. F., Tsien, J. Z., Izumi, Y., and Muglia, L. J. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 473-478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berger, S., Wolfer, D. P., Selbach, O., Alter, H., Erdmann, G., Reichardt, H. M., Chepkova, A. N., Welzl, H., Haas, H. L., Lipp, H. P., and Schutz, G. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 195-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eremina, V., Wong, M. A., Cui, S., Schwartz, L., and Quaggin, S. E. (2002) J. Am. Soc. Nephrol. 13 788-793 [DOI] [PubMed] [Google Scholar]
- 35.Mantero, F., and Boscaro, M. (1992) J. Steroid Biochem. Mol. Biol. 43 409-413 [DOI] [PubMed] [Google Scholar]
- 36.Kelly, J. J., Mangos, G., Williamson, P. M., and Whitworth, J. A. (1998) Clin. Exp. Pharmacol. Physiol. Suppl. 25 S51-S56 [DOI] [PubMed] [Google Scholar]
- 37.Trostel, K. A., and Osborn, J. W. (1992) Am. J. Physiol. 263 R1265-R1270 [DOI] [PubMed] [Google Scholar]
- 38.Walker, B. R., Best, R., Shackleton, C. H., Padfield, P. L., and Edwards, C. R. (1996) Hypertension 27 190-196 [DOI] [PubMed] [Google Scholar]
- 39.Baldwin, B. A., Hutchison, J. B., Parrott, R. F., and Steimer, T. (1979) J. Neurosci. Methods 1 243-248 [DOI] [PubMed] [Google Scholar]
- 40.Suter, D. E., and Schwartz, N. B. (1985) Endocrinology 117 855-859 [DOI] [PubMed] [Google Scholar]
- 41.Monder, C., Sakai, R. R., Blanchard, R. J., Blanchard, D. C., Lakshmi, V., Miroff, Y., Phillips, D. M., and Hardy, M. (1992) in Stress and Reproduction (Boublik, J. H., Funder, J. W., and Sheppard, K. E., eds) pp. 145-155, Raven Press, New York
- 42.Monder, C., Sakai, R. R., Miroff, Y., Blanchard, D. C., and Blanchard, R. J. (1994) Endocrinology 134 1193-1198 [DOI] [PubMed] [Google Scholar]
- 43.Hales, D. B., and Payne, A. H. (1989) Endocrinology 124 2099-2104 [DOI] [PubMed] [Google Scholar]
- 44.Orr, T. E., Taylor, M. F., Bhattacharyya, A. K., Collins, D. C., and Mann, D. R. (1994) J. Androl. 15 302-308 [PubMed] [Google Scholar]
- 45.Ge, R. S., Hardy, D. O., Catterall, J. F., and Hardy, M. P. (1997) Endocrinology 138 5089-5095 [DOI] [PubMed] [Google Scholar]
- 46.Bambino, T. H., and Hsueh, A. J. (1981) Endocrinology 108 2142-2148 [DOI] [PubMed] [Google Scholar]
- 47.Bain, P. A., Yoo, M., Clarke, T., Hammond, S. H., and Payne, A. H. (1991) Proc. Natl. Acad. Sci. U. S. A. 88 8870-8874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agular, B. M., and Vind, C. (1995) J. Steroid Biochem. Mol. Biol. 54 75-81 [DOI] [PubMed] [Google Scholar]
- 49.Agular, B. M., Vinggaard, A. M., and Vind, C. (1992) J. Steroid Biochem. Mol. Biol. 43 565-571 [DOI] [PubMed] [Google Scholar]
- 50.Ge, R. S., Dong, Q., Sottas, C. M., Chen, H., Zirkin, B. R., and Hardy, M. P. (2005) Biol. Reprod. 72 1405-1415 [DOI] [PubMed] [Google Scholar]
- 51.Huizenga, N. A., Koper, J. W., De Lange, P., Pols, H. A., Stolk, R. P., Burger, H., Grobbee, D. E., Brinkmann, A. O., De Jong, F. H., and Lamberts, S. W. (1998) J. Clin. Endocrinol. Metab. 83 144-151 [DOI] [PubMed] [Google Scholar]
- 52.Axelrod, L. (1976) Medicine (Baltimore) 55 39-65 [DOI] [PubMed] [Google Scholar]
- 53.Lamberts, S. W., Huizenga, A. T., de Lange, P., de Jong, F. H., and Koper, J. W. (1996) Steroids 61 157-160 [DOI] [PubMed] [Google Scholar]
- 54.Nagano, M., Nakamura, T., Niimi, S., Fujino, T., Nishimura, T., Murayama, N., Ishida, S., Ozawa, S., Saito, Y., and Sawada, J. (2002) Cancer Lett. 181 109-114 [DOI] [PubMed] [Google Scholar]
- 55.van Winsen, L. L., Hooper-van Veen, T., van Rossum, E. F., Polman, C. H., van den Berg, T. K., Koper, J. W., and Uitdehaag, B. M. (2005) J. Neuroimmunol. 167 150-156 [DOI] [PubMed] [Google Scholar]
- 56.Zhang, J., Tsai, F. T., and Geller, D. S. (2006) J. Mol. Endocrinol. 37 163-173 [DOI] [PubMed] [Google Scholar]