Abstract
Cells within tissues are continuously exposed to physical forces including hydrostatic pressure, shear stress, and compression and tension forces. Cells dynamically adapt to force by modifying their behaviour and remodelling their microenvironment. They also sense these forces through mechanoreceptors and respond by exerting reciprocal actomyosin- and cytoskeletal-dependent cell-generated force by a process termed ‘mechanoreciprocity’. Loss of mechanoreciprocity has been shown to promote the progression of disease, including cancer. Moreover, the mechanical properties of a tissue contribute to disease progression, compromise treatment and might also alter cancer risk. Thus, the changing force that cells experience needs to be considered when trying to understand the complex nature of tumorigenesis.
At a glance.
Cells within tissues are continuously exposed to physical forces, including hydrostatic pressure, shear stress and compression and tension forces. The nature of these forces can change in pathologies such as cardiovascular disease and cancer.
Cells sense force through mechanoreceptors and, regardless of the type of force applied, cells respond by exerting reciprocal actomyosin- and cytoskeleton-dependent cell-generated force by a process termed mechanoreciprocity.
Mechanoreciprocity maintains tensional homeostasis in the tissue and is necessary for development and tissue-specific differentiation. Its loss promotes disease progression, including liver fibrosis, atherosclerosis and cancer.
Cells dynamically adapt to force by modifying their behaviour and remodelling their microenvironment. This adaptation probably involves a combination of epigenetic chromatin remodelling events and direct physical links between the matrix and nucleus that regulate gene expression. These gene-regulatory processes are altered in diseases such as cancer.
Breast cancer is characterized by changes in cellular rheology and tissue level forces, a stiffening of the tissue and a progressive loss of tensional homeostasis that has been exploited to detect tumours. The mechanical properties of a tissue contribute to disease progression, compromise treatment and might also alter cancer risk.
Force modulates cell fate and directs tissue development and post-natal function. Although we know much about the biochemical pathways that direct cell behaviour, by comparison we know less about how force can regulate cell fate and tissue phenotype. Nevertheless, cells in tissues such as the heart, lung and skeleton encounter nanoscale to macroscale forces that are integral to their function. The nature of these tissue-associated forces can be parallel, such as the shear stress induced by blood flow on a vessel wall, or perpendicular, such as the compressive or tensile stress induced by weight bearing on bone. In fact, all cells, including those incorporated into traditionally mechanically static tissues, such as the breast or the brain, are exposed to isometric force or tension that is generated locally at the nanoscale level by cell–cell or cell–extracellular matrix (ECM) interactions. These nanoscale forces influence cell function through actomyosin contractility and actin dynamics, and it is increasingly clear that force collaborates with biochemical cues to modulate cell and tissue behaviour.
In this Review we summarize the current understanding of tensional homeostasis in tissue development, homeostasis and cancer, and identify important areas for investigation. Defining the role of force on cell and tissue behaviour depends on understanding what contributes to force generation in the tissue, how the cell senses and integrates exogenous mechanical signals within its tissue microenvironment, and thereafter how the cell coordinates its response as part of a multicellular, organized tissue structure within its three-dimensional ECM microenvironment. To focus our Review, we have detailed how force modulates the normal and malignant behaviour of mammary epithelial cells in the context of the breast, illustrating, where pertinent, major concepts with examples from experimental findings.
Forcing form and function
The importance of mechanical force in biological systems is illustrated by exploring its role in normal tissue development and function. The mechanical stress that a cell is subjected to is quantified in Pascals (Pa) and measured as force per unit area, or N per m2 (BOX 1). This mechanical stress or force, in turn, is perceived and integrated in the cell at the molecular level through mechanically responsive sensors that interface with biochemical signalling cascades to elicit a specific cellular response through mechano-effectors. For example, force and growth factor receptor signalling can each influence cell growth, survival, motility, differentiation, shape and gene expression by regulating the activity of RhoGTPases that modulate actomyosin contractility and actin dynamics1–7. Similarly, integrin-dependent extracellular-signal regulated kinase (Erk) signalling and focal adhesion assembly are regulated by both growth factor signalling and force from the ECM8,9 (BOX 2).
Force and embryogenesis
Force has a fundamental role in directing stem cell fate and in dictating embryonic development10–12. For instance, embryonic stem cells progressively stiffen as cells differentiate13, whereas stem cell shape and specification are influenced by Rho-dependent contractility that is modulated by the mechanical properties of the tissue microenvironment3,5. Indeed, mesenchymal stem cells undergo lineage selection in response to the elasticity of the matrix substrate. Soft matrices, similar to the brain, direct stem cells into a neurogenic lineage, whereas stiffer matrices, similar to muscle and newly deposited bone, direct them into myogenic and osteogenic lineages3. As development proceeds, tension fields mediated by cell compression that result from normal morphogenic movements also shape the embryo. Indeed, external micropipette-applied force, which mimics these developmental forces, drives nuclear translocation of the transcription factor Armadillo to activate the transcription of twist, which controls the formation of the dorsal–ventral axis in the early Drosphilia melanogaster embryo14. Tissue development depends not only on the precisely timed application of force, but also on its correct spatial localization, as in the distinctly patterned tissue domains that specify cell polarity, shape and motility in the trunk and head mesoderm in Xenopus laevis embryos15,16. By contrast, mislocalization of Rho and Rac, which regulate cell contractility, prevents blastula gastrulation15,16.
Force is essential for normal tissue-specific development, in which it orchestrates tissue organization and function, and regulates cell growth, survival and migration. The lung epithelium, for example, undergoes branching morphogenesis as a result of progressive end bud enlargement and expansion to form the respiratory tree17. Interestingly, like the branching of the adolescent mammary gland, branch patterning in the lung epithelium is dictated by localized remodelling of the ECM and the corresponding stretching of lung epithelial cells at these locations. Force also regulates the integrity of the final lung ductal tree, which is governed by the cyclic shear stress of fetal breathing movements18,19. Indeed, compromising Rho-dependent cytoskeletal tension perturbs basement membrane thickness, disrupts terminal bud formation and compromises lung epithelial duct organization20.
Adult tissue homeostasis and the ECM
A balance of forces is required to maintain adult tissue homeostasis. Skeletal health depends on mechanical loading, such that exercise increases the proteoglycan content of articular cartilage whereas reduced mobility leads to loss of proteoglycan content and exacerbates arthritis-associated joint degeneration21,22. Force also facilitates bone matrix deposition to accommodate skeletal loading such that immobilization of the organism, unilateral lower limb suspension or microgravity leads to loss of bone mineral density, which in turn compromises bone strength23,24. Similarly, vascular function is largely determined by fluid shear stress25,26. The shear stress induced by blood flow permits artery maturation by directing endothelial cells and their filamentous cytoskeletal networks to elongate and align with the direction of flow27.
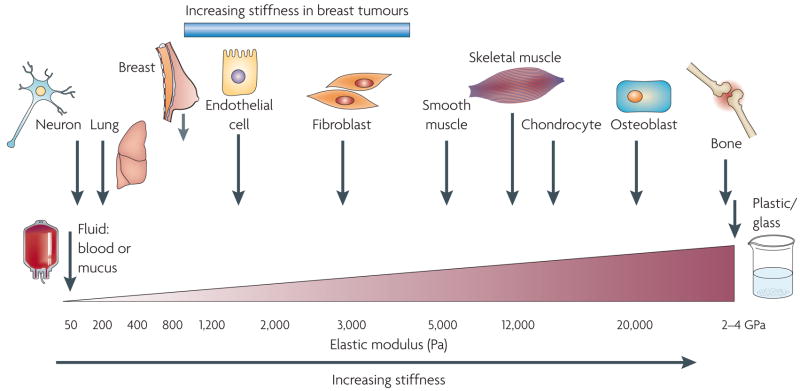
It is becoming increasingly apparent that each tissue has a characteristic ‘stiffness phenotype’ (FIG. 1) and that each cellular component within a tissue has a unique rheology and a stiffness optimum that can change over the course of development (as in lung branching morphogenesis), in response to function (as during mammary gland lactation) or in pathological situations (as in atherosclerotic plaque formation or in tumours)28,29. Furthermore, the physical properties of the ECM and cellular rheology can profoundly influence cellular behaviours as diverse as differentiation, tissue organization and cell migration6,30–32.
Figure 1. Cells are tuned to the materials properties of their matrix.
All cells, including those in traditionally mechanically static tissues, such as the breast or the brain, are exposed to isometric force or tension that is generated locally at the nanoscale level by cell–cell or cell–extracellular matrix interactions and that influences cell function through actomyosin contractility and actin dynamics. Moreover, each cell type is specifically tuned to the specific tissue in which it resides. The brain, for instance, is infinitely softer than bone tissue. Consequently, neural cell growth, survival and differentiation are favoured by a highly compliant matrix. By contrast, osteoblast differentiation and survival occurs optimally on stiffer extracellular matrices with material properties more similar to newly formed bone. Normal mammary epithelial cell growth, survival, differentiation and morphogenesis are optimally supported by interaction with a soft matrix. Following transformation, however, breast tissue becomes progressively stiffer and tumour cells become significantly more contractile and hyper-responsive to matrix compliance cues. Normalizing the tensional homeostasis of tumour cells, however, can revert them towards a non-malignant phenotype6, thereby illustrating the functional link between matrix materials properties, cellular tension and normal tissue behaviour. Importantly, however, although breast tumours are much stiffer than the normal breast, the materials properties of a breast tumour remain significantly softer than those of muscle or bone, emphasizing the critical association between tissue phenotype and matrix rigidity.
A cell within a tissue is subjected to isometric force through dynamic interactions with the ECM and its neighbouring cells, and these forces exert profound effects on cellular behaviour. For example, endothelial cells form branched capillary-like vessels when cultured within compliant gels, but form vessels with larger lumens in more rigid matrices. Compliance-dependent cell behaviour has also been observed in neural, muscle and mesenchymal cell populations. Therefore, ECM stiffness is an isometric force that exerts its effects gradually and chronically on cell behaviour, predominantly at the nanoscale level. An increase in ECM protein concentration, increased matrix crosslinking or parallel reorientation of matrix fibrils within a stromal matrix can stiffen a tissue locally to alter cell growth or direct cell migration, albeit to differing degrees. This phenomenon permits fine-tuning of cellular function within a heterogenous tissue.
Interstitial collagens are major contributors to tissue materials properties. Collagens undergo a myriad of post-translational modifications, including matrix metalloproteinase (MMP)-dependent cleavage, glycosylation and crosslinking, that modify their tensile strength and viscoelasticity. During extracellular processing, collagen propeptides are cleaved by specific endoproteinases. Thereafter, enzymes such as the lysyl oxidases (LOX) and the lysyl hydroxylases catalyse covalent intermolecular crosslinks between collagens and with elastin. LOX-mediated crosslinking increases insoluble matrix deposition, tissue tensile strength and matrix stiffness33. However, chronically increased LOX activity increases collagen crosslinking and this can stiffen heart muscle to compromise cardiac function34. Importantly, non-enzymatic collagen crosslinking, such as glycation and transglutamination, or increased biglycan and fibro-modulin proteoglycan levels also stiffen the matrix35. The excessive deposition of proteoglycans in injured lungs contributes to fibrosis by stiffening the parenchyma36, whereas inappropriate glycation-mediated crosslinking compromises wound healing and cardiac function in diabetic patients in whom glycation is increased owing to high blood glucose levels37,38. Therefore, isometric and active forces have crucial roles in tissue behaviour. Force directs the differentiation of stem cells, drives the assembly of differentiated tissues and maintains tissue homeostasis.
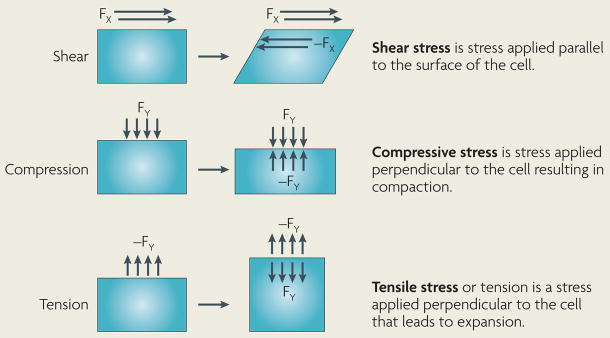
Box 1 | Types of forces experienced by a cell.
Normal physiological processes expose cells to a variety of mechanical stimuli including hydrostatic pressure, shear, compression and tensile force. The right-hand images in the figure depict the balance of forces once equilibrium is achieved following the application of a mechanical force. Newton’s third law states that for every action there is an equal and opposite reaction and, following this law, cells in vivo will respond to alterations in the mechanical properties of their surrounding matrix by adjusting their intracellular tension through the cytoskeletal network. Conversely, changes in cell tension will result in alterations in extracellular matrix (ECM) organization, thereby changing the mechanical properties of the ECM. Stress is defined as a normalized load, where the force or load is divided by the cross-sectional area available to support the load, and the units of stress are Newtons per square metre (N/m2) or Pascals (Pa). The deformation of a material in response to a given load varies with the geometry and the composition of the specimen. Strain is a normalized deformation, in which the change in length is divided by the original length of the specimen, and is a unitless quantity. We and others have previously measured the stiffness or Young’s modulus of tissues in vivo and reported values in units of Pascals6. Soft biological tissues can be described as viscoelastic materials. A viscous fluid resists shear flow and strain linearly with time under stress. An elastic solid undergoes deformation under stress and rapidly returns to its original state. Viscoelastic biological materials exhibit the characteristics of both a viscous fluid and an elastic solid.
Mechanotransduction and mechanoreciprocity
Given that cells are exposed to a myriad of active and isometric forces, it follows that cells must have derived an array of generic and specialized force-sensory mechanisms. Examples of specialized mechanosensors include the primary cilia in the hair cells of the inner ear and calcium-gated ion channels in cardiac muscle39,40. Activation of stretch-activated potassium channels41, activation and oligermization of transmembrane integrins, and remodelling of the cytoskeleton in response to shear flow are examples of conserved mechanosensory mechanisms42.
The current contention is that all force sensors directly undergo controllable molecular changes in response to force, regardless of their nature. This behaviour is illustrated by the sequential unfolding of p130Cas (also known as BCAR1) and conformational changes in the integrin-associated molecule talin 1 in response to force43,44. Elegant experiments demonstrated that direct application of a piconewton force can stimulate the mechanical extension of p130Cas, revealing a domain that is a substrate of Src family kinases45,46. Phosphorylation by Src family kinases subsequently activates the small GTPase RAP1 and initiates a sequence of events that propagates integrin signalling45,47. Force-induced conformational changes in talin 1 also reveal a binding site for vinculin, and force can modify extracellular fibronectin to alter integrin adhesion48, suggesting other plausible mechanisms by which force could link the ECM to the inside of the cell (FIG. 2a).
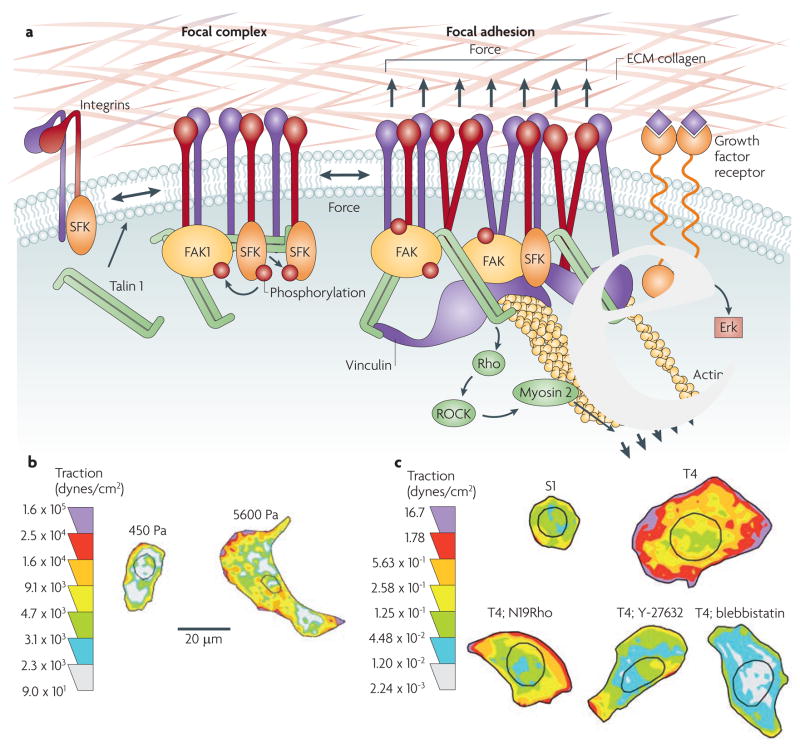
Figure 2. Mechanotransduction and focal adhesion maturation.
a | The majority of integrins exist at the plasma membrane in a resting, inactive state in which they can be activated by inside–out or outside–in cues. With regard to outside–in activation, when cells encounter a mechanically rigid matrix or are exposed to an exogenous force integrins become activated, which favours integrin oligomerization or clustering, talin 1 and p130Cas protein unfolding, vinculin–talin association, and Src and focal adhesion kinase (FAK) stimulation of RhoGTPase-dependent actomyosin contractility and actin remodelling. Focal adhesions mature with the recruitment of a repertoire of adhesion plaque proteins, including α-actinin to facilitate actin association, and adaptor proteins such as paxillin, which foster interactions between multiple signalling complexes to promote growth, migration and differentiation. b | Normal cells tune their contractility in response to matrix stiffness cues, but tumours exhibit altered tensional homeostasis. Cells exert actomyosin contractility and cytoskeleton-dependent force in response to matrix stiffness cues. These forces can be measured using traction force microscopy. Thus, non-malignant human mammary epithelial cells spread more and exert more force on a stiff matrix than on a soft matrix. c | By comparison, breast tumour cells (T4) are highly contractile and spread considerably more than their non-malignant counterparts (S1) in response to the same compliant matrix. Importantly, inhibiting RhoGTPase signalling in tumour cells, by expressing a dominant-negative N19Rho or treating tumours with an inhibitor of Rho-associated, coiled-coil-containing protein kinase (ROCK; Y-27632) or myosin 2 (blebbistatin), reduces tumour cell contractility and spreading to levels exhibited by non-malignant breast epithelial cells. These data illustrate the importance of Rho signalling and actomyosin contractility in cell force generation and show how transformation alters cell force sensing. The traction map is shown in pseudocolour indicating regions of low (grey) and high (purple) forces in dynes per cm2. ECM, extracellular matrix; SFK, Src family kinase. Reproduced, with permission, from REF. 6 © (2005) Elsevier Inc.
Once mechanical cues have been detected, cells must propagate and amplify the physical cue within the cell and translate the signal into either a transient response or sustained cellular behaviour. Integrins, by virtue of their extracellular interaction with the ECM and intracellular interaction with plaque proteins and the cytoskeleton, are an excellent example of one such ubiquitous mechanotransducer 49,50 (FIG. 2a). Either exogenous or endogenous force can activate integrins, facilitate their nucleation and clustering, and drive their maturation into focal adhesions6,51–55. Integrin oligomerization in turn facilitates RhoGTPase-dependent actomyosin contractility and cytoskeletal reinforcement6. Integrin clustering and cytoskeletal reinforcement lead to the phosphorylation of focal adhesion kinase (FAK) at tyrosine 397 (REF. 56), which stabilizes the focal adhesions through activation of small RhoGTPases and actin remodelling. The assembly of focal adhesions perpetuates downstream signalling through kinases and initiates cytoskeletal remodelling through the nucleation of an assortment of adhesion plaque proteins and signalling molecules, including Ras, Rac and Rho57,58. Ras couples force-dependent integrin signalling to MAPKs including Erk, as has been illustrated in lung epithelial cells in response to mechanical strain59 and increased Erk phosphorylation in endothelial cells in response to cyclic strain60. Importantly, however, what has yet to be determined is whether any cellular or extracellular protein whose activated conformation can be enhanced by force constitutes a viable mechanosignalling mechanism and, if so, what then dictates mechanospecificity. Indeed, do mechanohierarchies exist?
Force-dependent activation of signalling cascades allows cells to respond quickly to a dynamic force environment, and the same pathways also lead to sustained changes in cell behaviour. Force-activated Erk cooperates with other kinases, such as Src and FAK, to induce cell proliferation or sustain cell survival61, as shown for MAPK-dependent growth of keratinocytes in response to mechanical stretch62 and the load-dependent survival of osteocytes63. In addition to changes in cell growth and survival, compression stress affects microtubule dynamics64 to induce quantifiable changes in cell shape, whereas durotactic gradients of ECM stiffness30,65 direct cell motility and the migration of fibroblasts and smooth muscle cells. In response to mechanical loading, fibroblasts synthesize and secrete many ECM proteins, including fibronectin, tenascin and collagen, and direct matrix remodelling through the expression, secretion and activation of MMPs and crosslinking enzymes. These sustained cellular responses to force must be coordinated. One factor implicated in this orchestrated response is transforming growth factor-β (TGFβ). Mechanical force initiates post-translational activation of secreted TGFβ from a latent complex into the functional ligand66. TGFβ, in turn, can stimulate the production of matrix proteins and matrix-modifying enzymes such as MMPs and LOX that dramatically alter the characteristics of the extracellular stroma67. In extreme cases, chronic activation of TGFβ can even induce tissue fibrosis and disease in soft tissues such as the liver and kidney68. In this manner, cells can dramatically change the composition, topology and elasticity of their tissue microenvironment and alter their adhesions and cell shape and orientation to tune their behaviour according to the magnitude, direction and nature of applied mechanical stress.
Box 2 | Three-dimensional model systems to study the effect of force.
Three-dimensional cell culture models offer a distinct advantage over conventional two-dimensional systems because they recapitulate both the architecture and the phenotypical behaviour of differentiated tissues with reasonable fidelity. Three-dimensional model systems can be used to study force and its effects on cell behaviour in the context of an organized tissue structure in vitro. These systems use primary or immortalized cells and natural or synthetic hydrogels (for example, collagen I, reconstituted basement membrane, alginate, agarose, synthetic peptides and polyacrylamide). By various means, protein and polysaccharide gels can be manipulated to modify their mechanical properties. An increase in the total protein concentration of protein gels, such as collagen or fibrin, results in an increased stiffness of the polymerized network. In this case, the elastic modulus has been approximated to be proportional to the square of the protein concentration185. Free-floating or relaxed gels present a more compliant three-dimensional environment to cells than anchored or stressed gels and are more sensitive to cell force generation186 Glycation by the addition of reducing sugars such as glucose or ribose results in non-enzymatic crosslinking of collagen fibres that can further stiffen the three-dimensional collagen gels188. The stiffness of fibrin gels can be increased by the addition of salts at physiological pH or by activation of the plasma transglutaminase factor XIII188,189. Altering the protein concentration to change gel stiffness can introduce additional variables into the model system. The use of polyacrylamide gels allows for precise control of the gel stiffness while maintaining ligand density and chemical content and changing either the bis-acrylamide (a polyacrylamide crosslinking agent) or the acrylamide components of the gel can alter the gel mechanics190. Pelham and Wang pioneered polyacrylamide gels for cell culture less than 10 years ago and numerous investigators have used this model system with many different cell types to address the question of cell response to ECM force. This technique has proved exceptionally adaptable, such that gels of varying stiffness can be combined to resemble the mechanical properties of, for example, the alveolar basement membrane and breast stroma6,191.
Push-me-pull-you
Cells are not simply passive force recipients but also respond dynamically to externally applied force or stiff matrices with a proportional reciprocal cell-generated force. This reciprocal force response depends on actomyosin contractility and cytoskeletal remodelling. For instance, inside the cell, adaptor proteins associated with focal adhesions such as talin and α-actinin directly link the cytoplasmic domains of β integrin subunits with actin filaments69,70. Actin stress fibres polymerized at the focal adhesion act like viscoelastic cables and respond to the extracellular mechanical environment with myosin-induced cell contractility71,72. Cells anchor to and pull on ECM fibrils, creating intracellular tension73. This intracellular tension, which can be induced experimentally by local application of mechanical stress to the extracellular domains of integrins, redirects cytoskeletal reorganization and ultimately activates RhoGTPases to generate large traction forces that can be measured using traction force microscopy74 (FIG. 2b,c). Such approaches have revealed that cell-generated force or mechanoreciprocity can profoundly influence cell behaviour by enhancing cell spreading, growth, survival and motility6,30,65. Indeed, cellular tension and microrheology are finely tuned to the properties of their surrounding matrix, and the nature and magnitude of applied force they experience within their tissue microenvironment. The magnitude of cellular contractility reflects the cell type and state75,76. For instance, cells on stiff substrates tolerate excision of a single stress fibre by exerting greater myosin-dependent force, whereas the same manipulation in cells grown on a compliant substrate disrupts the cellular force balance and cell shape77. We have observed that normal mammary epithelial cells generate greater force and occupy more surface area on a stiff matrix (5,000 Pa) than similar cells interacting with a soft matrix of 140 Pa (FIG. 2b).
So, at the single cell level, cell-generated force can increase adhesion strength, enhance integrin-dependent signalling and drive cytoskeletal remodelling to change cell rheology and shape and modify cell behaviour. In multicellular tissues increased cell contractility can destabilize cell–cell adhesions and promote cell invasion to facilitate wound healing or drive MMP-dependent branching morphogenesis78,79. As well as changing cell shape and behaviour, mechanical forces can also alter gene expression.
Gene expression and force
Changes in microenvironment or cell behaviour that permit the long-term adaptation to exogenous forces or alterations in matrix compliance require a change in gene expression. Integrin expression, for example, is much higher in fibroblasts and epithelial cells that are grown on rigid substrates than those that are grown on compliant gels, and the expression of α5 integrin is induced following sustained exposure to a stiffer matrix80,81. So, how might force regulate gene expression? Many of the signalling networks that are activated in response to force, such as Erk and Jun N-terminal kinase (JNK), activate and induce nuclear translocation of transcription factors such as AP1, p53, signal transducer and activator of transcription 1 (STAT1), STAT3, MyC, CCAAT/enhancer-binding protein (C/EBP), cAMP response element-binding protein (CREB) and nuclear factor-κB (NF-κB)33,82–85. Therefore, force probably modifies cell fate by altering the activity of various adhesion and growth factor-dependent transcriptional networks. However, acinar morphogenesis within a compliant reconstituted basement membrane (rBM) or in response to mechanical loading is associated with the repression and induction of hundreds of genes, and we determined that human mammary epithelial cells (HMEC) respond to matrix stiffness by altering the expression of at least 1,500 genes that span multiple functional categories86 (K. C. Tsai et al., unpublished data). Likewise, although we showed that breast tumour progression in the HMT-3522 human breast cancer model is associated with specific genomic alterations, the accompanying gene expression profile differs markedly between those cells grown on either a rigid tissue culture plastic or stiff rBM-conjugated polyacrylamide gels and those within compliant rBM or on soft rBM-conjugated polyacrylamide gels87. This suggests that additional gene regulatory mechanisms, possibly linked to chromatin remodelling, must also be regulated by force.
A direct mechanical link from the ECM to nuclear chromatin could dynamically alter gene expression in response to exogenous force1 through a solid-state signalling mechanism that is governed by the principles of ‘tensegrity’ (tensional integrity). The tensegrity model implies that integrins are linked to the nucleus through the cytoskeleton, that an applied force is transmitted to the DNA through the cytoskeleton by nuclear lamins and nuclear envelope receptor complexes, and that this then modulates gene expression by inducing conformational changes in chromatin either by altering the nature of the protein complexes at the telomeres of chromosomes or by changing the activity of DNA remodelling enzymes88–92. Support for this paradigm has come from studies demonstrating how application of force on the integrin–ECM interface can induce nuclear and chromatin distortion93, that tension can alter DNA wrapping94, and that speared chromatin can be excised from the nucleus as a continuum that remains physically linked to the cytoskeleton and adhesion interface95. Alternately, epigenetic changes regulate gene expression during embryogenesis and tissue-specific development. Given that force also modulates these processes, it follows that mechanotransduction might influence chromatin remodelling to regulate histone acetylation and methylation. For example, HMEC morphogenesis and differentiation in a compliant rBM but not on a stiff two-dimensional substrate is associated with pronounced chromatin remodelling, changes in histone deacetylase (HDAC) expression and activity, and increased expression of the methyl-CpG-binding protein MECP2 (REFS 96,97) (Tsai et al., unpublished data). In addition, we and others have found that rBM compliance dictates the response of differentiated HMEC acini to the methylation inhibitor 5-azacytidine or the HDAC inhibitor trichostatin A. Only on compliant matrices do these inhibitors induce gene expression to sensitize a mammary epithelium to exogenous growth and death stimuli, coincident with a disruption of morphology and cytoskeletal organization96,97 (K. Levental, V.M.W. and N. Zahir, unpublished observations). These results indirectly implicate the properties of matrix materials in the control of cell shape, cytoskeleton morphology and chromatin remodelling.
Several studies have highlighted the interactions between force, Rho signalling, cell shape and histone acetylation98,99. Adhesion-induced changes in HMEC shape are associated with altered actin organization, RhoGTPase activity, actomyosin contractility and modified global patterns of chromatin histone acetylation6,100. Similarly, modifying fibroblast adhesion and changing cell shape alters cytoskeletal organization and shrinks the nucleus and nuclear lamina of cultured cells. These changes in the cytoskeleton and nuclear morphology are associated with impaired polymerase access to chromosomal territories and a concomitant reduction in gene transcription91,101–103. More convincingly, Rho-family GTPases indirectly regulate histone H4 acetylation by shifting the balance of cellular and nuclear pools of F and G actin, which in turn, modifies the association between serum response factor (SRF) and its co-activator MAL (also known as MKL1)104–106. These and other data argue convincingly that mechanical force regulates gene expression to alter cell behaviour either by directly altering the DNA or by modulating the function of chromatin remodelling molecules. The current challenge facing biologists then is to delineate the molecular mechanisms underlying these provocative phenomenological observations.
Changes in mechanical stress and cancer
Loss of tissue homeostasis is a hallmark of disease. Given the pluripotent role of force in tissue function, it is not surprising that multiple pathologies, including cancer, are characterized by compromised tensional homeostasis6,68,107. Indeed, tumours are often detected as a palpable ‘stiffening’ of the tissue, and approaches such as magnetic resonance imaging elastography and sono-elastography have been developed to exploit this observation to enhance cancer detection108,109. More provocatively, altered stromal–epithelial interactions precede and can even contribute to malignant transformation (K. Levantal and V.M.W., unpublished observations), and the desmoplastic stroma that is present in many solid tumours is typically significantly stiffer than normal6. This raises the interesting possibility that preventing tissue stiffening could impede cancer progression, and that genetically susceptible individuals predisposed to matrix stiffening might be at greater risk for tumours and could benefit from enhanced screening programmes37,110,111. To discuss these ideas further, we focus on the role of mechanical stimuli in the regulation of normal breast development and the implications these have for breast cancer.
The mechanics of mammary morphogenesis and maintenance
Force modulates all stages of breast development and is vital to the proper functioning of the differentiated tissue. Together with hormonal and growth cues, force specifies the architecture of the mature ductal tree and mediates efficient delivery of milk to the young. In mammals the breast is the source of nutrients and passive immunity for the offspring, so abnormalities in tensional homeostasis not only impair the structural organization and health of the tissue, but could also compromise the survival of the species. As such, understanding how force orchestrates the behaviour of such a crucial tissue as the breast should provide insight into how mechanics regulates the behaviour of other seemingly mechanically inert tissues.
The mammary gland comprises an organized ductal tree consisting of a single polarized layer of luminal epithelial cells that interact at their basal surface with a network of contractile myoepithelial cells (FIG. 3). Each intralobular ductal tree terminates in a cluster of alveoli, which comprise the basic structural unit of the breast. It is this basic acini unit that will differentiate to produce milk on exposure to lactogenic hormones112. Surrounding the ducts and alveoli of each lobule is the intralobular stroma, which is a loose connective tissue containing microvasculature, small lymphatic channels, adipocytes, resident fibroblasts and inflammatory cells113. Adjacent to and encompassing the intralobular stroma and ductal network is the interstitial stroma, which comprises over 80% of the human breast volume114,115. Unlike the loose and cellular intralobular stroma, the connective tissue of the interstitial stroma is dense, less cellular and contains variable proportions of ECM and adipose tissue.
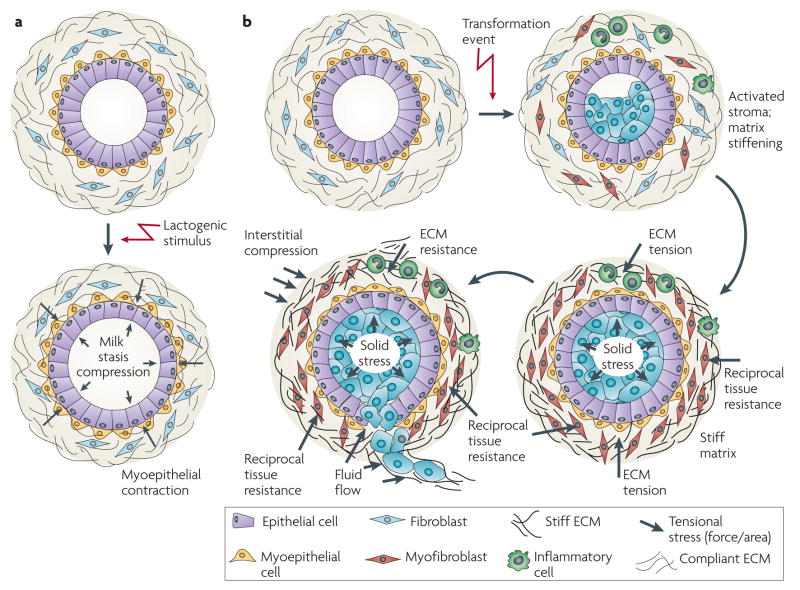
Figure 3. The normal mammary gland as a mechanically active tissue.
a | The developing breast is subjected to a number of forces that facilitate its normal function. During lactation, for instance, the normal breast experiences compressive stress on the luminal epithelial cells and the basement membrane owing to the accumulation of milk and alveolar distension. Upon sucking and oxytocin stimulation, epithelial cells encounter inward tensile stress as the myoepithelium contracts to force the milk out of the alveolar sacs. In the absence of this stimulus, milk will accumulate within the acinus and eventually exert an outward projecting compressive force on the surrounding epithelium. This compressive force is countered by a compensatory inward projecting resistance force and the combination of these two forces eventually compromises the integrity of the tight junctions between alveolar cells. Chronic exposure to these forces and perturbed tissue integrin sensitize the gland to apoptotic cues so that the gland undergoes involution accompanied by extensive remodelling of the epithelium and the cellular and extracellular components of the stroma. b | Transformation (blue cells) resulting from the accumulation of genetic and epigenetic alterations in the epithelium along with an altered stromal matrix leads to unchecked proliferation and enhanced survival of luminal epithelial cells within the ductal tree, which compromises normal ductal architecture. With prolonged growth and abnormal survival, the abnormal pre-neoplastic luminal mammary epithelial cells eventually expand to fill the breast ducts. The expanding luminal epithelial mass exerts outward projecting compression forces of increasing magnitude on the basement membrane and adjacent myoepithelium. These forces are countered by an inward projecting resistance force. Importantly, the pre-neoplastic lesion secretes a plethora of soluble factors that stimulate immune cell infiltration and activation of resident fibroblasts to induce a desomoplastic response in the breast stroma. The desmoplastic stroma, which is characterized by dramatic changes in the composition, post-translational modifications and topology of the extracellular matrix (ECM), stiffens over time. This rigid parenchyma exerts a progressively greater inward projecting resistance force on the expanding pre-neoplastic duct. Over time, the number of myoepithelial cells surrounding the pre-neoplastic mass decreases and the basement membrane thins, probably owing to increased matrix metalloproteinase (MMP) activity, decreased protein deposition and compromised assembly (adapted from REF. 128). In parallel, there is a build-up of interstitial fluid pressure contributed by a leaky vasculature and compromised lymphatic drainage. In response to their genetic modifications and the altered materials properties of the matrix, the pre-neoplastic luminal epithelial cells exhibit modified tensional homeostasis and respond to the combination of forces and stromal cues to invade the breast parenchyma. Some resident fibroblasts transdifferentiate into myofibroblasts and facilitate tumour migration and invasion by promoting the assembly of linearized collagen fibrils surrounding the distended pre-neoplastic epithelial ducts.
A highly organized ECM supports the tissue and cellular level architecture of the breast. Collagen IV, heparin proteoglycans, perlecan and various laminin isoforms comprise the basement membrane that surrounds the mammary epithelial cell (MEC) bilayer. Together these provide mechanical stress shielding that is crucial for functional integrity of the ductal tree116,117. The intralobular stromal matrix, which surrounds the ductal tree, is secreted primarily by stromal fibroblasts and is composed of structural matrix proteins — including collagens I and III, elastin, proteoglycans, glycosaminoglycans and glycoproteins — that interact to form a large complex network in the extracellular space that is contiguous with the basement membrane118. The organization, concentration and crosslinking of the structural components of the basement membrane and the intralobular matrix contribute to their material properties119. Together, the basement membrane, intralobular matrix and interstitial stroma are a continuum that cooperates to define the form and function of the breast through their ability to act as a physical scaffold, to function as a repository for growth factors and cytokines, and to provide specific biochemical and tensional cues through specialized cellular receptors. Thus, the ECM can be considered a highway by which MECs are able to communicate with one another and with stromal cells locally and distally through biochemical and mechanical cues.
Forces that operate from the nanoscale to the macroscale facilitate normal functioning of the differentiated breast. The nature and magnitude of these forces reflect the organization, composition, topology and post-translational modification state of the ECM and the organization of the ductal tree. Thus, the matrix surrounding the large ducts is more linear and stiffer, whereas the collagen surrounding the terminal ductal units is more relaxed and the matrix is more compliant (K. Levantal and V.M.W., unpublished observations). During lactation, the breast is subjected to compressive stress on the luminal and myoepithelial cells and the basement membrane owing to the accumulation of milk and distension of the ducts, which is facilitated by the highly compliant, relaxed collagen matrix surrounding the differentiated acini. Upon suckling, the luminal epithelial cells encounter inward projecting tensile stress as the myoepithelium contracts in response to oxytocin to force the milk out of the aveolar sacs and into the larger ducts to facilitate efficient feeding of the young. In the absence of the suckling stimulus, lactation ceases and milk accumulates within the acini, slowly exerting an outwardly projecting compressive force of increasing magnitude on the surrounding luminal epithelium and myoepithelium. With prolonged milk stasis and continued gland distension, this compressive force eventually compromises the integrity of the tight junctions between luminal alveolar cells, and the gland undergoes involution accompanied by extensive remodelling of the cellular and extracellular stroma120,121. Importantly, gland remodelling dramatically changes the composition and architecture of the stroma. The remodelled stroma consequently alters the signals and the force encountered by the MECs within the ducts and by so doing sets the stage for a subsequent round of epithelial proliferation and differentiation122. For example, primary cultures of murine MECs form polarized mammary acini with an endogenous basement membrane and differentiate in response to lactogenic hormones when embedded within a floating collagen gel. By contrast, these same cells will spread and continue proliferating in response to identical stimuli when interacting with a stiff two-dimensional scaffold or incorporated into a mechanically loaded collagen gel123. Similarly, immortalized MECs fail to express one of the major milk proteins, β-casein, unless they interact with a compliant basement membrane123–125.
The crucial role of matrix compliance in MEC morphogenesis was illustrated by studies using matrices with defined viscoelastic properties. HMECs embedded within collagen–rBM gels or interacting with rBM-crosslinked polyacrylamide gels with matrix compliance comparable to the normal murine mammary gland proliferated until they formed growth-arrested, polarized mammary acinus-like structures with a central lumen and an external endogenous basement membrane. When the matrix is progressively stiffened, cell growth is enhanced, cell–cell junction integrity is compromised and lumen formation is impeded. MECs interacting with the most rigid matrices form continuously growing, non-polarized, disorganized and invasive colonies that lack detectable cell–cell junction proteins and exhibit irregular cell shapes with detectable actin stress fibres. Whereas MECs interacting with the highly compliant matrix form nascent focal contacts, those within the stiff gels assemble mature focal adhesions with active FAK phosphorylated on Tyr397, vinculin and p130Cas (REF. 6). Importantly, when MECs engineered to express a constitutively active V14Rho or a mutant V737N integrin that promotes integrin clustering interact with a compliant basement membrane, they exert higher contractility, assemble focal adhesions and display tissue phenotypes characteristic of MECs interacting with a stiff matrix. Such observations underscore the importance of integrin signalling and Rho-dependent actomyosin contractility in multicellular tissue morphogenesis. This work also highlights the central role of active and isometric force in the functional integrity of soft tissues such as the breast, where small changes in matrix stiffness or mechanical cues can profoundly alter cell behaviour.
Cancer: forcing transformation
Epithelial cancers are characterized by an altered tissue tensional homeostasis that reflects differences in rheology and increased cell-generated force in the transformed cells126–128, increased compression force due to the solid state pressure exerted by the expanding tumour mass129, matrix stiffening due to the desmoplastic response6, and increased interstitial pressure due a leaky vasculature and poor lymphatic drainage130. For instance, transformed epithelial cells express vastly different intermediate filament profiles and cytoarchitecture to normal cells and consequently have an altered microrheology that could provide a distinct advantage to the cell during intravasation and extravasation of the vasculature, thereby facilitating cancer metastasis29,128,130. Transformed cells also show compromised mechanoreciprocity such that they often exert abnormally high force in response to a compliant matrix and these increased cell-generated forces disrupt cell–cell junction integrity, compromise tissue polarity, promote anchorage-independent survival and enhance invasion (FIG. 4). It is also plausible that altered cellular force could account for the increased invadopodia6 observed in transformed, invasive cells131. Increased cell contractility probably reflects increased expression and activity of RhoGTPases and Rho-associated, coiled-coil-containing protein kinase 1 (ROCK1), as well as high levels of growth factor-induced Erk activity. The increased cell-generated forces exhibited by tumours enhance their growth, survival and invasion by promoting focal adhesion maturation and signalling through actomyosin contractility6,128,130,132–135. The increased contractility of tumour cells and their associated stromal fibroblasts also induce tension-dependent matrix remodelling to promote the linear reorientation of collagen fibrils surrounding the invasive front of the tumour136,137. Rapidly migrating transformed mammary epithelial cells have been observed on prominent linear bundles of collagen fibres adjacent to blood vessels138–140.
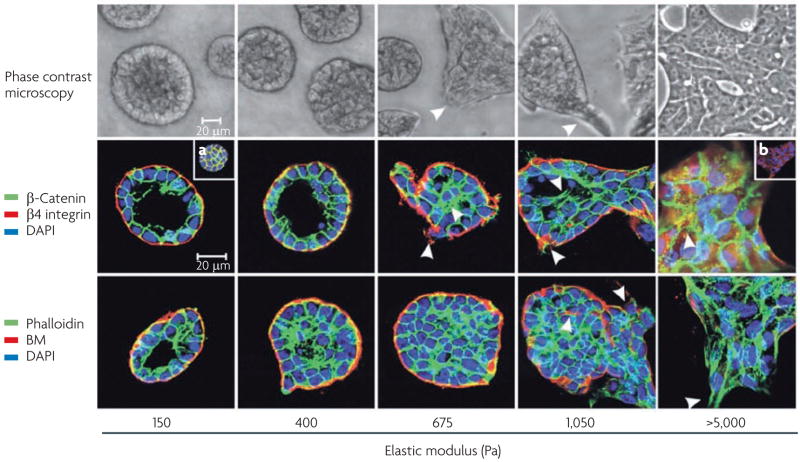
Figure 4. Matrix stiffness modulates cellular morphology and epidermal growth factor (EGF)-dependent growth.
Phase contrast microscopy and confocal immunofluorescence images of non-malignant immortalized human mammary epithelial cell (HMEC; MCF10A) colonies interacting with a three-dimensional reconstituted basement membrane (BM)-laminated polyacrylamide gel of increasing stiffness (150–5,000 Pa) showing colony morphogenesis after 20 days of culture. On compliant gels with materials properties similar to that measured in the normal murine mammary gland (150 Pa) non-malignant MECs proliferate for 6–12 days to eventually form growth-arrested, polarized acini analogous to the terminal ductal lobular units observed at the end buds of the differentiated breast. These structures have intact adherens junctions and insoluble cell–cell localized β-catenin before (main images) and after (inset a) Triton extraction, and polarity, as shown by the basal localization of (α6) β4 integrin, the apical–lateral localization of cortical actin (Phalloidin), and the assembly of an endogenous laminin 5 basement membrane. Incremental stiffening of the basement membrane gel progressively compromises tissue morphogenesis and alters EGF-dependent growth of these cells. Thus, colony size progressively increases with matrix stiffening, lumen formation is compromised, cell–cell junctions are disrupted, as revealed by loss of cell–cell-associated β-catenin (inset b), and tissue polarity is inhibited, as indicated by disorganized (α6) β4 integrin localization and loss of the endogenous laminin 5 basement membrane. Interestingly, actin stress fibres were not observed in the structures until the stiffness of the matrix reached 5,000 Pa, as has been observed in murine breast tumours in vivo6. The arrows indicate loss of the endogenous basement membrane and disruption of basal polarity. Reproduced, with permission, from REF. 6 © (2005) Elsevier Inc.
The expanding tumour mass exerts compressive stress on the surrounding tissue extracellular matrix, vasculature, lymphatics and interstitial space. The solid stress induced by tumour expansion could also promote tumour progression. For example, tumours in soft tissues such as the pancreas typically show compromised laminin and type IV collagen basement membrane organization and thinning that, when combined with outward projecting compression force, facilitates tumour cell invasion into the parenchyma6,141 (FIG. 3). Tumour-associated compression stress can induce tumour angiogenesis by directly increasing expression of vascular endothelial growth factor A (VEGFA) or by indirectly blocking the existing vasculature surrounding the tumour mass to promote hypoxia and VEGFA secretion142,143. In addition, compression can increase the interstitial pressure in the tumour to up to 10× that of normal tissue. This pressure induces the accumulation of fluid from leaky blood and lymphatic vessels144,145. Compression force can also shrink the interstitial space surrounding the ductal structures, which increases the local concentration of growth factors and cytokines to facilitate autocrine and paracrine signalling and promote tumour growth146. Tumour-associated changes in interstitial pressure and compressive stress also present real challenges for the treatment of solid tumours with chemotherapeutic drugs147.
Breast cancer progression is accompanied by a desmoplastic response that includes inflammatory cell infiltration, angiogenesis, fibroblast transdifferentiation and changes in ECM composition, integrity and topology114,148,149. The ECM remodelling observed in tumours includes increased deposition of fibronectin, tenascin, collagen types I, III and IV, and proteoglycans150–152, substantial MMP-dependent cleavage and increased levels of LOX-dependent matrix crosslinking33,153. In the normal breast, tightly controlled MMPs remodel the ECM to promote mammary gland growth or involution. In tumours, however, this stringent control of MMP expression and function is lost154. Overexpression of MMP3, MMP11, MMP12 and MMP13 have each been demonstrated in the tumour stroma, along with MMP2 in the transformed mammary epithelial cells155. Moreover, aberrant MMP activity is not merely symptomatic of a transformed tissue but rather has a causative role, as illustrated by the observation that polymorphisms in the human MMP3 promoter that increase its expression are associated with an increased tumour incidence156. Likewise, transgenic mice that overexpress MMP3 displayed marked desmoplasia and precocious branching of the mammary epithelium, and developed tumours with marked genetic abnormalities157. However, tumour progression is also associated with substantial post-translational modifications of matrix proteins including altered deposition of proteoglycans and increased expression and activity of collagen crosslinking enzymes such as LOX and LOXl158,159. We showed that experimentally induced breast tumour progression in transgenic mice is accompanied by a significant increase in reversible and irreversible collagen crosslinking, increased expression of LOX and an incremental increase in tissue and ECM stiffness (K. Levental et al., unpublished information). Inducing collagen crosslinking and stiffening either in three-dimensional collagen hydrogels or in vivo in a modified breast stroma promoted MEC transformation that was associated with increased mechanosignalling. Provocatively, inhibiting LOX-dependent collagen crosslinking tempered tissue desmoplasia, decreased tumour incidence, reduced tumour growth and reduced mechanotransduction in the mammary epithelium; thereby directly implicating changes in the properties of matrix materials in tumour evolution.
Tumour evolution is accompanied by dramatic changes in interstitial pressure and fluid flow. Fluid flow dynamics within soft tumour tissues has largely been ignored but is especially relevant to tissue development and tumour metastasis and for optimal treatment efficacy145. For instance, fluid flow facilitates lymphatic clearance and induces cytokine differentials that promote cell motility and invasion through the creation of chemotactic C-C chemokine receptor 7 (CCR7) gradients that are highly important for cancer cell metastasis through the lymphatics160. The increased interstitial pressure in an epithelial tumour mass, with fluids accumulating from leaky blood vessels and impaired lymphatic drainage, can greatly impede the delivery of tumour therapies130. Clearly, tumour cells are exposed to a myriad of altered mechanical forces that could dramatically modify their behaviour. A better understanding of how these force cues regulate tumour progression and metastasis and affect cancer therapy could significantly aid the development of improved treatments161.
Breast density and age: a new perspective
Clinicians have long recognized that there is a connection between breast density and breast cancer risk6,162–164. Increased mammographic density for instance, is associated with a four- to six-fold increase in the relative risk of developing breast cancer165,166. Unfortunately, however, deciphering the functional relationship between mammographic density and breast transformation has proved quite challenging111,167. For instance, although dense breasts have more collagen and increased cell density (reflected by a greater nuclear area), other factors such as altered levels of the tissue inhibitor of metalloprotease 3 (TIMP3) and insulin-like growth factor I (IGFI) are also associated with mammographic density and need to be considered165,168,169. In fact, the composition of the ECM differs in women with dense breasts, such that the proteoglycans lumican and decorin are often disproportionately increased in women with mammographically dense breasts165,169. Proteoglycan deposition often precedes fibrosis and may enhance tissue inflammation, raising the intriguing possibility that women with mammographically dense breasts could be more susceptible to chronic inflammation170. Interestingly, proteoglycans such as lumican and biglycan not only bind growth factors and maintain tissue hydration but also contribute crucially to the mechanical integrity of the stroma, suggesting that in some instances mammographic density could reflect a stiffer breast parenchyma36,171. Given that matrix stiffness can modify cell and tissue behaviour by altering adhesion and growth factor receptor signalling and cytoskeletal dynamics to change cell shape and tissue organization, it seems reasonable to predict that the increased breast cancer risk associated with dense breasts could be attributed in some instances to an aberrant tensional homeostasis in these tissues. In this regard, sonoelastography, which measures the stiffness of a tissue in real time in situ, might offer a tractable auxillary screening strategy to diagnose high-risk women who are identified initially using imaging mammography172 (FIG. 5).
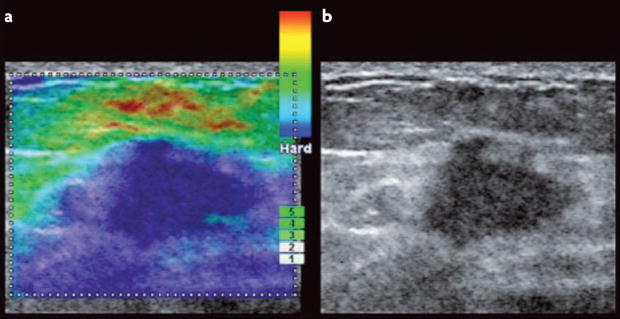
Figure 5. Imaging elastography of a breast tumour.
Tissue imaging elastography is a spatial ‘visual’ qualitative measurement of the stiffness of a tissue that is generated by extrapolating tissue viscoelastic characteristics from ultrasound wave reflection in real-time. Photographs of sonoelastography images compare an elastogram image (a) with a B mode ultrasound scan (b) of a breast tumour170. Ultrasound imaging elastography, as shown here, is an in situ mechanical imaging method that could improve the sensitivity and the specificity of breast cancer detection and may be a useful tool to advance our understanding of the link between mammographic density and the matrix materials properties of the breast. Image courtesy of A. Thomas & T. Fischer, Charité, Berlin, Germany.
As women age, mammographic density decreases yet cancer incidence rises169,173. Indeed, the post-menopausal breast has proportionately less collagen and more fatty tissue than the young breast, implying that older breast tissue must be softer169. Consistently, in old skin and bone, collagen deposition decreases and MMP-dependent degradation increases, and old bone and skin are mechanically weaker than their younger tissue counterparts174,175. How can we reconcile this seeming paradox between the increased cancer risk with age and the decreased mechanical strength of tissues? one explanation is that ageing is associated with a disproportionate increase in inappropriate post-translational modifications of ECM proteins, including increased collagen glycation and ultraviolet crosslinking, yielding old tissue matrices with less total collagen but a greater amount of disorganized collagen fibrils than young tissues. Consistently, although old skin has lower tensile properties (that is, is mechanically weaker) it is paradoxically stiffer (less elastic) and less functional than young skin176,177. Wound healing in old skin is severely compromised, which could be attributed to altered mechanical properties of the extracellular collagens178,179. Therefore, there is a positive association between age, matrix stiffening, aberrant matrix crosslinking and increased cancer incidence. Although the post-menopausal breast has less collagen, the collagen may be less mechanically elastic, stiffer, more disorganized and less functional, a possibility that now needs to be examined.
These findings underscore the need to understand the complex relationship between matrix remodelling and topology, and cell and tissue behaviour. Indeed, although hormone replacement therapy can increase breast density and tamoxifen treatment can reduce breast density, these mammographic changes do not always reflect modified cancer risk, emphasizing our need to develop additional metrics to understand the relationship between ECM remodelling and tissue phenotype23,180. Such insight would be highly beneficial for clarifying those issues encountered with the recent clinical trials of MMP inhibitors in cancer treatment181.
Summary
Realizing that force is a crucial determinant of tissue development, cell differentiation and homeostasis leads us to conclude that the loss of the ability to sense, respond and adapt appropriately to force contributes to disease. Indeed, we and others showed that pathological changes in cells and in the architecture, topology and material properties of their matrix microenvironments constitutes a positive feedback loop that propels carcinogenesis and other diseases. However, many questions still need to be resolved. Such issues include how the unique material properties of specific differentiated tissues are established and maintained, how cells coordinate their function and adaptation to external cues with their microenvironments, and how physical signals might interface with and modulate the activity of biochemical signalling pathways. Addressing these questions is particularly important if we are to understand lethal processes such as tumour metastasis, which clearly is profoundly influenced by the primary tissue microenvironment. Metastasis is also acutely regulated by the inherent cellular rheology and the forces that the cells experience during their metastatic spread, and is chronically regulated by the material properties of their targeted distal metastatic niche29,182. It may be that this niche is defined by proteins such as LOX or TGFβ, which have the capacity to modify tumour cell adhesion and the material properties of ECM in tissues targeted by these cells, respectively183,184. In this regard, recent work suggests that tumour cells select their metastatic microenvironments in part through compliance matching but also by pre-conditioning their metastatic niche. This raises a number of intriguing questions, including defining the part that mechanical force might play in modulating the function of tumour stem cells, why specific tumour types characteristically metastasize to distinct tissues, and whether tumour cells might be mechanically pre-conditioning their metastatic sites. Clearly, addressing such outstanding issues falls outside the realm of traditional cell biology approaches and instead requires the cooperative effort of biologists, materials scientists, physicists and engineers. Indeed, this exciting force ‘frontier’ is fertile territory for scientific exploration of development and cancer biology that will undoubtedly yield new insights into cancer evolution and identify novel anticancer therapeutic targets.
Acknowledgments
We apologize to the many authors whose work is not cited due to space limitations. A special thank you is extended to N. Zahir for her efforts on the text boxes, M. Paszek for his contribution to the traction force images in FIG. 1 and S. Cersosimo for administrative support. This work was supported by National Institutes of Health grant 7R01CA078731-07, Department of Defense Breast Cancer Research Era of Hope grant W81XWH-05-1-330 (BC044791), California Institute for Regenerative Medicine grant RS1-00449 and DOE grant A107165 to V.M.W., and a Sandler Family Foundation Award and National Institutes of Health grant RO3DE016868 to T.A.
- Rheology
The study of the deformation and flow of matter.
- Viscoelasticity
Soft biological tissues can be described as viscoelastic materials. A viscous fluid resists shear flow and strain linearly with time under stress. An elastic solid undergoes deformation under stress and rapidly returns to its original state. Viscoelastic biological materials exhibit characteristics of both a viscous fluid and an elastic solid.
- Endoproteinase
An enzyme that proteolytically cleaves peptides at internal amino acids.
- Durotactic
Directed movement of cells up or down the stiffness gradient of a biomaterial.
- Desmoplastic stroma
Stromal tissue responds to tumour cells with a characteristic desmoplasia resulting from fibroblast recruitment, collagen deposition and angiogenesis.
Biographies
Darci T. Butcher received her Honours bachelor degree in Genetics and her Ph.D. from the University of Western Ontario, Canada. Her postdoctoral studies in Valerie Weaver’s laboratory explored the role of cell and matrix tension in epigenetic regulation of mammary epithelial immortalization and tumour progression.
Tamara Alliston received her B.A. at Trinity University in San Antonio, Texas, USA and her Ph.D. at Baylor College of Medicine, Texas, followed by postdoctoral research at the University of California at San Francisco (UCSF). She is currently an assistant professor in the Department of Orthopaedic Surgery at UCSF and is affiliated with the Institute of Regeneration Medicine, the Departments of Bioengineering and Otolaryngology, and graduate programmes in Biomedical Sciences, Bioengineering, and Oral and Craniofacial Sciences. Her research focuses on mechanisms by which growth factors coordinately regulate cell differentiation and extracellular matrix material properties in skeletal development and disease.
Valerie M. Weaver received her Bachelor in Chemistry and Biochemistry from the University of Waterloo, Ontario, Canada and her Honours Bachelor summa cum laude and Ph.D. in Biochemistry from the University of Ottawa. After completing postdoctoral training with M. J. Bissell at the Lawrence Berkeley National Laboratory in Berkeley, California, USA she joined the faculty of Pathology at the University of Pennsylvania, Philadelphia, Pennsylvania as an Assistant Professor and was a member of the Institute for Medicine and Engineering. She relocated to UCSF where she is Associate Professor and Director of the Department of Surgery’s Center for Bioengineering and Tissue Regeneration. She has cross appointments in the Departments of Anatomy and Bioengineering and Therapeutics at UCSF and is Adjunct Associate Professor in the Department of Bioengineering at the University of Pennsylvania. Using an integrated, interdisciplinary approach her group studies the role of mechanical force and stromal–epithelial interactions in tissue development, tissue-specific differentiation and breast cancer.
Footnotes
DATABASES
National Cancer Institute Drug Dictionary: http://www.cancer.gov/drugdictionary/ 5-azacytidine|tamoxifen
UniProtKB: http://www.uniprot.orgβ5 integrin | β-casein | biglycan | BCAR1 | CCR7 | CREB | decorin | FAK | fibronectin | LOX | lumican | MAL | MECP2 | MMP2 | MMP3 | MMP11 | MMP12 | MMP13 | MYC | NF-κB | p53 | paxillin | RAP1 | SRF | STAT1 | STAT3 | talin 1 | tenascin | TGFβ | TIMP3 | TWIST1 | VEGFA | vinculin
References
- 1.Gieni RS, Hendzel MJ. Mechanotransduction from the ECM to the genome: are the pieces now in place? J Cell Biochem. 2008;104:1964–1987. doi: 10.1002/jcb.21364. [DOI] [PubMed] [Google Scholar]
- 2.Engler AJ, et al. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol. 2004;166:877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. Contractile myocytes were used to demonstrate that cells sense their mechanical environment. Myotubes form independently of matrix stiffness but myosin–actin striations emerge only on gels with stiffness typical of normal muscle, and not on matrices that are softer or stiffer. [DOI] [PubMed] [Google Scholar]
- 4.Georges PC, Miller WJ, Meaney DF, Sawyer ES, Janmey PA. Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophys J. 2006;90:3012–3018. doi: 10.1529/biophysj.105.073114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and rhoa regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 6.Paszek MJ, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. The first paper to describe tensional homeostasis regulation of the tumour phenotype and the molecular link between ECM stiffness, Rho-dependent cell contractility and oncogene-mediated transformation. [DOI] [PubMed] [Google Scholar]
- 7.Vial E, Sahai E, Marshall CJ. ERK–MAPK signaling coordinately regulates activity of Rac1 and RhoA for tumor cell motility. Cancer Cell. 2003;4:67–79. doi: 10.1016/s1535-6108(03)00162-4. [DOI] [PubMed] [Google Scholar]
- 8.Lee MK, Nikodem VM. Differential role of ERK in cAMP-induced Nurr1 expression in N2A and C6 cells. Neuroreport. 2004;15:99–102. doi: 10.1097/00001756-200401190-00020. [DOI] [PubMed] [Google Scholar]
- 9.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krieg M, et al. Tensile forces govern germ-layer organization in zebrafish. Nature Cell Biol. 2008;10:429–436. doi: 10.1038/ncb1705. This article defined the mechanical properties of progenitor cells of the ectoderm, mesoderm and endoderm in gastrulating zebrafish embryos and demonstrated that differential actomyosin-dependent cell–cortex tension is regulated by Nodal–TGFβ signalling. [DOI] [PubMed] [Google Scholar]
- 11.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nature Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Czirok A, Rongish BJ, Little CD. Extracellular matrix dynamics during vertebrate axis formation. Dev Biol. 2004;268:111–122. doi: 10.1016/j.ydbio.2003.09.040. [DOI] [PubMed] [Google Scholar]
- 13.Pajerowski JD, Dahl KN, Zhong FL, Sammak PJ, Discher DE. Physical plasticity of the nucleus in stem cell differentiation. Proc Natl Acad Sci USA. 2007;104:15619–15624. doi: 10.1073/pnas.0702576104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farge E. Mechanical induction of Twist in the Drosophila foregut/stomodeal primordium. Curr Biol. 2003;13:1365–1377. doi: 10.1016/s0960-9822(03)00576-1. [DOI] [PubMed] [Google Scholar]
- 15.Ren R, Nagel M, Tahinci E, Winklbauer R, Symes K. Migrating anterior mesoderm cells and intercalating trunk mesoderm cells have distinct responses to Rho and Rac during Xenopus gastrulation. Dev Dyn. 2006;235:1090–1099. doi: 10.1002/dvdy.20711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tahinci E, Symes K. Distinct functions of Rho and Rac are required for convergent extension during Xenopus gastrulation. Dev Biol. 2003;259:318–335. doi: 10.1016/s0012-1606(03)00206-9. [DOI] [PubMed] [Google Scholar]
- 17.Cardoso WV, Lu J. Regulation of early lung morphogenesis: questions, facts and controversies. Development. 2006;133:1611–1624. doi: 10.1242/dev.02310. [DOI] [PubMed] [Google Scholar]
- 18.Kitterman JA. The effects of mechanical forces on fetal lung growth. Clin Perinatol. 1996;23:727–740. [PubMed] [Google Scholar]
- 19.Liu M, Tanswell AK, Post M. Mechanical force-induced signal transduction in lung cells. Am J Physiol. 1999;277:L667–L683. doi: 10.1152/ajplung.1999.277.4.L667. [DOI] [PubMed] [Google Scholar]
- 20.Moore KA, et al. Control of basement membrane remodeling and epithelial branching morphogenesis in embryonic lung by Rho and cytoskeletal tension. Dev Dyn. 2005;232:268–281. doi: 10.1002/dvdy.20237. [DOI] [PubMed] [Google Scholar]
- 21.Bird JL, Platt D, Wells T, May SA, Bayliss MT. Exercise-induced changes in proteoglycan metabolism of equine articular cartilage. Equine Vet J. 2000;32:161–163. doi: 10.2746/042516400777591624. [DOI] [PubMed] [Google Scholar]
- 22.Haapala J, et al. Coordinated regulation of hyaluronan and aggrecan content in the articular cartilage of immobilized and exercised dogs. J Rheumatol. 1996;23:1586–1593. [PubMed] [Google Scholar]
- 23.Ebbesen EN, Thomsen JS, Mosekilde L. Nondestructive determination of iliac crest cancellous bone strength by pQCT. Bone. 1997;21:535–540. doi: 10.1016/s8756-3282(97)00196-8. [DOI] [PubMed] [Google Scholar]
- 24.Rittweger J, et al. Bone loss from the human distal tibia epiphysis during 24 days of unilateral lower limb suspension. J Physiol. 2006;577:331–337. doi: 10.1113/jphysiol.2006.115782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi M, Ishida T, Traub O, Corson MA, Berk BC. Mechanotransduction in endothelial cells: temporal signaling events in response to shear stress. J Vasc Res. 1997;34:212–219. doi: 10.1159/000159225. [DOI] [PubMed] [Google Scholar]
- 26.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davies PF, Remuzzi A, Gordon EJ, Dewey CF, Jr, Gimbrone M. A Jr Turbulent fluid shear stress induces vascular endothelial cell turnover. in vitro Proc Natl Acad Sci USA. 1986;83:2114–2117. doi: 10.1073/pnas.83.7.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guck J, et al. Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence. Biophys J. 2005;88:3689–369. doi: 10.1529/biophysj.104.045476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cross SE, Jin YS, Rao J, Gimzewski JK. Nanomechanical analysis of cells from cancer patients. Nature Nanotech. 2007;2:780–783. doi: 10.1038/nnano.2007.388. [DOI] [PubMed] [Google Scholar]
- 30.Wong JY, Velasco A, Rajagopalan P, Pham Q. Directed movement of vascular smooth muscle cells on gradient-compliant hydrogels. Langmuir. 2003;19:1908–1913. This article demonstrates the durotactic movement of cells along a stiffness gradient on polyacrylamide gels. [Google Scholar]
- 31.Gaudet C, et al. Influence of type I collagen surface density on fibroblast spreading, motility, and contractility. Biophys J. 2003;85:3329–3335. doi: 10.1016/S0006-3495(03)74752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 33.Payne SL, Hendrix MJ, Kirschmann DA. Paradoxical roles for lysyl oxidases in cancer — a prospect. J Cell Biochem. 2007;101:1338–1354. doi: 10.1002/jcb.21371. [DOI] [PubMed] [Google Scholar]
- 34.Sivakumar P, Gupta S, Sarkar S, Sen S. Upregulation of lysyl oxidase and MMPs during cardiac remodeling in human dilated cardiomyopathy. Mol Cell Biochem. 2008;307:159–167. doi: 10.1007/s11010-007-9595-2. [DOI] [PubMed] [Google Scholar]
- 35.Avery NC, Bailey AJ. The effects of the Maillard reaction on the physical properties and cell interactions of collagen. Pathol Biol (Paris) 2006;54:387–395. doi: 10.1016/j.patbio.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Ebihara T, Venkatesan N, Tanaka R, Ludwig MS. Changes in extracellular matrix and tissue viscoelasticity in bleomycin-induced lung fibrosis. Temporal aspects. Am J Respir Crit Care Med. 2000;162:1569–1576. doi: 10.1164/ajrccm.162.4.9912011. [DOI] [PubMed] [Google Scholar]
- 37.Susic D. Cross-link breakers as a new therapeutic approach to cardiovascular disease. Biochem Soc Trans. 2007;35:853–856. doi: 10.1042/BST0350853. [DOI] [PubMed] [Google Scholar]
- 38.Robins SP, et al. Increased skin collagen extractability and proportions of collagen type III are not normalized after 6 months healing of human excisional wounds. J Invest Dermatol. 2003;121:267–272. doi: 10.1046/j.1523-1747.2003.12373.x. [DOI] [PubMed] [Google Scholar]
- 39.Nayak GD, Ratnayaka HS, Goodyear RJ, Richardson GP. Development of the hair bundle and mechanotransduction. Int J Dev Biol. 2007;51:597–608. doi: 10.1387/ijdb.072392gn. [DOI] [PubMed] [Google Scholar]
- 40.Parker KK, Ingber DE. Extracellular matrix, mechanotransduction and structural hierarchies in heart tissue engineering. Philos Trans R Soc Lond B Biol Sci. 2007;362:1267–1279. doi: 10.1098/rstb.2007.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brakemeier S, Eichler I, Hopp H, Kohler R, Hoyer J. Up-regulation of endothelial stretch-activated cation channels by fluid shear stress. Cardiovasc Res. 2002;53:209–218. doi: 10.1016/s0008-6363(01)00476-x. [DOI] [PubMed] [Google Scholar]
- 42.Helmke BP, Rosen AB, Davies PF. Biophys J. 2003;84:2691–2699. doi: 10.1016/S0006-3495(03)75074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.del Pozo MA, et al. Integrins regulate Rac targeting by internalization of membrane domains. Science. 2004;303:839–842. doi: 10.1126/science.1092571. [DOI] [PubMed] [Google Scholar]
- 43.Chen Y, Dokholyan NV. Insights into allosteric control of vinculin function from its large scale conformational dynamics. J Biol Chem. 2006;281:29148–29154. doi: 10.1074/jbc.M605512200. The first computational study of the large-scale conformational dynamics of full-length vinculin. [DOI] [PubMed] [Google Scholar]
- 44.Defilippi P, Di Stefano P, Cabodi S. p130Cas: a versatile scaffold in signaling networks. Trends Cell Biol. 2006;16:257–263. doi: 10.1016/j.tcb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Tamada M, Sheetz MP, Sawada Y. Activation of a signaling cascade by cytoskeleton stretch. Dev Cell. 2004;7:709–718. doi: 10.1016/j.devcel.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 46.Sawada Y, et al. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. References 45 and 46 describe the changes in conformation of RAP1 and p130 Cas in response to mechanical stretch. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hattori M, Minato N. Rap1 GTPase: functions, regulation, and malignancy. J Biochem. 2003;134:479–484. doi: 10.1093/jb/mvg180. [DOI] [PubMed] [Google Scholar]
- 48.Friedland JC, Lee MH, Boettiger D. Mechanically activated integrin switch controls α5β1 function. Science. doi: 10.1126/science.1168441. (in the press) [DOI] [PubMed] [Google Scholar]
- 49.Ginsberg MH, Du X, Plow EF. Inside-out integrin signalling. Curr Opin Cell Biol. 1992;4:766–771. doi: 10.1016/0955-0674(92)90099-x. [DOI] [PubMed] [Google Scholar]
- 50.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 51.Galbraith CG, Yamada KM, Sheetz MP. The relationship between force and focal complex development. J Cell Biol. 2002;159:695–705. doi: 10.1083/jcb.200204153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 53.Riveline D, et al. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol. 2001;153:1175–1186. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tzima E, del Pozo MA, Shattil SJ, Chien S, Schwartz MA. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J. 2001;20:4639–4647. doi: 10.1093/emboj/20.17.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watanabe Y, Akaike T. Possible involvement of caspase-like family in maintenance of cytoskeleton integrity. J Cell Physiol. 1999;179:45–51. doi: 10.1002/(SICI)1097-4652(199904)179:1<45::AID-JCP6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 56.Shi Q, Boettiger D. A novel mode for integrin-mediated signaling: tethering is required for phosphorylation of FAK Y397. Mol Biol Cell. 2003;14:4306–4315. doi: 10.1091/mbc.E03-01-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clark EA, King WG, Brugge JS, Symons M, Hynes RO. Integrin-mediated signals regulated by members of the rho family of GTPases. J Cell Biol. 1998;142:573–586. doi: 10.1083/jcb.142.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cox EA, Sastry SK, Huttenlocher A. Integrin-mediated adhesion regulates cell polarity and membrane protrusion through the Rho family of GTPases. Mol Biol Cell. 2001;12:265–277. doi: 10.1091/mbc.12.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chess PR, Toia L, Finkelstein JN. Mechanical strain-induced proliferation and signaling in pulmonary epithelial H441 cells. Am J Physiol Lung Cell Mol Physiol. 2000;279:L43–51. doi: 10.1152/ajplung.2000.279.1.L43. [DOI] [PubMed] [Google Scholar]
- 60.Milkiewicz M, Mohammadzadeh F, Ispanovic E, Gee E, Haas TL. Static strain stimulates expression of matrix metalloproteinase-2 and VEGF in microvascular endothelium via JNK- and ERK-dependent pathways. J Cell Biochem. 2007;100:750–761. doi: 10.1002/jcb.21055. [DOI] [PubMed] [Google Scholar]
- 61.Chaturvedi LS, Marsh HM, Basson MD. Src and focal adhesion kinase mediate mechanical strain-induced proliferation and ERK1/2 phosphorylation in human H441 pulmonary epithelial cells. Am J Physiol Cell Physiol. 2007;292:C1701–C1713. doi: 10.1152/ajpcell.00529.2006. [DOI] [PubMed] [Google Scholar]
- 62.Kippenberger S, et al. Signaling of mechanical stretch in human keratinocytes via MAP kinases. J Invest Dermatol. 2000;114:408–412. doi: 10.1046/j.1523-1747.2000.00915.x. [DOI] [PubMed] [Google Scholar]
- 63.Plotkin LI, et al. Mechanical stimulation prevents osteocyte apoptosis: requirement of integrins, Src kinases, and ERKs. Am J Physiol Cell Physiol. 2005;289:C633–C643. doi: 10.1152/ajpcell.00278.2004. [DOI] [PubMed] [Google Scholar]
- 64.Dennerll TJ, Joshi HC, Steel VL, Buxbaum RE, Heidemann SR. Tension and compression in the cytoskeleton of PC-12 neurites. II: Quantitative measurements. J Cell Biol. 1988;107:665–674. doi: 10.1083/jcb.107.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang HB, Dembo M, Hanks SK, Wang Y. Focal adhesion kinase is involved in mechanosensing during fibroblast migration. Proc Natl Acad Sci USA. 2001;98:11295–11300. doi: 10.1073/pnas.201201198. This paper demonstrates that FAK is important for migrating fibroblasts to respond to mechanical force. FAK-null cells are unable to migrate at the same speed or in a sustained direction when compared with wild-type cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-β1 from the extracellular matrix. J Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. This paper demonstrates that myofibroblast contraction, through integrin activation, directly activates TGFβ from ECM stores. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heinemeier KM, et al. Expression of collagen and related growth factors in rat tendon and skeletal muscle in response to specific contraction types. J Physiol. 2007;582:1303–1316. doi: 10.1113/jphysiol.2007.127639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wells RG. The role of matrix stiffness in hepatic stellate cell activation and liver fibrosis. J Clin Gastroenterol. 2005;39:S158–161. doi: 10.1097/01.mcg.0000155516.02468.0f. [DOI] [PubMed] [Google Scholar]
- 69.Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix–cytoskeleton crosstalk. Nature Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- 70.Liu BP, Burridge K. Vav2 activates Rac1, Cdc42, and RhoA downstream from growth factor receptors but not β1 integrins. Mol Cell Biol. 2000;20:7160–7169. doi: 10.1128/mcb.20.19.7160-7169.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bresnick AR. Molecular mechanisms of nonmuscle myosin-II regulation. Curr Opin Cell Biol. 1999;11:26–33. doi: 10.1016/s0955-0674(99)80004-0. [DOI] [PubMed] [Google Scholar]
- 72.Mogilner A, Oster G. Force generation by actin polymerization II: the elastic ratchet and tethered filaments. Biophys J. 2003;84:1591–1605. doi: 10.1016/S0006-3495(03)74969-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beningo KA, Wang YL. Flexible substrata for the detection of cellular traction forces. Trends Cell Biol. 2002;12:79–84. doi: 10.1016/s0962-8924(01)02205-x. [DOI] [PubMed] [Google Scholar]
- 74.Dembo M, Wang YL. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys J. 1999;76:2307–2316. doi: 10.1016/S0006-3495(99)77386-8. This paper demonstrates a method to determine the traction forces exerted by a single fibroblast during steady locomotion, revealing that the lamellopodia generate larger traction forces than the body of the cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sirghi L, Ponti J, Broggi F, Rossi F. Probing elasticity and adhesion of live cells by atomic force microscopy indentation. Eur Biophys J. 2008;37:935–945. doi: 10.1007/s00249-008-0311-2. [DOI] [PubMed] [Google Scholar]
- 76.Solon J, Levental I, Sengupta K, Georges PC, Janmey PA. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys J. 2007;93:4453–4461. doi: 10.1529/biophysj.106.101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kumar S, et al. Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization, and extracellular matrix mechanics. Biophys J. 2006;90:3762–3773. doi: 10.1529/biophysj.105.071506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hamelers IH, et al. The Rac activator Tiam1 is required for α3β1-mediated laminin-5 deposition, cell spreading, and cell migration. J Cell Biol. 2005;171:871–881. doi: 10.1083/jcb.200509172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ewald AJ, Brenot A, Duong M, Chan BS, Werb Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev Cell. 2008;14:570–581. doi: 10.1016/j.devcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yeung T, et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 81.Delcommenne M, Streuli CH. Control of integrin expression by extracellular matrix. J Biol Chem. 1995;270:26794–26801. doi: 10.1074/jbc.270.45.26794. [DOI] [PubMed] [Google Scholar]
- 82.Triplett JW, O’Riley R, Tekulve K, Norvell SM, Pavalko FM. Mechanical loading by fluid shear stress enhances IGF-1 receptor signaling in osteoblasts in a PKCzeta-dependent manner. Mol Cell Biomech. 2007;4:13–25. [PubMed] [Google Scholar]
- 83.Reichelt J. Mechanotransduction of keratinocytes in culture and in the epidermis. Eur J Cell Biol. 2007;86:807–816. doi: 10.1016/j.ejcb.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 84.Chien S. Effects of disturbed flow on endothelial cells. Ann Biomed Eng. 2008;36:554–562. doi: 10.1007/s10439-007-9426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Avvisato CL, et al. Mechanical force modulates global gene expression and β-catenin signaling in colon cancer cells. J Cell Sci. 2007;120:2672–2682. doi: 10.1242/jcs.03476. [DOI] [PubMed] [Google Scholar]
- 86.Alcaraz J, et al. Laminin and biomimetic extracellular elasticity enhance functional differentiation in mammary epithelia. EMBO J. 2008;27:2829–2838. doi: 10.1038/emboj.2008.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rizki A, et al. A human breast cell model of preinvasive to invasive transition. Cancer Res. 2008;68:1378–1387. doi: 10.1158/0008-5472.CAN-07-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ingber DE. Tensegrity II. How structural networks influence cellular information processing networks. J Cell Sci. 2003;116:1397–1408. doi: 10.1242/jcs.00360. [DOI] [PubMed] [Google Scholar]
- 89.Ingber DE. Tensegrity I. Cell structure and hierarchical systems biology. J Cell Sci. 2003;116:1157–1173. doi: 10.1242/jcs.00359. [DOI] [PubMed] [Google Scholar]
- 90.Bloom S, Lockard VG, Bloom M. Intermediate filament-mediated stretch-induced changes in chromatin: a hypothesis for growth initiation in cardiac myocytes. J Mol Cell Cardiol. 1996;28:2123–2127. doi: 10.1006/jmcc.1996.0204. [DOI] [PubMed] [Google Scholar]
- 91.Molenaar C, et al. Visualizing telomere dynamics in living mammalian cells using PNA probes. EMBO J. 2003;22:6631–6641. doi: 10.1093/emboj/cdg633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bustamante C, Bryant Z, Smith SB. Ten years of tension: single-molecule DNA mechanics. Nature. 2003;421:423–427. doi: 10.1038/nature01405. [DOI] [PubMed] [Google Scholar]
- 93.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20:811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 94.Gore J, et al. Mechanochemical analysis of DNA gyrase using rotor bead tracking. Nature. 2006;439:100–104. doi: 10.1038/nature04319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maniotis AJ, Bojanowski K, Ingber DE. Mechanical continuity and reversible chromosome disassembly within intact genomes removed from living cells. Cell Biochem. 1997;65:114–130. [PubMed] [Google Scholar]
- 96.Plachot C, Lelievre SA. DNA methylation control of tissue polarity and cellular differentiation in the mammary epithelium. Exp Cell Res. 2004;298:122–132. doi: 10.1016/j.yexcr.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 97.Lelievre S, et al. Tissue phenotype is dependent on reciprocal interactions between the extracellular matrix and the structural organization of the nucleus. Proc Natl Acad Sci USA. 1998;95:14711–14716. doi: 10.1073/pnas.95.25.14711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim YB, et al. Cell adhesion status-dependent histone acetylation is regulated through intracellular contractility-related signaling activities. J Biol Chem. 2005;280:28357–28364. doi: 10.1074/jbc.M412608200. This paper shows a link between cell adhesion and contractility and a decrease in acetylation of histone H3 and higher HDAC activity, suggesting that histone modifications can be regulated by mechanical cues. [DOI] [PubMed] [Google Scholar]
- 99.Destaing O, et al. A novel Rho–mDia2–HDAC6 pathway controls podosome patterning through microtubule acetylation in osteoclasts. J Cell Sci. 2005;118:2901–2911. doi: 10.1242/jcs.02425. [DOI] [PubMed] [Google Scholar]
- 100.Le Beyec J, et al. Cell shape regulates global histone acetylation in human mammary epithelial cells. Exp Cell Res. 2007;313:3066–3075. doi: 10.1016/j.yexcr.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dalby MJ, Riehle MO, Sutherland DS, Agheli H, Curtis AS. Morphological and microarray analysis of human fibroblasts cultured on nanocolumns produced by colloidal lithography. Eur Cell Mater. 2005;9:1–8. doi: 10.22203/ecm.v009a01. discussion 8. [DOI] [PubMed] [Google Scholar]
- 102.Dalby MJ, et al. Nanomechanotransduction and interphase nuclear organization influence on genomic control. J Cell Biochem. 2007;102:1234–1244. doi: 10.1002/jcb.21354. This paper demonstrated that changes in cell shape owing to cell spreading altered the location of chromosomes within the nucleus and the chromosomal sites of regulated gene expression. [DOI] [PubMed] [Google Scholar]
- 103.Dalby MJ, et al. Group analysis of regulation of fibroblast genome on low-adhesion nanostructures. Biomaterials. 2007;28:1761–1769. doi: 10.1016/j.biomaterials.2006.11.049. [DOI] [PubMed] [Google Scholar]
- 104.Alberts AS, Geneste O, Treisman R. Activation of SRF-regulated chromosomal templates by Rho-family GTPases requires a signal that also induces H4 hyperacetylation. Cell. 1998;92:475–487. doi: 10.1016/s0092-8674(00)80941-1. [DOI] [PubMed] [Google Scholar]
- 105.Posern G, Miralles F, Guettler S, Treisman R. Mutant actins that stabilise F-actin use distinct mechanisms to activate the SRF coactivator MAL. EMBO J. 2004;23:3973–3983. doi: 10.1038/sj.emboj.7600404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vartiainen MK, Guettler S, Larijani B, Treisman R. Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science. 2007;316:1749–1752. doi: 10.1126/science.1141084. [DOI] [PubMed] [Google Scholar]
- 107.Davies PF, Spaan JA, Krams R. Shear stress biology of the endothelium. Ann Biomed Eng. 2005;33:1714–1718. doi: 10.1007/s10439-005-8774-0. [DOI] [PubMed] [Google Scholar]
- 108.Glaser KJ, Felmlee JP, Manduca A, Kannan Mariappan Y, Ehman RL. Stiffness-weighted magnetic resonance imaging. Magn Reson Med. 2006;55:59–67. doi: 10.1002/mrm.20748. [DOI] [PubMed] [Google Scholar]
- 109.Garra BS. Imaging and estimation of tissue elasticity by ultrasound. Ultrasound Q. 2007;23:255–268. doi: 10.1097/ruq.0b013e31815b7ed6. [DOI] [PubMed] [Google Scholar]
- 110.Reihsner R, Melling M, Pfeiler W, Menzel EJ. Alterations of biochemical and two-dimensional biomechanical properties of human skin in diabetes mellitus as compared to effects of in vitro non-enzymatic glycation. Clin Biomech (Bristol, Avon) 2000;15:379–386. doi: 10.1016/s0268-0033(99)00085-6. [DOI] [PubMed] [Google Scholar]
- 111.Stone J, et al. The heritability of mammographically dense and nondense breast tissue. Cancer Epidemiol Biomarkers Prev. 2006;15:612–617. doi: 10.1158/1055-9965.EPI-05-0127. [DOI] [PubMed] [Google Scholar]
- 112.Nelson CM, Vanduijn MM, Inman JL, Fletcher DA, Bissell MJ. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science. 2006;314:298–300. doi: 10.1126/science.1131000. This article demonstrates that tissue geometry is a crucial factor in establishing the morphogen microenvironments that dictate branch formation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Elston CW, Ellis IO, editors. The Breast. Vol. 13. Churchill Livingstone, Edinburgh; New York: 1998. pp. 356–384. [Google Scholar]
- 114.Ronnov-Jessen L, Petersen OW, Bissell MJ. Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev. 1996;76:69–125. doi: 10.1152/physrev.1996.76.1.69. [DOI] [PubMed] [Google Scholar]
- 115.Weaver VM, Fischer AH, Peterson OW, Bissell MJ. The importance of the microenvironment in breast cancer progression: recapitulation of mammary tumorigenesis using a unique human mammary epithelial cell model and a three-dimensional culture assay. Biochem Cell Biol. 1996;74:833–851. doi: 10.1139/o96-089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Timpl R. Macromolecular organization of basement membranes. Curr Opin Cell Biol. 1996;8:618–624. doi: 10.1016/s0955-0674(96)80102-5. [DOI] [PubMed] [Google Scholar]
- 117.Griffith LG, Swarz MA. Capturing complex 3D tissue physiology in vitro. Nature Rev Mol Cell Biol. 2006:7211–224. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 118.Kreis T, Vale R, editors. Guidebook to the ECM, Anchor, and Adhesion Proteins. Oxford Univ. Press; New York: 1999. [Google Scholar]
- 119.Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378–386. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 120.Green KA, Lund LR. ECM degrading proteases and tissue remodelling in the mammary gland. Bioessays. 2005;27:894–903. doi: 10.1002/bies.20281. [DOI] [PubMed] [Google Scholar]
- 121.Watson CJ. Post-lactational mammary gland regression: molecular basis and implications for breast cancer. Expert Rev Mol Med. 2006;8:1–15. doi: 10.1017/S1462399406000196. [DOI] [PubMed] [Google Scholar]
- 122.McDaniel SM, et al. Remodeling of the mammary microenvironment after lactation promotes breast tumor cell metastasis. Am J Pathol. 2006;168:608–620. doi: 10.2353/ajpath.2006.050677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105:223–235. doi: 10.1242/dev.105.2.223. This article demonstrates that growth of mammary epithelial cells in RBM permits the assembly of polarized alveolus-like structures that secrete milk proteins into the luminal space. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Weaver VM, et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. Blocking integrin function reverted tumour cells grown in three-dimensional culture to a normal phenotype, demonstrating that the ECM and its receptors determine the phenotype and can override genotype in this model system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li ML, et al. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proc Natl Acad Sci USA. 1987;84:136–140. doi: 10.1073/pnas.84.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bao G, Suresh S. Cell and molecular mechanics of biological materials. Nature Mater. 2003;2:715–725. doi: 10.1038/nmat1001. [DOI] [PubMed] [Google Scholar]
- 127.Samani A, Bishop J, Luginbuhl C, Plewes DB. Measuring the elastic modulus of ex vivo small tissue samples. Phys Med Biol. 2003;48:2183–2198. doi: 10.1088/0031-9155/48/14/310. [DOI] [PubMed] [Google Scholar]
- 128.Suresh S. Biomechanics and biophysics of cancer cells. Acta Biomater. 2007;3:413–438. doi: 10.1016/j.actbio.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Paszek MJ, Weaver VM. The tension mounts: mechanics meets morphogenesis and malignancy. J Mammary Gland Biol Neoplasia. 2004;9:325–342. doi: 10.1007/s10911-004-1404-x. [DOI] [PubMed] [Google Scholar]
- 130.Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000;60:2497–2503. This article demonstrates that increased elastic modulus resulting from increased ECM collagen content influences the resistance of tissues to macromolecule transport, including chemotherapeutic agents. [PubMed] [Google Scholar]
- 131.Dalby MJ, Riehle MO, Johnstone H, Affrossman S, Curtis AS. Investigating the limits of filopodial sensing: a brief report using SEM to image the interaction between 10 nm high nano-topography and fibroblast filopodia. Cell Biol Int. 2004;28:229–236. doi: 10.1016/j.cellbi.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 132.Chaw KC, Manimaran M, Tay FE, Swaminathan S. A quantitative observation and imaging of single tumor cell migration and deformation using a multi-gap microfluidic device representing the blood vessel. Microvasc Res. 2006;72:153–160. doi: 10.1016/j.mvr.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 133.Croft DR, et al. Conditional ROCK activation in vivo induces tumor cell dissemination and angiogenesis. Cancer Res. 2004;64:8994–9001. doi: 10.1158/0008-5472.CAN-04-2052. [DOI] [PubMed] [Google Scholar]
- 134.O’Brien LE, et al. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nature Cell Biol. 2001;3:831–838. doi: 10.1038/ncb0901-831. [DOI] [PubMed] [Google Scholar]
- 135.Wang F, et al. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci USA. 1998;95:14821–14826. doi: 10.1073/pnas.95.25.14821. This article demonstrates that the spatial organization of breast cells in three-dimensions is important for correct signalling through integrin and EGFR–MAPK pathways. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Provenzano PP, et al. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4:38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rhee S, Grinnell F. Fibroblast mechanics in 3D collagen matrices. Adv Drug Deliv Rev. 2007;59:1299–1305. doi: 10.1016/j.addr.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ingman WV, Wyckoff J, Gouon-Evans V, Condeelis J, Pollard JW. Macrophages promote collagen fibrillogenesis around terminal end buds of the developing mammary gland. Dev Dyn. 2006;235:3222–3229. doi: 10.1002/dvdy.20972. [DOI] [PubMed] [Google Scholar]
- 139.Provenzano PP, et al. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008;6:11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wyckoff JB, Pinner SE, Gschmeissner S, Condeelis JS, Sahai E. ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasionin vivo. Curr Biol. 2006;16:1515–1523. doi: 10.1016/j.cub.2006.05.065. This paper describes the mechanism by which cells move through a dense ECM without proteolysis by MMPs. The tumour cells generated actomyosin forces that deformed the collagen fibres to push through the ECM. [DOI] [PubMed] [Google Scholar]
- 141.Ingber DE, Madri JA, Jamieson JD. Role of basal lamina in neoplastic disorganization of tissue architecture. Proc Natl Acad Sci USA. 1981;78:3901–3905. doi: 10.1073/pnas.78.6.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Roose T, Netti PA, Munn LL, Boucher Y, Jain RK. Solid stress generated by spheroid growth estimated using a linear poroelasticity model small star, filled. Microvasc Res. 2003;66:204–212. doi: 10.1016/s0026-2862(03)00057-8. [DOI] [PubMed] [Google Scholar]
- 143.Harris AL. Hypoxia — a key regulatory factor in tumour growth. Nature Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 144.Jain RK. Transport of molecules, particles, and cells in solid tumors. Annu Rev Biomed Eng. 1999;1:241–263. doi: 10.1146/annurev.bioeng.1.1.241. [DOI] [PubMed] [Google Scholar]
- 145.Rutkowski JM, Swartz MA. A driving force for change: interstitial flow as a morphoregulator. Trends Cell Biol. 2007;17:44–50. doi: 10.1016/j.tcb.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 146.Tschumperlin DJ, et al. Mechanotransduction through growth-factor shedding into the extracellular space. Nature. 2004;429:83–86. doi: 10.1038/nature02543. This article demonstrates for the first time how compressive force can modify growth factor receptor signalling by increasing the local ligand concentration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nature Rev Cancer. 2006;6:583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 148.Walker RA. The complexities of breast cancer desmoplasia. Breast Cancer Res. 2001;3:143–145. doi: 10.1186/bcr287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Willis R. Pathology of Tumors. Butterworth and Company; London: 1967. [Google Scholar]
- 150.Goepel C, Buchmann J, Schultka R, Koelbl H. Tenascin — a marker for the malignant potential of preinvasive breast cancers. Gynecol Oncol. 2000;79:372–378. doi: 10.1006/gyno.2000.5978. [DOI] [PubMed] [Google Scholar]
- 151.Gorczyca W, Holm R, Nesland JM. Laminin production and fibronectin immunoreactivity in breast carcinomas. Anticancer Res. 1993;13:851–858. [PubMed] [Google Scholar]
- 152.Guarino M, Reale D, Micoli G. The extracellular matrix in sarcomatoid carcinomas of the breast. Virchows Arch A Pathol Anat Histopathol. 1993;423:131–136. doi: 10.1007/BF01606587. [DOI] [PubMed] [Google Scholar]
- 153.Rodriguez C, Rodriguez-Sinovas A, Martinez-Gonzalez J. Lysyl oxidase as a potential therapeutic target. Drug News Perspect. 2008;21:218–224. doi: 10.1358/dnp.2008.21.4.1213351. [DOI] [PubMed] [Google Scholar]
- 154.Strongin AY. Mislocalization and unconventional functions of cellular MMPs in cancer. Cancer Metastasis Rev. 2006;25:87–98. doi: 10.1007/s10555-006-7892-y. [DOI] [PubMed] [Google Scholar]
- 154.Jodele S, Blavier L, Yoon JM, DeClerck YA. Modifying the soil to affect the seed: role of stromal-derived matrix metalloproteinases in cancer progression. Cancer Metastasis Rev. 2006;25:35–43. doi: 10.1007/s10555-006-7887-8. [DOI] [PubMed] [Google Scholar]
- 156.Biondi ML, et al. MMP1 and MMP3 polymorphisms in promoter regions and cancer. Clin Chem. 2000;46:2023–2024. [PubMed] [Google Scholar]
- 157.Sternlicht MD, Safarians S, Rivera SP, Barsky SH. Characterizations of the extracellular matrix and proteinase inhibitor content of human myoepithelial tumors. Lab Invest. 1996;74:781–796. [PubMed] [Google Scholar]
- 158.Akiri G, et al. Lysyl oxidase-related protein-1 promotes tumor fibrosis and tumor progression in vivo. Cancer Res. 2003;63:1657–1666. [PubMed] [Google Scholar]
- 159.Decitre M, et al. Lysyl oxidase-like protein localizes to sites of de novo fibrinogenesis in fibrosis and in the early stromal reaction of ductal breast carcinomas. Lab Invest. 1998;78:143–151. [PubMed] [Google Scholar]
- 160.Shields JD, et al. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell. 2007;11:526–538. doi: 10.1016/j.ccr.2007.04.020. This paper demonstrates that interstitial flow establishes an autocrine CCR7 gradient that guides tumour cells to lymphatic vessels during metastasis. [DOI] [PubMed] [Google Scholar]
- 161.Lieber MM. Towards an understanding of the role of forces in carcinogenesis: a perspective with therapeutic implications. Riv Biol. 2006;99:131–160. [PubMed] [Google Scholar]
- 162.Wolfe JN. Risk for breast cancer development determined by mammographic parenchymal pattern. Cancer. 1976;37:2486–2492. doi: 10.1002/1097-0142(197605)37:5<2486::aid-cncr2820370542>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 163.Wolfe JN. Breast patterns as an index of risk for developing breast cancer. AJR Am J Roentgenol. 1976;126:1130–1137. doi: 10.2214/ajr.126.6.1130. References 162 and 163 were the first to describe the link between mammographic density and breast cancer risk. [DOI] [PubMed] [Google Scholar]
- 164.Couzin J. Breast cancer. Fine-tuning breast density measures. Science. 2005;309:1665. doi: 10.1126/science.309.5741.1665. [DOI] [PubMed] [Google Scholar]
- 165.Boyd NF, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 166.Boyd NF, et al. Mammographic densities and breast cancer risk. Breast Dis. 1998;10:113–126. doi: 10.3233/bd-1998-103-412. [DOI] [PubMed] [Google Scholar]
- 167.Boyd NF, et al. Heritability of mammographic density, a risk factor for breast cancer. N Engl J Med. 2002;347:886–894. doi: 10.1056/NEJMoa013390. [DOI] [PubMed] [Google Scholar]
- 168.Guo YP, et al. Growth factors and stromal matrix proteins associated with mammographic densities. Cancer Epidemiol Biomarkers Prev. 2001;10:243–248. [PubMed] [Google Scholar]
- 169.Li T, et al. The association of measured breast tissue characteristics with mammographic density and other risk factors for breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:343–349. doi: 10.1158/1055-9965.EPI-04-0490. This article demonstrated that collagen levels are increased in breast tissues with high mammographic density. [DOI] [PubMed] [Google Scholar]
- 170.Radisky ES, Radisky DC. Stromal induction of breast cancer: inflammation and invasion. Rev Endocr Metab Disord. 2007;8:279–287. doi: 10.1007/s11154-007-9037-1. [DOI] [PubMed] [Google Scholar]
- 171.Martin LJ, Boyd NF. Mammographic density. Potential mechanisms of breast cancer risk associated with mammographic density: hypotheses based on epidemiological evidence. Breast Cancer Res. 2008;10:201. doi: 10.1186/bcr1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Thomas A, et al. Real-time elastography--an advanced method of ultrasound: First results in 108 patients with breast lesions. Ultrasound Obstet Gynecol. 2006;28:335–340. doi: 10.1002/uog.2823. [DOI] [PubMed] [Google Scholar]
- 173.Woloshin S, Schwartz LM, Welch HG. The risk of death by age, sex, and smoking status in the United States: putting health risks in context. J Natl Cancer Inst. 2008;100:845–853. doi: 10.1093/jnci/djn124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tang SY, Zeenath U, Vashishth D. Effects of non-enzymatic glycation on cancellous bone fragility. Bone. 2007;40:1144–1151. doi: 10.1016/j.bone.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Varani J, et al. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J Invest Dermatol. 2000;114:480–486. doi: 10.1046/j.1523-1747.2000.00902.x. [DOI] [PubMed] [Google Scholar]
- 176.Gosain AK, Recinos RF, Agresti M, Khanna AK. TGF-β1, FGF-2, and receptor mRNA expression in suture mesenchyme and dura versus underlying brain in fusing and nonfusing mouse cranial sutures. Plast Reconstr Surg. 2004;113:1675–1684. doi: 10.1097/01.prs.0000117362.33347.43. [DOI] [PubMed] [Google Scholar]
- 177.Alexander H, Cook T. Variations with age in the mechanical properties of human skin in vivo. J Tissue Viability. 2006;16:6–11. doi: 10.1016/s0965-206x(06)63002-7. [DOI] [PubMed] [Google Scholar]
- 178.Agah A, Kyriakides TR, Letrondo N, Bjorkblom B, Bornstein P. Thrombospondin 2 levels are increased in aged mice: consequences for cutaneous wound healing and angiogenesis. Matrix Biol. 2004;22:539–547. doi: 10.1016/j.matbio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 179.Bornstein P, Agah A, Kyriakides TR. The role of thrombospondins 1 and 2 in the regulation of cell–matrix interactions, collagen fibril formation, and the response to injury. Int J Biochem Cell Biol. 2004;36:1115–1125. doi: 10.1016/j.biocel.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 180.Lasco A, et al. Effect of long-term treatment with raloxifene on mammary density in postmenopausal women. Menopause. 2006;13:787–792. doi: 10.1097/01.gme.0000233493.20712.ad. [DOI] [PubMed] [Google Scholar]
- 181.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 182.Psaila B, Kaplan RN, Port ER, Lyden D. Priming the ‘soil’ for breast cancer metastasis: the pre-metastatic niche. Breast Dis. 2006;26:65–74. doi: 10.3233/bd-2007-26106. [DOI] [PubMed] [Google Scholar]
- 183.Balooch G, et al. TGF-β regulates the mechanical properties and composition of bone matrix. Proc Natl Acad Sci USA. 2005;102:18813–18818. doi: 10.1073/pnas.0507417102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Erler JT, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 185.MacKintosh FC, Kas J, Janmey PA. Elasticity of semiflexible biopolymer networks. Phys Rev Lett. 1995;75:4425–4428. doi: 10.1103/PhysRevLett.75.4425. [DOI] [PubMed] [Google Scholar]
- 186.Halliday NL, Tomasek JJ. Mechanical properties of the extracellular matrix influence fibronectin fibril assembly in vitro. Exp Cell Res. 1995;217:109–117. doi: 10.1006/excr.1995.1069. [DOI] [PubMed] [Google Scholar]
- 187.Girton TS, Oegema TR, Tranquillo RT. Exploiting glycation to stiffen and strengthen tissue equivalents for tissue engineering. J Biomed Mater Res. 1999;46:87–92. doi: 10.1002/(sici)1097-4636(199907)46:1<87::aid-jbm10>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 188.Marx G. Elasticity of fibrin and protofibrin gels is differentially modulated by calcium and zinc. Thromb Haemost. 1988;59:500–503. [PubMed] [Google Scholar]
- 189.Carr ME, Jr, Gabriel DA, McDonagh J. Influence of factor XIII and fibronectin on fiber size and density in thrombin-induced fibrin gels. J Lab Clin Med. 1987;110:747–752. [PubMed] [Google Scholar]
- 190.Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci USA. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. This article illustrated the development of polyacrylamide gels of defined mechanical stiffness for use in tissue culture, facilitating the study of cell response to extracellular matrix force and demonstrated that focal adhesions are irregular and dynamic on flexible matrices and have normal morphology and stability on stiff matrices. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Debnath J, Brugge JS. Modelling glandular epithelial cancers in three-dimensional cultures. Nature Rev Cancer. 2005;5:675–688. doi: 10.1038/nrc1695. [DOI] [PubMed] [Google Scholar]