Abstract
This study was designed to examine the mechanism of heart rate (HR) responses elicited by the stimulation of hypothalamic paraventricular nucleus (PVN). Experiments were done in urethane-anesthetized, barodenervated, adult, male Wistar rats. Chemical stimulation of the PVN by unilateral microinjections of N-methyl-D-aspartic acid (NMDA) elicited increases in HR which were attenuated by bilateral vagotomy. PVN-induced tachycardia was also attenuated by the blockade of the spinal ionotropic glutamate receptors (iGLURs) which was accomplished by intrathecal injections at T9–T10 or direct application at T1–T4 of iGLUR antagonists. The blockade of spinal iGLURs combined with bilateral vagotomy completely blocked PVN-induced tachycardia. Blockade of GABA receptors in the medial nucleus tractus solitarius (mNTS) also attenuated the PVN-induced tachycardia. Complete blockade of PVN-induced tachycardia was also observed after the blockade of iGLURs in both the spinal cord and mNTS. Combination of the blockade of mNTS GABA receptors and spinal iGLURs also abolished PVN-induced tachycardia. PVN-induced tachycardia was not altered by the blockade of spinal vasopressin or oxytocin receptors at T1–T4. These results suggested that in barodenervated rats: 1) tachycardia elicited by the chemical stimulation of the PVN was mediated via both inhibition of vagal and activation of sympathetic outflows to the heart, 2) the vagal inhibition contributing to the PVN-induced tachycardia was mediated by the iGLURs and GABARs in the mNTS, 3) sympathetic activation contributing to the PVN-induced tachycardia was mediated via spinal iGLURs, and 4) spinal vasopressin and oxytocin receptors were not involved in the mediation of PVN-induced tachycardia.
Keywords: barodenervation, vagotomy, ionotropic glutamate receptor antagonists, GABA receptor antagonists, microinjections, intrathecal injection
1. Introduction
Emerging evidence indicates that the hypothalamic paraventricular nucleus (PVN) plays a significant role in controlling cardiovascular function (Badoer, 2001; Coote et al., 1998; Coote, 2004). Although a few reports indicate that chemical stimulation of the PVN elicits depressor responses (Kannan et al., 1988; Yamashita et al., 1987), there is a general consensus that chemical stimulation or disinhibition of the PVN elicits increases in blood pressure (BP) and renal sympathetic nerve activity (RSNA) (Chen et al., 2003; Kannan et al., 1989; Li et al., 2001, 2006). The responses to the PVN stimulation have been reported to be mediated via different pathways. For example, the PVN is known to project to the rostral ventrolateral medullary pressor area (RVLM) and chemical stimulation of the PVN has been reported to excite spinally projecting RVLM neurons (Pyner and Coote, 1999, 2000; Yang and Coote, 1998). Furthermore, excitation of spinally projecting RVLM neurons in response to the chemical stimulation of the PVN was mediated via glutamate in the RVLM (Yang et al., 2001). PVN is also known to project directly to the intermediolateral cell column of the spinal cord (IML) (Holstege, 1987). Chemical stimulation of this projection has been reported to elicit increases in BP and RSNA via the release of glutamate or vasopressin in the IML (Yang et al., 2002).
Divergent results have been reported for the heart rate (HR) responses to chemical stimulation of the PVN. For example, Darlington et al., (1989) have reported that chemical stimulation of the caudal PVN elicited bradycardia. In other studies, chemical stimulation or disinhibition of the PVN has been reported to elicit tachycardia (Chen et al., 2003; Li et al., 2001, 2006). The neural pathways and neurotransmitters mediating HR responses to the PVN stimulation have not been firmly established.
The nucleus tractus solitarius (NTS) is another area that is important in cardiovascular regulation (Sapru, 2004). It is well established that peripheral baroreceptor, chemoreceptor and cardiopulmonary afferents make their first synapse in the NTS. The secondary NTS neurons send an excitatory projection to the caudal ventrolateral medullary depressor area (CVLM), which, in turn, sends an inhibitory GABAergic projection to the RVLM (Guyenet, 2006; Madden and Sved, 2003; Sapru, 2002; Schreihofer and Guyenet, 2002; Willette et al., 1983, 1984).
The NTS is known to receive projections from the anterior, medial and lateral parvocellular cells of the PVN (Hardy, 2001; Luiten et al., 1985; Palkovits, 1999; Swanson and Sawchenko, 1983). We hypothesized that the projection from the PVN to the medial subnucleus of NTS (mNTS) may also play a role in mediating the HR responses to PVN stimulation. In a previous study we have reported that a tonically active glutamatergic projection from the PVN to the mNTS may serve as a restraint mechanism for pressor effects of PVN stimulation (Kawabe et al., 2008). Recent demonstration of abundance of glutamatergic neurons in the PVN is consistent with our report (Kawabe et al., 2008; Stocker et. al., 2006).
The first aim in the present study was to determine the mechanism by which the projection from the PVN to the mNTS may contribute to the tachycardic responses. Since it is established that glutamate (Gordon and Sved, 2002; Guyenet, 2006; Sapru, 2002, 2004; Talman et al., 1984) and GABA (Sved, 1994) are involved in the reflex regulation of cardiovascular function in the mNTS, we hypothesized that these neurotransmitters may play a role in this nucleus in mediating the tachycardic responses to the PVN stimulation. The second aim was to determine the neurotransmitter/receptor mechanisms in the spinal cord that mediate HR responses to the PVN stimulation. Since BP responses to the chemical stimulation of the PVN were also recorded, they were included in the results for comparison with HR responses.
2. Results
Baseline values for mean arterial pressure (MAP) and HR in non-barodenervated rats (n = 14) were 100 ± 5 mmHg and 400 ± 10 bpm, respectively. Barodenervation elicited increases in MAP (45 ± 5 mmHg) and HR (40 ± 10 bpm) which lasted for about 60 min. After this time, the baseline MAP and HR remained at 105 ± 5 mmHg and 410 ± 5 bpm, respectively, in barodenervated rats (n = 65). The recovery of MAP and HR in barodenervated rats has been attributed to the failure to maintain sustained elevation of sympathetic nerve activity (Osborn and England, 1990). In this study, barodenervated rats were used in all experiments, unless indicated otherwise, in order to avoid any effects of baroreceptor activation on PVN-induced cardiovascular responses. The following series of experiments were conducted to study the mechanism of PVN-induced tachycardic and pressor responses.
2.1. Effect of vagotomy
In all experiments, N-methyl-D-aspartic acid (NMDA, 10 mmol/L, 50 nL) was used for chemical stimulation of the PVN. We have previously reported that repeated NMDA microinjections into the PVN at 20 min intervals do not exhibit tachyphylaxis (Kawabe et al, 2008). In barodenervated rats (n = 5), unilateral microinjection of NMDA into the PVN elicited tachycardic responses. Bilateral vagotomy was done 20 min later. After an interval of 5–10 min, NMDA was again microinjected into the same PVN site. Bilateral vagotomy attenuated the tachycardic responses to the PVN stimulation significantly (Fig. 1A). PVN stimulation elicited pressor responses also; bilateral vagotomy did not alter these responses significantly (Fig. 1B).
Fig. 1.
A & B: Effect of vagotomy on the PVN-induced cardiovascular responses in barodenervated rats (n = 7). A: In this and other experiments, PVN was stimulated by microinjections of NMDA (10 mmol/L, 50 nL). Bilateral vagotomy significantly attenuated PVN-induced tachycardia; the increases in HR before and after the bilateral vagotomy were 40.7 ± 2.5 and 22.8 ± 1.5 beats/min (bpm), respectively (*P < 0.01). B: Bilateral vagotomy did not alter PVN-induced pressor responses significantly; increases in MAP before and after bilateral vagotomy were 35 ± 1.5 and 32.8 ± 2.8 mmHg, respectively (P > 0.05). C & D: Effect of vagotomy on PVN-induced cardiovascular responses in non-barodenervated rats (n = 5). C: Bilateral vagotomy did not alter significantly PVN-induced tachycardia; increases in HR before and after vagotomy were 22 ± 3 and 20 ± 4 bpm, respectively (P > 0.05). D: Bilateral vagotomy did not alter significantly PVN-induced pressor responses; the increases in MAP before and after bilateral vagotomy were 19 ± 3 and 17 ± 4 mmHg, respectively (P > 0.05). E & F: Effects of the mNTS GABAR blockade on PVN-induced cardiovascular responses in barodenervated rats (n = 7). E: Blockade of mNTS GABARs bilaterally significantly attenuated PVN-induced tachycardia; increases in HR before and after the bilateral microinjections of gabazine (0.2 mmol/L, 50 nL) and 2-hydroxysaclofen (10 mmol/L, 50 nL) into the mNTS, were 46.5 ± 3 and 25.7 ± 2.5 bpm, respectively (*P < 0.01). F: Bilateral blockade of mNTS GABARs did not alter PVN-induced pressor responses significantly; the increases in MAP before and after the blockade of mNTS GABARs were 39 ± 2 and 37 ± 2.5 mmHg, respectively (P > 0.05).
The same experimental protocol was used in a group of non-barodenervated rats (n = 5). In these rats, the tachycardic and pressor responses to the PVN stimulation were not altered significantly by bilateral vagotomy (Fig. 1 C and D). In this and other figures showing bar graphs, numerical data are presented in the legends.
In barodenervated rats, the onset, peak and duration of PVN-induced tachycardic responses were 5–15 sec, 2–3 min and 10–15 min, respectively. In non-barodenervated rats, corresponding times were10–25 sec, 3–5 min and 5–10 min, respectively. Relatively longer onset and peak times and shorter duration in non-barodenervated rats may be due to the presence of intact baroreflex.
Bilateral vagotomy increased baseline HR in barodenervated (20 ± 4 bpm) as well as non-barodenervated (24 ± 6 bpm) rats; the differences in HR increases were not statistically significant (P > 0.05).
2.2. Effect of mNTS GABAR blockade
In another group of barodenervated rats (n = 5), unilateral microinjections of NMDA into the PVN elicited usual tachycardic and pressor responses. Twenty min later, gabazine (0.2 mmol/L, 50 nL) and 2-hydroxysaclofen (10 mmol/L, 50 nL) were microinjected sequentially within 1 min into the mNTS on each side to block gamma-aminobutyric acid receptors (GABARs). These antagonists elicited decreases in HR (72 ± 11 bpm) and MAP (8 ± 6 mmHg). Within 10 min, when the BP recovered to the baseline, NMDA was again microinjected into the same PVN site. Tachycardic responses to the PVN stimulation were significantly attenuated after mNTS GABAR blockade (Fig. 1E). Pressor responses to the PVN stimulation were not altered by the blockade of GABARs in the mNTS (Fig. 1F).
2.3. Specificity of ionotropic glutamate receptor antagonists
D(−)-2-amino-7-phosphono-heptanoic acid (D-AP7) and 2,3-dioxo-6-nitro-1,2,3,4-tetrahydro-benzo[f]quinoxaline-7-sulfonamide disodium (NBQX) were used as antagonists for NMDA and non-NMDA receptors, respectively (Dhruva et al., 1998). We have previously shown that NBQX (2 mmol/L) and D-AP7 (5 mmol/L) did not alter responses to another unrelated agonist, carbachol (Kasamatsu et al., 2004). The same concentrations of NBQX and D-AP7 were used in our other experiments. Since vasopressin has been reported to be a neurotransmitter in the projection from the PVN to the spinal cord (Malpas and Coote, 1994), effects of the ionotropic glutamate receptor (iGLUR) antagonists on the cardiovascular responses to the intrathecal injection of (Arg8)-vasopressin (AVP) were also studied in another group of non-barodenervated rats (n = 4). Intrathecal injections of NBQX and D-AP7 were made sequentially within 1 min; the tip of the intrathecal cannula was at T9–T10 level. In this and other experiments, intrathecal injections of NBQX and D-AP7 elicited decreases in HR (52 ± 5 bpm) and MAP (35 ± 3 mmHg); the onset time was 1–2 min and maximum responses were observed within 5–10 min. Intrathecal injections of NBQX and D-AP7 did not alter the pressor and tachycardic responses elicited by the intrathecal injection of AVP (20 μmol/L, 20 μL); similar concentrations of AVP have been used by other investigators (Riphagen and Pittman, 1989; Yang et al., 2002). The increases in MAP induced by the intrathecal injection of AVP before and after the intrathecal injections of NBQX and D-AP7 were 18 ± 6 mmHg and 21 ± 5 mmHg, respectively (P > 0.05). In the same group of rats, AVP-induced the increases in HR before and after the intrathecal injections of NBQX and D-AP7 were 19 ± 8 bpm and 23 ± 5 bpm, respectively (P > 0.05).
2.4. Effect of spinal iGLUR blockade
In another group of barodenervated rats (n = 6), stimulation of the PVN by unilateral microinjections of NMDA elicited usual increases in HR and MAP. After 20 min, NBQX and D-AP7 were injected intrathecally at T9–T10. Presumably the antagonists delivered at T9–T10 blocked iGLURs in the entire spinal cord. Five to ten min later, the tachycardic responses to the PVN stimulation were significantly attenuated but not abolished (Fig. 2A). Pressor responses to the PVN stimulation were almost blocked after the blockade of spinal iGLURs (Fig. 2B).
Fig. 2.

A & B: Effect of spinal iGLUR blockade on PVN-induced cardiovascular responses in barodenervated rats (n = 6). A: Intrathecal injections of NBQX (2 mmol/L, 10 μL) and DAP-7 (5 mmol/L, 10 μL) at T9–T10 significantly attenuated PVN-induced tachycardia; HR increases before and after iGLUR blockade were 38 ± 5 and 17 ± 5 bpm, respectively (*P < 0.01). B: PVN-induced pressor responses were almost blocked; increases in MAP before and after iGLUR blockade were 33 ± 5 and 2 ± 2 mmHg, respectively (*P < 0.001). C & D: Effect of blockade of spinal iGLURs at T1–T4 on the PVN-induced cardiovascular responses in barodenervated rats (n = 5). C: PVN-induced tachycardia was attenuated; HR increases before and after iGLUR blockade were 46 ± 2 and 26 ± 3 bpm, respectively (*P < 0.01). D: PVN-induced pressor responses were not significantly attenuated; increases in MAP before and after iGLUR blockade were 38 ± 3 and 35 ± 3 mmHg, respectively (P > 0.05).
The effect of blockade of iGLURs in the upper thoracic cord on PVN-induced responses was studied in another group of barodenervated rats (n = 5). The spinal cord was exposed at C8-T5 level for direct application of drugs. Stimulation of the PVN by unilateral microinjection of NMDA elicited usual increases in HR and MAP. After 20 min, a pledget of tissue paper soaked in NBQX (2 mmol/L, 10 μL) and D-AP7 (5 mmol/L, 10 μL) was applied to the surface of the spinal cord at T1–T4 level (see Section 4.6). Five to 6 min later, the tachycardic responses to the PVN stimulation were significantly attenuated but not abolished (Fig. 2C). The pressor responses to the PVN stimulation were not altered significantly (Fig. 2D). These experiments indicated that attenuation of PVN-induced HR responses was similar in experiments in which the iGLURs were blocked in the entire spinal cord (by intrathecal injections delivered at T9–T10 level) or upper thoracic cord (by direct application of the antagonists at T1–T4 level). However, PVN-induced pressor responses were completely blocked when intrathecal injections of iGLUR antagonists were made at T9–T10 level (Fig. 2B) but they were not significantly altered when the iGLURs were blocked in the upper thoracic cord (Fig. 2D).
As mentioned in section 2.3, intrathecal injections of NBQX and D-AP7 at T9–T10 elicited decreases in baseline HR and MAP. Application of NBQX and D-AP7 directly to the upper thoracic cord at T1–T4 via a tissue paper pledget also decreased HR (36 ± 8 bpm) and MAP (12 ± 3 mmHg). Although the magnitudes of HR and MAP decreases were smaller, the onset time (1–2 min) and time to peak responses (5–10 min) was similar with intrathecal injections at T9–T10 and direct application at T1–T4 suggesting that these times were required for the drugs to diffuse to the IML. In this and other series of experiments, intrathecal injections of artificial cerebrospinal fluid (aCSF, 20 μL) at T9–T10 or application of tissue paper pledget soaked in aCSF (20 μL) at T1–T4 level did not alter the responses to PVN stimulation.
2.5. Effect of combined blockade of spinal and mNTS iGLURs
In another group of barodenervated rats (n = 5), unilateral microinjections of NMDA elicited usual increases in HR and MAP. After allowing an interval of 20 min, NBQX and D-AP7 were injected intrathecally at T9–T10 which was followed by bilateral microinjections of NBQX and D-AP7 (50 nL each) into the mNTS. The tachycardic and pressor responses to subsequent chemical stimulation of the PVN were almost completely blocked (Figs. 3 A and B).
Fig. 3.
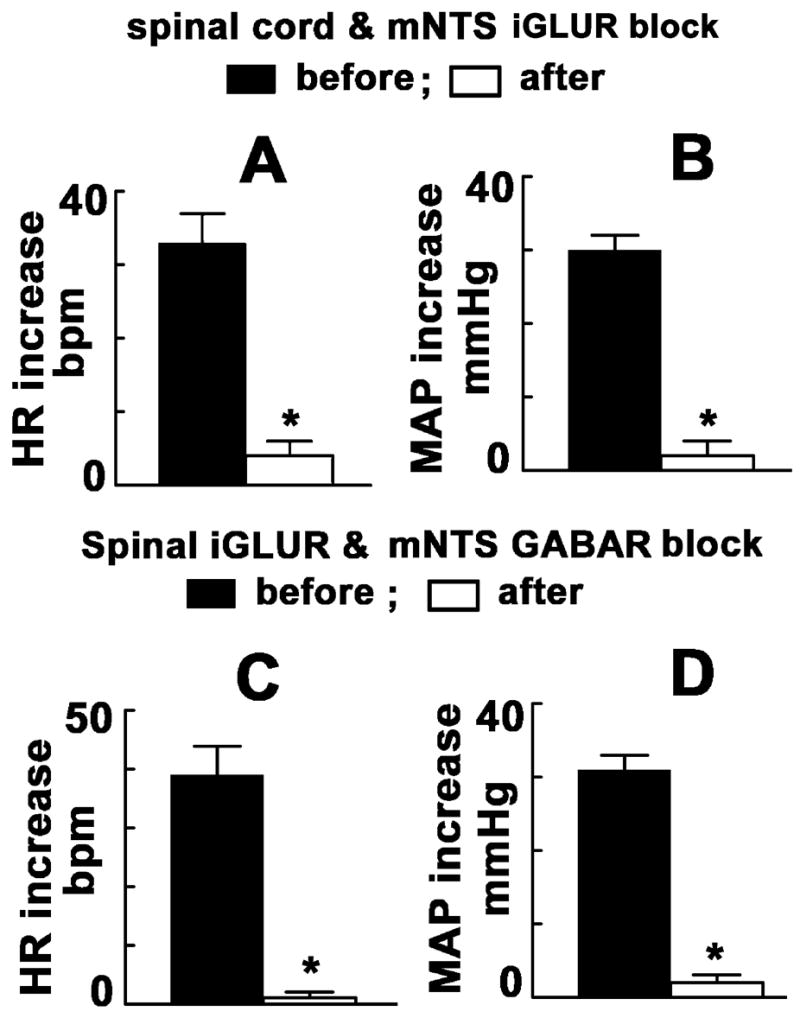
A & B: Effect of the combined blockade of spinal and mNTS iGLURs on PVN-induced cardiovascular responses in barodenervated rats (n = 5). A: NBQX and DAP-7 were administered intrathecally (T9–T10; 10 μL each) and microinjected bilaterally (50 nL) into the mNTS. PVN-induced tachycardia was almost blocked; increases in HR before and after the combined blockade were 33 ± 4 and 4 ± 2 bpm, respectively (*P < 0.01). B: PVN-induced pressor responses were almost blocked by the combined blockade; increases in MAP before and after the blockade were 30 ± 2 and 2 ± 2 mmHg, respectively (*P < 0.01). C & D: Effect of spinal iGLUR and mNTS GABAR block on PVN-induced cardiovascular responses in barodenervated rats (n = 5). C: NBQX and D-AP7 were injected intrathecally (T9–T10) and gabazine (2 mmol/L) and 2-hydroxysaclofen (100 mmol/L) were microinjected (50 nL each) into the mNTS. PVN-induced tachycardia was almost blocked; increases in HR before and after the blockade were 39 ± 5 and 1 ± 1 bpm, respectively (*P < 0.01). D: PVN-induced pressor responses were almost blocked; increases in MAP before and after the blockade were 31 ± 2 and 2 ± 1 mmHg, respectively (*P < 0.01).
2.6. Effect of combined blockade of spinal iGLURs and mNTS GABARs
Stimulation of the PVN by unilateral microinjection of NMDA elicited usual increases in HR and MAP in another group of barodenervated rats (n = 5). After 20 min, NBQX and D-AP7 were injected intrathecally at T9–T10 which was followed by bilateral microinjections of gabazine (2 mmol/L, 50 nL) and 2-hydroxysaclofen (100 mmol/L, 50 nL) into the mNTS. Combined blockade of spinal iGLURs and mNTS GABARs almost completely blocked the tachycardic and pressor responses to PVN stimulation (Figs. 3 C and D). Intrathecal injections of aCSF combined with bilateral microinjections of aCSF (100 nL) into the mNTS did not alter PVN-induced HR and MAP responses.
2.7. Effect of spinal iGLUR blockade combined with vagotomy
The effect of the blockade of spinal iGLURs combined with bilateral vagotomy on the PVN-induced cardiovascular responses was studied as follows:
The effect of the blockade of iGLURs in the entire spinal cord combined with bilateral vagotomy on the PVN-induced cardiovascular responses was studied in one group of barodenervated rats (n = 5). PVN stimulation by unilateral microinjection of NMDA elicited usual increases in HR and MAP. After 20 min, NBQX and D-AP7 were injected intrathecally at T9–T10 level. Five-ten min later, bilateral vagotomy was performed; there was an increase in baseline HR (42 ± 12 bpm). Five min later, the tachycardic and pressor responses to the PVN stimulation were completely blocked (Figs. 4 A and B). After the blockade of spinal iGLURs by intrathecal injections at T9–T10 combined with bilateral vagotomy, there was no recovery of PVN-induced cardiovascular responses even after 90 min.
Fig. 4.
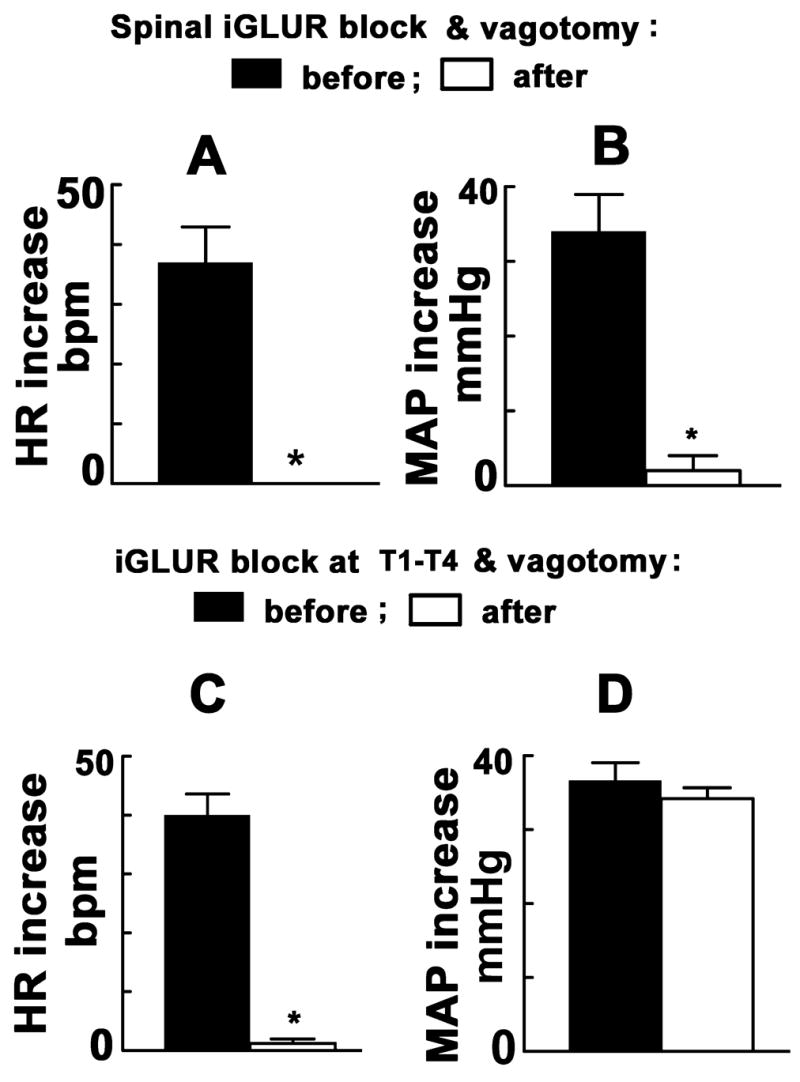
A & B: Effect of the spinal iGLUR block combined with bilateral vagotomy on the PVN-induced cardiovascular responses in barodenervated rats (n = 5). A: NBQX and DAP-7 were injected intrathecally (T9–T10) and bilateral vagotomy was performed. PVN-induced tachycardia was completely blocked; the increase in HR before these procedures was 37 ± 6 bpm. B: PVN-induced pressor responses were also almost blocked; increases in MAP before and after the blockade of spinal iGLURs combined with vagotomy were 34 ± 5 and 2 ± 2 mmHg, respectively (*P < 0.001). C & D: Effect of the blockade of iGLURs in the upper thoracic cord combined with bilateral vagotomy on the PVN-induced cardiovascular responses in barodenervated rats (n = 6). C: PVN-induced tachycardia was almost abolished by the blockade of iGLURs at T1–T4 combined with bilateral vagotomy; the increase in HR before and after these procedures were 40 ± 4 and 2 ± 1 bpm, respectively (*P < 0.001). D: PVN-induced pressor responses were not significantly altered; the increases in MAP before and after these procedures were 37 ± 3 and 34 ± 2 mmHg, respectively (P > 0.05).
The effects of the blockade of iGLURs in the upper thoracic cord combined with bilateral vagotomy on PVN-induced responses were studied in another group of barodenervated rats (n = 6). Stimulation of the PVN by unilateral microinjection of NMDA elicited usual increases in HR and MAP. After 20 min, bilateral vagotomy was performed; an increase in baseline HR (40 ± 10 bpm) was observed. Within 5–10 min, a tissue paper pledget soaked in NBQX and D-AP7 was applied at T1–T4 level. Five min later, the tachycardic responses to the PVN stimulation were almost completely blocked (Fig. 4C) while pressor responses were not altered significantly (Fig. 4D). The paper pledget soaked in the iGLUR blockers was removed after 10–15 min, the spinal surface was irrigated with aCSF and a fresh paper pledget soaked in aCSF was applied at T1–T4. Partial recovery of tachycardic responses to PVN-stimulation was observed after 60 min.
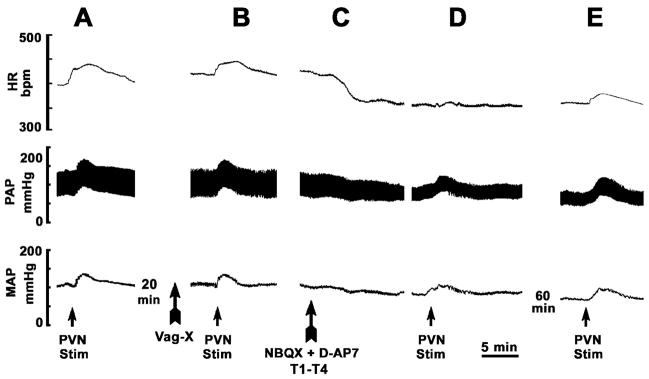
A typical tracing depicting the effects of the combination of bilateral vagotomy and blockade of iGLURs in the upper thoracic cord on PVN-induced cardiovascular responses in a barodenervated rat is shown in Fig. 5.
Fig. 5.
A tracing showing effect of the blockade of spinal iGLURs at T1–T4 and bilateral vagotomy on PVN-induced cardiovascular responses in a barodenervated rat. In each panel, top trace: heart rate (HR), middle trace: pulsatile arterial pressure (PAP) and bottom trace: mean arterial pressure (MAP). A: a unilateral microinjection of NMDA (10 mmol/L, 50 nL) into the PVN elicited increases in HR, PAP, and MAP. B: Twenty min later, bilateral vagotomy (Vag-X) was performed; the baseline HR increased. Vagotomy attenuated an increase in HR elicited by the PVN stimulation. C: Five min later, a paper pledget soaked in NBQX (2 mmol/L, 10 μL) and D-AP7 (5 mmol/L, 10 μL) was applied at T1–T4; baseline HR, PAP and MAP decreased and reached a nadir within 5–10 min. D: At this time, NMDA was again microinjected into the PVN; tachycardia was no longer observed while PAP and MAP increases persisted. E: PVN-induced tachycardia showed partial recovery within 60 min.
2.8. Effect of spinal oxytocin receptor blockade
The PVN-induced cardiovascular responses were not significantly altered by application of a tissue paper pledget soaked in a peptide oxytocin antagonist (0.05 mmol/L, 20 μL) at T1–T4 level (n = 5). The oxytocin peptide antagonist used (Section 4.8) has been shown to attenuate oxytocin-induced increase in RSNA (Yang et al., 2002). PVN-induced increases in HR before and after the application of this concentration of the oxytocin receptor (OTR) antagonist were 45 ± 3.5 and 44 ± 4 bpm, respectively (P > 0.05). PVN-induced increases in MAP before and after the application of this concentration of the OTR antagonist were 38 ± 2 and 36 ± 4 mmHg, respectively (P > 0.05). In another group of barodenervated rats (n = 6), a tissue paper pledget soaked in a higher concentration (0.1 mmol/L, 20 μL) of the OTR antagonist was applied at T1–T4 level; cardiovascular responses to subsequent PVN stimulation were not significantly altered (Fig. 6 A and B). Application of the OTR antagonist alone did not elicit any significant changes in baseline HR or MAP.
Fig. 6.
A & B: Effect of blockade of spinal oxytocin receptors (OTRs) on PVN-induced cardiovascular responses in barodenervated rats (n = 6). Application of a peptide oxytocin receptor antagonist (OTRA; 0.1 mM, 20 μL) at T1–T4 did not significantly alter the PVN-induced cardiovascular responses. A: HR increases before and after the spinal OTR blockade were 43 ± 3 and 44 ± 4 bpm, respectively (P > 0.05). B: Pressor responses before and after the spinal OTR blockade were 35 ± 5 and 37.5 ± 4 mmHg, respectively (P > 0.05). C & D: Effect of spinal vasopressin receptor (VPR) blockade on PVN-induced cardiovascular responses in barodenervated rats (n = 6). Application of a vasopressin receptor antagonist (VPRA; 0.1 mM, 20 μL) at T1–T4 did not significantly alter the PVN-induced cardiovascular responses. C: HR increases before and after the VPR blockade were 45 ± 2 and 46 ± 2 bpm, respectively (P > 0.05). D: Pressor responses before and after the VPR blockade were 42 ± 3 and 41 ± 3.5 mmHg, respectively (P > 0.05). See section 4.8 for OTR and VPR antagonists used.
2.9. Effect of spinal vasopressin receptor blockade
The PVN-induced cardiovascular responses were not significantly altered by application of a tissue paper pledget soaked in a vasopressin receptor (VPR) antagonist (0.05 mmol/L, 20 μL) at T1–T4 level (n = 5). This concentration of the VPR antagonist used (Section 4.8) has been shown to attenuate AVP-induced increase in RSNA (Yang et al., 2002). PVN-induced increases in HR before and after the application of this concentration of the VPR antagonist were 42 ± 4 and 40 ± 5 bpm, respectively (P > 0.05). PVN-induced increases in MAP before and after the application of this concentration of the VPR antagonist were 36 ± 2 and 34 ± 3 mmHg, respectively (P > 0.05). In another group of barodenervated rats (n = 6), a tissue paper pledget soaked in a higher concentration (0.1 mmol/L, 20 μL) of the VPR antagonist was applied at T1–T4 level; cardiovascular responses to subsequent PVN stimulation were not significantly altered (Fig. 6 C and D). Application of the VPR antagonist alone did not elicit any significant changes in baseline HR or MAP.
2.10. Histological identification of microinjection sites
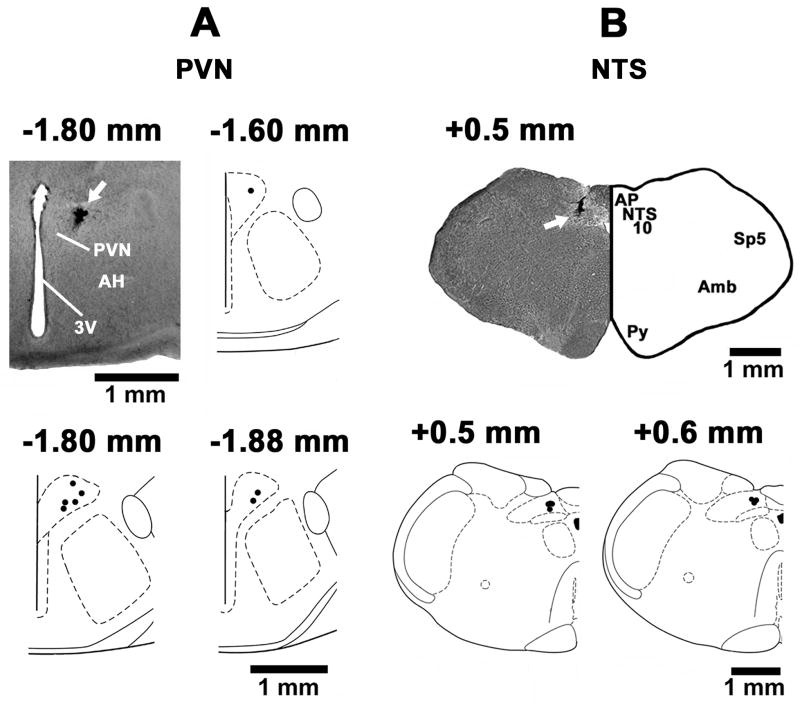
The PVN sites, where microinjections of NMDA elicited excitatory effects, were marked in 8 rats. A typical PVN site, marked with India ink (50 nL) and composite diagrams of the PVN microinjection sites at levels 1.6, 1.8 and 1.88 mm caudal to the bregma are shown in Fig. 7A. The mNTS sites, where iGLUR or GABAR antagonists were microinjected, were marked in 7 rats. A typical microinjection site in the mNTS, marked with India Ink (100 nL) and composite diagrams at levels 0.5 and 0.6 mm rostral to the calamus scriptorius are presented in Fig. 7B.
Fig. 7.
Histological identification of the microinjection sites. A: A coronal section at a level 1.8 mm caudal to the bregma showing a typical PVN microinjection site (arrow); the center of the spot was 0.5 mm lateral to the midline and 7.5 mm deep from dorsal surface of the cortex. Composite diagrams representing the PVN sites (n = 8) where NMDA was microinjected at levels 1.60, 1.8 and 1.88 mm caudal to the bregma. B: A coronal section at a level 0.5 mm rostral to the calamus scriptorius showing a typical mNTS microinjection site (arrow); the center of the spot was 0.58 mm lateral to the midline and 0.60 mm deep from the dorsal medullary surface. Composite diagrams representing the mNTS sites (n = 7) at levels 0.5 and 0.6 mm rostral to the calamus scriptorius. In the composite diagrams, each spot represents the microinjection site in one rat. Abbreviations: AH: anterior hypothalamic nucleus; Amb: nucleus ambiguus, AP: area postrema, NTS: nucleus tractus solitarius; PVN: hypothalamic paraventricular nucleus; Py: pyramids; Sp5: spinal trigeminal tract; 3V: 3rd ventricle; 10: dorsal motor nucleus of vagus.
3. Discussion
The following new observations were made in this study: 1) in barodenervated rats, chemical stimulation of the PVN, by microinjections of NMDA, elicited tachycardia which was mediated via both inhibition of vagal and activation of sympathetic outflow to the heart, 2) the vagal inhibition contributing to the tachycardia was mediated by both iGLURs and GABARs in the mNTS, 3) the sympathetic activation contributing to the tachycardia was mediated via spinal iGLURs, and 4) spinal vasopressin and oxytocin receptors did not play a role in mediating PVN-induced tachycardia.
In order to exclude the reflex effects on HR, barodenervated rats were used in all experiments unless indicated otherwise. Our results indicating that microinjections of NMDA into the PVN elicited pressor and tachycardic responses are in agreement with earlier reports (Li et al., 2001, 2006). Tachycardic responses to the microinjection of NMDA into the PVN were attenuated by bilateral vagotomy in barodenervated rats indicating that the inhibition of vagal outflow to the heart was partly responsible for this response. The tachycardic responses to the chemical stimulation of the PVN were also attenuated by the intrathecal injections of iGLUR antagonists indicating that activation of spinal iGLURs was partially responsible for this response. This result is consistent with the reports that the PVN projects either directly to the IML (Holstege, 1987) or via the RVLM (Pyner and Coote, 1999; 2000) and glutamate is one of the transmitters involved in mediating cardiovascular responses at the spinal level (Sundaram and Sapru, 1991; Yang et al., 2002).
In barodenervated rats, the blockade of spinal iGLURs induced by intrathecal injections at T9–T10 significantly attenuated, but did not abolish, the PVN-induced tachycardic responses. The specificity of iGLUR antagonists has been established previously (Dhruva et al., 1998). It may be argued that incomplete blockade of tachycardic responses may be due to inadequate blockade of iGLURs in the upper thoracic cord when the intrathecal injections are delivered at T9–T10. This scenario is unlikely because direct application of iGLUR antagonists at T1–T4, where sympathetic innervation to the heart originates (Sundaram et al., 1989), also resulted in partial blockade of PVN-induced tachycardia. The blockade of spinal iGLURs induced by intrathecal injections at T9–T10 completely blocked the PVN-induced pressor responses. However, the blockade of iGLURs in the upper thoracic cord did not alter these pressor responses significantly; this result is expected because sympathetic innervation to the systemic arterioles originates predominantly in the thoraco-lumbar cord at T5-L2 level.
The above-mentioned results indicated that in barodenervated rats both inhibition of vagal outflow and excitation of sympathetic outflow to the heart play a role in mediating the tachycardic responses to the chemical stimulation of the PVN. Indeed, combination of bilateral vagotomy with the blockade of iGLURs in the spinal cord completely blocked the tachycardia elicited by the chemical stimulation of the PVN in these rats. The PVN-induced tachycardia was almost completely blocked irrespective of whether the iGLUR antagonists were applied directly at T1–T4 or administered intrathecally at T9–T10.
In experiments in which spinal IGLURs were blocked by intrathecal injections of iGLUR antagonists at T9–T10 combined with bilateral vagotomy, no recovery of PVN-induced cardiovascular responses was observed even after 90 min. Since the volume of intrathecal injections containing the iGLUR antagonists was large (20 μL) relative to the volumes generally microinjected directly into the brain tissue, the antagonists may have lingered in the spinal CSF for longer time and exerted persistent blocking effects. Consistent with this possibility are the reports in which high levels of radioactivity were detected in the whole spinal cord even after 2 hours of intrathecal injections of radioactive opiates (Gustafsson et al., 1985) and analgesia lasted up to 5 hours after intrathecal injections of morphine (Nishio et al. 1989). On the other hand, when iGLURs were blocked at T1–T4 and bilateral vagotomy was performed, partial recovery of tachycardic responses to PVN-stimulation was observed after 60 min. Partial recovery in this situation may be attributed the removal of the antagonist-soaked paper pledget within 10–15 min, irrigation of the spinal surface by aCSF and application of a fresh paper pledget soaked in aCSF. Complete recovery of PVN-induced tachycardia was not expected in these experiments because the rats are vagotomized and bilateral vagotomy attenuated the PVN-induced increases in HR.
An excitatory amino acid (probably glutamate) is likely to be the main neurotransmitter at the spinal level mediating PVN-induced cardiovascular responses. Indirect projections from the PVN to the RVLM and RVLM to the IML may be involved in these responses. Indeed, electrophysiological studies have shown that the spinally projecting RVLM neurons were excited by the release of glutamate in the RVLM after the chemical stimulation of the PVN (Yang and Coote, 1998; Yang et al., 2001). Cardiovascular responses to the chemical stimulation of the PVN could also be mediated via a direct projection from the PVN to the IML (Holstege, 1987).
The vagal mechanisms mediating PVN-induced tachycardic responses involve the mNTS as indicated by the following results. The blockade of GABA receptors in the mNTS attenuated the tachycardic response to the PVN stimulation. The concentrations of the GABA receptor antagonists used were selected on the basis of our earlier report in which gabazine and 2-hydroxysaclofen completely blocked the maximum responses to the microinjections of muscimol and baclofen, respectively, in the mNTS (Kasamatsu et al., 2004). In the experiment involving GABA receptor blockade in the mNTS without intrathecal administration of NBQX and D-AP7 (Figs. 1 E and F), smaller concentrations of gabazine (0.2 mmol/L) and 2-hydroxysaclofen (10 mmol/L) were used because microinjections of higher concentrations of these antagonists elicited cardiac arrhythmias. In spite of the smaller concentrations of GABA receptor antagonists used, the tachycardic responses to the PVN stimulation were significantly attenuated.
It should be noted that due to close proximity of the dorsal motor nucleus of vagus (DMNV) to the mNTS, it is possible that drugs microinjected into the mNTS may have diffused to the DMNV. However, this nucleus has a minor role in the parasympathetic regulation of the heart rate and essentially no role in blood pressure regulation.
Combination of the blockade of mNTS GABARs and spinal iGLURs abolished the PVN-induced tachycardic responses. Combined blockade of iGLURs in both the mNTS and spinal cord also abolished PVN-induced tachycardia. These results suggested that both GABA and glutamate in the mNTS were involved in mediating the tachycardic responses to the PVN stimulation. It is well known that GABA containing neurons and axon terminals are plentiful within the NTS (Sved, 1994) and some of them make local connections (Izzo et al., 1992; Jia et al., 1996). It is possible that the chemical stimulation of the projection from the PVN to the mNTS (Kawabe et al., 2008) resulted in the release of glutamate in the mNTS and iGLURs on GABAergic neurons were stimulated with the resultant release of GABA in the mNTS. Released GABA may inhibit glutamatergic mNTS neurons projecting to the nucleus ambiguus and decrease vagal outflow to the heart causing tachycardia. Kunos and Varga (1995) have proposed a similar mechanism for tachycardia elicited by chemical stimulation of the dorsomedial nucleus of the hypothalamus. In other reports, activation of nociceptive afferents has been reported to excite GABAergic interneurons in the mNTS (Boscan et al., 2002; Pickering et al., 2003). Cardiac arrhythmias did not appear after the intrathecal blockade of iGLURs even when high concentrations of gabazine (2 mmol/L) and 2-hydroxysaclofen (100 mmol/L) were microinjected into the mNTS (Figs. 3 C and D). This observation suggested that GABA receptors in the mNTS may be important for the suppression of cardiac rhythm abnormalities induced by excitation of the sympathetic nervous system.
There was a difference in the effects of vagotomy on PVN-induced tachycardic responses in barodenervated and non-barodenervated rats. Tachycardic responses to the microinjection of NMDA into the PVN were attenuated by bilateral vagotomy in barodenervated rats but not in non-barodenervated rats. This difference in the effects of vagotomy can be explained as follows (see Fig. 8). In non-barodenervated rats, activity of vagal innervation to the heart is relatively increased due to tonic baroreceptor input to the glutamatergic mNTS neurons projecting to the nucleus ambiguus. Increase in the activity of the vagal innervation to the heart in these rats may also be contributed by baroreceptor activation caused by PVN-induced pressor response. Simultaneously, stimulation of the glutamatergic projection from the PVN to the mNTS may decrease vagal activity via activation of mNTS GABAergic neurons and consequent inhibition of glutamatergic mNTS neurons projecting to the nucleus ambiguus. Because of these two opposing effects on activity of vagal innervation to the heart in the non-barodenervated rats, the role of vagus nerves in the PVN control of HR may be minimized. Consistent with this possibility was the observation that bilateral vagotomy had no effect on PVN-induced tachycardia in non-barodenervated rats. On the other hand, in barodenervated rats, stimulation of the PVN induces a decrease in the vagal outflow to the heart via activation of GABAergic mNTS neurons projecting to the nucleus ambiguus. The decrease in vagal outflow remains unopposed because there is no baroreceptor input to the glutamatergic mNTS neurons projecting to the nucleus ambiguus. Therefore, bilateral vagotomy in this situation attenuated the tachycardic responses elicited by the PVN stimulation.
Fig. 8.
A schematic representation of pathways mediating PVN-induced HR responses. Abbreviations: CVLM = caudal ventrolateral medullary depressor area; IML = intermediolateral cell column of the spinal cord; nAmb = nucleus ambiguus; NTS = nucleus tractus solitarius; PVN = paraventricular hypothalamic nucleus; RVLM = rostral ventrolateral medullary pressor area; SG= sympathetic ganglion.
Vasopressin has been implicated as a neurotransmitter in the projection from the PVN to the IML. For example, intrathecal injections of vasopressin have been reported to increase RSNA and PVN-induced increases in MAP and RSNA were blocked by the intrathecal injections of a V1 vasopressin receptor antagonist (Malpas and Coote, 1994; Yang et al., 2002). Oxytocin has also been reported to increase RSNA when administered intrathecally (Yang et. al., 2002). However, vasopressin and oxytocin are not involved in the mediation of PVN-induced tachycardic and pressor responses at the spinal level based on our observations that application of VPR and OTR antagonists at T1–T4 failed to block PVN-induced tachycardia. The concentrations of VPR and OTR antagonists used in these experiments have been shown to block the effects of their respective agonists at the spinal level (Riphagen and Pittman, 1989; Yang et al., 2002). It may be argued that the failure of the VPR and OTR antagonists to attenuate PVN-induced tachycardia may be due the fact that the antagonists may not have reached the IML when they were directly applied to the spinal cord at T1–T4. This possibility is unlikely because iGLURs when applied in a similar manner attenuated the PVN-induced tachycardic responses. In this context, it should be noted that in our experiments, the concentrations of iGLUR antagonists administered intrathecally did not alter responses to intrathecally administered vasopressin further strengthening our conclusion that spinal vasopressin receptors do not participate in the PVN-induced tachycardic responses.
In summary, in barodenervated rats, the PVN-induced tachycardia was mediated by both activation of sympathetic and inhibition of vagal outflows to the heart (Fig. 8). The sympathetic activation was mediated by the stimulation of spinal iGLURs. This effect may be mediated via direct projections of the PVN to the IML or indirect projections from the PVN to the IML via the RVLM. Contribution of the release of catecholamines released from the adrenals in response to PVN-induced sympathetic activation in mediating cardiovascular responses cannot be excluded based on our results. The vagal inhibition was mediated via the activation of iGLURs and GABARs in the mNTS. Stimulation of a glutamatergic projection from the PVN to the mNTS (Kawabe et al., 2008; stocker et al., 2006) may have activated iGLURs in the mNTS which, in turn, may have activated mNTS GABA neurons. GABA release in the mNTS may have resulted in the inhibition of glutamatergic mNTS neurons projecting to the nucleus ambiguus which normally provide vagal innervation to the heart (Mendelowitz, 1999). Inhibition of the glutamatergic mNTS neurons projecting to the nucleus ambiguus may have resulted in a decrease in the activity of the nucleus ambiguus neurons with consequent decrease in the vagal outflow to the heart causing tachycardia.
4. Experimental Procedures
4.1. General procedures
Experiments were done in adult male Wistar rats (Charles River Laboratories, Wilmington, MA) weighing 300–350 g. All animals were housed under controlled conditions with a 12:12-h light-dark cycle. Food and water were available to the animals ad libitum. The experiments were performed according to the NIH guide for “The Care and Use of Laboratory Animals, 7th Edition, 1996” and with the approval of the Institutional Animal Care and Use Committee of this university.
The general procedures have been described in detail elsewhere (Kawabe et al., 2006). Briefly, the rats were anesthetized with inhalation of isoflurane (2–3% in 100% oxygen). The trachea was cannulated with polyethylene tubing (PE 240) and the rats were artificially ventilated using a rodent ventilator (model 683, Harvard Instruments, Holliston, MA). The tidal volume and frequency were adjusted on the ventilator to maintain the end tidal CO2 at 3.5–4.5%. One of the veins was cannulated and urethane (1.2–1.4 g/kg) was injected intravenously in 8–9 aliquots at 2-min intervals (total volume of the anesthetic solution was 0.4–0.5 mL injected over a period of about 15–18 min). Isoflurane inhalation was terminated as soon as urethane administration was completed. Absence of a BP response and/or withdrawal of the limb in response to pinching of a hind paw indicated that the rats were properly anesthetized. One of the arteries was cannulated for monitoring BP. HR was monitored by a tachograph that was triggered by the BP waves. MAP was derived electronically. Rectal temperature was maintained at 37 ± 0.5°C. All the tracings were recorded on a polygraph (model 7D, Grass Instruments, West Warwick, RI, USA).
4.2. Barodenervation
The carotid sinus nerves were sectioned at their junction with the glossopharyngeal nerves. Occasionally a tiny nerve from the carotid sinus region joined the pharyngeal branch of the vagus nerve; this nerve was also sectioned bilaterally. In the rat, the aortic nerves usually join the superior laryngeal nerves; therefore, the superior laryngeal nerves were sectioned bilaterally as they joined the vagus nerves just caudal to the nodose ganglia. Occasionally the aortic nerves joined the vagus nerves directly just below the nodose ganglia. In such cases, the aortic nerves were identified under an operating microscope and sectioned bilaterally. Subsequent bolus injections of phenylephrine (4–10 μg/kg, i.v.) failed to elicit reflex bradycardia and inhibition of sympathetic nerve activity indicating that the barodenervation was complete (Kawabe et al., 2008).
4.3. Vagotomy
To determine the role of parasympathetic innervation to the heart in mediating the HR responses elicited by microinjections of NMDA in the PVN, silk sutures were placed loosely around the vagus nerves bilaterally for subsequent identification and sectioning.
4.4. Microinjection technique
The details of this technique are described elsewhere (Kawabe et al., 2006). Briefly, the rats were placed in a prone position in a stereotaxic instrument with bite bar 11 mm below the interaural line. The bregma was visually identified and a small hole was drilled in the parietal bone. Multi-barreled glass-micropipettes (tip size 20–40 μm) were used for microinjections. The coordinates for microinjections into the PVN were: 1.5–2.0 mm caudal to the bregma, 0.4–0.5 mm lateral to the midline, and 7.7–8.0 mm deep from the dura. For microinjections into mNTS in the same rat, the coordinates were: 0.5–0.6 mm rostral to the calamus scriptorius, 0.5–0.6 mm lateral to the midline and 0.5–0.6 mm ventral to the dorsal medullary surface. The PVN was identified and stimulated by microinjections of NMDA (10 mmol/L, 50 nL) and the mNTS was identified by microinjecting L-glutamate (L-Glu; 5 mmol/L, 100 nL). The duration of microinjection was 10 sec. Controls for microinjections consisted of aCSF (pH 7.4).
4.5. Intrathecal injection
After incising the atlanto-occipital membrane, the tip of a polyethylene tubing (PE-10), connected to a Hamilton microsyringe and filled with drugs or aCSF, was inserted under the dura mater and advanced 6 cm caudally to T9–T10 level. The location of the cannula tip at T9–T10 was confirmed by post-mortem examination under a microscope. The volume of intrathecal injection was 20 μL and the duration of injection was 10 sec.
4.6. Application of drugs at T1–T4
The head of the rats was fixed prone in a stereotaxic instrument to which a rat-spinal unit was attached (David Kopf Instruments, model 980). One of the lower thoracic vertebrae (e.g., T8) and the pelvic bones were fixed rigidly in the spinal unit. The dorsal surface of the spinal cord, from C8 to T5 level, was exposed by laminectomy and the dura was sectioned. A pledget of tissue paper (4 mm long and 2.5 mm wide) was soaked in warm (37°C) aCSF and applied to the exposed spinal cord surface to prevent it from drying. Application of a drug to the upper thoracic cord was accomplished by soaking a different paper pledget in the drug solution (20 μL) and applying it to the spinal surface at T1–T4. After observing responses to application of a drug in this manner, the paper pledget was removed, the spinal cord surface irrigated with aCSF and a fresh aCSF-soaked paper pledget was applied.
4.7. Histological identification of microinjection sites
Typical sites of microinjections in the PVN and mNTS were marked by microinjections of diluted India ink (PVN: 50 nL; mNTS: 100 nL). The animals were perfused and fixed with 4% paraformaldehyde and serial sections of the medulla were cut (40 μm) in a vibratome, mounted on slides, dehydrated, cleared and stained with cresyl violet. The microinjection sites were identified under a microscope. The sections were photographed and compared with a standard atlas (Paxinos and Watson, 1986).
4.8. Drugs and chemicals
The following drugs and chemicals were used: (Arg8)-vasopressin (AVP), D(−)-2-amino-7-phosphono-heptanoic acid (D-AP7; NMDA receptor antagonist), gabazine bromide (GABAA receptor antagonist), 2-Hydroxysaclofen (GABAB receptor antagonist), [β-mercapto-β, β-cyclopentamethylenepropionyl1, O-Et-Ty2, Val4, Arg8]-vasopressin (V1a vasopressin receptor antagonist), L-glutamate monosodium, 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide disodium (NBQX disodium salt; non-NMDA receptor antagonist), N-methyl-D-aspartic acid (NMDA), L-phenylephrine hydrochloride, [d(CH2)51, Tyr(Me)2, Orn8]-oxytocin (peptide oxytocin antagonist), isoflurane, and urethane. All of the solutions for the microinjections were freshly prepared in aCSF (pH 7.4). Where applicable, the concentration of drugs refers to their salts. AVP, vasopressin receptor antagonist and peptide oxytocin antagonist were purchased from Bachem California Inc. (Torrance, CA, USA). Isoflurane was purchased from Baxter Pharmaceutical Products (Deerfield, IL, USA). All other drugs and chemicals were obtained from Sigma Chemicals (St. Louis, MO, USA).
4.9. Statistical Analyses
The means and standard error of the means (SEM) were calculated in different groups of rats for maximum changes in MAP and HR. Student’s paired t-test was used for comparisons of the maximum changes in these variables when the animal served as its own control. Student’s unpaired t–test was used for comparisons of maximum changes in MAP and HR between different groups of rats. In all cases, the differences were considered significant at P < 0.05.
Acknowledgments
This work was supported in part by N.I.H. grants HL24347 and HL076248 awarded to Dr. H. N. Sapru.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Badoer E. Hypothalamic paraventricular nucleus and cardiovascular regulation. Clin Exp Pharmacol Physiol. 2001;28:95–99. doi: 10.1046/j.1440-1681.2001.03413.x. [DOI] [PubMed] [Google Scholar]
- Boscan P, Kasparov S, Paton JFR. Somatic nociception activates NK1 receptors in the nucleus tractus solitarii to attenuate the baroreceptor cardiac reflex. Eur J Neurosci. 2002;16:907–920. doi: 10.1046/j.1460-9568.2002.02131.x. [DOI] [PubMed] [Google Scholar]
- Chen QH, Haywood JR, Toney GM. Sympathoexcitation by PVN-injected bicuculline requires activation of excitatory amino acid receptors. Hypertension. 2003;42:725–731. doi: 10.1161/01.HYP.0000085197.20043.44. [DOI] [PubMed] [Google Scholar]
- Coote JH. The hypothalamus and cardiovascular regulation. In: Dun NJ, Machado BH, Pilowsky PM, editors. Neural mechanisms of cardiovascular regulation. Kluwer Academic Publishers; Boston, MA, USA: 2004. pp. 117–146. [Google Scholar]
- Coote JH, Yang Z, Pyner S, Deering J. Control of sympathetic outflows by the hypothalamic paraventricular nucleus. Clin Exp Pharmacol Physiol. 1998;25:461–463. doi: 10.1111/j.1440-1681.1998.tb02235.x. [DOI] [PubMed] [Google Scholar]
- Darlington DN, Miyamoto M, Keil LC, Dallman MF. Paraventricular stimulation with glutamate elicits bradycardia and pituitary responses. Am J Physiol Regul Integr Comp Physiol. 1989;256:R112–R119. doi: 10.1152/ajpregu.1989.256.1.R112. [DOI] [PubMed] [Google Scholar]
- Dhruva A, Bhatnagar T, Sapru HN. Cardiovascular responses to microinjections of glutamate into the nTS of unanesthetized supracollicular decerebrate rats. Brain Res. 1998;801:88–100. doi: 10.1016/s0006-8993(98)00550-2. [DOI] [PubMed] [Google Scholar]
- Gordon FJ, Sved AF. Neurotransmitters in central cardiovascular regulation: Glutamate and GABA. Clin Exp Pharmacol Physiol. 2002;29:522–524. doi: 10.1046/j.1440-1681.2002.03666.x. [DOI] [PubMed] [Google Scholar]
- Gustafsson LL, Post C, Edvardsen B, Ramsay CH. Distribution of morphine and meperidine after intrathecal administration in rat and mouse. Anesthesiology. 1985;63:483–489. doi: 10.1097/00000542-198511000-00003. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- Hardy SGP. Hypothalamic projections to cardiovascular centers of the medulla. Brain Res. 2001;894:233–240. doi: 10.1016/s0006-8993(01)02053-4. [DOI] [PubMed] [Google Scholar]
- Holstege G. Some anatomical observations on the projections from the hypothalamus to brainstem and spinal cord: an HRP and autoradiographic tracing study in the cat. J Comp Neurol. 1987;260:98–126. doi: 10.1002/cne.902600109. [DOI] [PubMed] [Google Scholar]
- Izzo PN, Sykes RM, Spyer KM. Gamma-aminobutyric acid immunoreactive structures in the nucleus tractus solitarius: a light and electron microscopic study. Brain Res. 1992;591:69–78. doi: 10.1016/0006-8993(92)90979-j. [DOI] [PubMed] [Google Scholar]
- Jia HG, Wang BR, Rao ZR, Shi JW, Shigemoto R, Kaneko T, Mizuno N. GABAergic synapses upon neurons expressing substance P receptors in the nucleus of the solitary tract: an immunocytochemical electron microscope study in the rat. Neurosci Lett. 1996;210:49–52. doi: 10.1016/0304-3940(96)12654-9. [DOI] [PubMed] [Google Scholar]
- Kannan H, Niijima A, Yamashita H. Effects of stimulation of the hypothalamic paraventricular nucleus on blood pressure and renal sympathetic nerve activity. Brain Res Bull. 1988;20:779–783. doi: 10.1016/0361-9230(88)90091-3. [DOI] [PubMed] [Google Scholar]
- Kannan H, Hayashida Y, Yamashita H. Increase in sympathetic outflow by paraventricular nucleus stimulation in awake rats. Am J Physiol Regul Interg Comp Physiol. 1989;256:R1325–R1330. doi: 10.1152/ajpregu.1989.256.6.R1325. [DOI] [PubMed] [Google Scholar]
- Kasamatsu K, Chitravanshi VC, Sapru HN. Depressor and bradycardic responses to microinjections of endomorphin-2 into the NTS are mediated via ionotropic glutamate receptors. Am J Physiol Regul Integr Comp Physiol. 2004;287:R715–R728. doi: 10.1152/ajpregu.00642.2003. [DOI] [PubMed] [Google Scholar]
- Kawabe T, Chitravanshi VC, Kawabe K, Sapru HN. Cardiovascular effects of adrenocorticotropin microinjections into the rostral ventrolateral medullary pressor area of the rat. Brain Res. 2006;1102:117–126. doi: 10.1016/j.brainres.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Kawabe T, Chitravanshi VC, Kawabe K, Sapru HN. Cardiovascular function of a glutamatergic projection from the hypothalamic paraventricular nucleus to the nucleus tractus solitarius in the rat. Neuroscience. 2008;153:605–617. doi: 10.1016/j.neuroscience.2008.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunos G, Varga K. The tachycardia associated with the defense reaction involves activation of both GABAA and GABAB receptors in the nucleus tractus solitarii. Clin Exp Hypertens. 1995;17:91–100. doi: 10.3109/10641969509087057. [DOI] [PubMed] [Google Scholar]
- Li YF, Mayhan GW, Patel KP. NMDA-mediated increase in renal sympathetic nerve discharge within the PVN: role of nitric oxide. Am J Physiol Heart Circ Physiol. 2001;281:H2328–H2336. doi: 10.1152/ajpheart.2001.281.6.H2328. [DOI] [PubMed] [Google Scholar]
- Li YF, Jackson KL, Stern JE, Rabeler B, Patel KP. Interaction between glutamate and GABA systems in the integration of sympathetic outflow by the paraventricular nucleus of hypothalamus. Am J Physiol Heart Circ Physiol. 2006;291:H2847–H2856. doi: 10.1152/ajpheart.00625.2005. [DOI] [PubMed] [Google Scholar]
- Luiten PG, Ter Horst GJ, Karst H, Steffens AB. The course of paraventricular hypothalamic efferents to autonomic structures in medulla and spinal cord. Brain Res. 1985;329:374–378. doi: 10.1016/0006-8993(85)90554-2. [DOI] [PubMed] [Google Scholar]
- Madden CJ, Sved AF. Rostral ventrolateral medulla C1 neurons and cardiovascular regulation. Cell Mol Neurobiol. 2003;23:739–749. doi: 10.1023/A:1025000919468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malpas SC, Coote JH. Role of vasopressin in sympathetic response to paraventricular nucleus stimulation in anesthetized rats. Am J Physiol Regul Interg Comp Physiol. 1994;266:R228–R236. doi: 10.1152/ajpregu.1994.266.1.R228. [DOI] [PubMed] [Google Scholar]
- Mendelowitz D. Advances in parasympathetic control of heart rate and cardiac function. News in Physiol Sci. 1999;14:155–161. doi: 10.1152/physiologyonline.1999.14.4.155. [DOI] [PubMed] [Google Scholar]
- Nishio Y, Sinatra RS, Kitahata LM, Collins JG. Spinal cord distribution of 3H-morphine after intrathecal administration: relationship to analgesia. Anesth Analg. 1989;69:323–327. [PubMed] [Google Scholar]
- Osborn JW, England SK. Normalization of arterial pressure after barodenervation: role of pressure natriuresis. Am J Physiol Regul Integr Comp Physiol. 1990;259:R1172–R1180. doi: 10.1152/ajpregu.1990.259.6.R1172. [DOI] [PubMed] [Google Scholar]
- Palkovits M. Interconnections between the neuroendocrine hypothalamus and the central autonomic system. Front Neuroendocrinol; Geoffrey Harris Memorial Lecture; Kitakyushu, Japan. October 1998; 1999. pp. 270–295. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- Pickering AE, Boscan P, Paton JFR. Nociception attenuates parasympathetic but not sympathetic baroreflex via NK1 receptors in the rat nucleus tractus solitarii. J Physiol. 2003;551:589–599. doi: 10.1113/jphysiol.2003.046615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyner S, Coote JH. Identification of an efferent projection from the paraventricular nucleus of the hypothalamus terminating close to spinally projecting rostral ventrolateral medullary neurons. Neuroscience. 1999;88:949–957. doi: 10.1016/s0306-4522(98)00255-3. [DOI] [PubMed] [Google Scholar]
- Pyner S, Coote JH. Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience. 2000;100:549–556. doi: 10.1016/s0306-4522(00)00283-9. [DOI] [PubMed] [Google Scholar]
- Riphagen CL, Pittman QJ. Spinal arginine vasopressin elevates renal nerve activity in the rat. J Neuroendocrinol. 1989;1:339–344. doi: 10.1111/j.1365-2826.1989.tb00126.x. [DOI] [PubMed] [Google Scholar]
- Sapru HN. Glutamate circuits in selected medullo-spinal areas regulating cardiovascular function. Clin Exp Pharmacol Physiol. 2002;29:491–496. doi: 10.1046/j.1440-1681.2002.03661.x. [DOI] [PubMed] [Google Scholar]
- Sapru HN. Neurotransmitters in the nucleus tractus solitarius mediating cardiovascular function. In: Dun NJ, Machado BH, Pilowsky PM, editors. Neural mechanisms of cardiovascular regulation. Kluwer Academic Publishers; Boston, MA, USA: 2004. pp. 81–98. [Google Scholar]
- Schreihofer AM, Guyenet PG. The baroreflex and beyond: Control of sympathetic vasomotor tone by GABAergic neurons in the ventrolateral medulla. Clin Exp Pharmacol Physiol. 2002;29:514–521. doi: 10.1046/j.1440-1681.2002.03665.x. [DOI] [PubMed] [Google Scholar]
- Stocker SD, Simmons JR, Stornetta RL, Toney GM, Guyenet PG. Water deprivation activates a glutamatergic projection from the hypothalamic paraventricular nucleus to the rostral ventrolateral medulla. J Comp Neurol. 2006;494:673–685. doi: 10.1002/cne.20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram K, Murugaian J, Sapru HN. Cardiac responses to the microinjections of excitatory amino acids into the intermediolateral cell column of the rat spinal cord. Brain Res. 1989;482:12–22. doi: 10.1016/0006-8993(89)90537-4. [DOI] [PubMed] [Google Scholar]
- Sundaram K, Sapru HN. NMDA receptors in the intermediolateral column of the spinal cord mediate sympathoexcitatory responses elicited from the ventrolateral medullary pressor area. Brain Res. 1991;544:33–41. doi: 10.1016/0006-8993(91)90882-v. [DOI] [PubMed] [Google Scholar]
- Sved AF. GABA mediated neural transmission in mechanisms of cardiovascular control by the NTS. In: Barraco IRA, editor. Nucleus of the Solitary Tract. CRC Press; Boca Raton, FL, USA: 1994. pp. 245–253. [Google Scholar]
- Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Talman WT, Granata AR, Reis DJ. Glutamatergic mechanisms in the nucleus tractus solitarius in blood pressure control. Fed Proc. 1984;43:39–44. [PubMed] [Google Scholar]
- Willette RN, Krieger AJ, Barcas PP, Sapru HN. Medullary GABA receptors and the regulation of blood pressure in the rat. J Pharmacol Exp Ther. 1983;226:893–899. [PubMed] [Google Scholar]
- Willette RN, Barcas PP, Krieger AJ, Sapru HN. Endogenous GABAergic mechanisms in the medulla and the regulation of blood pressure. J Pharmacol Exp Ther. 1984;230:34–39. [PubMed] [Google Scholar]
- Yamashita H, Kannan H, Kasai M, Osaka T. Decrease in blood pressure by stimulation of the rat hypothalamic paraventricular nucleus with L-glutamate or weak current. J Auton Nerv Syst. 1987;19:229–234. doi: 10.1016/0165-1838(87)90069-5. [DOI] [PubMed] [Google Scholar]
- Yang Z, Coote JH. Influence of the hypothalamic paraventricular nucleus on cardiovascular neurons in the rostral ventrolateral medulla of the rat. J Physiol. 1998;513:521–530. doi: 10.1111/j.1469-7793.1998.521bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Bertram D, Coote JH. The role of glutamate and vasopressin in the excitation of RVL neurones by paraventricular neurones. Brain Res. 2001;908:99–103. doi: 10.1016/s0006-8993(01)02593-8. [DOI] [PubMed] [Google Scholar]
- Yang Z, Wheatley M, Coote JH. Neuropeptides, amines and amino acids as mediators of the sympathetic effects of paraventricular nucleus activation in the rat. Exp Physiol. 2002;87(6):663–674. doi: 10.1113/eph8702439. [DOI] [PubMed] [Google Scholar]