Abstract
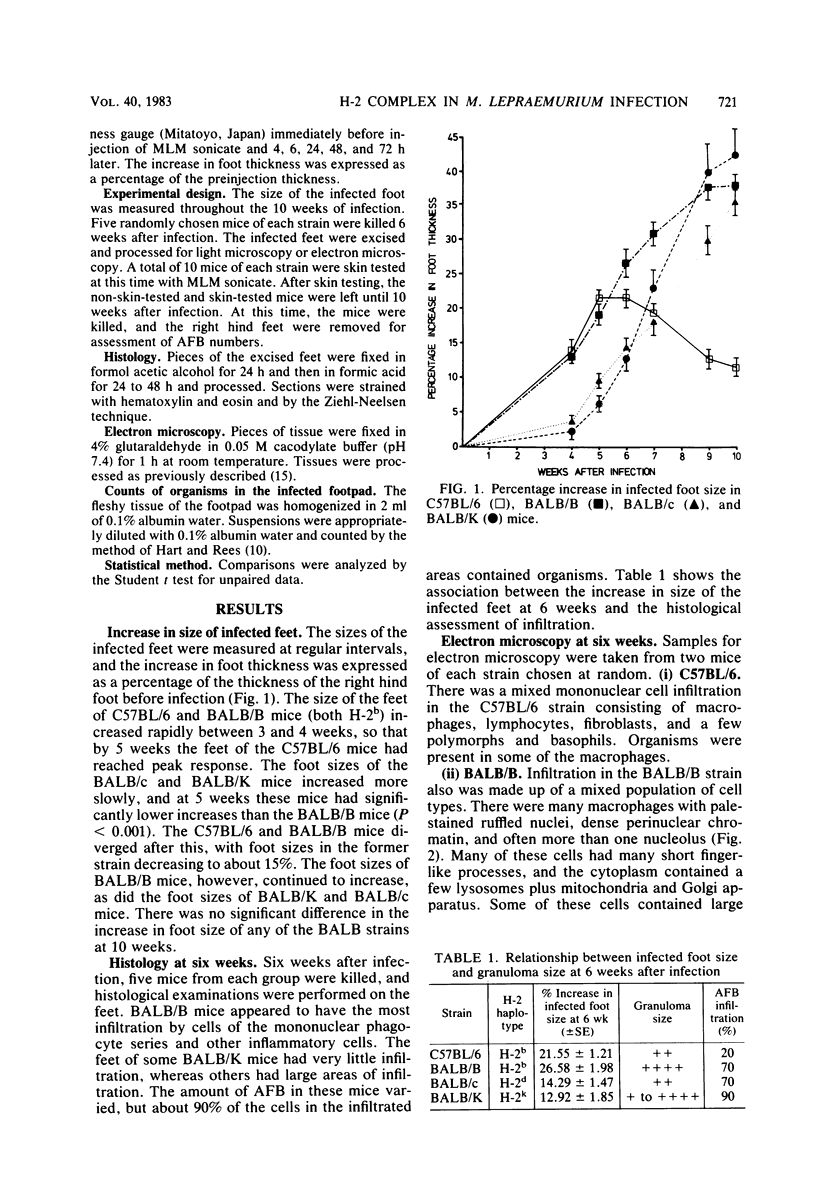
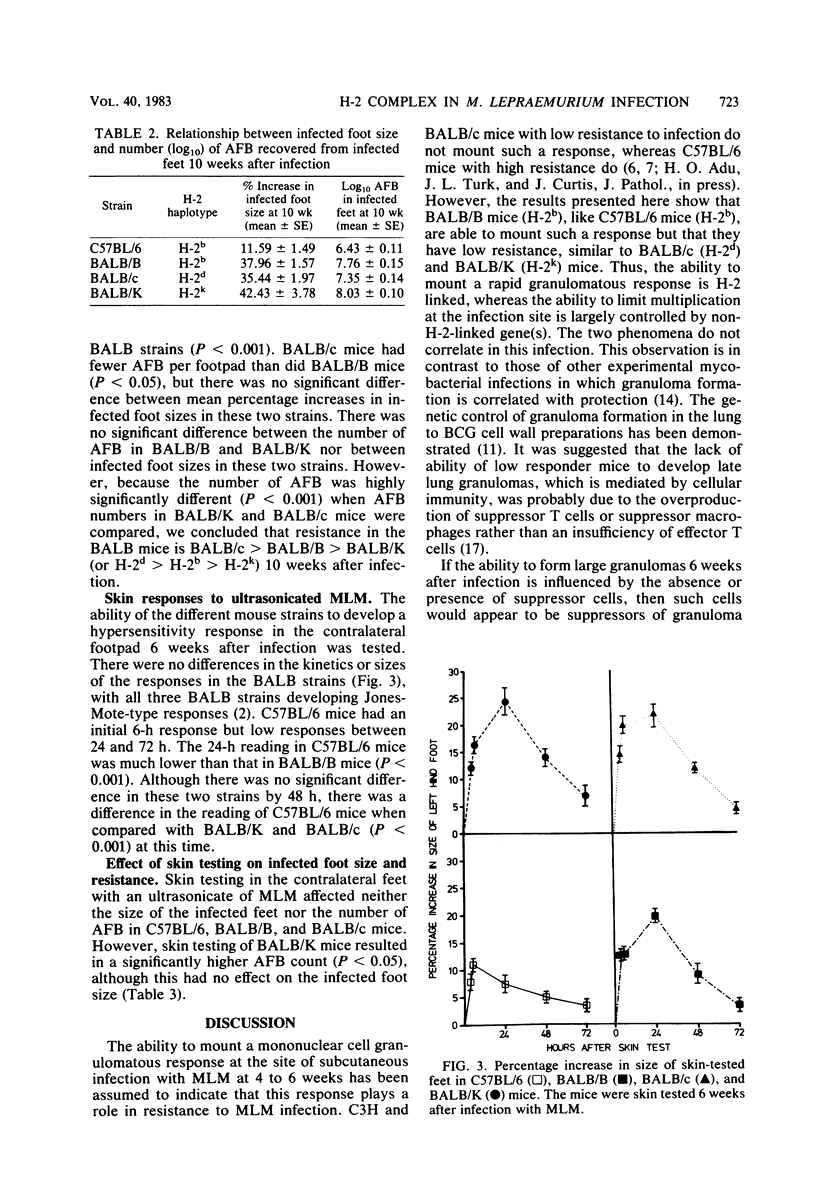
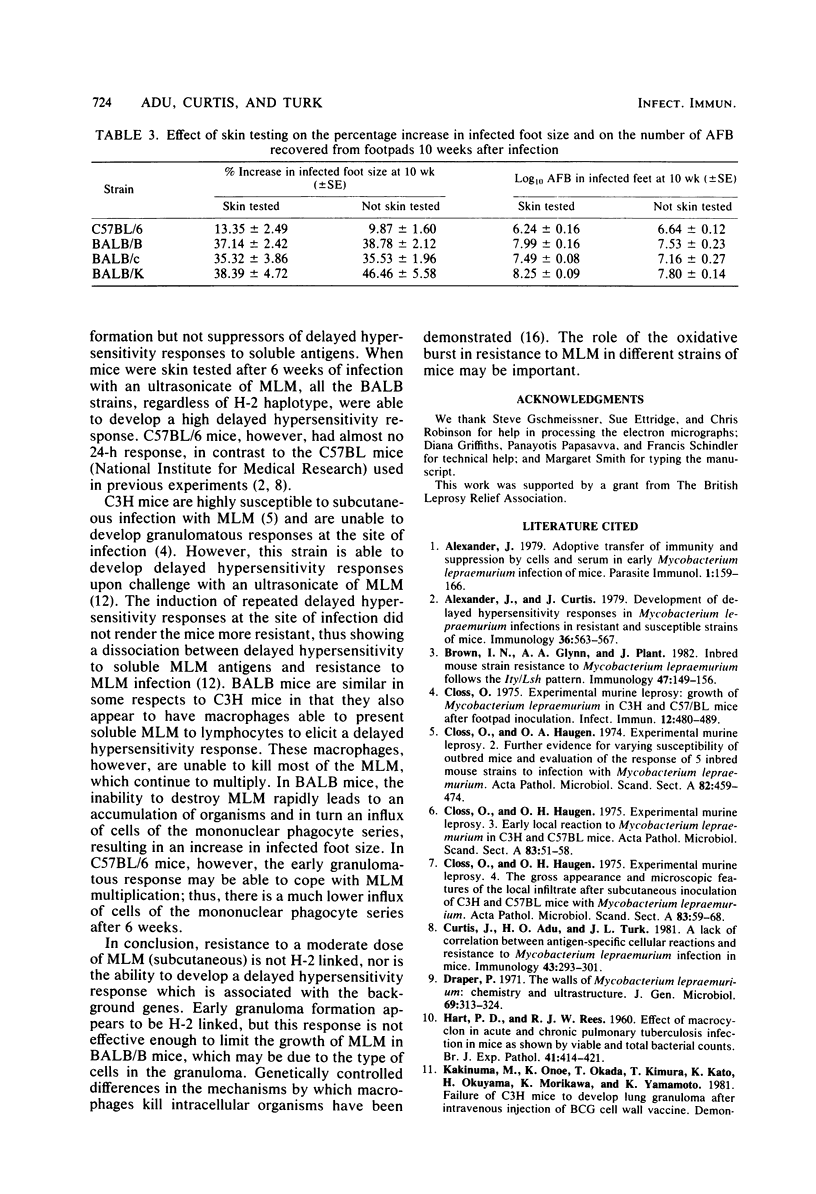
Resistance to a subcutaneous infection with a moderate dose of Mycobacterium lepraemurium was investigated in C57BL/6 mice and in three congenic strains with the BALB background (BALB/c, BALB/B, and BALB/K). Resistance after 10 weeks of infection was found not to be linked to the major histocompatibility complex. The ability to develop a delayed hypersensitivity response to an ultrasonicate of M. lepraemurium was associated with the background genes, and this ability had no influence on resistance to M. lepraemurium. Granuloma formation at the infection site in the early stages appeared to be linked to the H-2b haplotype. The types of cells involved in the granulomas were also investigated.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. Adoptive transfer of immunity and suppression by cells and serum in early Mycobacterium lepraemurium infections of mice. Parasite Immunol. 1979 Summer;1(2):159–166. doi: 10.1111/j.1365-3024.1979.tb00703.x. [DOI] [PubMed] [Google Scholar]
- Alexander J., Curtis J. Development of delayed hypersensitivity responses in Mycobacterium lepraemurium infections in resistant and susceptible strains of mice. Immunology. 1979 Mar;36(3):563–567. [PMC free article] [PubMed] [Google Scholar]
- Brown I. N., Glynn A. A., Plant J. Inbred mouse strain resistance to Mycobacterium lepraemurium follows the Ity/Lsh pattern. Immunology. 1982 Sep;47(1):149–156. [PMC free article] [PubMed] [Google Scholar]
- Closs O. Experimental murine leprosy: growth of Mycobacterium lepraemurium in C3H and C57/BL mice after footpad inoculation. Infect Immun. 1975 Sep;12(3):480–489. doi: 10.1128/iai.12.3.480-489.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Closs O., Haugen O. A. Experimental murine leprosy. 2. Further evidence for varying susceptibility of outbred mice and evaluation of the response of 5 inbred mouse strains to infection with Mycobacterium lepraemurium. Acta Pathol Microbiol Scand A. 1974 Jul;82(4):459–474. [PubMed] [Google Scholar]
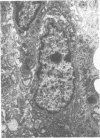
- Closs O., Haugen O. A. Experimental murine leprosy. 3. Early local reaction to mycobacterium lepraemurium in C3H and C57/BL mice. Acta Pathol Microbiol Scand A. 1975 Jan;83(1):51–58. [PubMed] [Google Scholar]
- Closs O., Haugen O. A. Experimental murine leprosy. 4. The gross appearance and microscopic features of the local infiltrate after subcutaneous inoculation of C3H and C57/BL mice with mycobacterium lepraemurium. Acta Pathol Microbiol Scand A. 1975 Jan;83(1):59–68. [PubMed] [Google Scholar]
- Curtis J., Adu H. O., Turk J. L. A lack of correlation between antigen-specific cellular reactions and resistance to Mycobacterium lepraemurium infection in mice. Immunology. 1981 Jun;43(2):293–301. [PMC free article] [PubMed] [Google Scholar]
- Draper P. The walls of Mycobacterium lepraemurium: chemistry and ultrastructure. J Gen Microbiol. 1971 Dec;69(3):313–324. doi: 10.1099/00221287-69-3-313. [DOI] [PubMed] [Google Scholar]
- HART P. D., REES R. J. Effect of macrocyclon in acute and chronic pulmonary tuberculous infection in mice as shown by viable and total bacterial counts. Br J Exp Pathol. 1960 Aug;41:414–421. [PMC free article] [PubMed] [Google Scholar]
- Kakinuma M., Onoé K., Okada M., Kimura T., Kato K., Okuyama H., Morikawa K., Yamamoto K. Failure of C3H mice to develop lung granuloma after intravenous injection of BCG cell wall vaccine. Demonstration of a defect in lymphoid cells. Immunology. 1981 May;43(1):1–9. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Løvik M., Closs O. Repeated delayed-type hypersensitivity reactions against Mycobacterium lepraemurium antigens at the infection site do not affect bacillary multiplication in C3H mice. Infect Immun. 1982 May;36(2):768–774. doi: 10.1128/iai.36.2.768-774.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribi E., Granger D. L., Milner K. C., Yamamoto K., Strain S. M., Parker R., Smith R. W., Brehmer W., Azuma I. Induction of resistance to tuberculosis in mice with defined components of mycobacteria and with some unrelated materials. Immunology. 1982 Jun;46(2):297–305. [PMC free article] [PubMed] [Google Scholar]
- Turk J. L., Badenoch-Jones P., Parker D. Ultrastructural observations on epithelioid cell granulomas induced by zirconium in the guinea-pig. J Pathol. 1978 Jan;124(1):45–49. doi: 10.1002/path.1711240110. [DOI] [PubMed] [Google Scholar]
- Wilson C. B., Tsai V., Remington J. S. Failure to trigger the oxidative metabolic burst by normal macrophages: possible mechanism for survival of intracellular pathogens. J Exp Med. 1980 Feb 1;151(2):328–346. doi: 10.1084/jem.151.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Kato K., Kakinuma M., Okuyama H., Azuma I. Further study on relationship of anti-tuberculous protection to lung granulomata produced by intravenous injections of synthetic 6-0-mycoloyl-N-acetyl-muramyl-L-alanyl-D-isoglutamine with or without specific antigens. Immunology. 1982 Jun;46(2):473–479. [PMC free article] [PubMed] [Google Scholar]