Abstract
The present report describes a young female patient with acute myocardial infarction and inflammatory lesions limited to proximal and midsegments of the left anterior descending coronary artery. Based on the presence of positive inflammatory markers, an angiographically confirmed coronary artery lesion and the young age of the patient, an atypical presentation of Takayasu arteritis was diagnosed.
Keywords: Aneurysm, Angiography, Complications, Myocardial infarction
Abstract
Le présent article décrit le cas d’une jeune femme ayant subi un infarctus aigu du myocarde et présentant des lésions inflammatoires limitées aux segments proximal et moyen de l’artère interventriculaire antérieure gauche. Compte tenu de la présence confirmée de marqueurs inflammatoires et d’une lésion coronarienne avérée à la coronarographie ainsi que du jeune âge de la patiente, un diagnostic de la maladie de Takayashu atypique a été posé.
Although the vast majority of acute coronary events result from atherosclerotic lesions in coronary arteries, some cases of myocardial infarction are related to nonatherosclerotic processes, such as coronary embolism, vasospasm, dissection, congenital abnormalities and vasculitis (1). Many systemic vasculitides, such as polyarteritis nodosa, giant cell arteritis, Wegener’s granulomatosis, Kawasaki disease, Takayasu arteritis (TA) and Behçet’s disease, may affect coronary vessels (1–4). Also, vasculitis may develop as a complication of some autoimmune rheumatic conditions such as systemic lupus erythematosus and rheumatoid arthritis.
Kawasaki disease, polyarteritis nodosa and TA are the most common causes of coronary vasculitis. Inflammatory thickening of coronary arteries may lead to their occlusion, and in some cases, aneurysms may develop as a result of the weakening of the vessel wall. Although easily identified if a patient presents with typical symptoms, these conditions may often produce diagnostic difficulties if they show atypical or incomplete clinical features.
The present report describes the case of a young woman diagnosed with acute myocardial infarction due to coronary vasculitis followed by the development of isolated aneurysms of the left anterior descending artery (LAD).
CASE PRESENTATION
A 23-year-old Caucasian woman was admitted to the cardiology department of Poznan University of Medical Sciences, Poznan, Poland, because of burning substernal pain that radiated to her left arm and lasted for approximately 6 h. Her medical history included arterial hypertension, smoking and three pregnancies, each with an uncomplicated labour. She did not report having any common colds, weakness, fever or weight loss within the past six months.
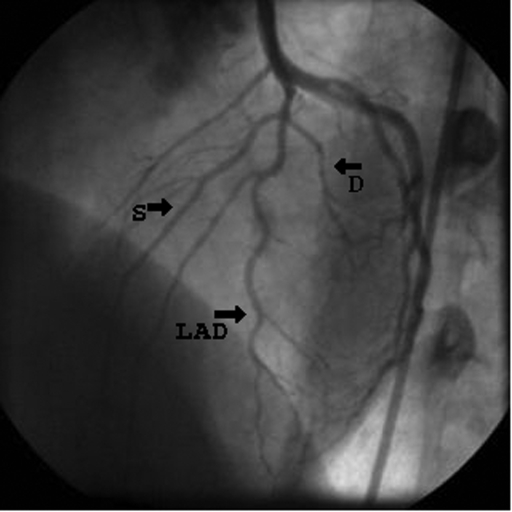
On admission, the patient was pale and diaphoretic. Her arterial blood pressure was 140/90 mmHg, pulse rate 100 beats/min, symmetrical over carotid and upper and lower extremity arteries. An electrocardiogram revealed sinus rhythm of 100 beats/min and ST-segment elevations in leads V1 to V5. Laboratory tests showed increased total plasma creatine kinase activity (911 U/L [normal values 25 U/L to 170 U/L]), creatine kinase (muscle and brain) activity (86 U/L [normal values 0 U/L to 25 U/L]), and her troponin I concentration was 3.42 μg/L (normal values 0 μg/L to 0.1 μg/L). Coronary angiography performed immediately after admission revealed a nonsignificant lesion (25%) in the proximal segment of the LAD and Thrombolysis In Myocardial Infarction (TIMI) grade 3 flow (Figure 1). Relief of the chest pain and resolution of ST-segment elevations were observed within a few hours of pharmacological therapy, which included intravenous infusions of heparin and nitroglycerin, as well as oral administration of acetylsalicylic acid, metoprolol, enalapril and simvastatin.
Figure 1).
Figure shows wall irregularities in the proximal segment of the left anterior descending artery (LAD) on day 1. Thrombolysis in Myocardial Infarction (TIMI) grade 3 flow was present (left anterior oblique cranial projection). D Diagonal branch; S Septal branch
Retrosternal pain followed by ST-segment elevation in leads V1 to V5 reappeared on day 4 of hospitalization. Coronary angiography performed several hours later showed wall irregularities, haziness and diffuse filling defects in the proximal and midsegments of the LAD, as well as TIMI grade 1 flow (Figure 2). Because coronary vasculitis with formation of intracoronary thrombi was suspected, intravenous administration of abciximab (ReoPro, Centocor and Eli Lilly, USA) and unfractioned heparin were initiated, resulting in an improvement of the patient’s general condition and resolution of ST segment changes on electrocardiogram within 2 h. Laboratory studies confirmed systemic inflammation: the patient’s white blood cell count was 17.4×109/L (normal values 3.6 ×109/L to 9.6 ×109/L), erythrocyte sedimentation rate was 43 mm/h (normal values 2 mm/h to 8 mm/h), level of C-reactive protein was 32.4 mg/L (normal values 2 mg/L to 5 mg/L) and serum fibrinogen concentration was 16.4 μmol/L (normal values 4.8 μmol/L to 12 μmol/L). Serological tests such as for venereal disease, rheumatoid factor, antineutrophil cytoplasmic antibodies, antinuclear antibodies, antistreptolysin O and antiphospholipid antibodies were negative. Echocardiographic evaluation of left ventricular (LV) function performed on day 4 revealed akinesia of the interventricular septum and hypokinesia of the apical segment of the anterior wall, as well as diminished LV ejection fraction of approximately 43%. Because TA was suspected, pulse therapy with cyclophosphamide (CTX) (600 mg administered intravenously) followed by orally administered prednisone (40 mg/24 h) were added to the standard treatment.
Figure 2).
Figure shows more proximal narrowing, early aneurysmal dilation (arrow) and clot on day 4. Thrombolysis in Myocardial Infarction (TIMI) grade 1 flow was present (left anterior oblique cranial projection). LAD Left anterior descending artery; D Diagonal branch; S Septal branch
Digital subtraction arteriography showed no lesions in the thoracic or abdominal aorta, or in its main branches, while an angiographic follow-up performed on day 14 revealed two aneurysms localized in the proximal and midsegments of the LAD (Figure 3). Because the patient was asymptomatic and hemodynamically stable, she was discharged two days later. On discharge, acetylsalicylic acid, enalapril, simvastatin and metoprolol were prescribed. Pulse therapy with CTX was continued, and the patient received three additional CTX infusions six, 12 and 20 weeks after discharge. The dose of prednisone was tapered, and the treatment was discontinued after six months.
Figure 3).
Figure shows the resolved clot on day 14; remarkable aneurysms (arrows) are localized in the proximal and midsegments of the left anterior descending artery (LAD), with streaming or filling defects. TIMI grade 3 flow was present (left anterior oblique cranial projection). D Diagonal branch; S Septal branch
The patient did not experience further episodes of chest pain or other cardiovascular complications during the 12-month follow-up. Echocardiographic examinations at month 6 and month 12 showed gradual recovery of LV contractility, with limited hypokinesia of the interventricular septum, and the LV ejection fraction after one year was 58%. Laboratory tests after one year showed no abnormalities. However, despite the aggressive anti-inflammatory treatment and no evidence of systemic inflammation on follow-up, coronary angiography carried out 12 months later showed a slight increase in the size of the aneurysm localized in the midsegment of the LAD.
DISCUSSION
Elevated inflammatory markers, characteristic evolution of angiographic changes, and good response to glucocorticoids and CTX provided strong evidence that coronary vasculitis was the cause of myocardial infarction in our patient. The nature of the underlying condition was, however, less clear. In our opinion, the patient may have suffered from atypical Takayasu-type arteritis, presenting as isolated coronaritis. First described in 1908, TA is an inflammatory disease affecting the aorta, its major branches and the pulmonary arteries. It typically affects young women (younger than 40 years of age), and occurs quite frequently in Asians and Latin Americans, but is rare in Caucasians (5). The diagnosis of TA is mainly based on the presence of characteristic clinical features (pain adjacent to the inflamed arteries, carotid and clavicular bruits, asymmetrical upper-extremity blood pressures, diminished or absent upper-extremity pulses, hypertension and ischemic symptoms) and typical angiographic changes. Coronary arteries are involved in 9% to 11% of cases, and the lesions may present as stenosis or occlusion of proximal coronary segments involving the coronary ostia (type 1, most common), diffuse or focal coronary arteritis (type 2) and aneurysms of the coronary vessels (type 3, most rare) (6).
Although some of the clinical features in our patient (systemic inflammation, angiographically confirmed coronary artery lesion, evolution of the vascular changes, young age and female sex) match the characteristics of TA, the diagnostic criteria for the disease were not fulfilled. According to criteria reported by Sharma et al (7), the patient presented with three (erythrocyte sedimentation rate greater than 20 mm/h, documented lesion in a coronary artery and hypertension) of four required minor criteria. Also, two of six criteria that were proposed by the American College of Rheumatology (8), at least three of which were required, were fulfilled. However, other differential diagnoses, such polyarteritis nodosa, Kawasaki disease, Behçet’s disease, giant cell arteritis or syphilitic arteritis, were less likely and were not supported by the patient’s medical history, physical findings and laboratory data.
TA may present as an isolated coronary lesion in fewer than 5% of cases (6). It is known, however, that lesions in the aorta or its branches may develop even five to 20 years after the early ‘pre-pulseless’ stage of the disease. The present case is of special interest because it is the first reported demonstration of the rapid formation of isolated coronary aneurysms (within two weeks from the onset of the disease) in a patient suspected of having TA.
Treatment strategies to improve clinical outcomes in TA have ranged from pharmacological therapy, with steroid and immunosuppressive agents, to invasive procedures, such as coronary angioplasty, stenting and surgery. It must be stressed, however, that there is no consensus on how coronary lesions related to TA should be treated. The weakening of the wall of the affected vessels, as well as the increased risk of thrombosis, make invasive procedures problematic and result in a high rate of restenosis (9). Moreover, several studies have shown an increased risk of failure of surgical revascularization in patients with TA (10). Conversely, Suzuki et al (11) described the fairly long-term survival of a patient with a giant aneurysm of the left main coronary artery due to TA, without major cardiac complications.
In the reported case, we did not perform coronary angioplasty or stent implantation. Instead, we decided to introduce pharmacological treatment with abciximab, acetylsalicylic acid and heparin, which effectively re-established TIMI grade 3 flow in the patient’s infarct-related artery. In addition, the patient was given aggressive anti-inflammatory therapy with glucocorticoids and CTX, which was also effective: the markers of inflammation normalized within a few months. Unfortunately, the treatment did not prevent the formation of the LAD aneurysms. Because the aneurysms had slightly increased in size during the 12-month observation period, the patient will clearly require a close follow-up and repeated angiographic evaluations to establish the indications for possible surgical intervention.
REFERENCES
- 1.Mirza A. Myocardial infarction resulting from nonatherosclerotic coronary artery diseases. Am J Emerg Med. 2003;21:578–84. doi: 10.1016/s0735-6757(03)00104-9. [DOI] [PubMed] [Google Scholar]
- 2.Testro AG, Lazzari P. Giant cell arteritis presenting with scalp necrosis. Intern Med J. 2003;33:395–6. doi: 10.1046/j.1445-5994.2003.00432.x. [DOI] [PubMed] [Google Scholar]
- 3.Tezcan H, Yavuz S, Fak AS, Aker U, Direskeneli H. Coronary stent implantation in Behcet’s disease. Clin Exp Rheumatol. 2002;20:704–6. [PubMed] [Google Scholar]
- 4.Al-Hulaimi N, Al-Saileek A, Ahmed T, Al-Zaibag M, Pai RG, El-Widaa H. Mixed aneurysmal and obstructive coronary artery disease causing acute myocardial infarction in a young woman with Takayasu’s arteritis. Can J Cardiol. 2001;17:602–5. [PubMed] [Google Scholar]
- 5.Sharma BK, Jain S, Suri S, Numano F. Diagnostic criteria for Takayasu arteritis. Int J Cardiol. 1996;54(Suppl):S141–7. doi: 10.1016/s0167-5273(96)88783-3. [DOI] [PubMed] [Google Scholar]
- 6.Lie JT. Pathology of isolated nonclassical and catastrophic manifestations of Takayasu arteritis. Int J Cardiol. 1998;66(Suppl 1):S11–21. doi: 10.1016/s0167-5273(98)00144-2. [DOI] [PubMed] [Google Scholar]
- 7.Sharma BK, Siveski-Iliskovic N, Singal PK. Takayasu arteritis may be underdiagnosed in North America. Can J Cardiol. 1995;11:311–6. [PubMed] [Google Scholar]
- 8.Arend WP, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990;33:1129–34. doi: 10.1002/art.1780330811. [DOI] [PubMed] [Google Scholar]
- 9.Son JW, Koh KK, Dang Q, Choi IS, Shin EK. Recurrent restenosis following stent and rotational atherectomy of coronary artery stenosis in Takayasu’s arteritis. Int J Cardiol. 1998;65:295–300. doi: 10.1016/s0167-5273(98)00126-0. [DOI] [PubMed] [Google Scholar]
- 10.Pajari R, Hekali P, Harjola PT. Treatment of Takayasu’s arteritis: An analysis of 29 operated patients. Thorac Cardiovasc Surg. 1986;34:176–81. doi: 10.1055/s-2007-1020404. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki H, Daida H, Tanaka M, et al. Giant aneurysm of the left main coronary artery in Takayasu aortitis. Heart. 1999;81:214–7. doi: 10.1136/hrt.81.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]