Abstract
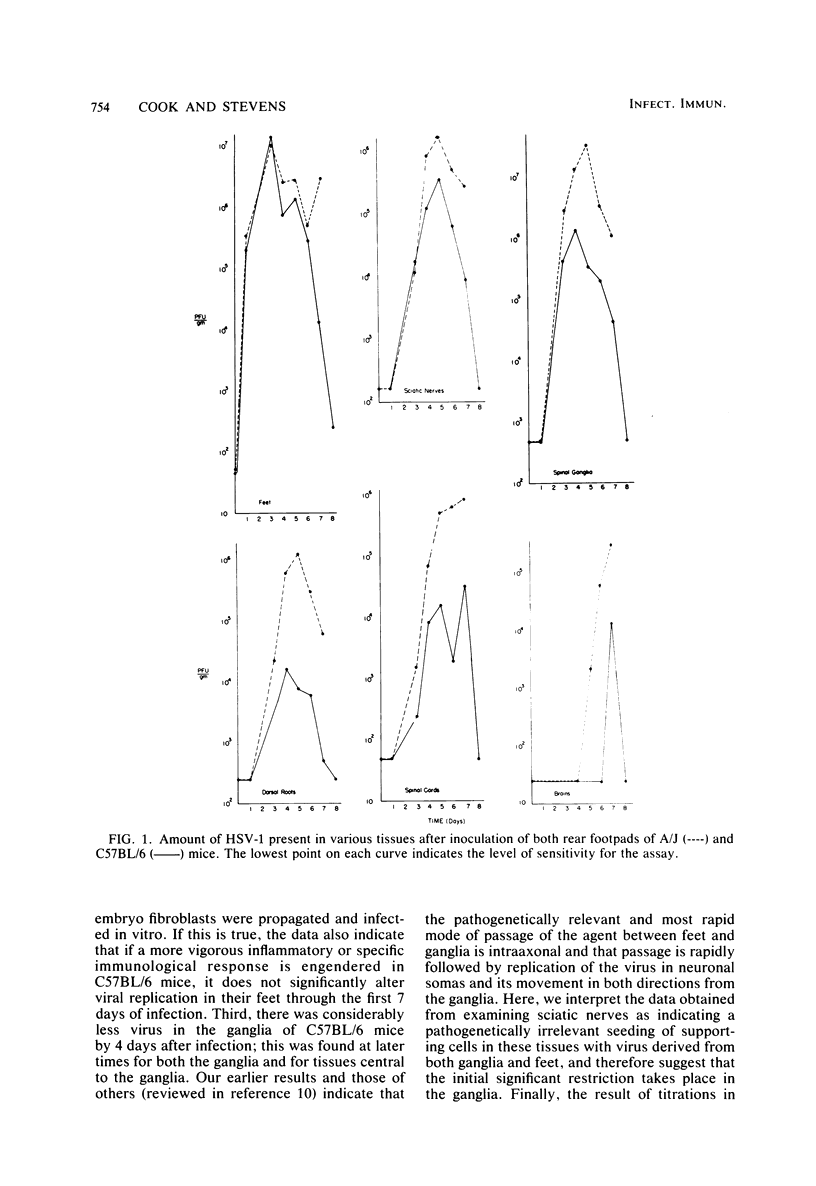
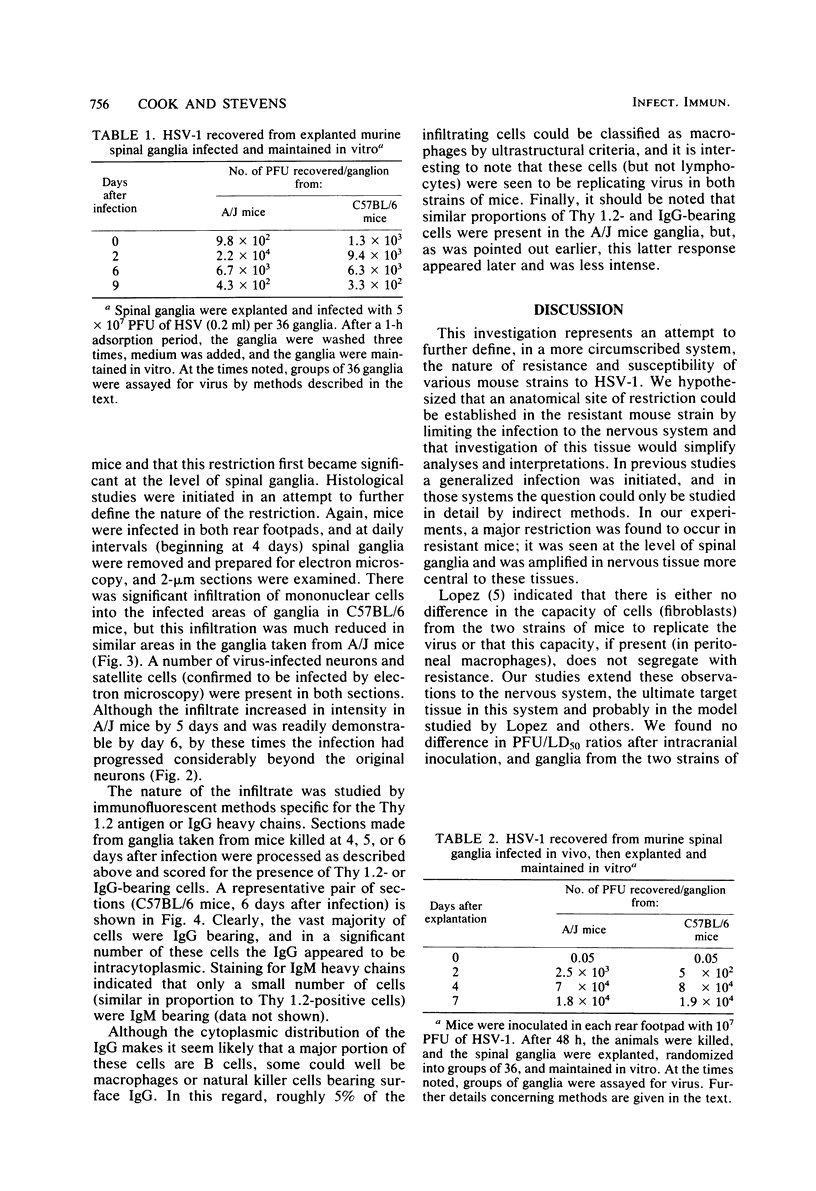
In an attempt to define the nature of the difference in the susceptibility of C57BL/6 (resistant) and A/J (susceptible) mice to herpes simplex virus type 1, we initiated a study of virus progression through the nervous system. After inoculation of virus in a rear footpad, C57BL/6 mice were found to be more than 500-fold more resistant, but resistance did not extend to pseudorabies virus. In additional investigations, it was found that the virus was selectively restricted at the level of spinal ganglia in C57BL/6 mice. No intrinsic difference in the ability of this tissue from either mouse strain to replicate virus was found. However, by 4 days after infection, morphological investigations indicated that a mononuclear cell infiltrate was present surrounding infected neurons and satellite cells both earlier and in greater numbers in the ganglia of C57BL/6 mice. Immunohistochemical methods showed that most of these cells did not express Thy 1.2 antigen, but the vast majority bore immunoglobulin G. The mechanism by which these infiltrating cells could restrict virus spread is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bang F. B. Genetics of resistance of animals to viruses: I. Introduction and studies in mice. Adv Virus Res. 1978;23:269–348. doi: 10.1016/S0065-3527(08)60102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook M. L., Stevens J. G. Pathogenesis of herpetic neuritis and ganglionitis in mice: evidence for intra-axonal transport of infection. Infect Immun. 1973 Feb;7(2):272–288. doi: 10.1128/iai.7.2.272-288.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Lipton H. L., Gonzalez-Scarano F. Central nervous system immunity in mice infected with theiler's virus. I. Local neutralizing antibody response. J Infect Dis. 1978 Feb;137(2):145–151. doi: 10.1093/infdis/137.2.145. [DOI] [PubMed] [Google Scholar]
- Lopez C. Genetics of natural resistance to herpesvirus infections in mice. Nature. 1975 Nov 13;258(5531):152–153. doi: 10.1038/258152a0. [DOI] [PubMed] [Google Scholar]
- Lopez C. Immunological nature of genetic resistance of mice to herpes simplex virus type 1 infection. IARC Sci Publ. 1978;(24 Pt 2):775–781. [PubMed] [Google Scholar]
- Lopez C. Resistance to HSV-1 in the mouse is governed by two major, independently segregating, non-H-2 loci. Immunogenetics. 1980 Jul;11(1):87–92. doi: 10.1007/BF01567772. [DOI] [PubMed] [Google Scholar]
- Lopez C., Ryshke R., Bennett M. Marrow-dependent cells depleted by 89Sr mediate genetic resistance to herpes simplex virus type 1 infection in mice. Infect Immun. 1980 Jun;28(3):1028–1032. doi: 10.1128/iai.28.3.1028-1032.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J. C., Whiteman M. D., Richter C. B. Susceptibility of inbred and outbred mouse strains to Sendai virus and prevalence of infection in laboratory rodents. Infect Immun. 1978 Jan;19(1):123–130. doi: 10.1128/iai.19.1.123-130.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G., Kado-Boll G. J., Haven C. B. Changes in nuclear basic proteins during pseudorabies virus infection. J Virol. 1969 May;3(5):490–497. doi: 10.1128/jvi.3.5.490-497.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G. Latent herpes simplex virus and the nervous system,. Curr Top Microbiol Immunol. 1975;70:31–50. doi: 10.1007/978-3-642-66101-3_2. [DOI] [PubMed] [Google Scholar]
- Watson K., Stevens J. G., Cook M. L., Subak-Sharpe J. H. Latency competence of thirteen HSV-1 temperature-sensitive mutants. J Gen Virol. 1980 Jul;49(1):149–159. doi: 10.1099/0022-1317-49-1-149. [DOI] [PubMed] [Google Scholar]
- Zawatzky R., Hilfenhaus J., Krichner H. Resistance of nude mice to herpes simplex virus and correlation with in vitro production of interferon. Cell Immunol. 1979 Oct;47(2):424–428. doi: 10.1016/0008-8749(79)90352-6. [DOI] [PubMed] [Google Scholar]
- Zawatzky R., Hilfenhaus J., Marcucci F., Kirchner H. Experimental infection of inbred mice with herpes simplex virus type 1. I. Investigation of humoral and cellular immunity and of interferon induction. J Gen Virol. 1981 Mar;53(Pt 1):31–38. doi: 10.1099/0022-1317-53-1-31. [DOI] [PubMed] [Google Scholar]





