Abstract
A copper-catalyzed α-functionalization of glycine derivatives and short peptides with nucleophiles is described. The present method provides ways to introduce functionalities such as aryl, vinyl, alkynyl, or indolyl specifically to the terminal glycine moieties of small peptides, which are normally difficult in peptide modifications. Furthermore, on functionalization, the configurations of other stereocenters in the peptides could be maintained. Because the functionalized peptides could easily be deprotected and carried onto the next coupling process, our approach provides a useful tool for the peptide-based biological research.
Keywords: amino acid, C–C bond formation, peptide modification
Recent advances in proteomics demands innovative methods to rapidly generate and modify peptides and amino acids. Direct and site-specific modification of amino acids and peptides takes advantage of the existing structure and provides a convenient way to generate large arrays of diverse amino acids and peptides for biomedical applications. For amino acid C modifications, known methods include: alkylation of α-carbanions (preformed by deprotonation with a strong base) (1–4), via radicals [α-bromination by N-bromosuccinimide (5, 6) or UV photolysis in the presence of di-tert-butyl peroxide (7)], the Claisen rearrangements (8, 9), and the recently reported palladium-catalyzed arylation of an amide (10–14). In the field of peptide synthesis, stepwise mounting of amino acids via solution and solid phase techniques has been prevalent ever since they were first developed (15, 16). In another scenario, direct site-specific C-functionalization of peptides provides an ideal approach that takes advantage of the preexisting peptides and provides rapid access to diverse peptide libraries for biological studies. Recently, by using enolate chemistry, O'Donnell (17–19) and Maruoka (3, 4, 20–22) reported an elegant method to introduce alkyl groups into activated N-terminal glycine unit of a short-chain peptide. However, a general method for site-specifically introducing various functional groups, leading to more elaborated functionalized peptides such as aryl peptides, vinyl peptides, or alkynyl peptides, still does not exist. This is largely because of the insurmountable difficulty in distinguishing the α-C–H bonds of each amino acid unit in peptides by using existing methods. Recently, we discovered that the α-C–H bond of tertiary amines or glycine derivatives can be alkylated by using a copper-catalyzed cross-coupling reaction (23–25). We also made the preliminary observation that glycine amides could be alkynylated in the presence of glycine ester to alkynylated glycine amide (23). An interesting and important nonproteinogenic class of amino acids is the arylglycines. It has attracted more and more attention because the frequency of isolating arylglycines has increased rapidly in the past few decades. For example, vancomycin (26–28), which was the first glycopeptide antibiotic discovered, contains a heptapeptide in which three of the amino acid residues are arylglycines. Besides that, arylglycines are important intermediates in the commercial production of β-lactam antibiotics. Phenylglycine (ampicillin, cefachlor) and p-hydroxyphenylglycine (amoxicillin, cefadroxil) are the predominant representatives in this family. According to World Health Organization (WHO) data, ampicillin and amoxicillin totally accounted for almost half of the β-lactam antibiotics produced globally in the year 2000 (29). Although the Strecker synthesis (30–32), the Ugi reaction (33–36) and the Petasis reaction (37–39) are important tools to construct arylglycine derivatives; direct arylation of glycine derivatives or glycine moieties in peptides would be more powerful in cases where the glycine moiety is already present. Herein, we wish to report the detailed study of a general method for site-specific C arylation, vinylation, alkynylation, and indolylation of α-C–H bonds of glycines and short peptides at the N terminus (Fig. 1).
Fig. 1.
C-functionalization of N terminus of peptides.
Results and Discussions
Alkynylation of Glycine Derivatives.
To find a general method to modify natural amino acids rapidly, we need a reaction system that can directly activate the α-C–H bonds of an amino amide with high chemo- and regioselectivity. The design of our methodology is to catalytically generate, in situ, an electrophilic glycine inter mediate, which can be intercepted by a nucleophile to form a α-functionalized glycine derivative.
By using N-PMP (p-methoxyphenyl) glycine amide derivatives as the amine substrate, phenylacetylene as the nucleophile, in the presence of CuBr as catalyst, TBHP as oxidant, the coupling reaction proceeded very well at room temperature. The best solvent was found to be dichloromethane; other nonchlorinated solvents such as THF, 1,6-dioxane, and toluene afforded low yields of the coupling product (Table 1). Under the optimized conditions, various glycine derivatives were coupled with aromatic alkynes (Table 2). Secondary (Table 2, entries 1, 2, 3, and 4) and tertiary (Table 2, entry 5) amides all reacted well. For the aromatic alkyne counterpart, 4-ethynylbiphenyl (Table 2, entry 6), 1-bromo-4-ethynylbenzene (Table 2, entry 7), and 4-ethynyltoluene (Table 2, entry 8) all afforded the corresponding products in good yields. However, 2-methoxyphenylacetylene (Table 2, entry 9) is less reactive than the other substrates, indicating that the steric hindrance on the alkyne retarded its reactivity. In the meantime, R1 being a substituted amine moiety is also very important for the success of this transformation. When R1 was switched to an OEt group (Table 2, entry 10), the coupling reaction did not occur at all at room temperature; whereas switching R1 to a phenyl group (Table 2, entry 11) afforded a mixture of unidentified compounds. This indicates that R1 being a substituted amine moiety could probably reduce the oxidation potential of the substrate and stabilize the imine intermediate being generated.
Table 1.
*Optimization of the coupling reaction between glycine derivative and phenylacetylene
*Reaction conditions: glycine derivative (0.10 mmol), alkyne (0.30 mmol), TBHP (18 μL, 5–6 M in decane), CuBr (0.01 mmol), CH2Cl2 (0.2 mL).
†NMR yields using an internal standard. DCE, dichloroethane; DME, dimethoxyethane; THF, tetrahydrofuran; NP, no product.
Table 2.
*Reaction conditions: glycine derivative (0.30 mmol), alkyne (0.90 mmol), TBHP (54 μL, 5–6 M in decane), CuBr (0.03 mmol), CH2Cl2 (0.5 mL).
†Isolated yields are based on amine, and NMR yields using an internal standard are given in parentheses. NA, not available; NR, no reaction; ND, not determined.
Arylation of Glycine Derivatives.
With the success of alkynylation, we then examined the C-functionalization with other nucleophiles. Among all of the examined nucleophiles, such as tributylphenyltin, trimethylphenylsilane, and phenylboronic acid, only phenylboronic acid afforded the desired arylation product. With 10 mol% CuBr and 1.0 equiv TBHP in 1,2-dichloroethane (DCE), the arylation reaction proceeded efficiently at 100 °C, affording the coupling product in 75% isolated yield, using a slight excess of N-PMP glycine amide (1.5 equiv, Table 3, entry 3). Other nonchlorinated solvents, such as THF, 1,6-dioxane, or toluene, afforded low yields of the coupling product (Table 3, entries 4–8). With this result in hand, we then briefly investigated the scope of this arylation reaction (Table 4). Aryl boronic acids bearing electron-donating groups (Table 4, entries 2 and 5), a weak electron-withdrawing group (Table 4, entry 4), or a steric hindered functional group (Table 4, entry 3) all afforded the corresponding coupling products in good yields. Heterocyclic boronic acids (Table 4, entries 7 and 9) and vinylboronic acid (Table 4, entry 8) underwent the coupling reaction smoothly as well. However, arylboronic acids bearing strong electron-withdrawing groups (Table 4, entries 10 and 11) were nonreactive under the optimized conditions. Other N-PMP glycine amide derivatives reacted equally well with arylboronic acids (Table 4, entries 12 and 13). However, the coupling reaction did not proceed at all when N-PMP glycine amides without hydrogen on the amide nitrogen (Table 4, entries 15 and 16) or an N-PMP glycine ester (Table 4, entry 14) was used. These results suggested a potential approach for site-specific functionalization of peptides via the current methods.
Table 3.
Optimization of the coupling reaction between glycine derivative and phenylboronic acid
*NMR yields using an internal standard. DCE, dichloroethane; DME, dimethoxyethane; THF, tetrahydrofuran; NP, no product.
Table 4.
Arylation of glycine amide*
*Reaction conditions; aryl boronic acid (0.20 mmol), glycine derivative (0.30 mmol), TBHP (36 μL, 5–6 M in decane), CuBr (0.02 mmol), DCE (0.5 mL).
†Isolated yields are based on aryl boronic acid, and NMR yields using an internal standard are given in parentheses. ND, not determined; NA, not available; NR, no reaction.
α-Functionalization of Peptides.
Having succeeded in the functionalization of glycine derivatives, we decided to tackle the more challenging task of functionalizing peptides. Considering that α-aryl peptides are more prevalent in nature and more important synthons in organic syntheses, we decided to focus on the arylation of peptides. Simple dipeptides (Table 5, entries 1–8) and tripeptides (Table 5, entries 12–19) all reacted with arylboronic acids, affording the coupling products in good yields in most cases. The scope of arylboronic acids is very similar to the one examined for N-PMP glycine amide. A dipeptide (Table 5, entry 2) and tripeptides (Table 5, entry 18 and 19) with an amino acid moiety other than glycine also afforded the cross-coupling products. Interestingly, similar diastereoselectivities were observed when the preexisting chiral center is either one (Table 5, entry 2) or two (Table 5, entry18 and 19) amino acid units away from the N-terminal glycine moiety.
Table 5.
α-Functionalization of dipeptides and tripepptides*
*Reaction conditions: aryl boronic acid (0.20 mmol), peptide (0.30 mmol), TBHP (36 μL, 5–6 M in decane), CuBr (0.02 mmol), DCE (0.5 mL).
†Isolated yields are based on aryl boronic acid, and NMR yields using an internal standard are given in parentheses.
‡d.r. (diastereomer ratio) was determined by HPLC analysis. d.r. of the product is 4:5.
§Reaction was performed using peptide as the limiting reagent at 70 °C, phenylacetylene was used at 3.0 equiv.
¶Reactions were performed using peptide as the limiting reagent at room temperature, indole was used at 1.5 equiv.
‖Reaction was performed using dipeptide as the limiting reagent at room temperature in DCM, diethyl zinc was used at 2 equiv.
**d.r. (diastereomer ratio) was determined by HPLC analysis. d.r. of the product is 3:5.
To examine the scope of this method for peptide modifications, other nucleophiles such as phenylacetylene (Table 5, entry 9 and 20) and indole (Table 5, entry 10 and 21) were also tested. The coupling reactions went very well at conditions even milder than with arylboronic acids. It should also be noted that all of the functionalizations occurred exclusively at the N terminus of the peptides without any scrambling on other amino acid moieties.
Importance of N-PMP Protecting Group.
As it is well known, there are other useful protecting groups for nitrogen compounds, such as benzyl, Boc (butoxycarbonyl), and Ts (p-toluene sulfonamide). Accordingly, the protected dipeptides with those protecting groups were synthesized and tested for the oxidative coupling reactions with phenyl boronic acid (Table 6). However, no desired coupling product was obtained by using those protecting groups, which illustrates the importance of the N-PMP group in the oxidative coupling process.
Table 6.
Tests of various protecting groups
*NMR yields using an internal standard. NP, no product.
Racemization Test.
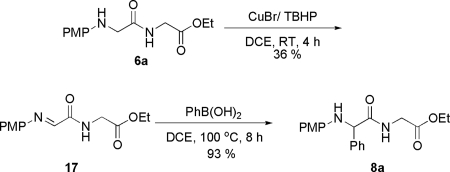
Traditional methods to functionalize amino acid derivatives are not applicable to peptide modifications due to not only the site-specificity issue, but also the fact that the most popular method to functionalize amino acid derivatives is via the enolate chemistry, which usually requires the use of an excess amount of strong bases such as potassium tert-butoxide or lithium diisopropyl amide (LDA). Therefore, the α-protons adjacent to the amides in most cases cannot tolerate such strong basic conditions and would racemize quickly. To test whether our present method can still maintain the preexisting chiral center on the peptide, we used the optically pure compound 6a. Under the standard reaction conditions, the coupling product 8a was generated without any race mization of the adjacent stereocenter (Scheme 1).*
Scheme 1.
Deprotection of PMP Group and Further Functionalization.
To test the compatibility of this functionalization method with state-of-the-art peptide syntheses, the functionalized glycine derivative 9 was deprotected readily by TCCA to afford the amine salt 10. Compound 10 could then be coupled with Fmoc-Gly efficiently by using HBTU/HOBt as coupling reagents to afford the desired peptide 11 (Scheme 2).
Scheme 2.
Mechanism of CDC Reactions.
For the arylation reaction, a tentative mechanism involving an iminol intermediate is proposed (Scheme 3). First, CuBr/TBHP initiated a dehydrogenative oxidation of 12 to give the imino amide intermediate 13, which will tautomerize to the iminol form 14. Then, the newly formed hydroxyl group from 14 coordinates with phenylboronic acid to give intermediate 15. After that, the phenyl group will be delivered to the imine bond. Final hydrolysis affords the α-aryl glycine derivative 16. Because of the presence of the PMP group, only the CH2 adjacent to the N terminus can be functionalized. This mechanism is consistent with the results that tertiary amides (Table 4, entries 15 and 16) did not react at all because of the lack of a hydrogen on the amides to tautomerize to the iminol form. It is also consistent with the absence of reactivity with N-PMP glycine ethyl ester (Table 4, entry 14). To support our proposed mechanism, the imino amide intermediate 17 was synthesized by oxidation of N-PMP dipeptide. Compound 17 was then heated with phenylboronic acid in DCE at 100 °C. Even in the absence of CuBr, the reaction still proceeded well, affording the final coupling product with good yields (Scheme 4).
Scheme 3.
Proposed mechanism for the arylation reaction.
Scheme 4.
Conclusion
An efficient way to functionalize glycine derivatives and short peptides with various nucleophiles is described. Alkynyl, aryl, vinyl, and indolyl can all be introduced to the α-position of the terminal glycine moieties. In the meantime, the configurations of other stereocenters in the peptide are maintained. The current method could also be easily integrated into subsequent peptide syntheses. With the advantages of site specificity, mild conditions, compatibility with different nucleophiles and simple experimental procedure, this peptide modification method is expected to provide synthetic pathways for the increasingly important proteomics and peptide-based pharmaceutical research.
Materials and Methods
General Information.
1H NMR spectra were recorded on Varian 300-, 400-, and 500-MHz spectrometers and the chemical shifts (δ) were reported in parts per million (ppm). The peak patterns are indicated as follows: s, singlet; d, doublet; t, triplet; dd, doublet of doublet; m, multiplet; q, quartet. The coupling constants, J, are reported in hertz (Hz). 13C NMR spectra were obtained at 75, 100, and 125 MHz and referenced to the internal solvent signals (central peak is 77.0 ppm in CDCl3 or 40.4 ppm in DMSO-d6). CDCl3 was used to get NMR spectra unless otherwise mentioned. HRMS were made by McGill University. Thin-layer chromatography was performed by using Sorbent Silica Gel 60 F254 TLC plates and visualized with UV light. Flash column chromatography was performed over SORBENT silica gel 30–60 μm. All reagents were weighed and handled in air at room temperature. All reagents were purchased from Aldrich, Strem, and Acros and used without further purification.
General Procedure for Preparation of PMP-Protected Glycine Derivatives; 2-(4-Methoxyphenylamino)-N-methylacetamide.
2-Bromoacetyl bromide (2.4 g, 1.2 mmol) in CH2Cl2 (10 mL) was added dropwise to a mixture of MeNH2 (1.0 g, 30 wt% in H2O, 1.0 mmol) and K2CO3 (1.66 g, 1.2 mmol) in CH2Cl2/H2O (30 mL/10 mL) at 0 °C. The mixture was then allowed to warm up to room temperature and stirred for 6 h. Then, the organic layer was separated and the aqueous layer was extracted with CH2Cl2 (3 × 5 mL). The organic layers were combined and dried over Na2SO4, and CH2Cl2 was removed in vacuo. Subsequently, EtOH (5 mL), p-anisidine (1.23 g, 1 mmol), and NaOAc (0.84 g, 1 mmol) were added to the residue. The resulting mixture was refluxed for 6 h and was filtered. The solvent of the filtrate was removed in vacuo. Recrystallization (CH2Cl2/hexanes) gave the pure product 2-(4-methoxyphenylamino)-N-methylacetamide (1.5 g, 80% yield).
General Procedure for the Preparation of PMP-Protected Peptide Derivatives; N-(N-p-Methoxyphenylglycyl)-Glycine Ethyl Ester.
SOCl2 (3.6 g, 30 mmol) was added slowly to EtOH (30 mL) at 0 °C. After stirring at this temperature for 10 min, glycine (0.75 g, 10 mmol) was added to the solution. Then, the reaction was stirred at 70 °C for 3 h. EtOH was removed in vacuo. The resulting solid was then mixed with CH2Cl2 (30 mL) and NEt3 (2.2 g, 22 mmol). The reaction mixture was cooled to −78 °C, and BrCH2COBr (2.0 g, 10 mmol) was added dropwise to the solution at this temperature. The solution was allowed to warm up to room temperature and the stirring was continued for 6 h. After that, the solution was washed with H2O (10 mL). The organic layer was dried over Na2SO4, and CH2Cl2 was removed in vacuo to afford BrCH2CONHCH2CO2Et (1.8 g, 81%). NaOAc (0.50 g, 6 mmol), p-anisole (0.74 g, 6 mmol), and BrCH2CONHCH2CO2Et (1.1 g, 5 mmol) were successively added to EtOH (4 mL). The reaction tube was heated at 80 °C for 6 h. EtOH was removed in vacuo and the residue was dissolved in CH2Cl2 (20 mL) and washed with H2O (5 mL). The organic layer was dried over Na2SO4, and CH2Cl2 was removed in vacuo. Flash column chromatography on silica gel by using ethyl acetate/hexanes (1:1) furnished the final product N-(N-p-methoxyphenylglycyl)-glycine ethyl ester (0.95 g, 72% yield).
General Procedure for the Alkynylation of Glycine and Peptide Derivatives.
2-(4-Methoxyphenylamino)-N-methyl acetamide (59 mg, 0.30 mmol), CuBr (4.2 mg, 0.03 mmol), phenylacetylene (90 mg, 0.90 mmol), TBHP (54 μL, 5–6 M in decane) were successively added into a test tube with CH2Cl2 (0.5 mL). The test tube was purged with argon. Then, the mixture was stirred for 15 h at room temperature, filtered through a small pad of silica gel, and concentrated in vacuo. Flash chromatography by using ethyl acetate/hexanes gradient eluent (1/4 to 1/2) furnished the final product (60 mg, 68% in yield).
General Procedure for the Arylation of Glycine and Peptide Derivatives.
N-PMP-Gly-Gly-OEt (80 mg, 0.30 mmol) and CuBr (2.8 mg, 0.02 mmol) were dissolved in DCE (0.5 mL), TBHP (36 μL, 5–6 M in decane) was then added. The solution was stirred at room temperature for 10 min, followed by the addition of PhB(OH)2 (25 mg, 0.2 mmol). The test tube was capped and stirred at 100 °C for 6 h. Then, the reaction mixture was filtered through a small pad of silica gel and concentrated in vacuo. Flash column chromatography on silica gel by using ethyl acetate/hexanes (1/5 to 1/3) furnished the final coupling product (52 mg, 77% yield).
General Procedure for the Deprotection of PMP-Gly(Ph)-OEt and Subsequent Coupling Reaction with Fmoc-Gly.
To a stirred solution of compound N-PMP-Gly(Ph)-OEt (27 mg, 0.1 mmol) in CH3CN/H2O (1 mL/1 mL), HCl (100 μL, 2M), and trichloroisocyanuric acid (TCCA) (12 mg, 0.1 mmol) were successively added. The reaction mixture was stirred at room temperature for 4 h, and CH3CN was later removed in vacuo. The aqueous solution was extracted with CH2Cl2 (2 × 1 mL), and water in the aqueous solution was then removed in vacuo at 40 °C. The resulting residue was used for the next step without further purification. The residue was dissolved in DMF (0.5 mL). Then HBTU (38 mg, 0.1 mmol), HOBt (14 mg, 0.1 mmol), N,N-diisopropyl ethyl amine(DIPEA) (25 μL, 0.25 mmol), and Fmoc-Gly (30 mg, 0.1 mmol) were successively added. The reaction mixture was stirred at room temperature for 10 h. H2O (5 mL) was added to quench the reaction. The product was extracted with EtOAc (3 × 2 mL). The EtOAc layer was dried over Na2SO4. After evaporation of the EtOAc in vacuo, the product was isolated by using flash column chromatography on silica gel eluting with ethyl acetate/NEt3 (100/3) (25 mg, 57% yield).
Supporting Information.
For general reaction procedures, diffraction details, and characterization of products, see supporting information (SI) Appendix.
Supplementary Material
Acknowledgments.
This work was supported by the Canada Research Chair (Tier I) Foundation (C.-J.L.), the Canada Foundation for Innovation, Natural Sciences and Engineering Research Council (Canada), and McGill University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0809052106/DCSupplemental.
The retention times of the two newly formed diastereomers were compared with the racemic compound by using HPLC analysis. No observation of the peak at 15.6 min indicates that the stereo center on the alanine moiety was not destroyed. Details of the experiment and explanations can be found in SI Appendix.
References
- 1.Beak P, Zajdel WJ, Reitz DB. Metalation and electrophilic substitution of amine derivatives adjacent to nitrogen: α-metallo amine synthetic equivalents. Chem Rev. 1984;84:471–523. [Google Scholar]
- 2.Meyers AI. Formamidines as precursors to α-amino carbanions and their application to asymmetric carbon-carbon bond-forming reactions. Aldrichim Acta. 1985;18:59–68. [Google Scholar]
- 3.Maruoka K, Ooi T. Enantioselective amino acid synthesis by chiral phase-transfer catalysis. Chem Rev. 2003;103:3013–3028. doi: 10.1021/cr020020e. [DOI] [PubMed] [Google Scholar]
- 4.Hashimoto T, Maruoka K. Recent development and application of chiral phase- transfer catalysts. Chem Rev. 2007;107:5656–5682. doi: 10.1021/cr068368n. [DOI] [PubMed] [Google Scholar]
- 5.Easton CJ, Scharfbillig IM, Tan EW. Selective modification of glycine residues in dipeptides. Tetrahedron Lett. 1988;29:1565–1568. [Google Scholar]
- 6.Easton CJ, Hutton CA, Rositano G, Tan EW. Regioselective functionalization of N-phthaloyl-substituted amino acid and peptide derivatives. J Org Chem. 1991;56:5614–5618. [Google Scholar]
- 7.Knowles HS, Hunt K, Parsons AF. Photochemical alkylation of glycine leading to phenylalanines. Tetrahedron Lett. 2000;41:7121–7124. [Google Scholar]
- 8.Kubel B, Hofle G, Steglich W. Hetero Cope rearrangements in cyclization of allyl and propargyl esters of N-acyl amino acids to oxazolin-5- ones. Angew Chem Int Ed. 1975;14:58–59. [Google Scholar]
- 9.Ireland RE, Mueller RH, Willard AK. Ester enolate claisen rearrangement: Stereochemical control through stereoselective enolate formation. J Am Chem Soc. 1976;98:2868–2877. [Google Scholar]
- 10.Lee S, Beare NA, Hartwig JF. Palladium-catalyzed α-arylation of esters and protected amino acids. J Am Chem Soc. 2001;123:8410–8411. doi: 10.1021/ja016032j. [DOI] [PubMed] [Google Scholar]
- 11.Gaertzen O, Buchwald SL. Palladium-catalyzed intramolecular α-arylation of α-amino acid esters. J Org Chem. 2002;67:465–475. doi: 10.1021/jo0107756. [DOI] [PubMed] [Google Scholar]
- 12.Liu XX, Hartwig JF. Palladium-catalyzed α-arylation of azlactones to form quaternary amino acid derivatives. Org Lett. 2003;5:1915–1918. doi: 10.1021/ol034570q. [DOI] [PubMed] [Google Scholar]
- 13.Culkin DA, Hartwig JF. Palladium-catalyzed α-arylation of carbonyl compounds and nitriles. Acc Chem Res. 2003;36:234–245. doi: 10.1021/ar0201106. [DOI] [PubMed] [Google Scholar]
- 14.Liu XX, Hartwig JF. Palladium-catalyzed arylation of trimethylsilyl enolates of esters and imides. High functional group tolerance and stereoselective synthesis of α-aryl carboxylic acid derivatives. J Am Chem Soc. 2004;126:5182–5191. doi: 10.1021/ja031544e. [DOI] [PubMed] [Google Scholar]
- 15.Gross E, Meienhofer J. The Peptides. Analysis, Synthesis, Biology. New York: Academic; 1981. [Google Scholar]
- 16.Stewart JM, Young JD. Solid Phase Peptide Synthesis. Rockford, IL: Pierce Chemical Co.; 1984. [Google Scholar]
- 17.O'Donnell MJ, Burkholder TP, Khau VV, Roeske RW, Tian Z. Selective Alkylation of Protected Peptide Derivatives by Phase-Transfer Catalysis. Pol J Chem. 1994;68:2477–2488. [Google Scholar]
- 18.O'Donnell MJ, Zhou CY, Scott WL. Solid-phase unnatural peptide synthesis (UPS) J Am Chem Soc. 1996;118:6070–6071. [Google Scholar]
- 19.O'Donnell MJ, Drew MD, Pottorf RS, Scott WL. UPS on weinreb resin: A facile solid-phase route to aldehyde and ketone derivatives of “unnatural” amino acids and peptides. J Comb Chem. 2000;2:172–181. doi: 10.1021/cc990071y. [DOI] [PubMed] [Google Scholar]
- 20.Ooi T, Tayama E, Maruoka K. Highly stereoselective N-terminal functionalization of small peptides by chiral phase-transfer catalysis. Angew Chem Int Ed. 2003;42:579–582. doi: 10.1002/anie.200390167. [DOI] [PubMed] [Google Scholar]
- 21.Maruoka K, Tayama E, Ooi T. Stereoselective terminal functionalization of small peptides for catalytic asymmetric synthesis of unnatural peptides. Proc Natl Acad Sci USA. 2004;101:5824–5829. doi: 10.1073/pnas.0307725101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ooi T, Maruoka K. Recent advances in asymmetric phase-transfer catalysis. Angew Chem Int Ed. 2007;46:4222–4266. doi: 10.1002/anie.200601737. [DOI] [PubMed] [Google Scholar]
- 23.Zhao L, Li C-J. Functionalizing glycine derivatives by direct C-C bond formation. Angew Chem Int Ed. 2008;47:7075–7078. doi: 10.1002/anie.200801367. [DOI] [PubMed] [Google Scholar]
- 24.Li Z, Li C-J. CuBr-catalyzed efficient alkynylation of sp3 C-H bonds adjacent to a nitrogen atom. J Am Chem Soc. 2004;126:11810–11811. doi: 10.1021/ja0460763. [DOI] [PubMed] [Google Scholar]
- 25.Li Z, Li C-J. Catalytic enantioselective alkynylation of prochiral sp3 C-H bonds adjacent to a nitrogen atom. Org Lett. 2004;6:4997–4999. doi: 10.1021/ol047814v. [DOI] [PubMed] [Google Scholar]
- 26.Perkins HR. Vancomycin and related antibiotics. Pharmacol Ther. 1982;16:181–197. doi: 10.1016/0163-7258(82)90053-5. [DOI] [PubMed] [Google Scholar]
- 27.Williams DH. Structural studies on some antibiotics of the vancomycin group, and on the antibiotic-receptor complexes, by proton NMR. Acc Chem Res. 1984;17:364–369. [Google Scholar]
- 28.Nagarajan R. Antibacterial activities and modes of action of vancomycin and related glycopeptides. Antimicrob Agents Chemother. 1991;35:605–609. doi: 10.1128/aac.35.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elander RP. Industrial production of beta-lactam antibiotics. Appl Microbiol Biotechnol Appl Microbiol Biotechnol. 2003;61:385–392. doi: 10.1007/s00253-003-1274-y. [DOI] [PubMed] [Google Scholar]
- 30.Yet L. Recent developments in catalytic asymmetric Strecker-type reactions. Angew Chem Int Ed. 2001;40:875–877. [PubMed] [Google Scholar]
- 31.Groger H. Catalytic enantioselective Strecker reactions and analogous syntheses. Chem Rev. 2003;103:2795–2827. doi: 10.1021/cr020038p. [DOI] [PubMed] [Google Scholar]
- 32.Connon SJ. The catalytic asymmetric strecker reaction: Ketimines continue to join the fold. Angew Chem Int Ed. 2008;47:1176–1178. doi: 10.1002/anie.200703879. [DOI] [PubMed] [Google Scholar]
- 33.Ugi I. Versuche Mit Isonitrilen. Angew Chem. 1959;71:386–386. [Google Scholar]
- 34.Ugi I, Steinbruckner C. Uber ein neues kondensations-prinzip. Angew Chem. 1960;72:267–268. [Google Scholar]
- 35.Ugi I. The α-addition of ammonium ions and anions to isonitriles accompanied by secondary reactions. Angew Chem Int Ed. 1962;1:8–21. [Google Scholar]
- 36.Domling A, Ugi I. Multicomponent reactions with isocyanides. Angew Chem Int Ed. 2000;39:3169–3210. doi: 10.1002/1521-3773(20000915)39:18<3168::aid-anie3168>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 37.Petasis NA, Akritopoulou I. The boronic acid Mannich reaction: A new method for the synthesis of geometrically pure allylamines. Tetrahedron Lett. 1993;34:583–586. [Google Scholar]
- 38.Petasis NA, Zavialov IA. A new and practical synthesis of α-amino acids from alkenyl boronic acids. J Am Chem Soc. 1997;119:445–446. [Google Scholar]
- 39.Petasis NA, Zavialov IA. Highly stereocontrolled one-step synthesis of anti-β-amino alcohols from organoboronic acids, amines, and α-hydroxy aldehydes. J Am Chem Soc. 1998;120:11798–11799. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.