Abstract
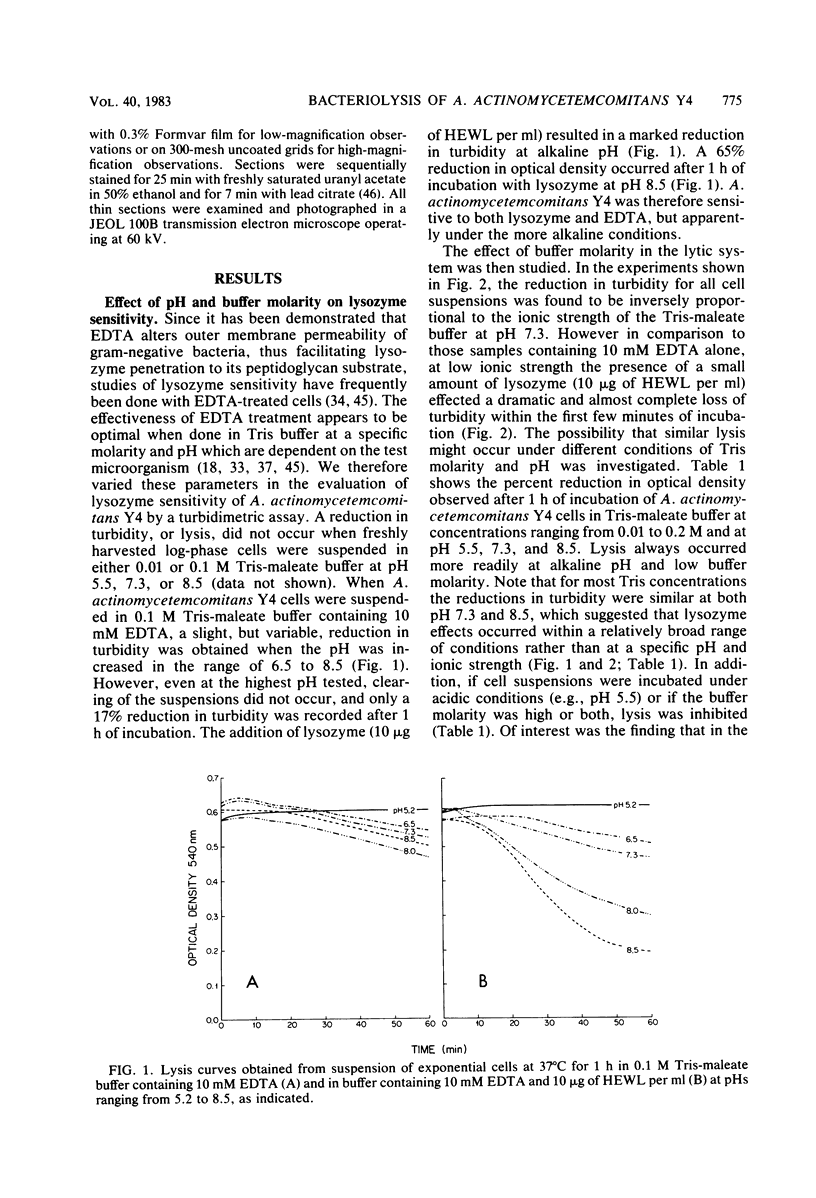
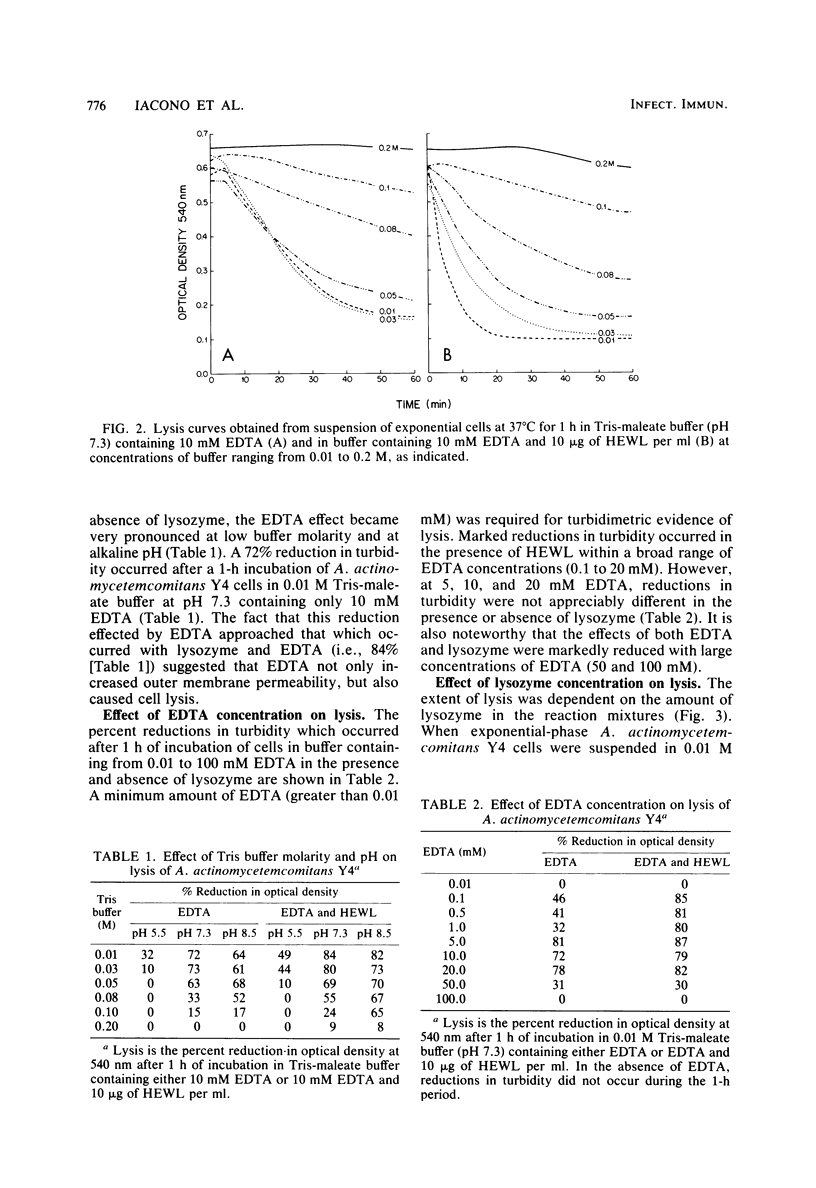
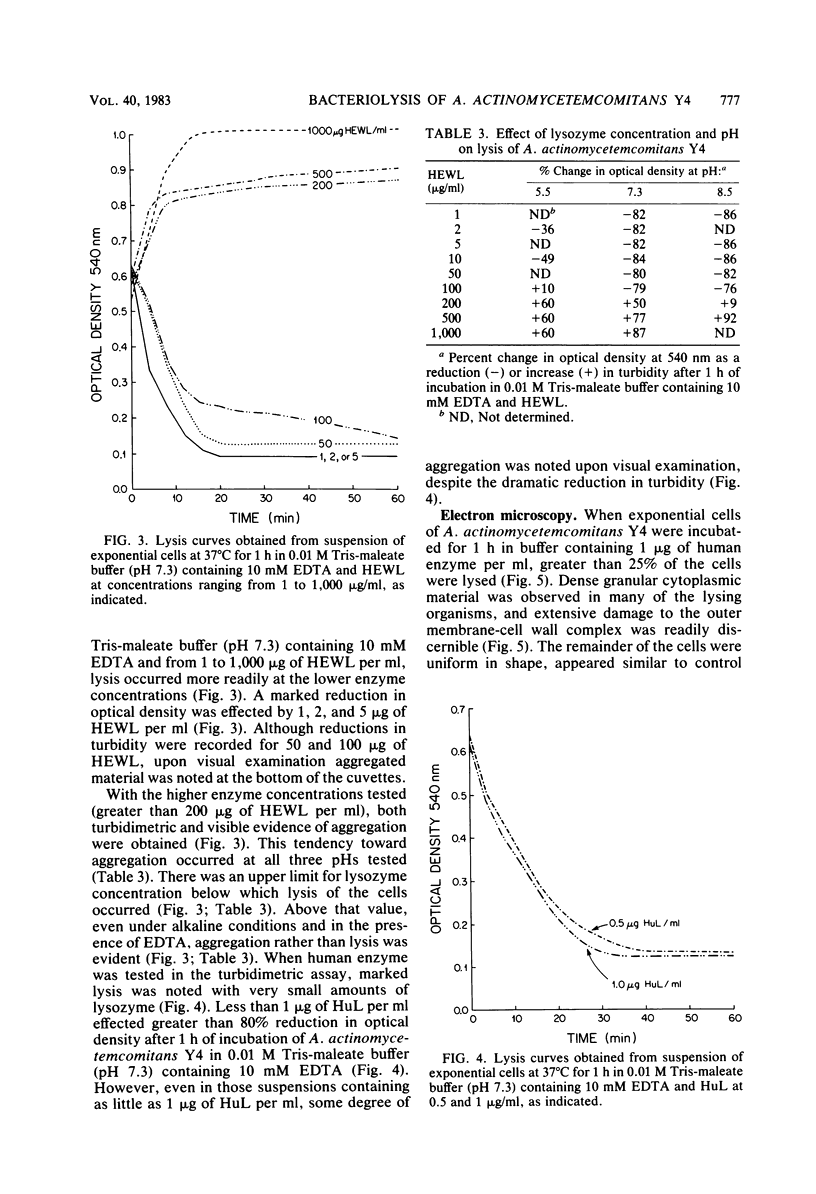
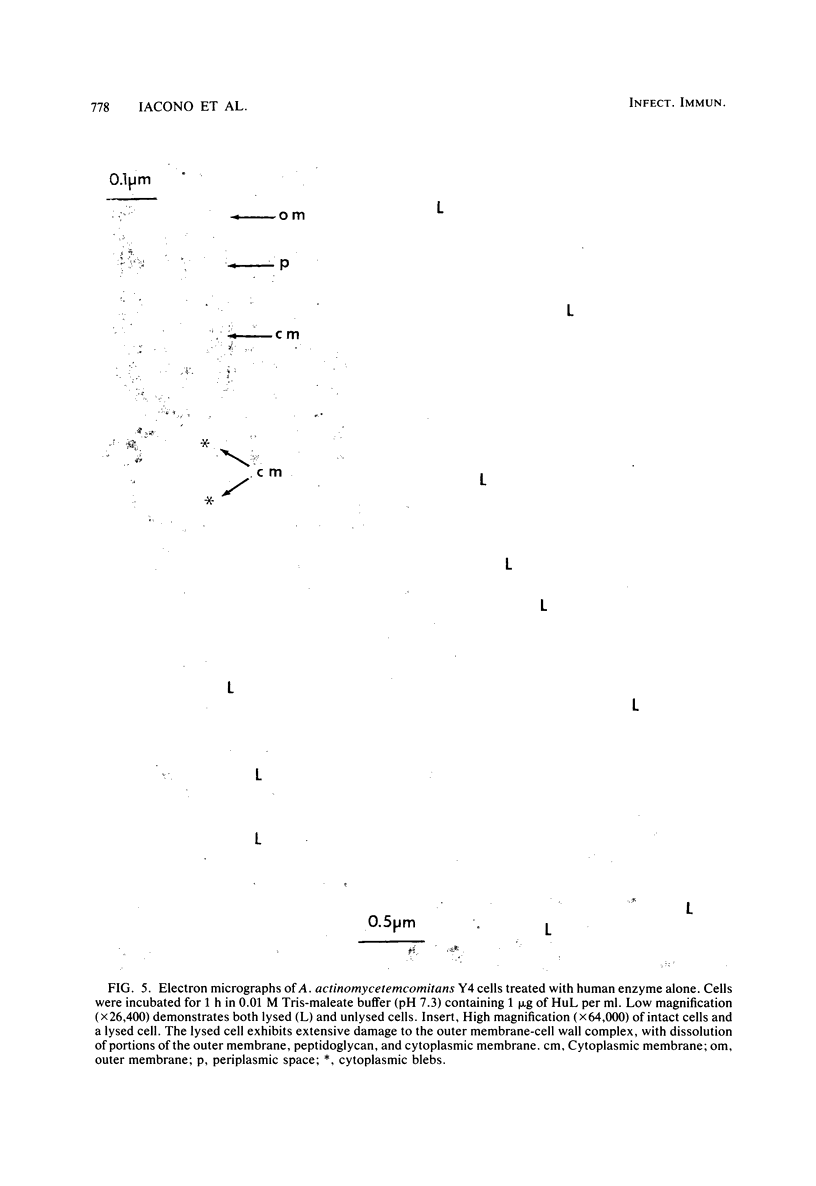
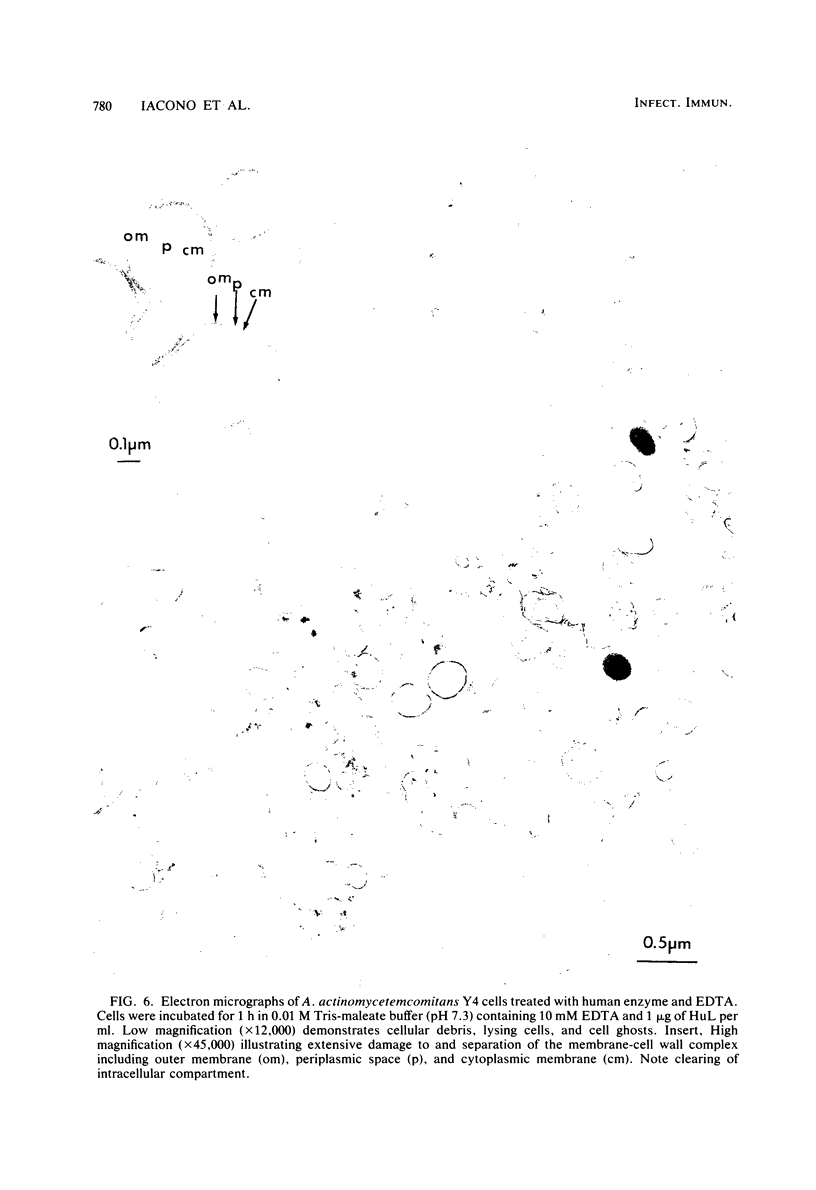
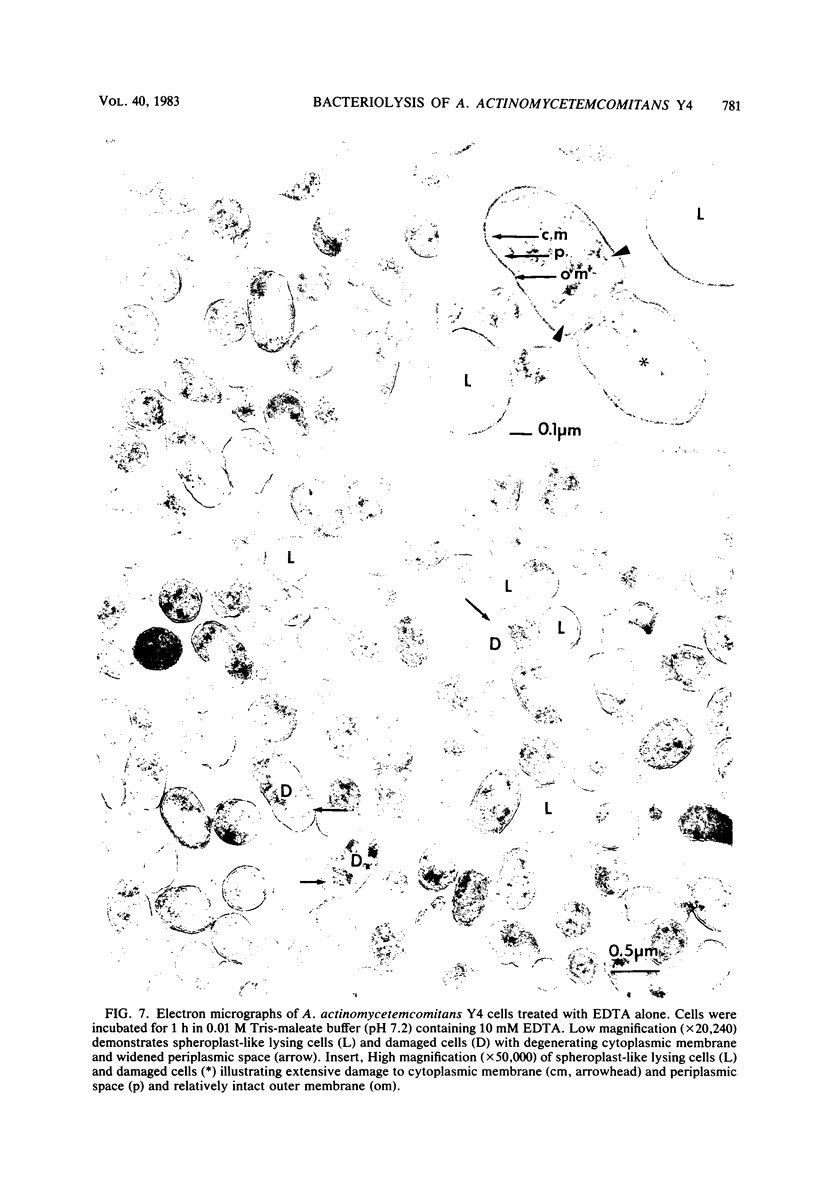
The ability of both human and hen egg white lysozymes to lyse Actinobacillus actinomycetemcomitans Y4 was investigated. Lysis was followed optically at 540 nm by measuring the percent reduction in turbidity of freshly harvested log-phase cells suspended in Tris-maleate buffers within a wide range of pH (5.2 to 8.5) and molarity (0.01 to 0.2 M) and containing various amounts of enzyme and EDTA. In several instances, treated microorganisms were subsequently examined in thin sections by electron microscopy. Reductions in turbidity and clearing of suspensions occurred with small amounts of lysozyme (less than 1 microgram) under relatively alkaline conditions and at low ionic strength and in the presence of small amounts of EDTA (greater than 0.01 mM). Under the most alkaline conditions, EDTA alone effected turbidity reductions similar to those observed in the presence of lysozyme, which suggested that EDTA not only increased outer membrane permeability but also caused cell lysis. Ultrastructural analysis did not always correspond to turbidimetric observations. Cell lysis was virtually complete in suspensions containing both lysozyme and EDTA. However, in contrast to turbidimetric findings, a significant percentage of cells (greater than 25%) was lysed in the presence of lysozyme alone. Furthermore, significant damage occurred in the presence of EDTA alone. Spheroplast-like cell ghosts were present which surrounded condensed cytoplasm or relatively clear spaces. These findings further support the concept of the requirement for electron microscopy to assess lytic damage in addition to turbidimetric and biochemical methods. Our results are the first to demonstrate the remarkable sensitivity of A. actinomycetemcomitans Y4 to lysozyme and to show that EDTA not only affects outer membrane permeability but effects cell lysis, possibly through activation of autolytic enzymes at the cytoplasmic membrane. The exquisite sensitivity of A. actinomycetemcomitans Y4 to lysis could be an important mechanism by which lysozyme participates in the regulation of this suspected periodontal pathogen.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki Y., Nakatani T., Nakayama K., Ito E. Occurrence of N-nonsubstituted glucosamine residues in peptidoglycan of lysozyme-resistant cell walls from Bacillus cereus. J Biol Chem. 1972 Oct 10;247(19):6312–6322. [PubMed] [Google Scholar]
- Attström R. Studies on neutrophil polymorphonuclear leukocytes at the dento-gingival junction in gingival health and disease. J Periodontal Res Suppl. 1971;8:1–15. [PubMed] [Google Scholar]
- BLADEN H. A., MERGENHAGEN S. E. ULTRASTRUCTURE OF VEILLONELLA AND MORPHOLOGICAL CORRELATION OF AN OUTER MEMBRANE WITH PARTICLES ASSOCIATED WITH ENDOTOXIC ACTIVITY. J Bacteriol. 1964 Nov;88:1482–1492. doi: 10.1128/jb.88.5.1482-1492.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRANDTZAEG P., MANN W. V., Jr A COMPARATIVE STUDY OF THE LYSOZYME ACTIVITY OF HUMAN GINGIVAL POCKET FLUID, SERUM, AND SALIVA. Acta Odontol Scand. 1964 Oct;22:441–455. doi: 10.3109/00016356409028217. [DOI] [PubMed] [Google Scholar]
- BRUMFITT W., WARDLAW A. C., PARK J. T. Development of lysozyme-resistance in Micrococcus lysodiekticus and its association with an increased O-acetyl content of the cell wall. Nature. 1958 Jun 28;181(4626):1783–1784. doi: 10.1038/1811783a0. [DOI] [PubMed] [Google Scholar]
- Bretz U., Baggiolini M. Biochemical and morphological characterization of azurophil and specific granules of human neutrophilic polymorphonuclear leukocytes. J Cell Biol. 1974 Oct;63(1):251–269. doi: 10.1083/jcb.63.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cainciola L. J., Genco R. J., Patters M. R., McKenna J., van Oss C. J. Defective polymorphonuclear leukocyte function in a human periodontal disease. Nature. 1977 Feb 3;265(5593):445–447. doi: 10.1038/265445a0. [DOI] [PubMed] [Google Scholar]
- Chopra I., Howe G. B., Ball P. R. Lysozyme-promoted association of protein I molecules in the outer membrane of Escherichia coli. J Bacteriol. 1977 Nov;132(2):411–418. doi: 10.1128/jb.132.2.411-418.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P., Bonney R. J., Humes J. L., Kuehl F. A. The role of macrophage secretory products in chronic inflammatory processes. J Invest Dermatol. 1980 May;74(5):292–296. doi: 10.1111/1523-1747.ep12543476. [DOI] [PubMed] [Google Scholar]
- Davies R. C., Neuberger A. Modification of lysine and arginine residues of lysozyme and the effect on enzymatic activity. Biochim Biophys Acta. 1969 Apr 22;178(2):306–317. doi: 10.1016/0005-2744(69)90398-2. [DOI] [PubMed] [Google Scholar]
- Davies R. C., Neuberger A., Wilson B. M. The dependence of lysozyme activity on pH and ionic strength. Biochim Biophys Acta. 1969 Apr 22;178(2):294–305. doi: 10.1016/0005-2744(69)90397-0. [DOI] [PubMed] [Google Scholar]
- Day D. F., Marceau-Day M. L., Ingram J. M. Protein-lipopolysaccharide interactions. 1. The reaction of lysozyme with Pseudomonas aeruginosa LPS. Can J Microbiol. 1978 Feb;24(2):196–199. doi: 10.1139/m78-035. [DOI] [PubMed] [Google Scholar]
- Duckworth D. H., Bevers E. M., Verkleij A. J., Op den Kamp J. A., van Deenen L. L. Action of phospholipase A2 and phospholipase C on Escherichia coli. Arch Biochem Biophys. 1974 Nov;165(1):379–387. doi: 10.1016/0003-9861(74)90176-3. [DOI] [PubMed] [Google Scholar]
- Elsbach P. Degradation of microorganisms by phagocytic cells. Rev Infect Dis. 1980 Jan-Feb;2(1):106–128. doi: 10.1093/clinids/2.1.106. [DOI] [PubMed] [Google Scholar]
- Genco R. J., Mergenhagen S. E. From the National Institute of Dental Research: summary of a workshop on leukocyte function in bacterial diseases with an emphasis on periodontal disease. J Infect Dis. 1979 May;139(5):604–612. doi: 10.1093/infdis/139.5.604. [DOI] [PubMed] [Google Scholar]
- Goldschmidt M. C., Wyss O. The role of tris in EDTA toxicity and lysozyme lysis. J Gen Microbiol. 1967 Jun;47(3):421–431. doi: 10.1099/00221287-47-3-421. [DOI] [PubMed] [Google Scholar]
- HOLDEN J. T., VANBALGOOY J. N. EFFECT OF NUTRITIONAL AND PHYSIOLOGICAL FACTORS ON THE REACTION BETWEEN LACTOBACILLUS PLANTARUM AND MURAMIDASE. Biochem Biophys Res Commun. 1965 May 3;19:401–406. doi: 10.1016/0006-291x(65)90136-1. [DOI] [PubMed] [Google Scholar]
- Hardaway K. L., Buller C. S. Effect of ethylenediaminetetraacetate on phospholipids and outer membrane function in Escherichia coli. J Bacteriol. 1979 Jan;137(1):62–68. doi: 10.1128/jb.137.1.62-68.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann R., Bock-Hennig S. B., Schwarz U. Murein hydrolases in the envelope of Escherichia coli. Properties in situ and solubilization from the envelope. Eur J Biochem. 1974 Jan 3;41(1):203–208. doi: 10.1111/j.1432-1033.1974.tb03261.x. [DOI] [PubMed] [Google Scholar]
- Holt S. C., Leadbetter E. R., Socransky S. S. Capnocytophaga: new genus of gram-negative gliding bacteria. II. Morphology and ultrastructure. Arch Microbiol. 1979 Jul;122(1):17–27. doi: 10.1007/BF00408041. [DOI] [PubMed] [Google Scholar]
- Iacono V. J., MacKay B. J., DiRienzo S., Pollock J. J. Selective antibacterial properties of lysozyme for oral microorganisms. Infect Immun. 1980 Aug;29(2):623–632. doi: 10.1128/iai.29.2.623-632.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvin R. T., MacAlister T. J., Costerton J. W. Tris(hydroxymethyl)aminomethane buffer modification of Escherichia coli outer membrane permeability. J Bacteriol. 1981 Mar;145(3):1397–1403. doi: 10.1128/jb.145.3.1397-1403.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving J. T., Newman M. G., Socransky S. S., Heely J. D. Histological changes in experimental periodontal disease in rats mono-infected with a gram-negative organism. Arch Oral Biol. 1975 Mar;20(3):219–220. doi: 10.1016/0003-9969(75)90013-8. [DOI] [PubMed] [Google Scholar]
- Johnston L. S., Neuhaus F. C. Spin-label assay for lysozyme. Anal Biochem. 1978 Mar;85(1):56–62. doi: 10.1016/0003-2697(78)90273-7. [DOI] [PubMed] [Google Scholar]
- Kitano K., Tomasz A. Triggering of autolytic cell wall degradation in Escherichia coli by beta-lactam antibiotics. Antimicrob Agents Chemother. 1979 Dec;16(6):838–848. doi: 10.1128/aac.16.6.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. H., Listgarten M. A., Hammond B. F. Comparative ultrastructure of leukotoxic and non-leukotoxic strains of Actinobacillus actinomycetemcomitans. J Periodontal Res. 1981 Jul;16(4):379–389. doi: 10.1111/j.1600-0765.1981.tb00989.x. [DOI] [PubMed] [Google Scholar]
- Leduc M., Kasra R., van Heijenoort J. Induction and control of the autolytic system of Escherichia coli. J Bacteriol. 1982 Oct;152(1):26–34. doi: 10.1128/jb.152.1.26-34.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffell M. S., Spitznagel J. K. Association of lactoferrin with lysozyme in granules of human polymorphonuclear leukocytes. Infect Immun. 1972 Nov;6(5):761–765. doi: 10.1128/iai.6.5.761-765.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem Biophys Res Commun. 1965 Nov 22;21(4):290–296. doi: 10.1016/0006-291x(65)90191-9. [DOI] [PubMed] [Google Scholar]
- Leive L. Studies on the permeability change produced in coliform bacteria by ethylenediaminetetraacetate. J Biol Chem. 1968 May 10;243(9):2373–2380. [PubMed] [Google Scholar]
- Leive L. The barrier function of the gram-negative envelope. Ann N Y Acad Sci. 1974 May 10;235(0):109–129. doi: 10.1111/j.1749-6632.1974.tb43261.x. [DOI] [PubMed] [Google Scholar]
- MacKay B. J., Iacono V. J., Zuckerman J. M., Osserman E. F., Pollock J. J. Quantitative recovery, selective removal and one-step purification of human parotid and leukemic lysozymes by immunoadsorption. Eur J Biochem. 1982 Dec;129(1):93–98. doi: 10.1111/j.1432-1033.1982.tb07025.x. [DOI] [PubMed] [Google Scholar]
- Martin B. F., Derby B. M., Budzilovich G. N., Ransohoff J. Brain abscess due to Actinobacillus actinomycetemcomitans. Neurology. 1967 Sep;17(9):833–837. doi: 10.1212/wnl.17.9.833. [DOI] [PubMed] [Google Scholar]
- NOLLER E. C., HARTSELL S. E. Bacteriolysis of Enterobacteriaceae. I. Lysis by four lytic systems utilizing lysozyme. J Bacteriol. 1961 Mar;81:482–491. doi: 10.1128/jb.81.3.482-491.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C. The role of amine buffers in EDTA toxicity and their effect on osmotic shock. J Gen Microbiol. 1969 Aug;57(2):215–220. doi: 10.1099/00221287-57-2-215. [DOI] [PubMed] [Google Scholar]
- Newman M. G., Socransky S. S. Predominant cultivable microbiota in periodontosis. J Periodontal Res. 1977 Mar;12(2):120–128. doi: 10.1111/j.1600-0765.1977.tb00114.x. [DOI] [PubMed] [Google Scholar]
- Nowotny A., Behling U. H., Hammond B., Lai C. H., Listgarten M., Pham P. H., Sanavi F. Release of toxic microvesicles by Actinobacillus actinomycetemcomitans. Infect Immun. 1982 Jul;37(1):151–154. doi: 10.1128/iai.37.1.151-154.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osserman E. F., Lawlor D. P. Serum and urinary lysozyme (muramidase) in monocytic and monomyelocytic leukemia. J Exp Med. 1966 Nov 1;124(5):921–952. doi: 10.1084/jem.124.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page R. C., Schroeder H. E. Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab Invest. 1976 Mar;34(3):235–249. [PubMed] [Google Scholar]
- REPASKE R. Lysis of gram-negative bacteria by lysozyme. Biochim Biophys Acta. 1956 Oct;22(1):189–191. doi: 10.1016/0006-3002(56)90240-2. [DOI] [PubMed] [Google Scholar]
- REPASKE R. Lysis of gram-negative organisms and the role of versene. Biochim Biophys Acta. 1958 Nov;30(2):225–232. doi: 10.1016/0006-3002(58)90044-1. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALTON M. R. The properties of lysozyme and its action on microorganisms. Bacteriol Rev. 1957 Jun;21(2):82–100. doi: 10.1128/br.21.2.82-100.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOPHIANOPOULOS A. J., RHODES C. K., HOLCOMB D. N., VAN HOLDE K. E. Physical studies of lysozyme. I. Characterization. J Biol Chem. 1962 Apr;237:1107–1112. [PubMed] [Google Scholar]
- Schindler P. R., Teuber M. Ultrastructural study of Salmonella typhimurium treated with membrane-active agents: specific reaction dansylchloride with cell envelope components. J Bacteriol. 1978 Jul;135(1):198–206. doi: 10.1128/jb.135.1.198-206.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scibienski R. J. Cellular parameters of the immunological memory induced by lysozyme-LPS mixtures and complexes. Immunol Commun. 1979;8(3):325–336. doi: 10.3109/08820137909050046. [DOI] [PubMed] [Google Scholar]
- Slots J., Reynolds H. S., Genco R. J. Actinobacillus actinomycetemcomitans in human periodontal disease: a cross-sectional microbiological investigation. Infect Immun. 1980 Sep;29(3):1013–1020. doi: 10.1128/iai.29.3.1013-1020.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J. The predominant cultivable microflora of advanced periodontitis. Scand J Dent Res. 1977 Jan-Feb;85(2):114–121. doi: 10.1111/j.1600-0722.1977.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Stevens R. H., Hammond B. F., Lai C. H. Characterization of an inducible bacteriophage from a leukotoxic strain of Actinobacillus actinomycetemcomitans. Infect Immun. 1982 Jan;35(1):343–349. doi: 10.1128/iai.35.1.343-349.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taichman N. S., Tsai C. C., Baehni P. C., Stoller N., McArthur W. P. Interaction of inflammatory cells and oral microorganisms. IV. In vitro release of lysosomal constituents from polymorphonuclear leukocytes exposed to supragingival and subgingival bacterial plaque. Infect Immun. 1977 Jun;16(3):1013–1023. doi: 10.1128/iai.16.3.1013-1023.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner A. C., Haffer C., Bratthall G. T., Visconti R. A., Socransky S. S. A study of the bacteria associated with advancing periodontitis in man. J Clin Periodontol. 1979 Oct;6(5):278–307. doi: 10.1111/j.1600-051x.1979.tb01931.x. [DOI] [PubMed] [Google Scholar]
- Tortosa M., Cho M. I., Wilkens T. J., Iacono V. J., Pollock J. J. Bacteriolysis of Veillonella alcalescens by lysozyme and inorganic anions present in saliva. Infect Immun. 1981 Jun;32(3):1261–1273. doi: 10.1128/iai.32.3.1261-1273.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. C., McArthur W. P., Baehni P. C., Hammond B. F., Taichman N. S. Extraction and partial characterization of a leukotoxin from a plaque-derived Gram-negative microorganism. Infect Immun. 1979 Jul;25(1):427–439. doi: 10.1128/iai.25.1.427-439.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandepitte J., De Geest H., Jousten P. Subacute bacterial endocarditis due to Actinobacillus actinomycetemcomitans. Report of a case with a review of the literature. J Clin Pathol. 1977 Sep;30(9):842–846. doi: 10.1136/jcp.30.9.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss J. G. Effects of organic cations on the gram-negative cell wall and their bactericidal activity with ethylenediaminetetra-acetate and surface active agents. J Gen Microbiol. 1967 Sep;48(3):391–400. doi: 10.1099/00221287-48-3-391. [DOI] [PubMed] [Google Scholar]
- Weiss J., Elsbach P., Olsson I., Odeberg H. Purification and characterization of a potent bactericidal and membrane active protein from the granules of human polymorphonuclear leukocytes. J Biol Chem. 1978 Apr 25;253(8):2664–2672. [PubMed] [Google Scholar]