Abstract
Background
Polybrominated diphenyl ethers (PBDEs) are flame-retardant chemicals that accumulate in human tissues and are potential toxicants. Concentrations of PBDEs in human tissues have increased recently, and body burdens in the U.S. and Canadian populations are higher than in any other region.
Objectives
Although metabolism in animal laboratory studies has been examined, no studies have explored the metabolism of these contaminants in human tissues. We undertook this study to determine whether PBDEs could be metabolized by human liver cells in vitro and to identify what types of metabolites are formed.
Methods
We exposed hepatocytes from three different donors (two cryopreserved batches and one fresh batch) to solutions containing 10 μM of either of two environmentally relevant and prominent PBDE congeners—BDE-99 or BDE-209—for periods of 24–72 hr. We also conducted gene expression analysis to provide information on potential induction of xenobiotic metabolizing enzymes.
Results
Exposing hepatocytes to BDE-99 resulted in the formation of 2,4,5-tribromo phenol, two monohydroxylated pentabrominated diphenyl ether metabolites, and a yet unidentified tetrabrominated metabolite. No hydroxylated or debrominated metabolites were observed in the cells exposed to BDE-209. This suggests that BDE-209 was not metabolized, that nonextractable, covalently protein-bound metabolites were formed, or that the exposure time was not long enough for BDE-209 to diffuse into the cell to be metabolized. However, we observed up-regulation of genes encoding for cytochrome P450 monooxygenase (CYP) 1A2, CYP3A4, deiodinase type 1, and glutathione S-transferase M1 in hepatocyes exposed to both BDE-99 and BDE-209.
Conclusions
Our in vitro results suggest that the human liver will likely metabolize some BDE congeners (e.g., BDE-99) in vivo. These metabolites have been shown to elicit greater toxicity than the parent BDE congeners in laboratory bioassays; thus, more research on body burdens and human health effects from these metabolites are warranted.
Keywords: brominated flame retardants, hepatocytes, metabolism, OH-PBDEs, polybrominated diphenyl ethers
Polybrominated diphenyl ethers (PBDEs) are a class of flame-retardant chemicals frequently applied to textiles, furniture, and electronic and electrical items. Large amounts of PBDEs have been produced and applied over the past few decades, resulting in widespread contamination of the environment and accumulation in food webs. Furthermore, because of their physico-chemical properties, PBDEs are persistent in the environment and bioaccumulate in both aquatic and terrestrial food webs (Alaee et al. 2003; Christensen et al. 2005; de Wit et al. 2006; Law et al. 2006).
A number of laboratory animal exposure studies have found significant species-specific differences in uptake kinetics, metabolism, and disposition of several different 14C-labeled and unlabeled PBDE congeners. For example, mice or rats exposed in vivo to 2,2′,4,4′,5-penta-bromodiphenyl ether (BDE-99) have been found to produce oxidative metabolites, such as hydroxylated BDE congeners (OH-BDE) (Chen et al. 2006; Hakk et al. 2002; Qiu et al. 2007). However, in vivo exposure of common carp (Cyprinus carpio) to BDE-99 resulted in significant formation and accumulation of a reductively debrominated metabolite, 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) (Stapleton et al. 2004). In addition, the extent of metabolism in these studies depends on the structure and bromine substitution of the BDE congener. BDE-99 appears to be metabolized to a greater extent than does BDE-47, 2,2′,4,4′,5,5′-hexabromodiphneyl ether (BDE-153), or 2,2′,3,3′,4,4′,5,5′,6,6′-deca-bromodiphenyl ether (BDE-209) (Chen et al. 2006; Morck et al. 2003; Staskal et al. 2006). Thus, these laboratory PBDE metabolism studies suggest that humans will accumulate and metabolize PBDEs; however, it is not clear how PBDEs are specifically metabolized in human tissues and what types of metabolites will be formed.
Studies have documented measurements of PBDEs in several different human populations, and their presence in tissues appears to be ubiquitous (Hites 2004; Schecter et al. 2003; Sjodin et al. 2001). The primary congeners detected in human tissues include BDE congeners 47, 99, and 153, which are the primary congeners found in a commercial mixture referred to as pentaBDE. To our knowledge, no studies have investigated the metabolism of BDE congeners in human tissues. Analyses of human sera have identified multiple OH-BDE congeners, suggesting that metabolism does occur (Athanasiadou et al. 2008); however, natural sources of OH-BDEs have also been identified in marine environments (Malmvarn et al. 2005). The formation of OH-BDE metabolites is of concern because greater adverse effects have been documented for the OH-BDEs relative to the PBDEs in laboratory studies. For example, OH-BDEs have been shown to significantly affect aromatase activity in human adrenocortical carcinoma cells, whereas PBDEs had no effect (Canton et al. 2005). In addition, OH-BDEs have an order of magnitude higher potency than do PBDEs in their ability to compete with thyroid hormones for binding sites on serum transporters (Hamers et al. 2006; Meerts et al. 2000, 2001).
We undertook the present study to determine whether PBDE metabolites could be detected after in vitro exposure to human hepatocytes. Our objective was to determine if reductively debrominated and/or OH metabolites of BDE congeners 99 and 209 (i.e., the primary congeners found in the pentaBDE and decaBDE commercial mixtures) would be produced by human hepatocytes. We also designed this study to examine the expression of genes coding for the enzymes potentially involved in the metabolism of PBDEs through oxidative and reductive pathways.
Materials and Methods
Chemicals and materials
The test compounds, BDE-99 (100 ± 4% purity) and BDE-209 (decabromodiphenyl ether, 98 ± 1% purity), were obtained from AccuStandard, Inc. (New Haven, CT, USA) and Sigma (St. Louis, MO, USA), respectively. We also obtained 2,4,6-tribromo phenol (99% purity) and rifampicin (95% purity) from Sigma. We purchased mono fluorinated PBDEs [4′-fluoro-2,3′,4,6-tetrabromodiphenyl ether (F-BDE-69; 98.2% purity) and 4-fluoro-2,3,3′,4,5,6-hexabromodiphenyl ether (F-BDE-160; 98.1% purity)], used as internal and surrogate standards, from Chiron (Trondheim, Norway) and 13C-labeled BDE-209 (decabromodiphenyl ether; > 98% purity), 13C-labeled 6-OH-BDE-47 (6-OH-2,2′,4,4′-tetrabromodiphenyl ether), and a mixture of eight methoxylated PBDEs (MeO-PBDEs; > 98% purity) from Wellington Laboratories (Guelph, Ontario, Canada). All solvents and other reagents used in these experiments were of analytical grade or higher. For all experiments, we used In Vitro Technologies (Celsis Inc., Baltimore, MD, USA) hepatocytes, culture medium, antibiotics, and collagen-coated culture plates.
Hepatocyte incubations
We used cultured hepatocytes from three individual donors: two cryopreserved (one male and one female) and one (male) “fresh” (shipped within 48 hr of the donor’s passing). Donor information, including sex, age, race, body mass index, alcohol use, tobacco use, drug use, medical history, medication use, cause of death, and measured metabolic activities (provided by supplier), are listed in Table 1.
Table 1.
Hepatocyte donor characteristics.
| Donor
|
|||
|---|---|---|---|
| Characteristic | 1 | 2 | 3 |
| State of shipped hepatocyes | Cryopreserved | Fresh | Cryopreserved |
| Lot no. | KQG | MHU-L-092507 | ONQ |
| Experimental repetitions (n ) | 2 | 1 | 1 |
| Sex | Female | Male | Male |
| Age (years) | 38 | 50 | 61 |
| Race | Caucasian | Caucasian | Caucasian |
| Body mass index | 38.6 | 34.4 | 42.9 |
| History of alcohol use | Yes | Yes | Yes |
| History of narcotic use | None reported | None reported | None reported |
| History of tobacco use | Yes | None reported | Yes |
| Relevant medical history | None reported | None reported | None reported |
| Relevant chronic medications | None reported | None reported | None reported |
| Cause of death | Cerebrovascular accident (stroke) | Head trauma | Head trauma |
| Initial viability (%) | 83.8 | 93a | 83.7 |
| Viable cell density (cells/mL) | 7.0 × 105 | NA | 7.0 × 105 |
| Confluence at 24 hr (%) | 80 | 70 | 50–60 |
| Metabolic activityb (pmol/106 cells/min) | |||
| Formation of 7-hydroxycoumarin | 49 | N/A | 66 |
| Formation of 7-hydroxycoumarin glucuronide | 191 | NA | 247 |
| Formation of 7-hydroxycoumarin sulfate | 12 | NA | 47 |
| Formation of 6β-hydroxytestosterone | 108 | NA | 60 |
| Formation of 4′-methylhydroxytolbutamide | 25 | NA | 18 |
NA, not available.
At time of plating (measured by supplier).
Provided by hepatocyte supplier.
Cryopreserved human hepatocytes arrived in 1-mL vials at −80°C in liquid nitrogen. Before thawing, we added 5.5 mL Torpedo Antibiotic Mix to 250 mL InVitroGRO CP Media and warmed the mixture to 37°C. We immersed frozen vials of hepatocytes in a 37°C water bath, gently shook them until thawed, and then added them to 5 mL of the medium–antibiotic mix. We determined cell viability by the trypan blue exclusion method. The initial viability of the cryopreserved hepatocytes after thawing was high (> 83%), and we plated cells in a 12-well plate at a density of 7.0 × 105 cells/mL. We incubated the cultures undisturbed for 24 hr to allow for cell adhesion. Afterward, we visually inspected confluence under a microscope (10×) and exchanged the plating media for experimental dosing media.
Fresh hepatocytes were seeded by the manufacturer (Celsis Inc., Baltimore, MD, USA) at a seeding density of 160,000 cells/cm2 and were shipped overnight at 4°C, pre-plated on a 12-well plate with the cell cultures immersed in a proprietary “shipping medium.” Upon arrival, we placed the hepatocytes in the incubator for 3 hr to equilibrate and then exchanged the shipping medium for experimental dosing media. Table 1 summarizes the conditions of the plated cell cultures, including initial cell viability, viable cell density on plates, and confluence before experimental dosing.
We used InVitroGRO HI Media (Celsis Inc.) with Torpedo Antibiotic Mix for dosing the hepatocytes and for maintaining the cell cultures for the remaining experiments. BDE-99, BDE-209, and rifampicin solutions were prepared in dimethyl sulfoxide (DMSO) at a concentration 100 times that of the desired final concentration in order to minimize hepatocyte exposure to the dosing vehicle. DMSO dosing solution was then diluted 1:100 in InVitroGRO HI Media to yield final concentrations of 10 μM for BDE-99 and BDE-209 and 2.5 μM for rifampicin. We conducted experimental dosing of the hepatocytes using three to four replicates (wells) per treatment. Wells were treated with media containing BDE-99, BDE-209, or clean media only (control). All three hepatocyte batches were exposed to BDE-99 and BDE-209 at a nominal concentration of 10 μM, equivalent to 10 nmol of each compound per well. Aliquots of the BDE dosing media were also incubated (in triplicate) alone on the well plates adjacent to the hepatocytes as controls and analyzed at the end of exposure to determine the exposure concentrations. Separate 12-well plates were used for the metabolism and gene expression analysis. We conducted gene expression analysis using only fresh hepatocytes because of insufficient recovery of RNA in the cryopreserved hepatocytes.
We conducted steps involving manipulation of cell cultures in a biological safety cabinet under sterile conditions. Plated cell cultures were maintained in a saturating humidity incubator at 37°C and 5% CO2 during incubations. Cryopreserved cell cultures were allowed to incubate with one dose of experimental media for 48 hr. Plates of fresh hepatocyte cultures used for the metabolism studies were treated once every 24 hr for 3 days to take advantage of the increased activity of fresh cells and potential increase in metabolite formation. During medium exchange in the fresh hepatocytes, we collected and pooled the contents from each well. Fresh hepatocyte cultures used for the gene expression analysis were allowed to incubate with one dose of the experimental media for 24 hr. After incubation, the hepatocytes were removed from the wells using 1 mL methanol (only wells used in the metabolism study) to disrupt cell membranes. The contents were subsequently transferred to clean glass test tubes for extraction.
Sample extraction
Hepatocytes and media were extracted using methods developed for the extraction of phenolic and neutral compounds from serum (Hovander et al. 2000). Briefly, samples were first spiked with three internal standards—F-BDE-160, 13C-labeled 6-OH-BDE-47, and 13C-labeled BDE-209—and extracted using methyl-tert-butyl ether:hexane (1:1). Lipids were removed from the extracts with concentrated sulfuric acid, and then the neutral and phenolic compounds were separated using a basic aqueous solution of potassium hydroxide. The phenolic fraction was derivatized with an ethereal solution of diazomethane to produce MeO metabolites for GC/MS analysis.
Sample analysis
We analyzed all samples using gas chromatography–mass spectrometry (GC/MS) operated in both electron-impact mode (GC/EI-MS) and electron-capture negative-ionization mode (GC/ECNI-MS). The GC/MS operating conditions have been described previously (Stapleton et al. 2008). We confirmed metabolites in both GC/EI-MS and GC/ECNI-MS modes. We monitored PBDEs, MeO-BDEs, and the fluorinated BDEs using the m/z responses of 79 and 81 (bromide ions), and BDE-209 and 13C-BDE-209 using m/z responses of 486.6, 484.6, 496.6, and 494.6 in GC/ECNI-MS mode. We analyzed OH-BDE metabolites by GC/ECNI-MS using responses of MeO-BDE calibration standards. The National Wildlife Research Centre provided further analysis of phenolic fractions for possible BDE-209 oxidative metabolites using an Alliance 2695 high-performance liquid chromatograph (Waters Corporation, Milford, MA, USA) connected to a Waters Quattro Ultima triple quadrupole mass spectrometer (LC/MS-MS). Because OH-nona-BDE congeners are not commercially available, we optimized all methods using the LC/MS-MS using 6-OH-BDE-90.
Gene expression analysis
We collected RNA only from the fresh hepatocytes after 24 hr of exposure to the dosing media. We did not examine gene expression in the cryopreserved hepatocytes because of low recovery of RNA. The analysis was conducted to determine the expression of several genes that encode potential biotransforming enzymes, including cytochrome P450 monooxygenase (CYP) 1A2, CYP3A4, deiodinase (DI) types 1 and 2, glutathione S-transferase (GST) M1, and GSTP1. Absolute transcript numbers were quantified using a Stratagene Mx3000P Real Time PCR (polymerase chain reaction) apparatus (Stratagene, La Jolla, CA). We developed protocols using SYBR Green I (Molecular Probes, Inc., Eugene, OR, USA). Because SYBR Green I can bind nonspecifically to all double-stranded DNA, optimization steps were performed to eliminate signals obtained from either primer-dimer complexes or other nonspecific products. We monitored the expression of CYP1A2 [GenBank accession no. NM_000761 (National Center for Biotechnology Information 2008)], CYP3A4 (NM_017460), DI1 (NM_000792), GSTM1 (NM_000561), and GSTP1 (NM_000852) using published primer sequences (Brasch-Andersen et al. 2004; Chanas et al. 2002; Lindell et al. 2003; Yamaori et al. 2005). We used glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a housekeeping gene control for data analysis following published protocols (Strehlau et al. 1997). Each reaction was performed in a 25-μL reaction mixture consisting of 12.5 μL 2× iTaq SYBR Green Supermix with ROX (Stratagene, Hercules, CA, USA), 0.2 μM reverse and forward primers, 1 μL template, and 9.5 μL deionized water. The thermal cycler program consisted of an initial denaturing step at 95°C for 3 min, followed by 40 cycles of 95°C for 30 sec, 62°C for CYP3A4 (56°C for CYP1A2, 62°C for DI1, 60°C for GSTM1, 57°C for GSTP1, and 62°C for GAPDH) for 30 sec, and 72°C for 30 sec. The absolute target transcript number was calculated based on a standard curve prepared as described previously (Pei et al. 2006). Statistical significance was determined by performing a paired Student’s t-test comparing transcript numbers between the negative control (no BDE) and each treatment.
QA/QC and data analysis
Recovery of surrogate standards F-BDE-69, 13C-6-OH-BDE-47, and 13C-BDE-209 averaged 86 ± 12%, 50 ± 7%, and 47 ± 44%, respectively. Analyte values were corrected for recovery. Laboratory blanks did contain minor amounts of BDE-99 (< 3 ng); however, given the high concentrations used in the dosing, blank correction was not necessary. Levels of all metabolites observed were below limits of detection (LODs) in all laboratory blanks and hepatocyte control samples. We defined LODs as three times the SD of the laboratory blanks. For congeners not detected in the blanks, we set the LOD at the instrumental limit of quantification. BDE, metabolite, and gene expression data were analyzed for statistical significance by performing paired Student’s t-tests. For gene expression, we compared transcript numbers between the negative control (no BDE) and each treatment. All statistical analyses were carried out using Microsoft Excel (Microsoft Corp., Redmond, WA, USA), with the statistical significance defined at α = 0.05.
Results and Discussion
Table 1 presents descriptive information regarding donor characteristics, handling of hepatocytes, and viability of cells. All experiments showed optimal confluence (i.e., > 70%) except one, which displayed a confluence of about 50–60%. We observed no apparent differences in cytotoxicity between exposed and control hepatocytes based on visual inspection under the microscope. This is consistent with a previously published study using human adrenocortical carcinoma cells exposed to a similar concentration of BDE-99 in which no cytotoxicity was measured with a mitochondrial toxicity test (Canton et al. 2006).
BDE dosing and recovery
The mass of BDE-99 and BDE-209 to which the cells were exposed was 10.47 ± 0.50 and 8.72 ± 0.25 nmol/well, respectively, using GC/ECNI-MS. We measured concentrations of BDEs in the neutral fractions of extracts collected from the hepatocytes. Because the fresh hepatocytes were repeatedly exposed (exposure media replenished once daily for 3 days), we performed a mass balance only on the cryopreserved hepatocytes that were dosed once. After the 48-hr incubation, we recovered 9.62 ± 0.11 and 8.28 ± 0.16 nmol BDE-99 and BDE-209, respectively, in the cryopreserved hepatocyte wells. Therefore, it appears that approximately 8% of the BDE-99 mass was unrecovered, but only 3% of BDE-209 mass. The greater amount of unrecovered mass of BDE-99 is likely attributed to metabolism, since we observed metabolites of BDE-99. Most of the BDE-209 mass was recovered, which suggests that little to no metabolism occurred. We could not estimate the fraction of BDE mass that actually diffused into the cells and was available for metabolism. It is possible that diffusion of BDE-209 into hepatocytes is a slow process and that we did not provide adequate time to observe induction of enzymes (e.g., CYPs) and subsequent metabolism. We did not perform additional experiments with various dosing levels of BDEs and different exposure periods; our primary focus was to determine if metabolism was consistent among hepatocytes harvested from three different individuals, providing insight into expected metabolic capability among the general population. Furthermore, the high dose we used in this study increased the likelihood of detecting metabolites. The exposure used in this study (~ 10 μM) is relatively high and not environmentally relevant for human exposure. Concentrations of total BDEs in human blood and milk typically average about ≤ 0.5 nM (Schecter et al. 2003, 2005; Sjodin et al. 2004), yet BDE levels measured in adipose tissue are higher, with a mean value of 132 nmol/kg adipose tissue being reported for BDE-99 (Johnson-Restrepo et al. 2005).
Metabolite identification
We observed no reductively debrominated metabolites in the neutral extracts isolated from the hepatocytes exposed to either BDE-99 or BDE-209, indicating that the metabolic reductive debromination does not occur or that the exposure period of this assay was too small. This suggests that reductive debromination is not likely to be a substantial metabolic pathway in human liver tissue. These results are in contrast to several in vivo studies and one in vitro study using liver subcellular fractions that showed significant reductive debromination of BDE congeners 99, 183, and 209 in fish, rodents, and birds (Huwe and Smith 2007; Kierkegaard et al. 1999; Stapleton et al. 2004; Tomy et al. 2004; Van den Steen et al. 2007).
To determine whether oxidative metabolites were being formed during our experiment, we isolated the phenolic fraction of the extract, which we then derivatized to methyl analogs and analyzed using GC/ECNI-MS. Extracts from the phenolic fraction of hepatocytes exposed to BDE-209 revealed no oxidative metabolites. We hypothesized that steric hindrance from the large number of bromine atoms substantially decreases the degree of derivatization of any potential nona-OH-BDE metabolites to their MeO analogs. To examine this possibility, we analyzed the phenolic fractions using LC/MS-MS (see “Materials and Methods”). LC/MS-MS analyses of these fractions for the [M+→Br−] multiple reaction monitoring transition of OH derivatives of penta-, hexa-, hepta-, octa-, and nona-BDE analytes were all below LOD (< 0.05 ng/mL). Thus, no specific metabolites of BDE-209 were identified in this study. It is possible that metabolism led to reactive intermediates (e.g., arene oxides) that covalently bound BDE-209 to cellular lipids and/or proteins, which are not recovered during the extraction process. This has indeed been observed in rodent exposure studies using radiolabeled BDEs (Hakk et al. 2002; Morck et al. 2003). Further studies using radiolabeled BDE-209 are needed to determine if metabolism leading to covalent binding is occurring in human hepatocytes.
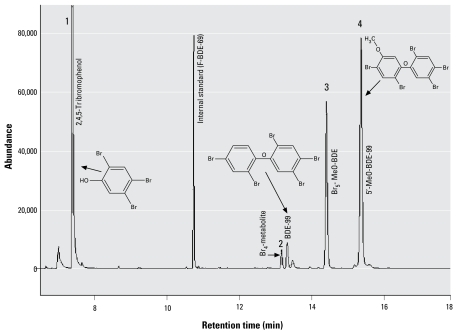
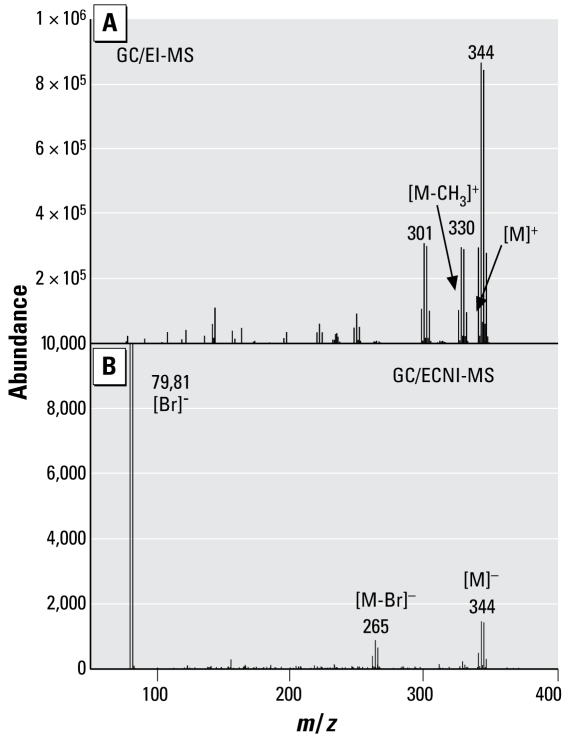
In contrast to the BDE-209 exposure, we observed several oxidative metabolites in all hepatocytes exposed to BDE-99. As shown in Figure 1, four metabolites were identified. Because a liquid/liquid extraction technique was used to separate the neutral and phenolic fractions, a small proportion of BDE-99 was identified in the phenolic fraction; however, the mass of BDE-99 in the phenolic fraction accounted for < 0.09% of the initial dose. Metabolite 1 has been identified as a tribromophenol and is likely 2,4,5-tribromophenol. Figure 2 presents the GC/EI-MS and GC/ECNI-MS mass spectra for this bromo phenol compound. We also used the NIST 2005 MS library (National Institute of Standards and Technology; Gaithersburg, MD, USA) to compare the metabolite mass spectra with all spectra available in the GC/EI-MS database. The NIST library confirmed a 98.8% match with the methyl derivative of 2,4,6-tribromophenol, also known as 1,3,5-tribromo-2-methoxy-benzene. We purchased a commercial standard of 2,4,6-tribromophenol, which we derivatized and compared with peak 1. Although the retention time of metabolite 1 was 0.5 min later than the 2,4,6-tribromophenol derivative, the molecular ion and ion fragment clusters were very similar. The elution time for a compound containing a meta-substituted bromine will typically be later than the elution time for a compound containing an ortho-substituted bromine, as exemplified by the earlier retention times of BDE-100 (2,2,4,4′,6-BDE) relative to BDE-99 on a DB-5 capillary column (Korytar et al. 2005; La Guardia et al. 2006). Thus, it is likely that metabolite 1 is 2,4,5-tribromophenol, which would be formed by a simple cleavage at the ether linkage. Previous studies in which rats were exposed to BDE-99 identified 2,4,5-tribromophenol and its glucuronide and sulfate conjugates in rat urine (Chen et al. 2006). However, an earlier study identified no brominated phenols in rats exposed to BDE-99 (Hakk et al. 2002).
Figure 1.
GC/ECNI-MS chromatogram (m/z 79 and 81) of the derivatized phenolic fraction isolated from fresh hepatocytes incubated with 10 μM BDE-99, identifying the four metabolites 1–4.
Figure 2.
GC/MS full-scan spectra (molecular weight, 342 Da) detected in fresh hepatocytes exposed to 10 μM BDE-99. (A) EI mode. (B) ECNI mode.
Using 2,4,6-tribromophenol as a standard, we measured the concentrations of 2,4,5-tribromophenol in all hepatocyte incubations; the mean values are presented in Table 2. The mean concentration of tribromophenol was an order of magnitude higher in fresh hepatocytes than in cryopreserved hepatocytes and is likely a result of the repeated dosing of the fresh hepatocytes.
Table 2.
Metabolite concentrations (pmol/well, mean ± SD) measured in hepatocytes after BDE-99 exposure.
| Donor
|
|||
|---|---|---|---|
| Metabolite | 1 (n = 6) | 2 (n = 4) | 3 (n = 4) |
| 2,4,5-Tribromophenola | 9.31 ± 1.6 | 96.3 ± 2.3 | 8.36 ± 0.24 |
| 5′-OH-BDE-99 | 20.8 ± 2.5 | 302.9 ± 41.4 | 11.2 ± 0.2 |
| Penta-OH-BDE | 23.3 ± 2.6 | 155.6 ± 12.2 | 13.6 ± 0.7 |
| Tetra metabolite 1b | NQ | 18.7 ± 1.1 | NQ |
NQ, not quantified; n, number of replicates/wells.
Estimated using response of 2,4,6-tribromophenol.
Estimated assuming GC/MS response of tetra-OH-BDEs.
We identified two metabolites (metabolites 3 and 4 in Figure 1) as the methyl derivative of mono-OH-pentabrominated diphenyl ethers (Br5-MeO-BDE and 5′-MeO-BDE-99). We made a positive identification using a commercially available standard for 5′-MeO-BDE-99 and confirmed it in both GC/ECNI-MS and GC/EI-MS modes. The molecular ion (M+ = m/z 594) and primary ion fragment cluster (M-158 = m/z 432) for pentabrominated MeO-BDE congeners were identified for both metabolites using GC/EI-MS mode. The structure of the first eluting Br5-MeO-BDE compound could not be positively identified because of a lack of standards for all pentabrominated MeO-BDEs. However, we can exclude the following compounds for which we have standards: 2,2′,4,4′,6-pentabromo-5′-methoxydiphenyl ether (5′-MeO-BDE-100), 2,2′,4,5,5′-pentabromo-4′-methoxydiphenyl ether (4′-MeO-BDE-101), and 2,2′,4,5′,6- pentabromo-4′-methoxydiphenyl ether (4′-MeO-BDE-103). Given that laboratory exposure studies using polychlorinated biphenyls and PBDEs have typically found oxidative metabolism primarily in the meta or para positions (Athanasiadou et al. 2008; Letcher et al. 2001; Malmberg et al. 2005; Qiu et al. 2007), it is possible that metabolite 3 is 2,2′,4,4′,5-pentabromo-3-methoxydiphenyl ether (3-MeO-BDE-99). Mean concentrations of the two pentabrominated MeO-BDE congeners are presented in Table 2, and were two to three times higher than the concentration of 2,4,5-tribromophenol. The fresh hepatocytes also contained higher concentrations of the OH metabolites relative to the cryopreserved hepatocytes; this is likely due to the repeated dosing of the fresh hepatocytes. We also found 5′-OH-BDE-99 at higher concentrations than the first eluting pentahydroxy-BDE metabolite in the fresh hepatocytes, whereas in the cryopreserved hepatocytes 5′-OH-BDE-99 was equivalent or lower in concentration. The reasons for this are unclear at this time.
The structure of metabolite 2 has not been identified. However, because of the molecular ion clusters observed in GC/ECNI-MS full scan, this metabolite likely contains four bromine atoms. Sensitivity was not sufficient to allow an analysis by full-scan GC/EI-MS needed for determining the molecular mass. Previous exposure studies with rats and mice have identified oxidative debrominated metabolites (e.g., OH-tetrabromo-BDEs) after exposure to BDE-99 in vivo (Chen et al. 2006; Hakk et al. 2002; Qiu et al. 2007). Therefore, it is possible that metabolite 2 is a tetra-OH-BDE.
mRNA expression
To investigate the potential involvement of several metabolizing enzymes, we investigated the mRNA expression of genes encoding these enzymes. Because our previous in vitro experiments with fish liver tissue found significant reductive debromination of BDEs by an unknown pathway (Benedict et al. 2007; Stapleton et al. 2006), we decided to examine the regulation of several enzymes that are involved in reductive pathways (e.g., DIs, GSTs, and CYPs): CYP1A2, DI1, DI2, GSTM1, and GSTP1. We investigated CYP1A2 rather than CYP1A1 because previous experiments have found no up-regulation of CYP1A1 genes in humans; however, data from Barber et al. (2006) suggested that lower concentrations of BDE-99 may result in the up-regulation of CYP1A2 in human MCF-7 breast cancer cells. A 2.5-μM solution of rifampicin was used as a positive control because this compound is a significant up-regulator of CYP3A4 (Nishimura et al. 2007).
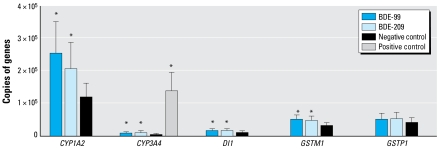
Figure 3 shows the absolute transcript number of each target gene for cells exposed to BDE-99 and BDE-209 relative to control cells. Statistical analysis shows that CYP1A2, CYP3A4, DI1, and GSTM1 were significantly (p < 0.05) up-regulated after exposure to both BDE-99 and BDE-209; however, the up-regulation was minor compared with the up-regulation of CYP3A4 from rifampicin (positive control). The up-regulation of CYP1A2, CYP3A4, DI1, and GSTM1 in BDE-99–exposed hepatocytes was 2.1-, 2.2-, 1.6-, and 1.6-fold, respectively, and was comparable with the up-regulation observed in BDE-209–exposed hepatocytes. There was no significant effect on either GSTP1 or DI2 (data not shown) with either BDE-99 or BDE-209. The up-regulation of the CYP genes and the formation of several oxidative metabolites of BDE-99 support a role for CYP-mediated metabolism.
Figure 3.
mRNA expression (mean ± SD; n = 3) of several genes encoding for potential biotransforming enzymes in fresh hepatocytes exposed to BDEs or rifampicin (positive control).
*p < 0.05 relative to negative control.
DIs are membrane-bound enzymes that catalyze the deiodination of thyroid hormone, and three subtypes of DI (DIs 1–3) have been reported (Kohrle 1999). After dietary exposure to PBDEs, circulating levels of the thyroid hormone thyroxine (T4) have been reduced in mice (Hallgren et al. 2001), rats (Hallgren et al. 2001; Zhou et al. 2001, 2002), birds (Fernie et al. 2005), and fish (Tomy et al. 2004). One possible explanation for these observations is that PBDEs induce up-regulation of DI1 and/or DI2, thereby increasing deiodination of T4 and reducing circulating T4. However, our results demonstrate that expression of DI1 (Figure 3) is minimally affected after exposure to PBDEs and that DI2 was not detected, as we expected because of reports that human liver tissues do not express DI2 activity (Hulbert 2000; Kohrle 1999). Another likely explanation is that the formation of the OH metabolites was responsible for the up-regulation of these genes because the addition of the OH group increases the structural similarities between PBDEs and T4. In fact, microsomal conversion of BDE-99 has been shown to lead to increased competition with T4 for binding to the transporter transthyretin, suggesting that PBDE hydroxylation leads to increased structural similarities and competition with T4 (Meerts et al. 2000). Thus, these data demonstrate that metabolism of BDE-99 may involve multiple pathways and that cytochrome P450, as a mono oxygenase, likely participates in the metabolism of BDE-99.
Conclusion
This study demonstrates that BDE-99, and perhaps other BDE congeners, is metabolized by human liver cells, primarily through oxidative pathways. These observations are very similar to results found in previous rodent exposure studies. This is particularly similar to a study by Chen et al. (2006), which found 2,4,5-tribromophenol, one mono-tetra-OH-BDE, and two mono-OH-BDE-99 metabolites in the feces of rats exposed to BDE-99 in vivo. In contrast, our results differ significantly from metabolism studies on fish liver cells, which found that metabolism occurred primarily through reductive pathways (Benedict et al. 2007; Stapleton et al. 2004, 2006). It may be the absence of DI2 in human liver cells and the high activity of this enzyme in fish liver tissue (Eales et al. 1999; Orozco et al. 1997) that is responsible for this difference. Further studies are warranted to determine whether human DI2 enzyme, found primarily in brain tissues, can reductively debrominate PBDEs, because several studies have found reductively debrominated metabolites of BDE-209 in laboratory-exposed rats (Huwe and Smith 2007), lactating cows (Kierkegaard et al. 2007), and occupationally exposed workers (Thuresson et al. 2005, 2006). Regardless, the oxidative metabolites observed in this study should be measured in human serum in the future because studies have demonstrated increased toxicity from these oxidative metabolites.
Footnotes
We thank T. Moeller (Celsis Inc.) for assistance and advice during these experiments, and S. Chu (National Wildlife Research Centre, Environment Canada, Carleton University) for expert liquid chromatography-mass spectrometry analysis for possible OH-containing metabolites from BDE-209. We also thank L. Birnbaum for assistance and advice in designing these experiments.
References
- Alaee M, Arias P, Sjodin A, Bergman A. An overview of commercially used brominated flame retardants, their applications, their use patterns in different countries/regions and possible modes of release. Environ Int. 2003;29(6):683–689. doi: 10.1016/S0160-4120(03)00121-1. [DOI] [PubMed] [Google Scholar]
- Athanasiadou M, Cuadra SN, Marsh G, Bergman A, Jakobsson K. Polybrominated diphenyl ethers (PBDEs) and bioaccumulative hydroxylated PBDE metabolites in young humans from Managua, Nicaragua. Environ Health Perspect. 2008;116:400–408. doi: 10.1289/ehp.10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber JL, Walsh MJ, Hewitt R, Jones KC, Martin FL. Low-dose treatment with polybrominated diphenyl ethers (PBDEs) induce altered characteristics in MCF-7 cells. Mutagenesis. 2006;21(5):351–360. doi: 10.1093/mutage/gel038. [DOI] [PubMed] [Google Scholar]
- Benedict RT, Stapleton HM, Letcher RJ, Mitchelmore CL. Debromination of polybrominated diphenyl ether-99 (BDE- 99) in carp (Cyprinus carpio) microflora and microsomes. Chemosphere. 2007;69(6):987–993. doi: 10.1016/j.chemosphere.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Brasch-Andersen C, Christiansen L, Tan QH, Haagerup A, Vestbo J, Kruse TA. Possible gene dosage effect of glutathione-S-transferases on atopic asthma: using real-time PCR for quantification of GSTM1 and GSTT1 gene copy numbers. Hum Mutat. 2004;24(3):208–214. doi: 10.1002/humu.20074. [DOI] [PubMed] [Google Scholar]
- Canton RF, Sanderson JT, Letcher RJ, Bergman A, van den Berg M. Inhibition and induction of aromatase (CYP19) activity by brominated flame retardants in H295R human adrenocortical carcinoma cells. Toxicol Sci. 2005;88(2):447–455. doi: 10.1093/toxsci/kfi325. [DOI] [PubMed] [Google Scholar]
- Canton RF, Sanderson JT, Nijmeijer S, Bergman A, Letcher RJ, van den Berg M. In vitro effects of brominated flame retardants and metabolites on CYP17 catalytic activity: a novel mechanism of action? Toxicol Appl Pharmacol. 2006;216(2):274–281. doi: 10.1016/j.taap.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Chanas SA, Jiang Q, McMahon M, McWalter GK, McLellan LI, Elcombe CR, et al. Loss of the Nrf2 transcription factor causes a marked reduction in constitutive and inducible expression of the glutathione S-transferase Gsta1, Gsta2, Gstm1, Gstm2, Gstm3 and Gstm4 genes in the livers of male and female mice. Biochem J. 2002;365:405–416. doi: 10.1042/BJ20020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LJ, Lebetkin EH, Sanders JM, Burka LT. Metabolism and disposition of 2,2′,4,4′,5-pentabromodiphenyl ether (BDE99) following a single or repeated administration to rats or mice. Xenobiotica. 2006;36(6):515–534. doi: 10.1080/00498250600674477. [DOI] [PubMed] [Google Scholar]
- Christensen JR, Macduffee M, Macdonald RW, Whiticar M, Ross PS. Persistent organic pollutants in British Columbia grizzly bears: consequence of divergent diets. Environ Sci Technol. 2005;39(18):6952–6960. doi: 10.1021/es050749f. [DOI] [PubMed] [Google Scholar]
- de Wit CA, Alaee M, Muir DCG. Levels and trends of brominated flame retardants in the Arctic. Chemosphere. 2006;64(2):209–233. doi: 10.1016/j.chemosphere.2005.12.029. [DOI] [PubMed] [Google Scholar]
- Eales JG, Brown S, Cyr DG, Adams BA, Finnson KR. Deiodination as an index of chemical disruption of thyroid hormone homeostasis and thyroidal status in fish. In: Henshel DS, Black MC, Harrass MC, editors. Environmental Toxicology and Risk Assessment: Standardization of Biomarkers for Endocrine Disruption and Environmental Assessments. West Conshohocken, PA: American Society for Testing and Materials; 1999. pp. 136–164. [Google Scholar]
- Fernie KJ, Shutt JL, Mayne G, Hoffman D, Letcher RJ, Drouillard KG, et al. Exposure to polybrominated diphenyl ethers (PBDEs): changes in thyroid, vitamin A, glutathione homeostasis, and oxidative stress in American kestrels (Falco sparverius) Toxicol Sci. 2005;88(2):375–383. doi: 10.1093/toxsci/kfi295. [DOI] [PubMed] [Google Scholar]
- Hakk H, Larsen G, Klasson-Wehler E. Tissue disposition, excretion and metabolism of 2,2′,4,4′,5-pentabromo-diphenyl ether (BDE-99) in the male Sprague-Dawley rat. Xenobiotica. 2002;32(5):369–382. doi: 10.1080/00498250110119117. [DOI] [PubMed] [Google Scholar]
- Hallgren S, Sinjari T, Hakansson H, Darnerud PO. Effects of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) on thyroid hormone and vitamin A levels in rats and mice. Arch Toxicol. 2001;75(4):200–208. doi: 10.1007/s002040000208. [DOI] [PubMed] [Google Scholar]
- Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Kester MHA, Andersson PL, et al. In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol Sci. 2006;92(1):157–173. doi: 10.1093/toxsci/kfj187. [DOI] [PubMed] [Google Scholar]
- Hites RA. Polybrominated diphenyl ethers in the environment and in people: a meta-analysis of concentrations. Environ Sci Technol. 2004;38(4):945–956. doi: 10.1021/es035082g. [DOI] [PubMed] [Google Scholar]
- Hovander L, Athanasiadou M, Asplund L, Jensen S, Wehler EK. Extraction and cleanup methods for analysis of phenolic and neutral organohalogens in plasma. J Anal Toxicol. 2000;24(8):696–703. doi: 10.1093/jat/24.8.696. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ. Thyroid hormones and their effects: a new perspective. Biol Rev. 2000;75(4):519–631. doi: 10.1017/s146479310000556x. [DOI] [PubMed] [Google Scholar]
- Huwe JK, Smith DJ. Accumulation, whole-body depletion, and debromination of decabromodiphenyl ether in male Sprague-Dawley rats following dietary exposure. Environ Sci Technol. 2007;41(7):2371–2377. doi: 10.1021/es061954d. [DOI] [PubMed] [Google Scholar]
- Johnson-Restrepo B, Kannan K, Rapaport DP, Rodan BD. Polybrominated diphenyl ethers and polychlorinated biphenyls in human adipose tissue from New York. Environ Sci Technol. 2005;39(14):5177–5182. doi: 10.1021/es050399x. [DOI] [PubMed] [Google Scholar]
- Kierkegaard A, Asplund L, de Wit CA, McLachlan MS, Thomas GO, Sweetman AJ, et al. Fate of higher brominated PBDEs in lactating cows. Environ Sci Technol. 2007;41(2):417–423. doi: 10.1021/es0619197. [DOI] [PubMed] [Google Scholar]
- Kierkegaard A, Balk L, Tjärnlund U, de Wit CA, Jansson B. Dietary uptake and biological effects of decabromodiphenyl ether in rainbow trout (Oncorhynchus mykiss) Environ Sci Technol. 1999;33(10):1612–1617. [Google Scholar]
- Kohrle J. Local activation and inactivation of thyroid hormones: the deiodinase family. Mol Cell Endocrinol. 1999;151(1–2):103–119. doi: 10.1016/s0303-7207(99)00040-4. [DOI] [PubMed] [Google Scholar]
- Korytar P, Covaci A, de Boer J, Gelbin A, Brinkman UAT. Retention-time database of 126 polybrominated diphenyl ether congeners and two Bromkal technical mixtures on seven capillary gas chromatographic columns. J Chromatogr A. 2005;1065(2):239–249. doi: 10.1016/j.chroma.2004.12.059. [DOI] [PubMed] [Google Scholar]
- La Guardia MJ, Hale RC, Harvey E. Detailed polybrominated diphenyl ether (PBDE) congener composition of the widely used penta-, octa-, and deca-PBDE technical flame-retardant mixtures. Environ Sci Technol. 2006;40(20):6247–6254. doi: 10.1021/es060630m. [DOI] [PubMed] [Google Scholar]
- Law K, Halldorson T, Danell R, Stern G, Gewurtz S, Alaee M, et al. Bioaccumulation and trophic transfer of some brominated flame retardants in a Lake Winnipeg (Canada) food web. Environ Toxicol Chem. 2006;25(8):2177–2186. doi: 10.1897/05-500r.1. [DOI] [PubMed] [Google Scholar]
- Letcher RJ, Klasson-Wehler E, Bergman A. Methyl sulfone and hydroxylated metabolites of polychlorinated biphenyls. In: Paasivirta J, editor. The Handbook of Environmental Chemistry. Berlin: Springer-Verlag; 2001. pp. 315–359. [Google Scholar]
- Lindell M, Karlsson MO, Lennernas H, Pahlman L, Lang MA. Variable expression of CYP and Pgp genes in the human small intestine. Eur J Clin Invest. 2003;33(6):493–499. doi: 10.1046/j.1365-2362.2003.01154.x. [DOI] [PubMed] [Google Scholar]
- Malmberg T, Athanasiadou M, Marsh G, Brandt I, Bergmant A. Identification of hydroxylated polybrominated diphenyl ether metabolites in blood plasma from polybrominated diphenyl ether exposed rats. Environ Sci Technol. 2005;39(14):5342–5348. doi: 10.1021/es050574+. [DOI] [PubMed] [Google Scholar]
- Malmvarn A, Marsh G, Kautsky L, Athanasiadou M, Bergman A, Asplund L. Hydroxylated and methoxylated brominated diphenyl ethers in the red algae Ceramium tenuicorne and blue mussels from the Baltic Sea. Environ Sci Technol. 2005;39(9):2990–2997. doi: 10.1021/es0482886. [DOI] [PubMed] [Google Scholar]
- Meerts I, Letcher RJ, Hoving S, Marsh G, Bergman A, Lemmen JG, et al. In vitro estrogenicity of polybrominated diphenyl ethers, hydroxylated PBDEs, and polybrominated bisphenol A compounds. Environ Health Perspect. 2001;109:399–407. doi: 10.1289/ehp.01109399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerts I, van Zanden JJ, Luijks EAC, van Leeuwen-Bol I, Marsh G, Jakobsson E, et al. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol Sci. 2000;56(1):95–104. doi: 10.1093/toxsci/56.1.95. [DOI] [PubMed] [Google Scholar]
- Morck A, Hakk H, Orn U, Wehler EK. Decabromodiphenyl ether in the rat: absorption, distribution, metabolism, and excretion. Drug Metab Dispos. 2003;31(7):900–907. doi: 10.1124/dmd.31.7.900. [DOI] [PubMed] [Google Scholar]
- National Center for Biotechnology Information. GenBank Overview. 2008. [[accessed 18 December 2008]]. Available: http://www.ncbi.nlm.nih.gov/Genbank/index.html.
- Nishimura M, Koeda A, Suganuma Y, Suzuki E, Shimizu T, Nakayama M, et al. Comparison of inducibility of CYP1A and CYP3A mRNAs by prototypical inducers in primary cultures of human, cynomolgus monkey, and rat hepatocytes. Drug Metab Pharmacokinet. 2007;22(3):178–186. doi: 10.2133/dmpk.22.178. [DOI] [PubMed] [Google Scholar]
- Orozco A, Silva JE, Valverde C. Rainbow trout liver expresses two iodothyronine phenolic ring deiodinase pathways with the characteristics of mammalian types I and II 5′-deiodinases. Endocrinology. 1997;138(1):254–258. doi: 10.1210/endo.138.1.4878. [DOI] [PubMed] [Google Scholar]
- Pei RT, Kim SC, Carlson KH, Pruden A. Effect of river landscape on the sediment concentrations of antibiotics and corresponding antibiotic resistance genes (ARG) Water Res. 2006;40(12):2427–2435. doi: 10.1016/j.watres.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Qiu X, Mercado-Feliciano M, Bigsby RM, Hites RA. Measurement of polybrominated diphenyl ethers and metabolites in mouse plasma after exposure to a commercial pentabromodiphenyl ether mixture. Environ Health Perspect. 2007;115:1052–1058. doi: 10.1289/ehp.10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A, Papke O, Tung KC, Joseph J, Harris TR, Dahlgren J. Polybrominated diphenyl ether flame retardants in the US population: current levels, temporal trends, and comparison with dioxins, dibenzofurans, and polychlorinated biphenyls. J Occup Environ Med. 2005;47(3):199–211. doi: 10.1097/01.jom.0000158704.27536.d2. [DOI] [PubMed] [Google Scholar]
- Schecter A, Pavuk M, Päpke O, Ryan JJ, Birnbaum L, Rosen R. Polybrominated diphenyl ethers (PBDEs) in U.S. mothers’ milk. Environ Health Perspect. 2003;111:1723–1729. doi: 10.1289/ehp.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjodin A, Jones RS, Focant JF, Lapeza C, Wang RY, McGahee EE, et al. Retrospective time-trend study of polybrominated diphenyl ether and polybrominated and polychlorinated biphenyl levels in human serum from the United States. Environ Health Perspect. 2004;112:654–658. doi: 10.1289/ehp.112-1241957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjodin A, Patterson DG, Bergman A. Brominated flame retardants in serum from US blood donors. Environ Sci Technol. 2001;35(19):3830–3833. doi: 10.1021/es010815n. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Kelly SM, Allen JG, McClean MD, Webster TF. Measurement of polybrominated diphenyl ethers on hand wipes: estimating exposure from hand-to-mouth contact. Environ Sci Technol. 2008;42(9):3329–3334. doi: 10.1021/es7029625. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Letcher RJ, Baker JE. Debromination of polybrominated diphenyl ether congeners BDE 99 and BDE 183 in the intestinal tract of the common carp (Cyprinus carpio) Environ Sci Technol. 2004;38(4):1054–1061. doi: 10.1021/es0348804. [DOI] [PubMed] [Google Scholar]
- Staskal DF, Hakk H, Bauer D, Diliberto JJ, Birnbaum LS. Toxicokinetics of polybrominated diphenyl ether congeners 47, 99, 100, and 153 in mice. Toxicol Sci. 2006;94(1):28–37. doi: 10.1093/toxsci/kfl091. [DOI] [PubMed] [Google Scholar]
- Strehlau J, Pavlakis M, Lipman M, Shapiro M, Vasconcellos L, Harmon W, et al. Quantitative detection of immune activation transcripts as a diagnostic tool in kidney transplantation. Proc Natl Acad Sci USA. 1997;94(2):695–700. doi: 10.1073/pnas.94.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuresson K, Bergman A, Jakobsson K. Occupational exposure to commercial decabromodiphenyl ether in workers manufacturing or handling flame-retarded rubber. Environ Sci Technol. 2005;39(7):1980–1986. doi: 10.1021/es048511z. [DOI] [PubMed] [Google Scholar]
- Thuresson K, Hoglund P, Hagmar L, Sjodin A, Bergman A, Jakobsson K. Apparent half-lives of hepta- to deca-brominated diphenyl ethers in human serum as determined in occupationally exposed workers. Environ Health Perspect. 2006;114:176–181. doi: 10.1289/ehp.8350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomy GT, Palace VP, Halldorson T, Braekevelt E, Danell R, Wautier K, et al. Bioaccumulation, biotransformation, and biochemical effects of brominated diphenyl ethers in juvenile lake trout (Salvelinus namaycush) Environ Sci Technol. 2004;38(5):1496–1504. doi: 10.1021/es035070v. [DOI] [PubMed] [Google Scholar]
- Van den Steen E, Covaci A, Jaspers VLB, Dauwe T, Voorspoels S, Eens M, et al. Accumulation, tissue-specific distribution and debromination of decabromodiphenyl ether (BDE 209) in European starlings (Sturnus vulgaris) Environ Pollut. 2007;148(2):648–653. doi: 10.1016/j.envpol.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Yamaori S, Yamazaki H, Iwano S, Kiyotani K, Matsumura K, Saito T, et al. Ethnic differences between Japanese and Caucasians in the expression levels of mRNAs for CYP3A4, CYP3A5 and CYP3A7: lack of co-regulation of the expression of CYP3A in Japanese livers. Xenobiotica. 2005;35(1):69–83. doi: 10.1080/00498250400021796. [DOI] [PubMed] [Google Scholar]
- Zhou T, Ross DG, DeVito MJ, Crofton KM. Effects of short-term in vivo exposure to polybrominated diphenyl ethers on thyroid hormones and hepatic enzyme activities in weanling rats. Toxicol Sci. 2001;61(1):76–82. doi: 10.1093/toxsci/61.1.76. [DOI] [PubMed] [Google Scholar]
- Zhou T, Taylor MM, DeVito MJ, Crofton KA. Developmental exposure to brominated diphenyl ethers results in thyroid hormone disruption. Toxicol Sci. 2002;66(1):105–116. doi: 10.1093/toxsci/66.1.105. [DOI] [PubMed] [Google Scholar]