Abstract
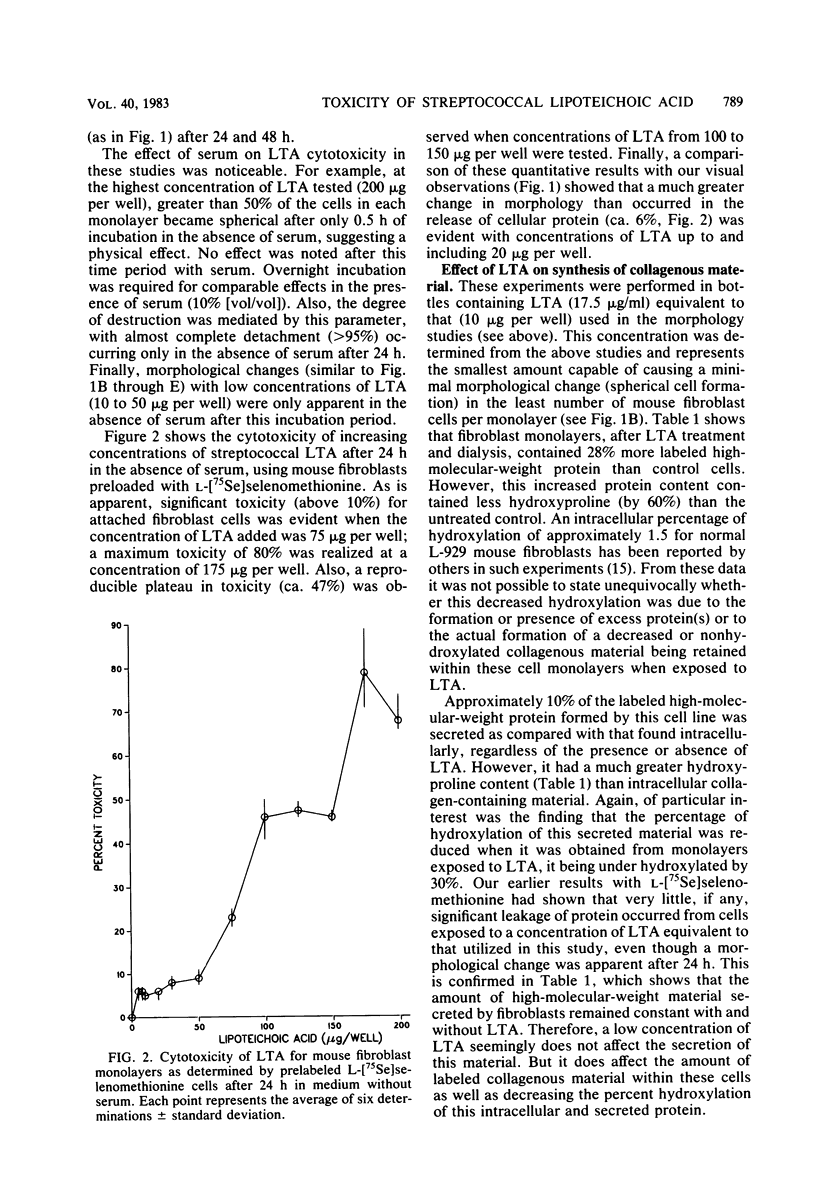
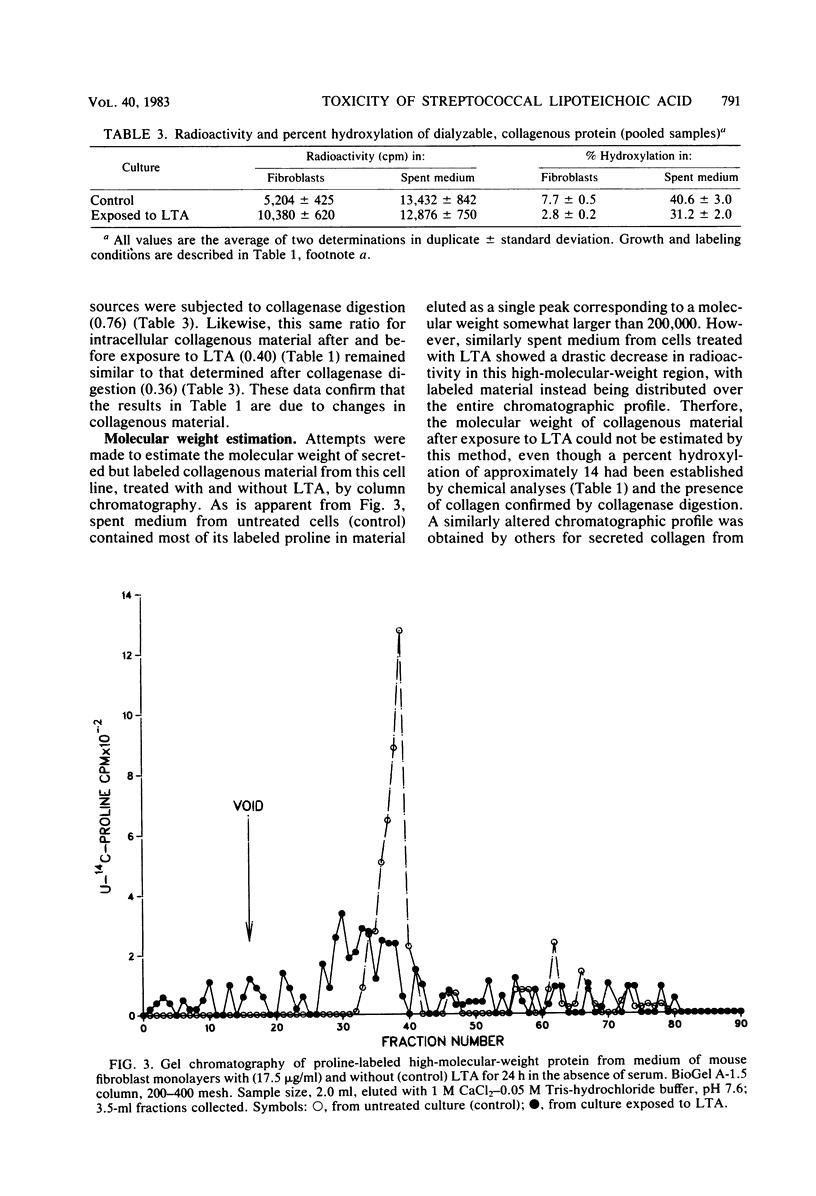
The toxicity of lipoteichoic acid (LTA) from Streptococcus pyogenes type 12 was investigated by using mouse fibroblasts in culture in the absence of serum. Morphologically, while low concentrations of LTA elicited a subtle effect characterized by progressive cellular degeneration with practically no release of protein, larger concentrations (greater than 50 micrograms/ml) of this amphiphile resulted in rapid death of cell monolayers. Metabolic studies utilized a concentration of LTA (17.5 micrograms/ml) which caused the smallest change in cell morphology in the least number of mouse fibroblast cells per monolayer. Under these conditions, cell monolayers showed an increase of 450% in their content of collagenous protein after exposure to LTA. However, the amount of such material secreted remained unchanged. Also, changes in the type of collagenous protein formed were observed after exposure to LTA. Collagenous protein accumulating intracellularly was found to be practically hydroxyproline-free. However, collagenous protein secreted by this cell line showed a significantly reduced content of hydroxyproline as compared with control cells unexposed to this coccal membrane component. Column chromatographic studies confirmed that the collagenous protein secreted by monolayers exposed to LTA was defective (under hydroxylated). It was concluded that LTA does not affect the amount of collagenous protein secreted. However, it does increase the amount of this protein formed and retained by this cell line as well as causing a reduction in the hydroxylation of proline in both intracellular and secreted collagenous material. A possible relationship between abnormal basement membrane morphology and disturbed collagen synthesis in post-streptococcal glomerulonephritis as related to LTA is discussed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Booth B. A., Polak K. L., Uitto J. Collagen biosynthesis by human skin fibroblasts. I. Optimization of the culture conditions for synthesis of type I and type III procollagens. Biochim Biophys Acta. 1980 Mar 28;607(1):145–160. doi: 10.1016/0005-2787(80)90228-2. [DOI] [PubMed] [Google Scholar]
- Brooks C. G. Studies on the microcytotoxicity test. III. Comparison of [75Se]selenomethionine with [3H]proline, Na2, 51CrO4 and [125I]iododeoxyuridine for pre-labelling target cells in long-term cytotoxicity tests. J Immunol Methods. 1978;22(1-2):23–36. doi: 10.1016/0022-1759(78)90055-8. [DOI] [PubMed] [Google Scholar]
- DeVuono J., Panos C. Effect of L-form Streptococcus pyogenes and of lipoteichoic acid on human cells in tissue culture. Infect Immun. 1978 Oct;22(1):255–265. doi: 10.1128/iai.22.1.255-265.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg I. Mechanisms of cell and tissue injury induced by group A streptococci: relation to poststreptococcal sequelae. J Infect Dis. 1972 Sep;126(3):294–340. doi: 10.1093/infdis/126.3.294. [DOI] [PubMed] [Google Scholar]
- Hausmann E., Lüderitz O., Knox K., Weinfeld N. Structural requirements for bone resorption by endotoxin and lipoteichoic acid. J Dent Res. 1975 Jun;54(SPEC):B94–B99. doi: 10.1177/00220345750540023401. [DOI] [PubMed] [Google Scholar]
- Jimenez S., Rosenbloom J. Decreased thermal stability of collagens containing analogs of proline or lysine. Arch Biochem Biophys. 1974 Aug;163(2):459–465. doi: 10.1016/0003-9861(74)90502-5. [DOI] [PubMed] [Google Scholar]
- Juva K., Prockop D. J. Modified procedure for the assay of H-3-or C-14-labeled hydroxyproline. Anal Biochem. 1966 Apr;15(1):77–83. doi: 10.1016/0003-2697(66)90249-1. [DOI] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Jefferson W., Campbell G. L. Cell membrane-binding properties of group A streptococcal lipoteichoic acid. J Exp Med. 1975 May 1;141(5):990–1003. doi: 10.1084/jem.141.5.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERKOFSKY B., PROCKOP D. J. A method for the simultaneous measurement of the radioactivity of proline-C14 and hydroxyproline-C14 in biological materials. Anal Biochem. 1962 Nov;4:400–406. doi: 10.1016/0003-2697(62)90141-0. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B. The effect of ascorbic acid on collagen polypeptide synthesis and proline hydroxylation during the growth of cultured fibroblasts. Arch Biochem Biophys. 1972 Sep;152(1):318–328. doi: 10.1016/0003-9861(72)90221-4. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I., Tuderman L., Guzman N. A. The biosynthesis of collagen and its disorders (first of two parts). N Engl J Med. 1979 Jul 5;301(1):13–23. doi: 10.1056/NEJM197907053010104. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I., Tuderman L., Guzman N. A. The biosynthesis of collagen and its disorders (second of two parts). N Engl J Med. 1979 Jul 12;301(2):77–85. doi: 10.1056/NEJM197907123010204. [DOI] [PubMed] [Google Scholar]
- Ramaley P. B., Rosenbloom J. Inhibition of proline and lysine hydroxylation prevents normal extrusion of collagen by 3T6 fibroblasts in culture. FEBS Lett. 1971 Jun 2;15(1):59–64. doi: 10.1016/0014-5793(71)80079-0. [DOI] [PubMed] [Google Scholar]
- Simpson W. A., Dale J. B., Beachey E. H. Cytotoxicity of the glycolipid region of streptococcal lipoteichoic acid for cultures of human heart cells. J Lab Clin Med. 1982 Jan;99(1):118–126. [PubMed] [Google Scholar]
- Simpson W. A., Ofek I., Beachey E. H. Binding of streptococcal lipoteichoic acid to the fatty acid binding sites on serum albumin. J Biol Chem. 1980 Jul 10;255(13):6092–6097. [PubMed] [Google Scholar]
- Slabyj B. M., Panos C. Membrane lipoteichoic acid of Streptococcus pyogenes and its stabilized L-form and the effect of two antibiotics upon its cellular content. J Bacteriol. 1976 Aug;127(2):855–862. doi: 10.1128/jb.127.2.855-862.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabyj B. M., Panos C. Teichoic acid of a stabilized L-form of Streptococcus pyogenes. J Bacteriol. 1973 Jun;114(3):934–942. doi: 10.1128/jb.114.3.934-942.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltersdorff R. L., Fiedel B. A., Jackson R. W. Induction of nephrocalcinosis in rabbit kidneys after long-term exposure to a streptococcal teichoic acid. Infect Immun. 1977 Sep;17(3):665–667. doi: 10.1128/iai.17.3.665-667.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]



