Abstract
RIC-3 belongs to a conserved family of proteins influencing nicotinic acetylcholine receptor (nAChR) maturation. RIC-3 proteins are integral membrane proteins residing in the endoplasmic reticulum (ER), and containing a C-terminal coiled-coil domain (CC-I). Conservation of CC-I in all RIC-3 family members indicates its importance; however, previous studies could not show its function. To examine the role of CC-I, we studied effects of its deletion on Caenorhabditis elegans nAChRs in vivo. Presence of CC-I promoted maturation of particular nAChRs expressed in body-wall muscle, whereas it was not required for other nAChR subtypes expressed in neurons or pharyngeal muscles. This effect is receptor-specific, because it could be reproduced after heterologous expression. Consistently, coimmunoprecipitation analysis showed that CC-I enhances the interaction of RIC-3 with a nAChR that requires CC-I in vivo; thus CC-I appears to enhance affinity of RIC-3 to specific nAChRs. However, we found that this function of CC-I is redundant with functions of sequences downstream to CC-I, potentially a second coiled-coil. Alternative splicing in both vertebrates and invertebrates generates RIC-3 transcripts that lack the entire C-terminus, or only CC-I. Thus, our results suggest that RIC-3 alternative splicing enables subtype specific regulation of nAChR maturation.
INTRODUCTION
Nicotinic acetylcholine receptors (nAChR) are a family of cys-loop ligand-gated ion channels expressed in muscles and neurons. nAChRs mediate excitation in the neuromuscular junction, and many additional roles in the peripheral and CNS, including influences on memory and cognition, and a central role in nicotine addiction (Cordero-Erausquin et al., 2000). The diverse functions of nAChRs are associated with diverse expression patterns of receptor subunits encoded by a large family of genes: 16 in mammals and as many as 29 in Caenorhabditis elegans (Jones et al., 2007). These subunits assemble to form many different receptors, having diverse subunit composition, properties, cellular localization, and functions. Each subunit traverses the membrane four times and is modified by glycosylation and by disulphide bond formation (Numa et al., 1983; Green and Claudio, 1993; Keller and Taylor, 1999). Folding, posttranslational modifications, and assembly of nAChR subunits mostly occur in the endoplasmic reticulum (ER; Merlie and Lindstrom, 1983). Complexity of this process suggests assistance by various cellular factors. One such factor is the ER-resident protein, RIC-3.
RIC-3, first identified in C. elegans, and its orthologues, influence maturation of multiple nAChRs, in vertebrates and invertebrates, in muscles and neurons (Halevi et al., 2002; Halevi et al., 2003). All members of this family encode for two membrane-spanning domains followed by one or more coiled-coil domains. The first coiled-coil domain (CC-I) is conserved among RIC-3 family members, whereas the second coiled-coil domain (CC-II) is only found in nematode species (Halevi et al., 2003). Members of the RIC-3 family specifically affect maturation of nAChRs; effects of RIC-3 on other ligand-gated ion channels have only been demonstrated for 5-HT3 receptors, receptors that are closely related to nAChRs (Halevi et al., 2003; Cheng et al., 2005). Specifically in C. elegans, RIC-3 is known to enhance expression of four different receptors: the neuronal DEG-3/DES-2 receptor, the pharyngeal muscle EAT-2 receptor, and the two body-wall muscle receptors: the hetero-pentameric levamisole sensitive receptor (L-AChR) and the homopentameric nicotine-sensitive receptor (N-AChR, comprising ACR-16 subunits). Other members of the cys-loop ligand-gated ion channel family not belonging to the nAChR family, such as the body-wall muscle GABA receptor and the pharyngeal muscle avermectin sensitive receptor, are unaffected by the presence of RIC-3 (Halevi et al., 2002). Thus RIC-3 although enhancing maturation of multiple and diverse nAChRs demonstrates considerable specificity in its effects.
Studies on the C. elegans and human RIC-3 genes demonstrated significant effects on maturation, surface expression, steady-state levels, and functional properties of coexpressed nAChRs (Halevi et al., 2003; Cheng et al., 2005; Williams et al., 2005; Cohen Ben-Ami et al., 2005; Gottschalk and Schafer, 2006). In most studies, RIC-3 was shown to enhance expression of coexpressed nAChRs; however, RIC-3 coexpression with specific receptors in certain experimental systems reduced receptor expression (Halevi et al., 2003; Castillo et al., 2005; Cheng et al., 2005; Lansdell et al., 2005). Thus RIC-3 orthologues have multiple and diverse effects on nAChR maturation, but the mechanisms enabling these effects are unknown. To better understand RIC-3's mechanism of action, several studies examined the function of RIC-3 domains. One consistent finding in these studies was that deletion of CC-I does not interfere with RIC-3 function (Castillo et al., 2005; Cohen Ben-Ami et al., 2005; Lansdell et al., 2008). Interestingly, both mammals and Drosophila express alternatively spliced transcripts of RIC-3 lacking either the entire C-terminal sequence downstream to the membrane-spanning domains or only the conserved coiled-coil domain (Halevi et al., 2003; Lansdell et al., 2008; Seredenina et al., 2008), yet the significance of conservation of the coiled-coil domain and of the alternative splicing mechanisms eliminating is presently not known.
To better understand the functional significance of the C. elegans CC-I we here examine its role in vivo on the four native receptors known to require RIC-3 for their expression (Halevi et al., 2002). Our work shows that the RIC-3 CC-I is differentially required, having significant effects on maturation of specific nAChRs (L-AChR and ACR-16 [N-AChR]), independent of the expression system. We also show that presence of CC-I enhances interaction of RIC-3 with ACR-16. However, those effects on RIC-3 function are only seen when both CC-I and sequences downstream to it are deleted, demonstrating redundant functions of this domain. Our results suggest that alternative splicing producing RIC-3 isoforms lacking either the entire C-terminal sequence downstream to the transmembrane domains (including CC-I), or CC-I only, enable receptor-specific regulation of nAChR maturation.
MATERIALS AND METHODS
Nematode Growth and Analysis
Wild type (N2 Bristol) and all other strains were grown on NGM plates seeded with OP50 at 20°C (Wood, 1988). Levamisole assays were done on 10 L4 animals per NGM plate containing the indicated concentration of levamisole. Animals were examined for paralysis after 1-h incubation at 20°C and after gentle prodding. Pumping rate was observed under high magnification, and animals whose pumping rate was lower then 1 pump per second were considered pumping-defective (normal pumping rate is >3 pumps/s); 10 adult animals were observed in each plate; L4 animals were picked for overnight growth on fresh plates at 20°C before each experiment. Degenerations were examined on newly hatched animals using Nomarski optics. Pictures of RIC-3:green fluorescent protein (GFP) distribution were taken using a CCD camera (Hamamatsu ORCA ER; Bridgewater, NJ) and a SimplePCI image acquisition and analysis program (Compix, Sewickley, PA) was used to analyze signal intensity. Staining of synaptic UNC-29 was done using a rabbit polyclonal antibody raised against the C-terminus (peptide CLDRLKEKYDTASNIP) and an AlexaFluor568-labeled secondary goat anti-rabbit antibody, following an established protocol (Duerr et al., 2001). Fluorescence corresponding to UNC-29 staining of the anterior ventral nerve cord was quantified using line scans and ImageJ software (http://rsb.info.nih.gov/ij/; Gottschalk and Schafer, 2006). As a control for staining irregularities, and for normal presynaptic morphology, animals were counterstained with mouse monoclonal anti-UNC-17 antibodies (a gift from J. Rand, Oklahoma Medical Research Foundation, Oklahoma City, OK) and AlexaFluor488-labeled secondary goat anti-mouse antibodies. Also UNC-17 fluorescence was analyzed, and UNC-29 fluorescence was normalized to the signals of UNC-17 in the wild-type background. In all experiments “n” is number of animals or plates (for levamisole assays) examined, and “N” is the number of independent experiments. Results are given as average ± SE of means. Significance was examined using one-way Anova, or Student's t test for two samples, as indicated.
Molecular Biology and Biochemical Analysis
RIC-3 deletion mutants contain RIC-3 cDNA fragments having end-points as previously described (Cohen Ben-Ami et al., 2005). These RIC-3 fragments were inserted upstream or downstream and in-frame to a GFP coding sequence and in between the noncoding regulatory regions of a ric-3 genomic rescuing clone. This genomic clone was tagged with GFP in between the two coiled-coil domains and used in the C. elegans experiments as control (Halevi et al., 2002). RIC-3 ΔCC-I constructs for in vivo and oocyte analysis were generated as part of this study and lack only CC-I with flanking residue (16 amino acids upstream and three amino acids downstream to CC-I; Cohen Ben-Ami et al., 2005). These constructs were injected at 5 ng/μl together with rol-6 DNA (pRF4) into ric-3(md1181) animals or with dpy-20 coding DNA into dpy-20(e1282)ric-3(md1181) animals. One transgene expressing RIC-3 TM downstream of the GFP reporter and rol-6 was integrated (Mitani, 1995), outcrossed, and used for immunohistochemistry and degeneration analysis. For degeneration assays the same transgene was crossed into a deg-3(u662)ric-3(md1181) background. Coimmunoprecipitations and Western analysis on Xenopus laevis oocyte extracts were done as previously described (Cohen Ben-Ami et al., 2005). Images of coimmunoprecipitation were obtained using Fuji Film LAS-3000 (Tokyo, Japan) and quantified using TINA 2.10g software (Raytest, Straubenhardt, Germany).
Electrophysiology of C. elegans Muscle
Electrophysiology of C. elegans body-wall muscles was done as previously described (Nagel et al., 2005; Richmond and Jorgensen, 1999). Analysis of trangenics was done on fluorescent muscles to avoid noise due to mosaic expression of transgenes. Transgenics used in this analysis, RIC-3 minimal and RIC-3 TM, have the GFP fusion downstream to RIC-3 and were injected into a dpy-20(e1282)ric-3(md1181) background. Nicotine (10−3 M) and levamisole (5 × 10−4 M) were pressure-applied onto dissected muscle cells, and corresponding inward currents were measured in whole-cell mode, patch-clamped at a holding potential of −60 mV, using an EPC10 amplifier with head stage, and analyzed by pulse software (HEKA Electronics, Bellmore, NY; EPC 10 patch clamp amplifier; S/N: 520328) connected to a standard HEKA pipette holder for glass capillaries (outer diameter: 1.0 mm).
Heterologous Expression and Electrophysiological Analysis
An EcoRI fragment containing the entire ACR-16 open reading frame (ORF) from a cDNA clone kindly provided by David Sattelle (MRC, Cambridge, United Kingdom) was inserted into a BamHI site of pGEMH19, an oocytes expression vector having Xenopus globin untranslated regions. For coimmunoprecipitations a 6Xmyc tag was inserted downstream and in-frame to the ACR-16 ORF. Plasmids for expression of GFP-tagged RIC-3, RIC-3 TM, and RIC-3 minimal were previously described (Cohen Ben-Ami et al., 2005). Transcription and injection of cRNAs into oocytes was previously described. Effects of coexpressing RIC-3 or RIC-3 deletion mutants on ACR-16 were examined as previously done for the DEG-3/DES-2 receptor (Cohen Ben-Ami et al., 2005).
RESULTS
The RIC-3 Conserved Coiled-Coil Domain (CC-I) Is Required for Maturation of the Body-Wall Muscle L-AChR
Previous studies have shown that a small 118-amino acid-long fragment encompassing the RIC-3 membrane-spanning domains but lacking the coiled-coil domains (RIC-3 TM) is sufficient for RIC-3 function in X. laevis oocytes (Cohen Ben-Ami et al., 2005). This finding was surprising in view of the conservation of CC-I in all RIC-3 homologues. Thus we decided to investigate the effects of this truncated RIC-3 TM fragment in C. elegans on native nAChRs known to require RIC-3 for their maturation. Among these receptors, the body-wall muscle L-AChR is the easiest to assay, because its agonist levamisole via activation of L-AChR produces muscle contraction that causes paralysis (Lewis et al., 1980). To examine effects of RIC-3 TM on L-AChR, we expressed it in a ric-3(md1181) background, a representative ric-3 loss-of-function homozygous mutant previously described by Halevi et al. (2002) and used levamisole to assay for function of L-AChR. This analysis (Figure 1A) shows that RIC-3 TM does not rescue the levamisole insensitivity of ric-3(md1181) mutants (Miller et al., 1996). Thus RIC-3 TM, unlike wild-type RIC-3 does not promote maturation of L-AChR.
Figure 1.
L-AChR maturation requires the RIC-3 C-terminal domains. (A) Levamisole-sensitivity assays (1 mM) on transgenics expressing the different RIC-3 deletion mutants. Given is the percent per plate of paralyzed animals. n = 9–14 N = 3–6, 10 animals per plate. Levamisole sensitivity of ric-3(md1181) animals expressing RIC-3 TM or RIC-3 ΔC′ is not significantly different from the sensitivity of ric-3(md1181) animals and is significantly different from wild-type (WT) animals and from ric-3(md1181) animals expressing full-length (wild-type) RIC-3, RIC-3 minimal (min), or RIC-3 ΔCC-II. Significant differences are also seen between ric-3(md1181) animals expressing RIC-3 ΔCC-I and all other strains examined (p < 0.01, one-way Anova). (B) Schematic representation of the domain organization in RIC-3 and RIC-3 deletion mutants. Arrowheads point to the position of the GFP tag; two arrowheads are seen for RIC-3 constructs having two different tagged versions.
RIC-3 TM lacks both the entire C-terminus containing the two coiled-coil domains and a short N-terminal sequence just upstream to the first transmembrane domain; thus, we needed to identify the domain responsible for the observed defect. For this purpose, we generated additional RIC-3 derivatives (Figure 1B): ΔCC-II lacks sequences downstream of CC-I, including the second nonconserved coiled-coil (CC-II); ΔCC-I lacks only CC-I and few flanking residues; ΔC′ lacks the entire C-terminal sequence downstream of the membrane-spanning domains, i.e., both CC-I and CC-II; and minimal RIC-3 lacks the nonconserved N-terminal sequences upstream of the first membrane-spanning domain, as well as nonconserved sequences downstream to CC-I that include CC-II (and thus contains only the conserved RIC-3 domains; Cohen Ben-Ami et al., 2005). Levamisole sensitivity assays of transgenic animals expressing these RIC-3 derivatives in a ric-3(md1181) background showed that only elimination of the entire C-terminal sequences downstream of the membrane-spanning domains as in the ΔC′ or TM constructs leads to severe L-AChR maturation defect. However, deletion of only CC-I (ΔCC-I) or only of the sequences downstream to it including CC-II (ΔCC-II) reproduce this effect only partially or not at all, suggesting that the two regions function redundantly. Deletion of CC-I alone, however, does lead to significantly reduced levamisole sensitivity, suggesting this domain has a more important role relative to the region downstream to CC-I (Figure 1A).
Sequence analysis does not suggest an obvious candidate for a domain downstream to CC-I that is functioning redundantly with it: 159 amino acids are deleted in ΔCC-II, only 68 amino acids within it code for CC-II, and they contain no obvious stretches of homology with mammalian RIC-3 orthologues. In addition, although redundancy between CC-I and CC-II is likely, no clearly recognizable coiled-coil domains are found in vertebrate species downstream of CC-I.
It was previously shown that a ric-3 loss-of-function allele reduces synaptic expression of L-AChR subunits (Gottschalk and Schafer, 2006). However, RIC-3 was also shown to enable functional expression without changes in surface expression of the α7 nAChR (Williams et al., 2005). Thus we examined the effects of RIC-3 TM on synaptic expression of the L-AChR subunit, UNC-29, using immunohistochemistry (Figure 2). Quantification of UNC-29 synaptic staining showed a large reduction in UNC-29 synaptic expression in RIC-3 TM expressing transgenic animals relative to wild type. This correlates with the greatly reduced levamisole sensitivity (Figure 1A) and suggests that RIC-3 TM is unable to promote maturation and surface expression of L-AChR. It is, however, important to note that synaptic expression of L-AChR in the presence of only RIC-3 TM, although greatly reduced, is significantly higher then synaptic expression in ric-3(md1181) mutants. Thus the RIC-3 TM protein retains low residual function in this assay.
Figure 2.
Effects of RIC-3 TM on synaptic UNC-29 expression. Immunohistochemical analysis of synaptic UNC-29 (a L-AChR subunit) expression levels in wild-type, ric-3(md1181), ric-3(md1181) expressing RIC-3 TM from an integrated transgene, and unc-29(x29); acr-16(ok789) double mutants. Top, representative images of UNC-29 staining in the respective strains. Arrowheads indicate the nerve ring and arrows the dorsal nerve cord, where L-AChR-containing synapses are found. Bottom, quantification of images (number of animals examined is indicated in each bar). Intensity of the staining is normalized to staining of UNC-17, a presynaptic marker. Significant differences are indicated (***p < 0.001, **p < 0.01, *p < 0.05; t test). Scale bar, 10 μm.
CC-I–mediated Interactions Are Required for RIC-3 Function But Have No Effect on Quantity or Distribution of RIC-3
Coiled-coil domains are known to mediate protein–protein interactions. Thus the suggested importance of CC-I and sequences downstream to it, possibly CC-II, for L-AChR maturation suggests that coiled-coil domain-mediated interactions may play a role in L-AChR maturation. To understand the function of these interactions, we examined effects of deleting CC-I and/or CC-II on RIC-3 quantity and distribution. For this purpose, RIC-3 deletion mutants were tagged with GFP. Tagging of wild-type RIC-3 either at the N- terminus or between the two coiled-coil domains affected function similarly and did not largely interfere with L-AChR maturation (85 ± 9.8 or 95 ± 2.6%, respectively, of transgenic animals were paralyzed on levamisole). In addition RIC-3 TM tagged with GFP at either the N- or C-termini similarly affected function in the levamisole assay (2.5 ± 2.5 or 5 ± 1.5%, respectively). Here we show results for C-terminally tagged RIC-3 derivatives. This analysis (Figure 3B) shows no correlation between expression level of RIC-3 protein deletion mutants and their function as assayed by sensitivity to levamisole (Figure 1A). Nor did we observe distribution defects in these mutants correlating with functional defects. Specifically, all RIC-3 mutants have a membranous ER-like distribution in body-wall muscles (Figure 3A). To examine whether RIC-3 TM mutants are aberrantly trafficked to synaptic regions (i.e., at punctate sites along the ventral or dorsal cords of C. elegans where neuromuscular junctions are located) we also examined distribution of this deletion mutant when expressed from a muscle-specific promoter (Pmyo-3; Fire and Waterston, 1989). Again, this analysis showed neither significant distribution changes, nor could we detect the RIC-3 deletion mutants in neuromuscular junctions (results not shown). One difference that we did observe is that mutant proteins lacking CC-I are less often found in puncta (Figure 3A, arrows). However, because puncta are not seen in some low-copy number wild-type RIC-3::GFP transgenes that are fully functional (not shown) we do not think that they are required for function of RIC-3. Instead we suggest that the observed puncta represent RIC-3 aggregates whose formation requires presence of CC-I as previously proposed by Cheng et al. (Cheng et al., 2007).
Figure 3.
Distribution and quantity of RIC-3 deletion mutants. (A) Representative pictures showing distribution of RIC-3 deletion mutants in the body-wall muscles. Arrows point to representative puncta. (B) Expression level of each deletion mutant was quantified based on fluorescence intensity of the GFP tag; 10–15 animals for each transgenic strain. Scale bar, 5 μm.
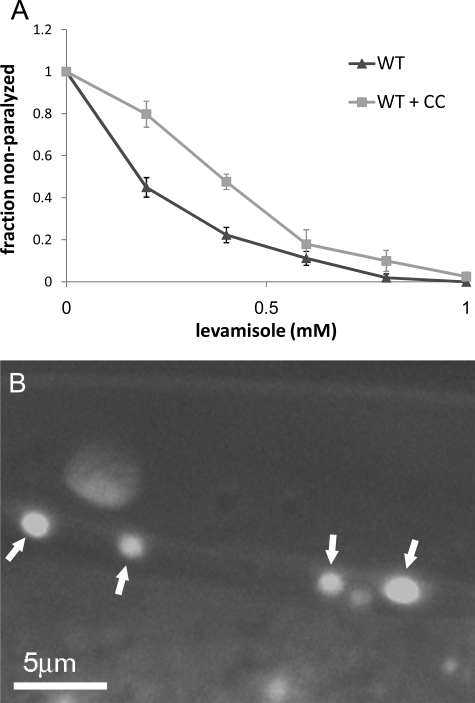
Results presented above suggest that presence of CC-I does not affect functional distribution or stability of RIC-3. However, coiled-coil domain-mediated interactions may still underlie the L-AChR maturation defects. To examine whether CC-I–mediated interactions play a role in L-AChR maturation, we examined the effects of overexpressing this domain in a wild-type background. Analysis of CC-I overexpressing transgenic animals shows a small but significant decrease in L-AChR maturation as assayed by levamisole sensitivity (Figure 4A). Thus overexpression of CC-I produces a dominant negative effect, suggesting that this domain competes with native RIC-3 CC-I for a yet unidentified interaction. We suggest that such competition reduces L-AChR maturation because the interaction mediated by the CC-I is important for this process. To further analyze the role of this domain on RIC-3 localization, we also looked at distribution of this domain when expressed alone. This analysis shows that CC-I does not contain sequences that can direct it to the ER, because GFP-tagged CC-I is diffusely distributed in the cytosol and nucleus of RIC-3 expressing neurons. Distribution of this protein in muscles was hard to visualize, probably because of its diffuse distribution (Figure 4B).
Figure 4.
Effects and distribution of the CC-I. (A) Levamisole dose response for wild-type (WT, ▴) and wild-type overexpressing CC-I fused to GFP (▩). Given is the fraction per plate of animals moving in the presence of different concentrations of levamisole. n = 4–14, 10 animals per plate for each point. Differences are significant (p < 0.01) for levamisole concentrations 0.2 mM and 0.4 using the Student's t test for two samples. (B) Representative image showing distribution of CC-I when expressed under the RIC-3 promoter in wild type. Arrows indicate ventral cord neurons.
Receptor-specific Effects of RIC-3 CC-I
Results presented above showing importance of CC-I for L-AChR maturation are in contradiction to previous results from several heterologous expression systems. Thus we wanted to examine whether our results were due to differences between C. elegans cells and heterologous hosts. For this purpose, we first examined ability of RIC-3 TM to rescue neuronal degeneration associated with mutations in the DEG-3/DES-2 nAChR subunit deg-3(u662). RIC-3 enhances function and trafficking of DEG-3/DES-2 receptors in C. elegans neurons and after expression in X. laevis oocytes (Halevi et al., 2002). Previously we have shown that RIC-3 TM enhances maturation of DEG-3/DES-2 nAChRs similarly to wild-type RIC-3 in X. laevis oocytes (Cohen Ben-Ami et al., 2005). Here we show that RIC-3 TM also enables the degeneration promoting activity of DEG-3(u662) containing nAChRs in C. elegans neurons (Figure 5A). Thus RIC-3 TM promotes maturation of DEG-3–containing receptors in C. elegans neurons, similar to its effects on the DEG-3/DES-2 receptor in X. laevis oocytes (Cohen Ben-Ami et al., 2005). However, we note that RIC-3 TM mediated rescue of the ric-3(md1181) mutation in head neurons, but not in tail neurons, is incomplete (Figure 5A), suggesting differences between DEG-3–containing receptors in head neurons relative to tail neurons.
Figure 5.
Rescue of DEG-3(u662) and EAT-2 nAChR maturation by RIC-3 TM. Different RIC-3 derivates were examined for their ability to rescue function of a ric-3(md1181) mutant. (A) Number of DEG-3(u662)-dependent degenerations in deg-3(u662) mutants, ric-3(md1181)deg-3(u662) mutants expressing RIC-3 TM, and ric-3(md1181)deg-3(u662) mutants. Numbers are given for tail (black), head (gray), and total (light gray), in early L1 larva. N = 2–3 and n = 20–30 animals each. Rescue by RIC-3 TM is significant relative to deg-3(u662)ric-3(md1181) for total, head, and tail numbers of degenerating cells, p < 0.01. Rescue by RIC-3 TM is not significantly different from control [deg-3(u662) having wild-type copies of ric-3] for total and tail degenerations; however, for head degenerations a significant difference is seen, suggesting incomplete rescue (p < 0.01). One-way Anova was done on each group (head, tail, and total) separately. (B) Pharyngeal pumping rate in ric-3(md1181), ric-3(md1181) expressing: RIC-3 TM, RIC-3 ΔCC-I, RIC-3 minimal, or wild-type RIC-3, and wild-type animals (WT). Animals pumping at a rate less than one pump per second are considered pumping-defective. N = 3–4 and n = 12–15, 10 animals per plate. Rescue of pumping by expression of all RIC-3 derivatives is significant at p < 0.01. No significant differences were found between wild-type animals or any of the RIC-3–expressing strains; one-way Anova.
To further examine the role of the conserved coiled-coil domain on maturation of native nAChRs we looked at the ability of RIC-3 TM to rescue activity of EAT-2, a pharyngeal nAChR. Again, expression of RIC-3 TM in a ric-3 loss-of-function mutant, ric-3(md1181), rescues nAChR maturation as seen using pharyngeal pumping as an assay for EAT-2 maturation. No significant differences were found between the function of RIC-3 TM and the function of full length RIC-3, RIC-3 minimal, or RIC-3 ΔCC-I when using pumping as an assay (Figure 5B).
In addition, we used electrophysiology to look at functional expression of the two body-wall muscle nAChRs (Figure 6). This analysis shows that maturation of ACR-16, the nicotine-sensitive body-wall muscle nAChR (ACR-16), like maturation of L-AChR, is greatly reduced in ric-3(md1181) animals expressing RIC-3 TM when compared with wild-type animals or with ric-3(md1181) animals expressing RIC-3 minimal (which differs from RIC-3 TM only in containing CC-I). However, this analysis also shows that very low L-AChR and ACR-16 maturation promoting activity is retained in transgenic animals expressing RIC-3 TM (Figure 6), a result that is in agreement with our findings for UNC-29 (L-AChR subunit) synaptic staining (Figure 2).
Figure 6.
RIC-3 TM does not rescue functional expression of body-wall muscle receptors. Inward currents were recorded in patch-clamped body-wall muscle cells in the whole-cell voltage-clamp mode. Agonists levamisole (A), and nicotine (B) were applied as indicated. Above-average peak currents, normalized to the currents in wild type from the indicated number of individual animals (levamisole: wild-type (WT) n = 11, ric-3(md1181) n = 7, RIC-3 TM n = 6, and RIC-3 minimal n = 7; nicotine: wild-type (WT) n = 11, ric-3(md1181) n = 7, RIC-3 TM n = 7, and RIC-3 minimal n = 7). Currents in RIC-3 minimal are not significantly different then currents in wild type but are significantly different from currents in RIC-3 TM transgenics (p < 0.01, t test). A significant difference is also seen between RIC-3 TM and ric-3(md1181); p < 0.001, t test; for levamisole-dependent responses but not for nicotine-dependent responses. Below representative original current traces for mutants, transgenics, and wild type.
Overall, our results show that in C. elegans deletion of CC-I together with sequences downstream to it greatly reduces maturation of the two body-wall muscle nAChRs (L-AChR and ACR-16) but has little or no effect on maturation of the neuronal DEG-3 and pharyngeal muscle EAT-2–containing nAChRs.
Deletion of the C-Terminus Significantly Reduces Effects of RIC-3 on ACR-16 after Heterologous Expression
Because the two nAChRs that required presence of the C-terminal domains of RIC-3 (L-AChR and ACR-16) are expressed in the same cells, i.e., body-wall muscles, we could not determine whether the C-terminal domains function in a cell- or receptor-specific manner. Thus we decided to examine effects of deleting CC-I alone or deleting the entire C-terminal domain on expression of the body-wall muscle receptors when expressed in X. laevis oocytes. For this purpose we focused on ACR-16 (N-AChR), a homomeric receptor previously shown to express in X. laevis oocytes (Ballivet et al., 1996; Touroutine et al., 2005). Indeed expression of this subunit in oocytes produces small but reproducible acetylcholine gated currents (55 ± 6.07 nA, n = 52, N = 6; Figure 7, A and C). Coexpression of ACR-16 with wild-type RIC-3 leads to a very large (124-fold) increase in current amplitudes that is similar in magnitude to the effects of RIC-3 on ACR-16 currents in C. elegans muscles [Figure 6: RIC-3 TM: 4.3-fold; RIC-3: 41-fold increase in currents compared with ric-3(md1181)] and that is significantly higher (ninefold) than effects of RIC-3 TM (Figure 7A). Differences between the effects of wild-type RIC-3 and RIC-3 TM on ACR-16 peak amplitudes are even greater when these effects are normalized to RIC-3 expression levels, as examined by Western analysis, i.e., a 23-fold difference (Figure 7B). In contrast, in X. laevis oocytes, RIC-3 TM enhances current amplitudes of DEG-3/DES-2 receptors to an extent similar to that of wild-type RIC-3, as shown by our previous results (Cohen Ben-Ami et al., 2005). Thus we conclude that RIC-3 TM–dependent defects are receptor-specific.
Figure 7.
Effects of wild-type RIC-3 and RIC-3 deletion mutants on ACR-16–dependent currents in X. laevis oocytes. (A) Average normalized current amplitudes elicited by 1 mM acetylcholine from oocytes expressing ACR-16, ACR-16 + RIC-3 TM, ACR-16 + RIC-3 minimal, ACR-16 + RIC-3 ΔCC-I, or ACR-16 + wild-type RIC-3 (0.1 μg/μl each). Peak current amplitude for each oocyte was normalized to the average current amplitude for ACR-16-expressing oocytes from the same experiment. N = 2–4 and n = 20–33 each. Effects of wild-type RIC-3 and RIC-3 TM on ACR-16 current amplitudes and the difference between them are significant; p < 0.01. No significant difference is seen between effects of wild-type RIC-3, RIC-3 minimal, and RIC-3 ΔCC-I; one-way Anova. (B) Representative Western analysis showing the relative expression of RIC-3 and RIC-3 deletion mutants. Anti-GFP antibodies were used to detect RIC-3, RIC-3 TM, and RIC-3 minimal, or anti-RIC-3 antibodies (directed to CC-II) were used to detect RIC-3 and RIC-3 ΔCC-I. On average expression of RIC-3 deletion mutants is higher than expression of wild-type RIC-3 2.74 ± 0.63- (N = 3), 6.07 ± 1.95- (N = 2). and 2.79 ± 0.11-fold (N = 2) difference for the TM, minimal, or ΔCC-I proteins, respectively. (C) Representative current traces from oocytes expressing the indicated proteins.
To further examine which domain is responsible for the reduced effects of RIC-3 TM, we also analyzed the effects of expressing ACR-16 with RIC-3 minimal or with RIC-3 ΔCC-I. This analysis shows that both RIC-3 deletion mutants enhance ACR-16 current amplitudes to an extent similar to that of wild-type RIC-3 (Figure 7, A and C). Effects of RIC-3 minimal on ACR-16 current amplitudes and the difference between these effects and the effects of RIC-3 TM are similar to what was seen in C. elegans muscles (Figure 6). This is in agreement with our previous suggestion that deletion of CC-I is responsible for the different effects of these two mutant proteins. However, the similarity between effects of RIC-3 ΔCC-I and RIC-3 minimal on ACR-16 current amplitudes does not reproduce the difference in effects of these two mutants as assayed by levamisole sensitivity in vivo (cf. Figures 1 and 7). Whether this difference between oocytes and C. elegans is due to differences in the assay or to differences in expression levels is unclear.
The RIC-3 C-Terminal Domain Enhances Interactions of RIC-3 with ACR-16
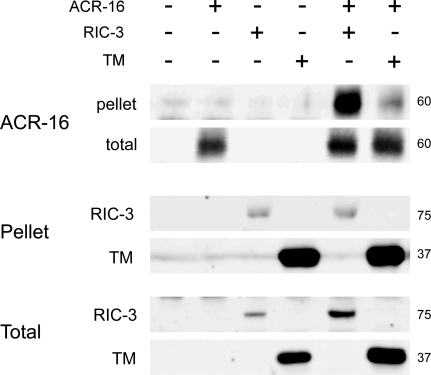
Deletion of the RIC-3 C-terminal domain leads to a dramatic decrease in the effects of RIC-3 on ACR-16 expression. Such reduction can be a result of decreased interaction of this deletion mutant with ACR-16 or to decreased efficacy of the interaction. To examine these possibilities, we used coimmunoprecipitation to look at effects of deleting the C-terminus on interaction of RIC-3 with ACR-16. For this purpose we quantified the amount of tagged ACR-16 found in pellets precipitated by antibodies directed to tagged wild-type RIC-3 or RIC-3 TM. This analysis shows reduced interaction of RIC-3 TM with ACR-16 relative to interaction of wild-type RIC-3 with ACR-16 (Figure 8). To better determine the relative quantity of ACR-16 + RIC-3 or ACR-16 + RIC-3 TM complexes, we normalized ACR-16 levels in the precipitates to levels of RIC-3 or RIC-3 TM in the same precipitate (we note that precipitation efficiency of RIC-3 in this experiment is significantly lower then precipitation efficiency of RIC-3 TM). After this normalization we see a 100-fold higher level of ACR-16 in precipitates from wild-type RIC-3–expressing oocytes relative to oocytes expressing RIC-3 TM. Thus reduced interaction of RIC-3 TM with ACR-16 relative to the interaction of wild-type RIC-3 with ACR-16 can explain the reduced effects of this mutant on ACR-16 expression.
Figure 8.
Interactions of RIC-3 and RIC-3 TM with ACR-16. Coimmunoprecipitation from control oocytes and oocytes expressing ACR-16; RIC-3; RIC-3 TM; ACR-16 and RIC-3, ACR-16; and RIC-3 TM (10 oocytes each). Precipitation was done using anti-GFP antibodies directed at RIC-3:GFP and RIC-3 TM:GFP, and precipitates were analyzed using antibodies against myc to detect myc tagged ACR-16 or using GFP to quantify levels of RIC-3 and RIC-3 TM in the precipitate. Also shown is the level of ACR-16, RIC-3, and RIC-3 TM expression in the same oocytes (total). Numbers on right indicate protein size in kDa.
DISCUSSION
RIC-3 is the founding member of a conserved family of proteins affecting nAChR maturation. All members of this family have a C-terminal coiled-coil domain (Halevi et al., 2003). Conservation of this domain, which is known to enable protein–protein interactions, suggested that interactions mediated by it are important for nAChR maturation. However, RIC-3 derivatives lacking the entire C-terminal domain or only the conserved coiled-coil domain, as a result of alternative splicing or of in vitro–generated deletions, had an effect on nAChR maturation similar to that of wild-type RIC-3 (Cohen Ben-Ami et al., 2005; Lansdell et al., 2008; Seredenina et al., 2008). RIC-3 derivatives lacking the C-terminal coiled coil domains can also physically interact with nAChRs (Cohen Ben-Ami et al., 2005; Lansdell et al., 2008 and results describe here). Thus these derivatives contain sequences mediating interactions with nAChRs. Previously, on the basis of the high sequence conservation within the second RIC-3 transmembrane domain, we suggested that this domain is the one mediating RIC-3's interaction with multiple nAChRs (Cohen Ben-Ami et al., 2005). Support for this suggestion comes from identification of conserved residues within the second RIC-3 membrane-spanning domain that when mutated greatly reduced the interaction of RIC-3 TM with nAChRs (Cohen Ben-Ami and Treinin, unpublished data); this finding, although confirming the importance of the second transmembrane domain in mediating interactions of RIC-3 with nAChRs, cannot be used to rule out contributions by additional domains such as the coiled-coil domains.
To better understand the function of the conserved coiled-coil domain (CC-I), we examined its role in C. elegans on native receptors known to require RIC-3 for their maturation. This analysis shows differential effects of deleting CC-I. Specifically, deletion of CC-I together with sequences downstream to it led to dramatic reduction in the effects of RIC-3 on the two body-wall muscle nAChRs (L-AChR and ACR-16), while producing small or no reduction in effects of RIC-3 on maturation of two other nAChRs (EAT-2 and DEG-3/DES-2). We also show that a receptor that requires the C-terminal domains for its maturation does so irrespective of the expression system. Specifically, ACR-16 maturation is greatly enhanced by the presence of CC-I both in C. elegans and in X. laevis oocytes. Thus our work shows that CC-I enhances the interaction of RIC-3 with specific receptors. In addition, we show that CC-I functions redundantly with sequences downstream to it. These downstream sequences contain another coiled-coil domain (CC-II), a likely candidate for the domain functioning redundantly with CC-I. The region downstream to CC-I, however, neither contains obvious stretches of homology between C. elegans and human RIC-3, nor does it contain a second coiled-coil domain in RIC-3 orthologs (Halevi et al., 2003). Nevertheless, functional homology is likely, as analysis of human RIC-3 showed that deletion of the entire C-terminus interferes with RIC-3 function, whereas deletion of only CC-I did not (Castillo et al., 2005). Additional evidence for the presence of functionally important sequences within this region is that alignment of multiple vertebrate RIC-3 homologues shows large stretches of sequence conservation downstream of CC-I (Halevi et al., 2003 and data not shown).
Coiled-coil domains are known to mediate protein–protein interactions. Our work shows that presence of CC-I enhances RIC-3's interaction with the ACR-16 nAChR. This enhancement may be a result of direct interactions of CC-I with ACR-16 or of an indirect effect of CC-I–mediated interactions on affinity of RIC-3 to specific nAChRs. Such an indirect effect, via coiled-coil domain–mediated oligomerization, enhances the interaction of TRAF (tumor necrosis factor receptor-associated factor) proteins with the CD40 receptor (Pullen et al., 1999).
RIC-3 homologues were shown to affect maturation of multiple nAChRs, suggesting a conserved mechanism of interaction. However, effects of RIC-3 homologues depend on both the receptor and the experimental system (Halevi et al., 2003; Cheng et al., 2005; Castillo et al., 2005; Lansdell et al., 2005). For example, we now show a 100-fold increase in ACR-16–dependent currents in the presence of RIC-3, whereas our previous work has shown at most a 10-fold increase in DEG-3/DES-2–dependent currents in the presence of RIC-3 and in the same expression system, X. laevis oocytes (Cohen Ben-Ami et al., 2005). In addition we show that the two receptors differ in their requirement for the RIC-3 C-terminal domains, domains that are not required for effects of RIC-3 on DEG-3/DES-2 maturation (Cohen Ben-Ami et al., 2005) but are required for full effects of RIC-3 on ACR-16 maturation. Thus our work suggests receptor-specific differences in the interactions of RIC-3 and RIC-3 domains with nAChRs. Interestingly, both vertebrates and invertebrates (Drosophila) use alternative splicing to generate RIC-3 isoforms lacking the C-terminal domains (Halevi et al., 2003; Lansdell et al., 2008 Seredenina et al., 2008). Although regulation of this alternative splicing events has not been studied in detail, differences in expression levels of alternatively spliced human ric-3 transcripts in different tissues were shown (Halevi et al., 2003). Our results suggest that regulation of this alternative splicing may serve as a mechanism for regulating surface expression of specific nAChRs.
ACKNOWLEDGMENTS
We thank David Sattelle for the ACR-16 clone, James Rand for the anti-UNC-17 mAb. This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG; SFB 628 and CEF-MC) to A.G. and the Israel Science Foundation Grant 379/06 to M.T.
Abbreviations used:
- nAChR
nicotinic acetylcholine receptor
- ER
endoplasmic reticulum.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-08-0851) on December 30, 2008.
REFERENCES
- Ballivet M., Alliod C., Bertrand S., Bertrand D. Nicotinic acetylcholine receptors in the nematode Caenorhabditis elegans. J. Mol. Biol. 1996;258:261–269. doi: 10.1006/jmbi.1996.0248. [DOI] [PubMed] [Google Scholar]
- Castillo M., Mulet J., Gutierrez L. M., Ortiz J. A., Castelan F., Gerber S., Sala S., Sala F., Criado M. Dual role of the RIC-3 protein in trafficking of serotonin and nicotinic acetylcholine receptors. J. Biol. Chem. 2005;280:27062–27068. doi: 10.1074/jbc.M503746200. [DOI] [PubMed] [Google Scholar]
- Cheng A., Bollan K. A., Greenwood S. M., Irving A. J., Connolly C. N. Differential subcellular localization of RIC-3 isoforms and their role in determining 5-HT3 receptor composition. J. Biol. Chem. 2007;282:26158–26166. doi: 10.1074/jbc.M703899200. [DOI] [PubMed] [Google Scholar]
- Cheng A., McDonald N. A., Connolly C. N. Cell surface expression of 5-hydroxytryptamine type 3 receptors is promoted by RIC-3. J. Biol. Chem. 2005;280:22502–22507. doi: 10.1074/jbc.M414341200. [DOI] [PubMed] [Google Scholar]
- Cohen Ben-Ami H., Yassin L., Farah H., Michaeli A., Eshel M., Treinin M. RIC-3 affects properties and quantity of nicotinic acetylcholine receptors via a mechanism that does not require the coiled-coil domains. J. Biol. Chem. 2005;280:28053–28060. doi: 10.1074/jbc.M504369200. [DOI] [PubMed] [Google Scholar]
- Cordero-Erausquin M., Marubio L. M., Klink R., Changeux J. P. Nicotinic receptor function: new perspectives from knockout mice. Trends Pharmacol. Sci. 2000;21:211–217. doi: 10.1016/s0165-6147(00)01489-9. [DOI] [PubMed] [Google Scholar]
- Duerr J. S., Gaskin J., Rand J. B. Identified neurons in C. elegans coexpress vesicular transporters for acetylcholine and monoamines. Am. J. Physiol. Cell Physiol. 2001;280:C1616–C1622. doi: 10.1152/ajpcell.2001.280.6.C1616. [DOI] [PubMed] [Google Scholar]
- Fire A., Waterston R. H. Proper expression of myosin genes in transgenic nematodes. EMBO J. 1989;8:3419–3428. doi: 10.1002/j.1460-2075.1989.tb08506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk A., Schafer W. R. Visualization of integral and peripheral surface proteins in live Caenorhabditis elegans. J. Neurosci. Methods. 2006;154:68–79. doi: 10.1016/j.jneumeth.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Green W. N., Claudio T. Acetylcholine receptor assembly: subunit folding and oligomerization occur sequentially. Cell. 1993;74:57–69. doi: 10.1016/0092-8674(93)90294-z. [DOI] [PubMed] [Google Scholar]
- Halevi S., McKay J., Palfreyman M., Yassin L., Eshel M., Jorgensen E., Treinin M. The C. elegans ric-3 gene is required for maturation of nicotinic acetylcholine receptors. EMBO J. 2002;21:1012–1020. doi: 10.1093/emboj/21.5.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevi S., Yassin L., Eshel M., Sala F., Sala S., Criado M., Treinin M. Conservation within the RIC-3 gene family: effectors of nAChR expression. J. Biol. Chem. 2003;278:34411–34417. doi: 10.1074/jbc.M300170200. [DOI] [PubMed] [Google Scholar]
- Jones A. K., Davis P., Hodgkin J., Sattelle D. B. The nicotinic acetylcholine receptor gene family of the nematode Caenorhabditis elegans: an update on nomenclature. Invertebrate Neurosci. 2007;7:129–131. doi: 10.1007/s10158-007-0049-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S. H., Taylor P. Determinants responsible for assembly of the nicotinic acetylcholine receptor. J. Gen. Physiol. 1999;113:171–176. doi: 10.1085/jgp.113.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansdell S. J., Collins T., Yabe A., Gee V. J., Gibb A. G., Millar N. S. Host cell specific effects of the nicotinic acetylcholine receptor chaperone RIC-3 revealed by a comparison of human and Drosophila RIC-3 homologs. J. Neurochem. 2008;105:1573–1581. doi: 10.1111/j.1471-4159.2008.05235.x. [DOI] [PubMed] [Google Scholar]
- Lansdell S. J., Gee V. J., Harkness P. C., Doward A.I., Baker E. R., Gibb A. J., Millar N. S. RIC-3 enhances functional expression of multiple nicotinic acetylcholine receptor subtypes in mammalian cells. Mol. Pharmacol. 2005;68:1431–1438. doi: 10.1124/mol.105.017459. [DOI] [PubMed] [Google Scholar]
- Lewis J. A., Wu C. H., Berg H., Levine J. H. The genetics of levamisole resistance in the nematode Caenorhabditis elegans. Genetics. 1980;95:905–928. doi: 10.1093/genetics/95.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlie J. P., Lindstrom J. Assembly in vivo of mouse muscle acetylcholine receptor: identification of an α subunit species that may be an assembly intermediate. Cell. 1983;34:747–757. doi: 10.1016/0092-8674(83)90531-7. [DOI] [PubMed] [Google Scholar]
- Miller K. G., Alfonso A., Nguyen M., Crowell J. A., Johnson C. D., Rand J. B. A genetic selection for Caenorhabditis elegans synaptic transmission mutants. Proc. Natl. Acad. Sci. USA. 1996;93:12593–12598. doi: 10.1073/pnas.93.22.12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani S. Genetic regulation of mec-3 expression implicated in the specification of the mechanosensory neuron cell types in Caenorhabditis elegans. Dev. Growth Differ. 1995;37:551–557. doi: 10.1046/j.1440-169X.1995.t01-4-00010.x. [DOI] [PubMed] [Google Scholar]
- Nagel G., Brauner M., Liewald J. F., Adeishvili N., Bamberg E., Gottschalk A. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr. Biol. 2005;15:2279–2284. doi: 10.1016/j.cub.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Numa S., Noda M., Takahashi H., Tanabe T., Toyosato M., Furutani Y., Kikyotani S. Molecular structure of the nicotinic acetylcholine receptor. Cold Spring Harbor Symp. Quant. Biol. 1983;48(1):57–69. doi: 10.1101/sqb.1983.048.01.008. [DOI] [PubMed] [Google Scholar]
- Pullen S. S., Labadia M. E., Ingraham R. H., McWhirter S. M., Everdeen D. S., Alber T., Crute J. J., Kehry M. R. High-Affinity interactions of tumor necrosis factor receptor-associated factors (TRAFs) and CD40 require TRAF trimerization and CD40 multimerization. Biochemistry. 1999;38:10168–10177. doi: 10.1021/bi9909905. [DOI] [PubMed] [Google Scholar]
- Richmond J. E., Jorgensen E. M. One GABA and two acetylcholine receptors function at the C. elegans neuromuscular junction. Nature neuroscience. 1999;2:791–797. doi: 10.1038/12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seredenina T., Ferraro T., Terstappen G. C., Caricasole A., Roncarati R. Molecular cloning and characterization of a novel human variant of RIC-3, a putative chaperone of nicotinic acetylcholine receptors. Biosci. Rep. 2008 doi: 10.1042/BSR20080055. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Touroutine D., Fox R. M., Von Stetina S. E., Burdina A., Miller D. M., Richmond J. E. acr-16 encodes an essential subunit of the levamisole-resistant nicotinic receptor at the Caenorhabditis elegans neuromuscular junction. J. Biol. Chem. 2005;280:27013–27021. doi: 10.1074/jbc.M502818200. [DOI] [PubMed] [Google Scholar]
- Williams M. E., Burton B., Urrutia A., Shcherbatko A., Chavez-Noriega L. E., Cohen C. J., Aiyar J. Ric-3 promotes functional expression of the nicotinic acetylcholine receptor alpha7 subunit in mammalian cells. J. Biol. Chem. 2005;280:1257–1263. doi: 10.1074/jbc.M410039200. [DOI] [PubMed] [Google Scholar]
- Wood W. B. The nematode Caenorhabditis elegans. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]