Abstract
The newly described yeast endosomal sorting complexes required for transport (ESCRT) protein increased sodium tolerance-1 (Ist1p) binds the late-acting ESCRT proteins Did2p/charged MVB protein (CHMP) 1 and Vps4p and exhibits synthetic vacuolar protein sorting defects when combined with mutations in the Vta1p/LIP5–Vps60p/CHMP5 complex. Here, we report that human IST1 also functions in the ESCRT pathway and is required for efficient abscission during HeLa cell cytokinesis. IST1 binding interactions with VPS4, CHMP1, LIP5, and ESCRT-I were characterized, and the IST1–VPS4 interaction was investigated in detail. Mutational and NMR spectroscopic studies revealed that the IST1 terminus contains two distinct MIT interacting motifs (MIM1 and MIM2) that wrap around and bind in different groves of the MIT helical bundle. IST1, CHMP1, and VPS4 were recruited to the midbodies of dividing cells, and depleting either IST1 or CHMP1 proteins blocked VPS4 recruitment and abscission. In contrast, IST1 depletion did not inhibit human immunodeficiency virus-1 budding. Thus, IST1 and CHMP1 act together to recruit and modulate specific VPS4 activities required during the final stages of cell division.
INTRODUCTION
The human endosomal sorting complexes required for transport (ESCRT) pathway functions in the terminal stages of cytokinesis (Carlton and Martin-Serrano, 2007), enveloped virus budding (Bieniasz, 2006; Fujii et al., 2007), and intralumenal vesicle formation at the multivesicular body (MVB) (Hurley and Emr, 2006; Gill et al., 2007; Nickerson et al., 2007). Although mechanistic details are lacking, all three of these processes terminate with the severing of thin, cytoplasm-filled membrane “necks,” suggesting that the ESCRT pathway may provide the terminal membrane fission activity. Most ESCRT pathway components function as subunits of a series of multiprotein complexes, termed ESCRT-0, -I, -II, and -III, which assemble into membrane-associated protein networks at sites of action. The VPS4 ATPase also functions late in the ESCRT pathway, where it can act in concert with the adaptor/activator protein Vta1p/LIP5 to release the assembled ESCRT machinery from the membrane. There is mounting evidence that the ESCRT-III and VPS4 complexes may work together to mediate key aspects of membrane deformation and fission (e.g., Hanson et al., 2008), and these complexes have therefore become a focus of interest in the ESCRT pathway.
Yeast express four essential “core” ESCRT-III proteins: Vps32p/Snf7p/charged MVB protein (CHMP)4, Vps20p/CHMP6, Vps2p/CHMP2, and Vps24p/CHMP3 (human protein names are in capital letters), as well as two “accessory” ESCRT-III-like proteins (Did2p/CHMP1 and Vps60/CHMP5) that seem to play regulatory roles (Babst et al., 2002). Although the ESCRT pathway is conserved across eukaryotes, the ESCRT-III subunits have diversified considerably in mammals, and humans express at least 11 different ESCRT-III-like proteins. These 11 proteins can be subdivided into six families (CHMP1–6) that correspond to the six yeast ESCRT-III–like proteins, as well as a seventh family (CHMP7) not present in yeast. At membrane sites of action, various ESCRT-III subunits associate into large copolymers that contain multiple copies of each ESCRT-III subunit (Babst et al., 2002). Deposition of ESCRT-III subunits is probably associated with conformational changes that promote oligomerization, membrane binding, and cofactor recruitment (Lin et al., 2005; Muziol et al., 2006; Zamborlini et al., 2006; Shim et al., 2007; Lata et al., 2008). Clues to the functions of ESCRT-III complexes seem to be provided by the recent observation that overexpression of CHMP4 subunits induces the extrusion of thin membrane tubules that extend out from the plasma membrane (Hanson et al., 2008). The CHMP4 proteins form circular filaments that surround the bases and extend up into the tubules. Thus, these experiments suggest the possibility that authentic ESCRT-III lattices may consist of concentric rings or helices of ESCRT-III filaments that encircle the necks of extruding MVB vesicles and viral particles.
The assembled ESCRT-III lattice recruits VPS4 and other cofactors required for cargo deubiquitylation, vesicle fission, and release of the assembled ESCRT machinery. VPS4 is a type 1 AAA ATPase that assembles into a double-ringed complex with six (Yu et al., 2008) or seven (Hartmann et al., 2008) subunits per ring. The Vta1p/LIP5 cofactor stimulates subunit assembly and ATPase activity by contacting the VPS4 β domains and forming a supercomplex of ∼12 Vps4p:6 Vta1p (Scott et al., 2005a; Azmi et al., 2006; Lottridge et al., 2006; Xiao et al., 2008; Yu et al., 2008). This supercomplex binds the ESCRT-III lattice through a series of interactions with MIT domains on both Vta1p/LIP5 (2 MIT domains/subunit) and VPS4 (1 MIT domain/subunit) (reviewed in Hurley and Yang, 2008). MIT domains are three helix bundles that can bind ESCRT-III proteins and other partners through one of two distinct types of MIT interacting motifs. Type 1 MIT interacting motifs (MIM1) form amphipathic helices that bind in the groove between MIT helices 2 and 3 (Obita et al., 2007; Stuchell-Brereton et al., 2007), whereas type 2 MIT interacting motifs (MIM2) are extended, proline-rich strands that bind in the groove between MIT helices 1 and 3 (Kieffer et al., 2008). Once the VPS4 complex is recruited and assembled, VPS4 uses the energy of ATP hydrolysis to drive disassembly of the ESCRT-III lattice, and this activity may also be mechanistically coupled to vesicle extrusion, membrane fission, or both.
Very recent studies in Saccharomyces cerevisiae have led to the identification and characterization of a new ESCRT pathway member, increased sodium tolerance-1 (Ist1p) (Dimaano et al., 2008; Rue et al., 2008). Ist1p binds both Vps4p and the ESCRT-III–like protein Did2p/CHMP1 (Krogan et al., 2006; Dimaano et al., 2008; Rue et al., 2008). Did2p/CHMP1, in turn, can bind other ESCRT-III subunits, and Ist1p can therefore help connect the ESCRT-III and Vps4p ATPase complexes (Nickerson et al., 2006; Tsang et al., 2006). Yeast cells lacking Ist1p exhibit only modest MVB cargo sorting defects, but these defects are synthetically enhanced in the absence of Vta1p/LIP5 or Vps60p/CHMP5, or in the presence of temperature-sensitive Vps4p mutants (but not in the absence of Did2p/CHMP1) (Dimaano et al., 2008; Rue et al., 2008). These observations suggest that Ist1p and Did2p/CHMP1 form a complex that can regulate Vps4p activity and that this complex may either act in concert with the Vta1p/LIP5–Vps60p/CHMP5 complex or, alternatively, that the Ist1p-Did2p/CHMP1 and Vta1p/LIP5–Vps60p/CHMP5 complexes may perform redundant functions. Ist1p has clear mammalian homologues, and in this study we have examined the protein interactions and biological functions of human IST1, with the goal of identifying human ESCRT functions that require IST1.
MATERIALS AND METHODS
Human IST1
A series of different human IST1 isoforms and polymorphisms have been documented previously (e.g., http://beta.uniprot.org/uniprot/P53990). The numbering schemes and experiments described in this article all used a 366-amino acid protein that corresponds to IST1 isoform 1, with a common two amino acid polymorphism at position 237. We sequenced cDNAs encoding this isoform from human spleen, brain, and lung cells, and this isoform comigrates with the major endogenous IST1 isoform in 293T cells (data not shown).
Expression Vectors
Expression vectors are summarized in Supplemental Table 1, A and B.
Antibodies
Antibodies used for Western blotting and immunofluorescence are summarized in Supplemental Table 1D.
Cell Cultures
HeLa, 293T, and COS7 cells were maintained in DMEM supplemented with 10% fetal calf serum. For immunofluorescence experiments, HeLa or COS7 cells were seeded onto coverslips and cotransfected at 50–60% confluence. For virion production, 293T cells were seeded in six-well plates and cotransfected at 50–60% confluence.
Yeast Two-Hybrid Assays
Two-hybrid vectors used in library screens and in directed two hybrid experiments are listed in Supplemental Table 1C. Automated yeast two-hybrid screens were performed as described previously (Garrus et al., 2001), using cDNA prey libraries from macrophages and from breast and prostate cancer cell lines, and interactions are summarized in Table 1E. Candidate genes were subcloned and retested using the Matchmaker GAL4 Yeast Two-Hybrid 3 system (Clontech, Mountain View, CA).
Coprecipitation Experiments with One-STrEP-FLAG (OSF)-tagged Proteins
293T cells were seeded (3 × 106 cells/55-cm2 dish) and cotransfected with 3 μg of each relevant expression plasmid (polyethylenimine 25,000 kDa; Polysciences, Warrington, PA) (Durocher et al., 2002). Cells were harvested 48 h after transfection by incubation in 300 μl of lysis buffer (50 mM Tris, pH 7.4, and 150 mM NaCl) supplemented with proteinase inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) and 1% Triton X-100. Lysates were clarified by centrifugation (18,000 × g; 10 min; 4°C), and OSF-tagged proteins were affinity purified by incubation with StrepTactin Sepharose for 2 h (40 μl of slurry; IBA, Gottingen Germany). The matrix was washed four times in wash buffer (20 mM Tris, pH 7.4, and 150 mM NaCl) supplemented with 0.1% Triton X-100, and bound proteins were detected by Western blotting.
IST1 Expression and Purification
IST1 was expressed in Escherichia coli and purified to homogeneity as described in Supplemental Figure S1D and in the figure caption to Supplemental Figure S1. Recombinant IST1 was shown to be monomeric by analytical centrifugation (Supplemental Figure S1E) and was used as an antigen to prepare anti-IST1 antibodies.
GST Fusion Proteins for Binding Assays
Glutathione transferase (GST), GST-IST1, and GST-Vps4A MIT proteins were expressed in 50-ml E. coli cultures in autoinduction media for 6 h at 37°, followed by 18 h at 23°C (Studier, 2005). Cells were pelleted, resuspended in 4 ml of binding buffer (150 mM NaCl, 1 mM dithiothreitol [DTT], 50 mM Tris, pH 8.0, and 0.2% NP-40), and lysed by lysozyme treatment (1.2 mg) and sonication (3 × 15 s). Insoluble proteins and debris were removed by centrifugation, and the soluble supernatants were used immediately in binding and biosensor experiments.
ESCRT-I Binding Experiments
For each binding reaction, 20 μl of soluble E. coli extract expressing the appropriate GST-IST1 protein or GST control was coincubated with 80 μg of purified recombinant ESCRT-I complex for 1 h at 4°C in binding buffer (100 μl total volume, 6 μM final ESCRT-I concentration and approximate equimolar IST1, as analyzed by SDS-polyacrylamide gel electrophoresis [PAGE]). GST fusion proteins and bound proteins were captured on 25 μl of glutathione-Sepharose slurry (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom), unbound ESCRT-I complexes were removed by washing with binding buffer, and bound proteins were released by boiling in SDS-PAGE loading buffer, and analyzed by 12% SDS-PAGE with Coomassie Blue staining and by Western blotting. ESCRT-I complexes used in these studies were expressed and purified from SF21 insect cells and were a gift from Angela Katsuyama (University of Utah). The expression and purification protocol will be described elsewhere.
Biosensor Binding Experiments
Biosensor binding experiments were performed as described previously (Scott et al., 2005b), with purified VPS4A and VPS4B MIT domains binding to immobilized GST-IST1 proteins. Running buffer was: 20 mM Tris, pH 8.0, 150 mM NaCl, 0.005% Tween, 0.1 mg/ml bovine serum albumin, and 1 mM DTT, at 25°C. All binding isotherms were fit to simple 1:1 binding models, and estimated dissociation constants are given in Supplemental Table 1F.
Nuclear Magnetic Resonance (NMR) Spectroscopic Titrations
15N-labeled VPS4A MIT (150 nmol) was prepared in 0.6 ml of NMR buffer containing 25 mM sodium phosphate, pH 5.75, 50 mM NaCl, 1.0 mM EDTA, 40 μM sodium azide, 90% H2O, and 10% D2O (Stuchell-Brereton et al., 2007). N-Acetyl– and C-amide—modified, IST1-derived peptides (MIM1: IST1321-339 and MIM2: IST1340-366) were dissolved in NMR buffer and quantified by amino acid analysis. Two-dimensional [15N,1H]-HSQC spectra of VPS4A MIT were monitored during titrations of 0–1.2 equivalents of MIM1 or MIM2 peptide. Fast exchanging NH resonances were tracked between the assigned unbound (Scott et al., 2005b) and MIM1/2 saturated states, and NH assignments were confirmed using NH-NH nuclear Overhauser effects from three-dimensional [1H,15N,1H] nuclear Overhauser effect spectroscopy spectra. The 1.2 equivalent titration points resulted in >98% saturation of VPS4A MIT based on equilibrium disassociation constants determined from biosensor studies. All NMR experiments were recorded at 25°C on an Inova 600 MHz NMR spectrometer (Varian, Palo Alto, CA) equipped with a triple-resonance cryogenic probe. Data were processed with FELIX 2004 (Felix NMR, San Diego, CA), and analyzed with SPARKY (T. D. Goddard and D. G. Kneller, University of California, San Francisco). Cut-offs for shifted residues shown in Figure 2D were as follows: MIM1 (top, red: Δδ > 0.10 ppm, Δδ = ((ΔδHN)2 + (ΔδN/6.5)2)0.5) or MIM2 (middle, orange: Δδ > 0.12 ppm).
Figure 2.
IST1 Interacts with VPS4A MIT. (A) Yeast two-hybrid interactions between IST1 and designated VPS4A constructs. Two-hybrid interactions (top) or cotransformation controls (bottom) are shown. Note that IST1 showed a positive two-hybrid interaction with the VPS4A MIT domain but not the VPS4A ATPase cassette (compare lanes 2 and 3 and 4). (B) IST1 has two distinct MIM elements. Top, alignment of IST1321-366 (top) with CHMP6 MIM2 and CHMP1A MIM1 (middle), and with MIM consensus sequences (bottom). Bottom, biosensor binding isotherms showing pure VPS4A MIT binding to GST-IST1 proteins captured from E. coli extracts. Estimated dissociation constants (micromolar) for VPS4A MIT binding were as follows: IST1, 1.1 (black); IST1303-366, 0.8 (red); IST1323-339, 12.4 (blue); IST1340-366, 26 (green); and IST11-189, >1500 (orange). Background binding of VPS4A MIT to control GST surfaces was negligible (data not shown). (C) Mutational analyses of IST1 MIM1 and MIM2. Biosensor binding isotherms showing purified VPS4A MIT binding to GST-IST1 proteins captured from E. coli extracts. Estimated dissociation constants (micromolar) for VPS4A MIT binding were as follows: IST1, 1.1 (black); IST1L325D, 8; IST1L328D, 16; IST1L355A, 17; IST1L362A, 9 (single mutants are in blue and purple shades); IST1L325D/L355A, 88; IST1L328D/L355A, 502; and IST1L328/L362, 432 (double mutants are in red and orange shades). (D) NMR chemical shift mapping of the IST1 MIM1 and MIM2 binding sites on VPS4A MIT. Figures show residues shifted by binding of IST1 MIM1 mapped onto space filling models of the VPS4A MIT domain. Binding modes of the structurally characterized CHMP1A MIM1 (top; green) and CHMP6 (middle; blue) elements are shown for reference. The bottom panel shows a structure-based cartoon model of the VPS4A MIT-IST1321-366 complex, with the three VPS4A MIT helices depicted in gray, and the IST1 MIM1 and MIM2 elements depicted in green and blue, respectively. (E) LIP5 binds the MIM1/MIM2 region of IST1. Wild-type (WT) Myc-IST1 (lanes 1 and 2) or Myc-IST1L328D/L355A (lanes 3 and 4) coprecipitations with empty vector controls (lanes 1 and 3) or with OSF-LIP5 (lanes 2 and 4).
Human Immunodeficiency Virus (HIV-1) Vector Release and Infectivity
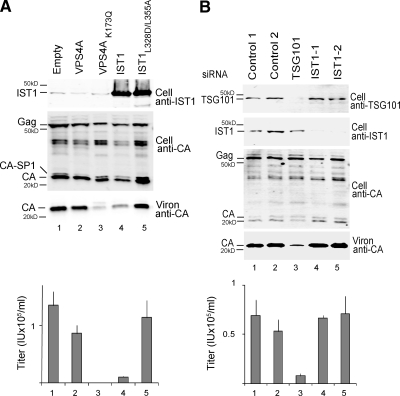
293T cells were seeded in six-well plates at 5 × 105 cells per well and cotransfected with the pcDNA-IST or pEGFP-VPS4 expression constructs and an HIV-1 vector system (pCMVΔR8.2, pWPTS-nlsLacZ, and phCMV-VSVG). Briefly, 8 μl of Lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad, CA) was combined with 1 μg of pCMVΔR8.2, 1 μg of pWPTS-nlsLacZ, 0.36 μg of phCMV-VSVG, and 0.75 μg of pcDNA-IST or pEGFP-VPS4A plasmid per well according to manufacturer's instructions. Virions were harvested from the supernatant 42 h after transfection, and analyzed for infectivity or pelleted through a 20% sucrose cushion for Western blotting. Cells were lysed in radioimmunoprecipitation assay buffer for Western blotting of intracellular proteins and visualized on an Odyssey scanner (Li-Cor Biosciences, Lincoln, NE). Vector titers were determined by transducing HeLa-M cells seeded in 96-well plates at 5 × 103 cells per well. Vector titers in Figure 3 are from triplicate measurements, and error bars indicate SDs. Note that the experiment shown in Figure 3B (293T cells) was also performed in HeLa M cells with similar results (data not shown).
Figure 3.
IST1 overexpression inhibits HIV budding but IST1 depletion does not. (A) IST1 overexpression reduces HIV-1 release and titer. Protein levels (Western blots) and vector titers (infectious units per milliliter; bottom) upon coexpression of an HIV-1 vector with an empty vector (lane 1, negative control) or with vectors expressing GFP-VPS4A (lane 2, negative control), GFP-Vps4AK173Q (lane 3, positive control), IST1 (lane 4), or IST1L328D/L355A (lane 5). Western blots show intracellular IST1 (top, anti-IST1), intracellular HIV Gag proteins (middle, anti-CA), and virion-associated CA proteins (bottom, anti-CA). (B) IST1 depletion does not reduce HIV-1 vector release or titer. Protein levels (Western blots) and vector titers (bottom) upon coexpression of an HIV-1 vector with two irrelevant siRNAs (lanes 1 and 2, negative controls), an siRNA against TSG101 (positive control, lane 3), or two different siRNAs against IST1 (lanes 4 and 5). Western blots show intracellular TSG101 (panel 1, anti-TSG101), intracellular IST1 (panel 2, anti-IST1), intracellular HIV Gag proteins (panel 3, anti-CA), and virion-associated CA proteins (panel 4, anti-CA).
Small Interferring RNA (siRNA) Depletion of Endogenous IST1, CHMP1, and VPS4 Proteins
For virus release assays, 293T cells were seeded (2 × 105 cells/well, 6-well plates; t = 0), and siRNA depletion experiments were performed with the following time course: t = 24 h, first siRNA transfection (10 nM siRNA, Lipofectamine RNAiMax; Invitrogen); t = 48 h, media change and second siRNA cotransfection with HIV-1 vector (Lipofectamine 2000; Invitrogen); and t = 96 h, harvest cells and supernatants for analysis. For cytokinesis assays, HeLa cells were seeded (0.7 × 104 cells/well, 6-well plates; t = 0), and siRNA depletion experiments were performed with the following time course: t = 24 h, first siRNA transfection (10 nM siRNA, Lipofectamine RNAiMax; Invitrogen); t = 36 h, media change and second siRNA transfection; t = 48 h media change and third siRNA transfection; and t = 72 h analyze cells by 1) fixing and staining (for fluorescence assays) or 2) harvesting using trypsin treatment for Western blotting or fluorescence-activated cell sorting analyses (with propidium iodide staining used in the latter case). siRNA duplexes matched 19 nt sites in target genes (with 3′ dTT overhangs on both strands) starting at the following coding strand nucleotides: IST1-1, 490; IST1-2, 385; CHMP1A-1, 56; CHMP1A-2, 275; CHMP1B-1, 83; CHMP1B-2, 335; VPS4A, 199; and VPS4B, 425.
Scoring Cytokinesis Defects by Direct Visualization
siRNA-transfected HeLa cells were fixed with 3% paraformaldehyde, stained with SYTOX-Green and anti γ-tubulin, and manually scored for multiple nuclei or cytokinesis arrest by using fluorescence microscopy. For each data point, three groups of 200 cells were blinded and scored, and error bars represent SDs.
Expression of siRNA-resistant IST1 in Cells Depleted of Endogenous IST1
HeLa cells in six-well plates were transfected with IST1 expression vectors (2 μg/well, Lipofectamine 2000; Invitrogen) and siRNA (10 nM, Lipofectamine RNAi MAX; Invitrogen), following the time course t = 0, cells seeded at 4 × 105 cells/well; t = 24 h, IST1 vector; t = 32 h, media change + IST1 vector; t = 44 h, trypsin treatment and reseed reseeded on an 18-mm glass coverslip (∼3 × 104 cells); t = 50 h, siRNA; t = 56 h, media change + siRNA; t = 68 h, media change + siRNA; and t = 98 h, cells fixed and stained with anti-α-tubulin and SYTOX-Green (Invitrogen) and analyzed.
Time-Lapse Microscopy
siRNA-transfected HeLa cells were cultured in a 37°C microscope chamber (Okolab, San Francisco, CA) with 5% CO2 and observed by phase contrast on an Olympus IX81 microscope (10× objective). Images were acquired every 7 min with MetaMorph version 6.2r6 software (Molecular Devices, Sunnyvale, CA).
Immunofluorescence Imaging
To image endogenous proteins, HeLa cells were grown on coverslips as described above, fixed 24 h after the third siRNA transfection with 3% paraformaldehyde in phosphate-buffered saline, and then stained with anti-α-tubulin and the indicated antibodies. To image exogenously expressed proteins, ∼3 × 104 cells on glass coverslips were transfected using Lipofectamine 2000 with 0.5 μg of pcDNA3.1 MycHis(−)A vectors expressing wild-type or mutant IST1-Myc and CHMP1A-Myc proteins, and fixed after 36 h. Confocal immunofluorescence images were acquired using FluoView software on a FV300 IX81 microscope (Olympus, Tokyo, Japan), and single confocal slices are shown. Midbody localization data for endogenous proteins were quantified by averaging two separate experiments, with n ∼20 midbodies per experiment.
RESULTS
Human IST1 Binds ESCRT-I, CHMP1, LIP5, and VPS4
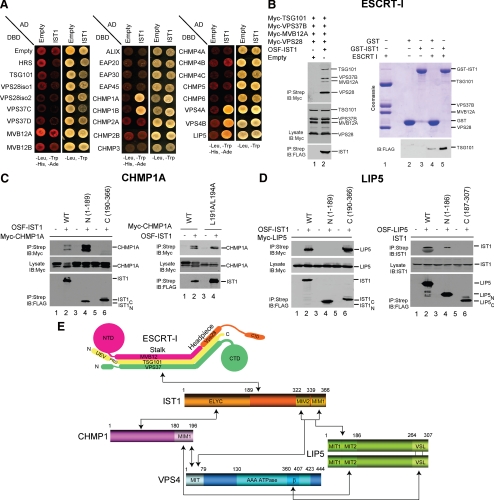
Extensive library and directed yeast two-hybrid screens were performed to identify potential binding partners for human IST1. These experiments identified six potential IST1 binding partners: VPS37B, LIP5, both known CHMP1 proteins (CHMP1A and CHMP1B), and both known VPS4 proteins (VPS4A and VPS4B) (Figure 1A and Supplemental Table 1). As noted above, yeast Ist1p also binds Did2p/CHMP1 and Vps4p (Krogan et al., 2006; Dimaano et al., 2008; Rue et al., 2008), and these IST1 interactions are therefore conserved from yeast to human. In contrast, IST1 interactions with the VPS37B subunit of the ESCRT-I complex and with the VPS4 activator Vta1p/LIP5 have not been reported previously. These novel interactions are of interest because they suggest that IST1 may engage the upstream ESCRT-I complex and may link the two known VPS4 regulatory complexes; Did2p/CHMP1-IST1 and Vta1p/LIP5-Vps60p/CHMP5. Each of the different two hybrid interactions was therefore confirmed and studied further at the protein level.
Figure 1.
IST1 binding interactions. (A) Yeast two-hybrid interactions of IST1 with other human ESCRT pathway proteins. IST1-AD fusions (columns 2, IST1) or control AD constructs (columns 1, Empty) were coexpressed with control DBD constructs (row 1, Empty) or ESCRT protein–DBD fusions (rows 2–26) and tested for positive yeast two-hybrid interactions (left) or cotransformation (control, right). IST1 showed positive interactions with CHMP1A, CHMP1B, VPS4A, VPS4B, and LIP5 in this assay. (B) IST1 binds ESCRT-I. Left, ESCRT-I coprecipitations with an empty vector control (lane 1) or One-STrEP-FLAG(OSF)-IST1 (lane 2). All four ESCRT-I subunits were coexpressed with N-terminal Myc epitope tags in these experiments. Right, binding of pure recombinant ESCRT-I complexes to GST-IST1 in E. coli extracts. Top right, Coomassie-stained SDS-PAGE gel showing pure recombinant ESCRT-I (lane 1), GST or GST-IST1 control extracts (lanes 2 and 3), and ESCRT-I pull-down reactions (lanes 4 and 5). Bottom right, Western blot showing matrix binding levels of the FLAG-TSG101/ESCRT-I subunit in the reactions corresponding to lanes 2–5 of the top panel. (C) IST1 binds CHMP1A. Left, Myc-CHMP1A coprecipitations with empty vector controls (lanes 1, 3, and 5), OSF-IST1 (lane 2), OSF-IST11-189 (lane 4), or OSF-IST1190-366 (lane 6). Right, Myc-CHMP1A (lanes 1 and 2) and Myc-CHMP1AL191A/L184A (lanes 3 and 4) coprecipitations with empty vector controls (lanes 1 and 3) and OSF-IST1 (lanes 2 and 4). (D) IST1 binds LIP5. Left, Myc-LIP5 coprecipitations with empty vector controls (lanes 1, 3, and 5), or OSF-IST1 (lane 2), OSF-IST11-189 (lane 4) or OSF-IST190-366 (lane 6). Right, endogenous IST1 coprecipitations with empty vector controls (lanes 1, 3, and 5), OSF-LIP5 (lane 2), OSF-LIP51-186 (lane 4), or OSF-LIP5197-307 (lane 6). (E) Summary of IST1 protein interactions. Domain abbreviations: VSL, Vta1/SBP1/LIP5 domain; UEV, Ubiquitin E2 Variant domain; PRD, proline-rich domain; NTD/CTD, N- and C-terminal domains; β, β-domain; AAA ATPase, ATPases associated with a variety of cellular activities.
IST1–ESCRT-I Interactions
VPS37B-IST1 interactions were identified in multiple automated yeast two-hybrid screens that used the VPS37B subunit of ESCRT-I as baits, and the smallest interacting fragments detected were VPS37B50-170 and IST164-279 (Supplemental Table 1E). IST1 can be divided into three regions based on sequence analyses: a helical N-terminal region termed the ELYC domain (residues 1-189) (Dimaano et al., 2008), a central proline-rich linker (190-322), and a C-terminal MIM region (323–366) that binds MIT domains (see below). Thus, VPS37B binds a conserved central region found in most IST1 isoforms that spans the C-terminal end of the ELYC domain and the central, proline-rich central region.
The IST1–ESCRT-I interaction was not studied further using yeast two-hybrid methods because 1) human ESCRT-I normally functions as a stable heterotetrameric complex, 2) Gal4 DNA binding domain (DBD) fusions with full-length VPS37A and VPS37B proteins activated transcription in the absence of binding partners (data not shown), and 3) VPS37C-DBD and VPS37D-DBD proteins did not show significant two-hybrid interactions with full-length IST1-AD (Figure 1A). Instead, we tested whether OSF-IST1 could coprecipitate heterotetrameric ESCRT-I complexes created by simultaneous overexpression of the four Myc-tagged ESCRT-I subunits in 293T cells (Morita et al., 2007a). As shown in the left panels of Figure 1B, all four ESCRT-I subunits coprecipitated with OSF-IST1 but not with a control matrix, thus confirming that full-length human IST1 can bind ESCRT-I within cells. Indeed, IST1 bound ESCRT-I complexes reconstituted with all four known VPS37 isoforms, indicating that IST1 binding is a property shared by many different ESCRT-I complexes (Supplemental Figure S1A).
Binding experiments with recombinant proteins were performed to characterize the interaction further and to test whether IST1 bound directly to ESCRT-I. As shown in the right panel of Figure 1B, pure recombinant ESCRT-I complex bound preferentially to recombinant GST-IST1 that was expressed in E. coli and captured on a glutathione matrix (compare lanes 4 and 5). However, ESCRT-I binding could only be detected by Western blotting (bottom right panel), and under conditions in which there was some background ESCRT-I binding to an immobilized GST control, indicating that the binding interaction was weak and substoichiometric. In spite of these limitations, our experiments indicate that IST1 can bind ESCRT-I, and they suggest the possibility that IST1 may help bridge the early acting ESCRT-I complex and the downstream ESCRT-III and VPS4 complexes.
IST1–CHMP1 Interactions
IST1 exhibited yeast two-hybrid interactions with both human CHMP proteins (Figure 1A), and these interactions also occurred in cells, as confirmed by coprecipitation experiments (Figure 1C). Specifically, Myc-CHMP1A coprecipitated with OSF-IST1 (Figure 1C, panel 1, compare lanes 1 and 2), but it did not bind control matrices lacking OSF-IST1 (lanes 1, 3, and 5). It was also noteworthy that IST1 overexpression reduced the accumulation of posttranslationally modified CHMP1A proteins (compare the protein banding patterns of CHMP1A-Myc proteins in the lysates from lanes 1 and 2). We (von Schwedler et al., 2003) and others (Howard et al., 2001) have shown previously that posttranslationally modified CHMP1A proteins are enriched on the membrane-associated fraction, and our experiments therefore indicate that IST1 overexpression alters CHMP1A modification or localization.
OSF-tagged fragments spanning the N- and C-terminal halves of IST1 both expressed well in 293T cells, but only the N-terminal ELYC domain (IST1-189) bound CHMP1A-Myc (Figure 1C, panel 1, compare lanes 4 and 6), in good agreement with analogous studies in the yeast system (Dimaano et al., 2008). Moreover, CHMP1A bound better to the ELYC domain alone than to full-length IST1, despite comparable expression levels (compare lanes 2 and 4), suggesting that IST1 may exist in an autoinhibited or foldback conformation that is relieved by removal of C-terminal elements. CHMP1A also binds VPS4A/B and Vta1p/LIP5, both of which contain MIT domains that bind MIM1 elements located at the Did2p/CHMP1 C termini (Lottridge et al., 2006; Stuchell-Brereton et al., 2007; Azmi et al., 2008), and the C-terminal end of Did2p is required to recruit Ist1p to yeast endosomes (Dimaano et al., 2008). We therefore tested whether IST1 binds the C-terminal MIM1 element of CHMP1A. However, L191/L194 point mutations within the terminal CHMP1A MIM1 helix that block VPS4 binding did not inhibit IST1 binding (Figure 1C, panel 2), indicating that IST1 must bind CHMP1A in a different manner.
IST1–LIP5 Interactions
Our two-hybrid screens also indicated that LIP5 might be an IST1 binding partner (Figure 1A), and this interaction was again confirmed in cells by using coprecipitation experiments. As shown in Figure 1D, Myc-LIP5 coprecipitated with OSF-IST1 (panel 1, lane 2), but it did not bind control matrices that lacked OSF-IST1 (lanes 1, 3, and 5). Similarly, endogenous IST1 bound OSF-LIP5 (panel 2, lane 2) but not control matrices (lanes 1, 3, and 5). Thus, IST1–LIP5 interactions were detected in both orientations and also with endogenous IST1. Structural studies of the yeast LIP5 homologue Vta1p indicate that LIP5 also has three distinct regions: an N-terminal domain composed of tandem MIT domains (residues 1–186), a central proline-rich linker (187–264), and a C-terminal VSL dimerization domain (265–307) (Xiao et al., 2008). Deletion analyses revealed that the IST1–LIP5 interaction was mediated by elements within the C-terminal half of IST1 and the N-terminal half of LIP5 (Figure 1D, panels 1 and 2, compare lanes 4 and 6 in each case). Thus, two proteins known to modulate VPS4 activity, Vta1/LIP5 and IST1, bind one another, and this interaction involves the LIP5 MIT region binding the C-terminal end of IST1.
Characterization of IST1–VPS4 Interactions
Yeast Ist1p regulates Vps4p activity in vitro and in vivo (Dimaano et al., 2008; Rue et al., 2008), and the human VPS4–IST1 interaction was therefore a major focus of our studies. The VPS4-IST1 interaction was initially detected by two hybrid methods (Figure 1A), and it was confirmed by coprecipitation experiments (Supplemental Figure S1B, compare lanes 1 and 2). Two-hybrid mapping experiments indicated that IST1 bound the N-terminal MIT domain of VPS4A (Figure 2A, lane 3, and Supplemental Table 1E), and this was also confirmed by GST pull-down experiments with purified recombinant proteins (Supplemental Figure S1C). A VPS4A fragment that lacked the MIT domain also coprecipitated IST1, suggesting that IST1 may also bind the VPS4A AAA ATPase cassette (Supplemental Figure S1B, compare lanes 3 and 4). However, the equivalent interaction was not detected in two-hybrid assays (Figure 2A, lane 4), and our data therefore do not establish this second interaction mode unambiguously.
As shown in Figure 2B, biosensor binding experiments revealed that IST1 bound the VPS4A and VPS4B MIT domains with dissociation constants of 1.1 ± 0.3 and 4.2 ± 0.8 μM, respectively (black curve in Figure 2B and Supplemental Table 1F). The IST1 ELYC domain did not bind detectably (orange curve), whereas IST1303-366 bound with full affinity (0.8 ± 0.2 μM; red curve) and formed a stable 1:1 complex with VPS4A MIT as analyzed by gel filtration chromatography (data not shown). These experiments indicated that the VPS4 MIT binding site is located within the C-terminal 64 residues of IST1. Sequence analyses revealed that the IST1 C terminus contains distinct sites that resemble each of the two known MIT interaction motifs, with residues 325–329 resembling the core of an MIM2 motif and residues 353–363 resembling the core of an MIM1 motif (Figure 2B). Moreover, IST1 fragments spanning the putative MIM2 (IST1323-339) and MIM1 (IST1340-366) elements both bound VPS4A MIT, with dissociation constants of 12 and 26 μM, respectively (blue and green curves). Similarly, separate inactivating point mutations in the VPS4 MIT binding grooves for MIM1 (L64D) and MIM2 (V13D) each reduced, but did not eliminate IST1303-366 binding (Kd = 25 and 20 μM, respectively, Supplemental Table 1F). Thus, IST1 has two distinct MIT interacting motifs that can bind simultaneously to two different grooves of VPS4A MIT to create full affinity binding.
Mutational analyses were performed to characterize the VPS4A MIT–IST1 interaction further and to identify point mutants that could be used to test the functional importance of MIM1 and MIM2. Based upon previous biochemical and structural studies, the L355A and L362A point mutations were expected to impair the MIM1–MIT interaction (Obita et al., 2007; Stuchell-Brereton et al., 2007), and the L325D and L328D mutations were expected to impair the MIM2–MIT interaction (Kieffer et al., 2008). As shown in Figure 2C, each of the four IST1 single point mutations reduced VPS4A MIT binding, with reductions ranging between 7- and 16-fold (purple and blue curves). Moreover, binding was reduced even further (80- to 500-fold) by double point mutations that impaired both MIM motifs simultaneously (red and orange curves), again indicating that both MIM elements contribute to VPS4A MIT binding.
NMR Chemical Shift Footprinting of VPS4A MIT–IST1 MIM Interactions
NMR chemical shift footprinting experiments were performed to test whether the two IST1 MIM elements bound the VPS4A MIT domain as expected. As illustrated in Figure 2D, a peptide that spanned the IST1 MIM1 element alone (IST1340-366) induced VPS4A MIT amide proton chemical shifts in residues clustered about the helix 2/3 groove. This chemical shift footprint (top; red) matched the expected binding site for an MIM1 helix (green). Similarly, a peptide that spanned the IST1 MIM2 element alone (IST1321-339) induced amide proton chemical shifts in residues clustered about the helix 1/3 groove of VPS4A MIT. This footprint (middle; orange) was again consistent with the expected MIM2–MIT interaction (blue), although in this case the presence of additional shifts suggested that the MIM2 strand may also contact the helix 2/3 loop. Assuming IST1 binds in the same manner as the structurally characterized VPS4A MIT–MIM1 and MIT–MIM2 complexes (Obita et al., 2007; Stuchell-Brereton et al., 2007; Kieffer et al., 2008), the IST1 MIM2 element will bind as an extended strand in the helix 1/3 groove and the MIM1 element will bind as an amphipathic helix in the helix 2/3 groove. Both elements will be oriented parallel to MIT helix 3, which requires the intervening polypeptide spacer to traverse the long axis of the three helix bundle (Figure 2D, bottom).
The IST1 MIM1 Element Binds LIP5 MIT Domain(s)
The N-terminal domain of Vta1p contains a tandem array of MIT domains, and both MIT domains are apparently also present in the human homologue LIP5 (Xiao et al., 2008). The mapping studies shown in Figure 1D suggested that either or both of the IST1 MIM1 and/or MIM2 elements might bind either or both of the LIP5 MIT domains (Xiao et al., 2008). In support of this model, point mutations that inactivated both MIM elements within Myc-IST1 (L328A/L355A) blocked all detectable OSF-LIP5 binding in a coprecipitation experiment (Figure 1E, top, compare lanes 2 and 4). Thus, LIP5 and IST1 also bind one another through MIT–MIM interaction(s).
IST1 Overexpression Inhibits HIV Budding, Whereas IST1 Depletion Does Not
One important function of the ESCRT pathway is to mediate the release of HIV-1 and other enveloped viruses (Bieniasz, 2006; Fujii et al., 2007). We therefore tested whether altering IST1 levels affected HIV-1 release and infectivity. In yeast, overexpression of Ist1p inhibits ESCRT pathway function (Dimaano et al., 2008), and we therefore began by testing the effect of IST1 overexpression on release of the HIV-1 vector pCMVΔR8.2. In a negative control, HIV-1 virion release was only modestly reduced by overexpression of the wild-type VPS4A protein, as assayed by the release of virion-associated HIV-1 CA protein (Figure 3A, compare lanes 1 and 2) and by infectious titer (Figure 3A, bottom, compare lanes 1 and 2). In contrast, virion release was efficiently blocked by expression of a VPS4A point mutant (VPS4AK173Q) that lacked ATP binding activity (positive control, compare lanes 1 and 3 in Figure 3A, 450-fold titer reduction). Importantly, IST1 overexpression also reduced the levels of virion release (panel 3, compare lanes 1 and 4) and reduced the titer (Figure 3A, bottom, compare lanes 1 and 4; 13-fold titer reduction). In contrast, overexpression of an IST1 mutant with inactivating mutations in both MIM1 and MIM2 (IST1L328D/L355A) neither inhibited HIV-1 release nor reduced viral titers (bottom two panels, lanes 5). We therefore conclude that IST1 overexpression reduces HIV-1 virion release by inhibiting the ESCRT pathway and that this reduction requires the MIT binding activity of IST1.
siRNA depletion experiments were also performed to test whether IST1 plays a direct role in HIV-1 virion release (Figure 3B). Two different siRNAs were used to target IST1, and both reduced the levels of endogenous IST1 protein to nearly undetectable levels (panel 2, compare lanes 1, 3, 4, and 5). However, this reduction in intracellular IST1 did not affect virion release (panel 4, compare lanes 1 and 2 with lanes 3 and 4) or infectious titer (bottom). A positive control behaved as expected because siRNA depletion of the ESCRT-I subunit, TSG101, reduced virion production and infectious titers (compare lanes 1 and 2 with lane 3; ∼9-fold titer reduction). Moreover, IST1 depletion had other significant biological effects, indicating that the protein was depleted to functionally significant levels (see below). We therefore conclude that IST1 does not play a critical role in ESCRT-mediated release of HIV-1.
IST1 Is Required for Efficient HeLa Cell Cytokinesis
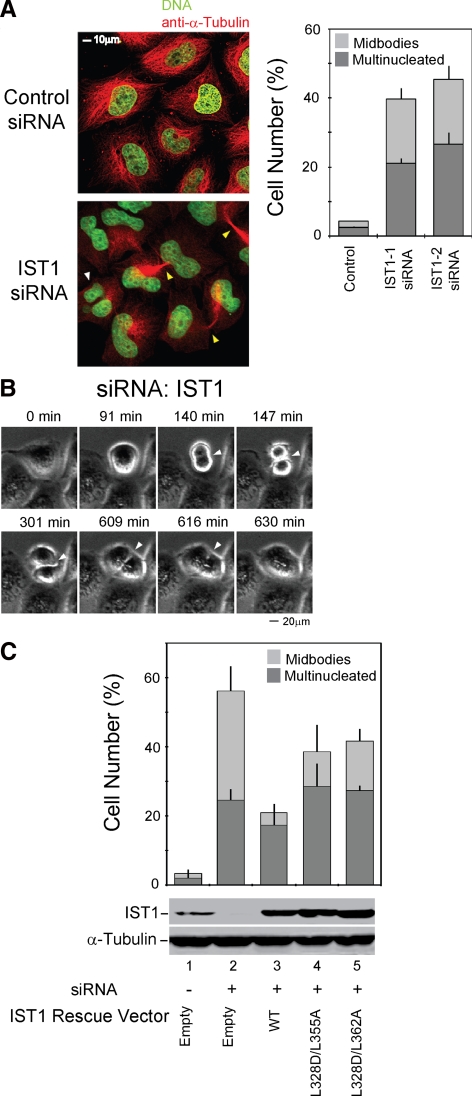
Components of the ESCRT pathway also play important roles in helping to facilitate the abscission stage of cytokinesis (Carlton and Martin-Serrano, 2007; Morita et al., 2007b). We therefore tested whether IST1 is functionally required for efficient HeLa cell cytokinesis. As shown in Figure 4A, IST1 depletion led to substantial enrichment of cells arrested during cytokinesis (bottom, yellow arrows) and in the number of multinuclear cells (white arrow). Both siRNAs tested induced substantial increases in the percentage of cells arrested in cytokinesis (right, 19 ± 3 and 19 ± 4 vs. 1.8 ± 0.2% for a control siRNA) and with multiple nuclei (21 ± 1 and 27 ± 4 vs. 2.5 ± 0.3% for a control siRNA). Flow cytometry was also used to assay for cytokinesis defects, and these experiments again confirmed that IST1 depletion significantly increased the numbers of cells with 4N and 8N DNA contents (Supplemental Figure S2A).
Figure 4.
IST1 is required for efficient cytokinesis. (A) IST1 depletion causes cells to arrest during cytokinesis and to accumulate multiple nuclei. Left, confocal immunofluorescence images of HeLa cells treated with a control siRNA (TOP) or an siRNA against IST1 (BOTTOM). Microtubules (anti-α-Tubulin, red) and nuclei (SYTOX-green) were stained for reference; the white arrowhead highlights a multinuclear cell, and yellow arrowheads highlight cells arrested in cytokinesis. Right, Quantification of cells with multiple nuclei (dark bars) or with midbodies (light bars) after treatment with the irrelevant control siRNA or with either of the two different siRNAs against IST1. (B) IST1 depletion blocks the abscission stage of cytokinesis. Time-lapse, phase contrast images of a dividing cell depleted of IST1. The figure shows a cell depleted of IST1 that arrests late in cytokinesis (t = 147 min; elapsed times are provided above the panels), remains tethered via a midbody for an extended period (t = 147–609 min), and then eventually recoalesces to form a multinucleated cell (t = 616 min). A full movie is provided in Supplemental Figure S3. (C) Rescue of cytokinesis defects with siRNA-resistant IST1 constructs. Percentages of cells with multiple nuclei (dark bars) or with midbodies (light bars) after depletion of endogenous IST1 (lane 2) or after depletion of IST1 and re-expression of exogenous wild-type IST1 (lane 3) or IST1 constructs with two different sets of MIM1/MIM2 mutations (lanes 4 and 5). Lane 1 shows cells that were treated with a control siRNA and cotransfected with an empty expression vector. Western blots (below) show cellular IST1 levels and anti-α-tubulin loading controls.
Time-lapse images of dividing cells revealed that IST1 depletion caused cells to arrest during the abscission stage of cytokinesis and remain tethered together through their midbody for variable times before eventually recoalescing into single cells with multiple nuclei (Figure 4B and movie in Supplemental Figure S3). This phenotype was clear and striking, as illustrated for the single dividing cell in Figure 4B, which initiates cleavage furrow ingression at ∼140 min, proceeds to midbody formation at ∼147 min, and then remains arrested at that stage for >8 h before the cleavage furrow finally dissolves to form a single cell with multiple nuclei (∼616 min). This phenotype resembles the strong abscission phenotypes observed upon depletion of other ESCRT factors such as ALIX, and we therefore conclude that IST1 acts in concert with other components of the ESCRT pathway to facilitate abscission.
The IST1 MIM Elements Contribute to the Efficiency of Cytokinesis
To rule out off target effects and to establish a system for analyzing IST1 mutants, we also tested whether expression of an siRNA-resistant IST1 construct could rescue the cytokinesis defects in cells depleted of endogenous IST1 (Figure 4C). As expected, IST1 depletion again induced cytokinesis arrest (32 ± 7%) and multiple nuclei (25 ± 3%), compared with cells treated with a control siRNA (1 ± 1 and 2 ± 2%, respectively; Figure 4C, compare lanes 1 and 2). These defects were corrected in the majority of cells by re-expression of the wild-type IST1 protein from an siRNA-resistant construct (4 ± 2 and 17 ± 4%, respectively; compare lanes 2 and 3). It is likely that failure to rescue the cytokinesis defects completely is primarily due to variations in IST1 expression, which are substantial at the single cell level (data not shown).
We also tested whether IST1 mutants that lacked functional MIM1 and MIM2 elements could rescue the cytokinesis defects induced by IST1 depletion. As shown in Figure 4C, siRNA resistant IST1L328D/L355A and IST1L328D/L362A mutants rescued the two cytokinesis defects less well than the wild-type IST1 control, indicating that the IST1 MIM elements contribute to IST1 function (compare lanes 4 and 5 with lane 2). Some rescue was evident, however, indicating that MIM1 and MIM2 are probably not the only functionally relevant elements within IST1.
CHMP1A Depletion Also Impairs Cytokinesis
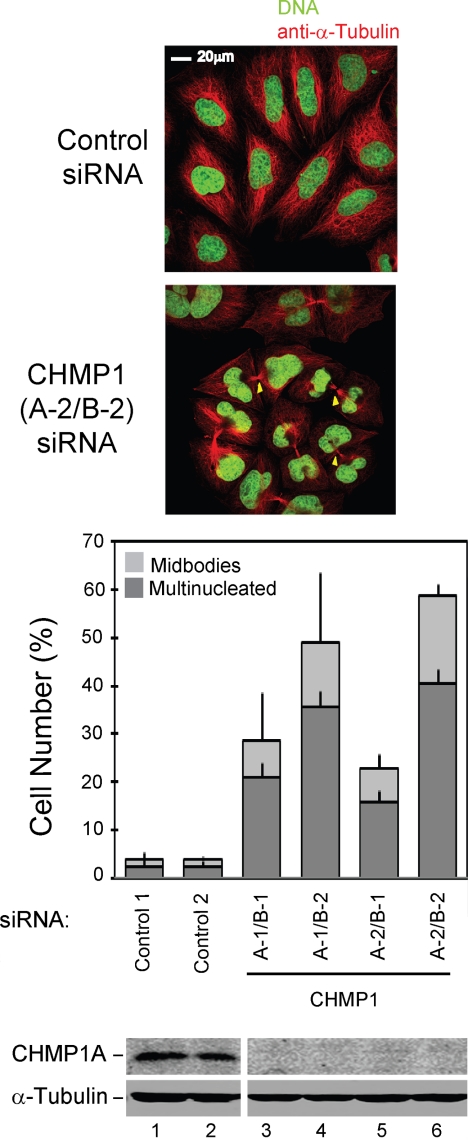
IST1 also binds the CHMP1 proteins, and we therefore tested whether cytokinesis was inhibited by depletion of the two human CHMP1 proteins. Pairs of siRNAs that simultaneously targeted both CHMP1A and CHMP1B were cotransfected to deplete both human CHMP1 proteins in case they were functionally redundant. As shown in Figure 5 and Supplemental S2B, all four siRNA combinations induced cytokinesis arrest and accumulation of multinucleated cells, indicating that CHMP1 proteins are also required for efficient HeLa cell cytokinesis. Western blots confirmed that both siRNAs efficiently depleted CHMP1A (Figure 5, bottom right). CHMP1B depletion could not be tested directly because antibodies are not yet available, but strong cytokinesis defects were observed for both of the CHMP1B siRNA tested. Stronger phenotypes were consistently observed for the CHMP1B-2 siRNA, presumably because this siRNA depleted CHMP1B more completely than the CHMP1B-1 siRNA. Finally, in addition to the abscission defects described above, some combinations of CHMP1 siRNAs also caused cells to accumulate aberrantly shaped nuclei and with large numbers of small, fragmented nuclei (Figure 5; data not shown). This observation raises the intriguing possibility that CHMP1 proteins (and possibly other ESCRT pathway components) may play additional roles in chromosome dynamics and/or other aspects of cell division. We are currently characterizing this new phenotype more systematically, and the results will be reported elsewhere.
Figure 5.
CHMP1 proteins are required for efficient cytokinesis. Simultaneous depletion of CHMP1A and CHMP1B causes cells to arrest during cytokinesis and to accumulate multiple nuclei. Top, confocal immunofluorescence images are analogous to those shown in Figure 4A, except that cells in the bottom panel were treated with siRNAs targeting both CHMP1A and CHMP1B. Bottom, quantification of cells with multiple nuclei (dark bars) or with midbodies (light bars). These experiments are analogous to those shown in Figure 4C, except that 1) cells were treated with different pairs of siRNAs that targeted both CHMP1A and CHMP1B, and 2) only CHMP1A protein levels were examined because CHMP1B antibodies are not yet available.
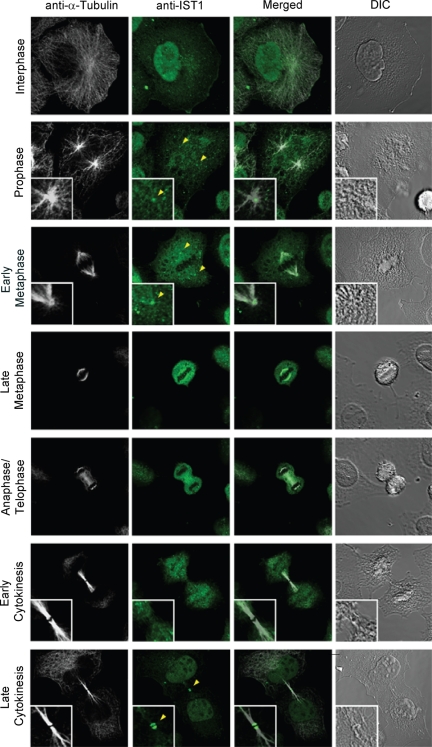
IST1 Concentrates within the Midbodies of Dividing Cells
Many ESCRT components are recruited to the midbody at late stages of cytokinesis; therefore, we examined the localization of IST1 at different stages of the cell cycle. Endogenous IST1 localized both diffusely and in small puncta throughout nondividing cells (Figure 6, row 1). IST1 remained diffusely distributed and in puncta during prophase and early metaphase, but it also clearly concentrated at the microtubule organizing center. IST1 was less clearly visible at the centrosome during anaphase, telophase, and early cytokinesis, but IST1-positive puncta frequently concentrated in the region of the emerging midbody (see row 5 and panel 2; data not shown). Finally, IST1 showed up at the Flemming body in late cytokinesis, after midbody thinning but before abscission. IST1 staining was typically observed at both ends of the Flemming body (see row 7, panel 3 inset). This double ring localization pattern matches the patterns seen for VPS4 proteins and also for a series of ESCRT-III–like proteins, including CHMP1A (Morita et al., 2007b; Supplemental Figure S6). In principle, this staining pattern could reflect concentration of IST1 in rings at either end of the Flemming body, although we cannot rule out the alternative possibility that IST1 is also present at the center of the Flemming body, but it is inefficiently stained owing to the dense protein environment.
Figure 6.
IST1 localization changes through the cell cycle. Representative images showing localization of endogenous HeLa IST1 (anti-IST1, green, column 2) at different stages of the cell cycle. Magnified insets show IST1 concentrated at the microtubule-organizing center/centrosome during prophase and early metaphase (rows 2 and 3) and at the midbody late in cytokinesis (row 7). Corresponding images show the localization of microtubules (anti-α-Tubulin, white, column 1), merged images (column 3), and differential interference contrast (DIC) images (column 4).
Although IST1 trafficking remains to be studied in detail, our data are consistent with a model in which IST1 is distributed at multiple sites in the cell during interphase, including within the nucleus, and probably also at endosomes and centrosomes. A subset of the IST1 is concentrated at centrosomes at the beginning of mitosis, and this subset may then be transported on vesicles from the centrosome to the Flemming body, where it concentrates at late telophase, immediately before abscission. Thus, IST1, CHMP1, and VPS4 colocalize together at Flemming bodies where they could help to coordinate final stages of abscission.
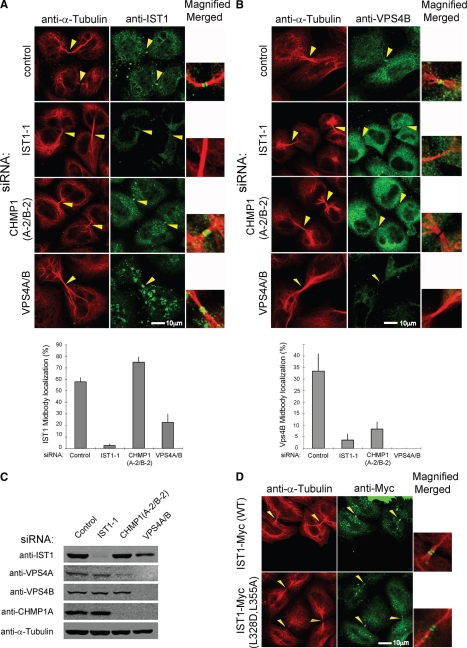
siRNA depletion experiments were performed to examine the requirements for IST1 recruitment to midbodies. As expected, IST1 concentrated in most of the observable midbodies of dividing cells (Figure 7A, row 1). This observation was quantified by counting the numbers of midbodies with IST1 (Figure 7A, bottom). In these experiments, we did not attempt to distinguish between “immature” and “mature” midbodies, and IST1 was therefore observable in most (58 ± 4%), but not all midbodies. As expected, depletion of IST1 abrogated staining of nuclei, cytoplasmic puncta, and midbodies (row 2 and bottom). Under these conditions, midbody localization was seen in just 2.4 ± 1.7% of dividing cells, demonstrating the specificity of the IST1 antibody and the efficiency of IST1 depletion. Cells depleted of IST1 did still exhibit low level cytoplasmic staining; therefore, we cannot rule out the possibility that some of the diffuse cytoplasmic staining seen in wild-type cells may be nonspecific. Simultaneous depletion of both human CHMP1 proteins actually increased the efficiency of IST1 midbody localization slightly (row 3, midbody localization seen in 75 ± 5% of dividing cells), demonstrating that CHMP1 proteins were not required to recruit IST1 to the midbody. Interestingly, this result differs from the requirements for endosomal localization of yeast Ist1p, in which Did2p/CHMP1 is required to recruit Ist1p to endosomes (Dimaano et al., 2008; Rue et al., 2008). It was also noteworthy that CHMP1A/B depletion reduced the overall levels of VPS4A (but not VPS4B) (Figure 7C), although residual VPS4A could still be detected both by immunofluorescence (Figure 7B, row 3) and by Western blotting (Figure 7C).
Figure 7.
Requirements for the midbody localization of IST1 and VPS4. (A) Requirements for IST1 localization to the Flemming bodies of dividing cells. Doubled-labeled confocal immunofluorescence images showing the localization of endogenous IST1 in dividing HeLa cells (anti-IST1, green, column 2); microtubules (anti-α-tubulin, red, column 1); and merged, magnified views (column 3). IST1 localization patterns are shown in the presence of a control siRNA (row 1) and in cells depleted of IST1 (row 2), CHMP1A and CHMP1B (row 3), or VPS4A and VPS4B (row 4). Percentages of midbodies with observable IST1 under the different conditions are quantified below. Note that IST1 concentrated in double ring structures within midbodies (arrowheads) except when IST1 itself was depleted. (B) Requirements for VPS4B localization to the Flemming bodies of dividing cells. Doubled-labeled confocal immunofluorescence images showing localization of endogenous VPS4B in dividing HeLa cells (anti-VPS4B, green, column 2), microtubules (anti-α-tubulin, red, column 1), and merged, magnified views (column 3). Percentages of midbodies with observable VPS4B under the different conditions are quantified below. Note that VPS4B was not efficiently recruited to midbodies (arrowheads) when either IST1, CHMP1, or VPS4 proteins were depleted. (C) Western blots showing the relative levels of IST1, VPS4, CHMP1, and α-Tubulin proteins under the different experimental conditions shown in A and B. Note that CHMP1A/B depletion reduced VPS4A levels and that depletion of VPS4A/B reduced CHMP1A to undetectable levels. (D) IST1 mutations that inhibit VPS4 MIT binding do not inhibit midbody localization. Both wild-type IST1-Myc protein (row 1, column 2) and an IST1 mutant that lacks the ability to bind VPS4 MIT domains (IST1L328D,L355A-Myc, row 2, column 2) localized to the midbodies of dividing cells. Microtubule localization (anti-α-tubulin, red, column 1) and merged, magnified views (column 3) are also shown for reference.
We have also recently developed an siRNA system that can be used to deplete both known human VPS4 ATPases from human cells (Kieffer et al., 2008). Simultaneous depletion of VPS4A and VPS4B reduced the frequency of IST1 recruitment to midbodies by ∼threefold (Figure 7A, row 4, IST1 localization observed in the midbodies of 22 ± 8% of dividing cells). However, these experiments were difficult to interpret unambiguously for several reasons. First, VPS4 depletion also reduced overall IST1 levels slightly and reduced CHMP1A to undetectable levels, indicating that these two proteins (and presumably also CHMP1B) are less stable in the absence of VPS4. Second, VPS4 depletion also altered the cytoplasmic distribution of IST1, which may indirectly affect recruitment of IST1 to the midbody. Under steady-state conditions, many ESCRT components are transiently recruited to endosomal membranes in which they function in MVB vesicle formation and are then released from the membrane by the action of the VPS4 ATPases (Babst et al., 1997; Babst et al., 1998). Depletion of VPS4 therefore induces the accumulation of aberrant endosomal compartments (termed class E compartments) that can trap ESCRT components on their surfaces. This phenomenon was evident in our experiments, as depletion of VPS4 proteins caused IST1 to concentrate in larger cytoplasmic puncta that corresponded to class E compartments (row 4, column 2; data not shown). Similarly, VPS4 depletion increased the percentage of IST1 in the insoluble/membrane-bound fraction of cell extracts (data not shown), and overexpression of VPS4A mutants that lacked ATPase activity also caused IST1 to accumulate on class E compartments (Supplemental Figure S4). These observations suggest that IST1, like other ESCRT factors, may function in MVB vesicle formation and be released by the action of the VPS4 ATPases. Thus, the moderate reduction in midbody localization of IST1 seen upon depletion of VPS4 may be an indirect effect arising from the trapping of cellular IST1 on class E compartments.
To test the requirement for VPS4 in IST1 localization further, we examined the localization of IST1L328D,L355A, a mutant that cannot bind VPS4 MIT domains. As expected, wild-type IST1-Myc concentrated at mature midbodies, indicating that the exogenously expressed, Myc-tagged protein localized normally (Figure 7D, row 1). Similarly, the IST1L328D,L355A-Myc mutant also localized to midbodies, indicating that MIM1 and MIM2 interactions were not required for IST1 localization (Figure 7D, row 2). Thus, our experiments indicate that neither CHMP1 nor VPS4 proteins are required to localize IST1 to the midbody.
IST1 and CHMP1 Proteins Help Recruit VPS4 to the Midbodies of Dividing Cells
Analogous siRNA depletion experiments were used to test whether IST1 or CHMP1 proteins were required to localize VPS4 to the midbodies of dividing cells (Figure 7B and Supplemental Figure S5). In this case, the data unambiguously demonstrated that both IST1 and CHMP1 help recruit VPS4 to midbodies. Specifically, VPS4B was observed at the midbodies of 33 ± 7% of dividing cells treated with a control siRNA (Figure 7B, row 1; and bottom) but was rarely seen at the midbodies of dividing cells depleted of IST1 (row 2, localization at 4 ± 3% of midbodies) or of CHMP1 (row 3, 8 ± 3% of midbodies). As expected, VPS4B was not observed at midbodies when VPS4A and VPS4B were depleted, demonstrating the specificity of the VPS4B antibody and the efficiency of VPS4 depletion. Very similar data were obtained for analogous experiments analyzing VPS4A localization (Supplemental Figure S5), indicating that VPS4A and VPS4B have similar requirements for midbody localization. Thus, both IST1 and CHMP1 are required to recruit VPS4 proteins to the midbodies of dividing cells.
DISCUSSION
Our experiments reveal that CHMP1 and IST1 form a complex that modulates a subset of VPS4 activities in mammalian cells. IST1 and CHMP1 bind one another, and both display C-terminal MIM elements that bind VPS4 MIT domains (Stuchell-Brereton et al., 2007; this work). Endogenous VPS4 and IST1 both localize to midbodies during cell division (Carlton and Martin-Serrano, 2007; Morita et al., 2007b; this work), as do epitope-tagged CHMP1 proteins (Supplemental Figure S6; Morita et al., 2007b). Most importantly, depletion of either IST1 or CHMP1 proteins strongly inhibited HeLa cell division at the abscission stage. Together, these observations imply that CHMP1, IST1, and VPS4 bind one another and function together at the midbody during HeLa cell division, providing the first nonsynthetic cellular phenotypes for either IST1 or CHMP1 proteins. In contrast, IST1 depletion does not inhibit HIV-1 budding, demonstrating that some VPS4-dependent ESCRT pathway functions do not require IST1.
Our studies show that MIT domains from VPS4 and Vta1p/LIP5 (and possibly other proteins) bind MIM elements located at the C-terminal end of IST1. Detailed characterization of the VPS4A MIT–IST1 interaction revealed a novel binding mode in which IST1 MIM1 and MIM2 elements bind in adjacent grooves of the three helix bundle of VPS4A MIT. The two elements cooperate to increase the interaction affinity, and the measured dissociation constant (Kd = 1 μM) is nearly an order of magnitude tighter than the best “single-mode” MIM interactions characterized to date (Obita et al., 2007; Stuchell-Brereton et al., 2007; Kieffer et al., 2008). Moreover, the two IST1 MIM elements fill both binding grooves of the VPS4 MIT domain and should therefore competitively inhibit VPS4 binding to ESCRT-III proteins of both MIM classes.
We find that VPS4 proteins do not localize efficiently to midbodies in the absence of either IST1 or CHMP1 proteins. Thus, one important function of the IST1/CHMP1 complex is to recruit VPS4 proteins to midbodies. It is also likely that the IST1/CHMP1 complex helps regulate other VPS4 activities, although it is not yet clear precisely what IST1 does or how its activities relate to those of the other known VPS4 modulator, Vta1/LIP5 (Scott et al., 2005a; Azmi et al., 2006; Lottridge et al., 2006; Dimaano et al., 2008; Rue et al., 2008; Xiao et al., 2008; Yu et al., 2008). It has been proposed that binary complexes of Did2p/CHMP1-IST1 and Vta1p/LIP5-Vps60p/CHMP5 may regulate VPS4 in functionally redundant manners, which could explain why strong MVB sorting phenotypes are only observed when yeast lack subunits of both complexes (Rue et al., 2008). This model can also rationalize why HIV-1 budding is reduced upon depletion of LIP5 but not IST1 (Ward et al., 2005; this work) because LIP5 could modulate VPS4 activities in HIV-1 budding, whereas IST1 could perform similar functions in cytokinesis. We note, however, that Vta1p/LIP5 and IST1 have very different biochemical properties, because they differ in domain structure and oligomeric state (Xiao et al., 2008), and they also bind VPS4 in different ways (Scott et al., 2005a; Yu et al., 2008; this work). Moreover, our experiments show that IST1 and LIP5 bind one another, suggesting that they may work together in some contexts. It is therefore alternatively possible that IST1 and Vta1p/LIP5 modulate distinct, but complementary VPS4 activities.
One interesting possibility is that Vta1p/LIP5 complexes may favor fully assembled and enzymatically active VPS4 state(s), whereas IST1 complexes favor state(s) in which the enzyme does not fully engage the ESCRT-III lattice. All available data indicate that Vta1p/LIP5 favors enzymatically active VPS4 states. Vta1p/LIP5 stimulates VPS4 assembly and ATPase activity in vitro (Scott et al., 2005a; Azmi et al., 2006, 2008; Lottridge et al., 2006; Xiao et al., 2008; Yu et al., 2008) and enhances substrate binding activity by contributing additional MIT domains that can bind multiple different ESCRT-III proteins (Scott et al., 2005a; Azmi et al., 2006; Lottridge et al., 2006; Shim et al., 2008; Xiao et al., 2008; Yu et al., 2008). In contrast, IST1 activities are less clear because the protein contributes positively to overall ESCRT pathway function (Dimaano et al., 2008; Rue et al., 2008; this work), yet paradoxically inhibits several known VPS4 activities. As discussed above, tight binding of IST1 MIM elements to VPS4 MIT domains should competitively inhibit VPS4 binding to ESCRT-III substrates. Furthermore, pure recombinant Ist1p forms a 1:1 complex with Vps4p that inhibits higher order enzyme assembly and ATPase activity (Dimaano et al., 2008). Similarly, elevated IST1 levels dominantly inhibit ESCRT pathway activities in vivo, including HIV-1 release (Figure 3A) and MVB protein sorting (Dimaano et al., 2008).
Possible functions for the IST1-Did2/CHMP1 complex that would be consistent with our observations include helping to recruit VPS4 to specific sites of action (Nickerson et al., 2006; Dimaano et al., 2008; Rue et al., 2008; this work), helping to disrupt the inactive cytoplasmic VPS4 dimer (Babst et al., 1998; Scott et al., 2005b), helping VPS4 to engage the ESCRT-III lattice, and/or helping to release or weaken VPS4–substrate interactions, perhaps as VPS4 enzymes act processively on the ESCRT-III lattice or after the terminal membrane fission event. Thus, we speculate that the IST1/CHMP1 and LIP5/CHMP5 complexes may modulate distinct VPS4 activities during the full ESCRT cycle and that specific VPS4-dependent functions such as cytokinesis and virus budding are more or less dependent on these different activities. These issues will clearly require further study, and our demonstration that both IST1 and CHMP1 perform essential functions during abscission provides a tractable biological system for analyzing their activities.
Supplementary Material
ACKNOWLEDGMENTS
We thank Debbie Eckert, David Myszka, and Chris Rodesch for assistance with analytical ultracentrifugation, biosensor, and immunofluorescence experiments; Angela Katsuyama for the generous gift of recombinant ESCRT-I; and Collin Kieffer for helpful discussions. Funding was provided by the Israeli Science Foundation (to M. B.) and National Institutes of Health grant AI-51174 (to W.I.S.).
Abbreviations used:
- CHMP
charged MVB protein
- ESCRT
endosomal sorting complexes required for transport
- IST1
increased sodium tolerance-1
- MVB
multivesicular body
- VPS
vacuolar protein sorting.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-05-0475) on January 7, 2009.
REFERENCES
- Azmi I., Davies B., Dimaano C., Payne J., Eckert D., Babst M., Katzmann D. J. Recycling of ESCRTs by the AAA-ATPase Vps4 is regulated by a conserved VSL region in Vta1. J. Cell Biol. 2006;172:705–717. doi: 10.1083/jcb.200508166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azmi I. F., Davies B. A., Xiao J., Babst M., Xu Z., Katzmann D. J. ESCRT-III family members stimulate Vps4 ATPase activity directly or via Vta1. Dev. Cell. 2008;14:50–61. doi: 10.1016/j.devcel.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Babst M., Katzmann D., Estepa-Sabal E., Meerloo T., Emr S. Escrt-III. An endosome-associated heterooligomeric protein complex required for mvb sorting. Dev. Cell. 2002;3:271–282. doi: 10.1016/s1534-5807(02)00220-4. [DOI] [PubMed] [Google Scholar]
- Babst M., Sato T. K., Banta L. M., Emr S. D. Endosomal transport function in yeast requires a novel AAA-type ATPase, Vps4p. EMBO J. 1997;16:1820–1831. doi: 10.1093/emboj/16.8.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babst M., Wendland B., Estepa E. J., Emr S. D. The Vps4p AAA ATPase regulates membrane association of a Vps protein complex required for normal endosome function. EMBO J. 1998;17:2982–2993. doi: 10.1093/emboj/17.11.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieniasz P. D. Late budding domains and host proteins in enveloped virus release. Virology. 2006;344:55–63. doi: 10.1016/j.virol.2005.09.044. [DOI] [PubMed] [Google Scholar]
- Carlton J. G., Martin-Serrano J. Parallels between cytokinesis and retroviral budding: a role for the ESCRT machinery. Science. 2007;316:1908–1912. doi: 10.1126/science.1143422. [DOI] [PubMed] [Google Scholar]
- Dimaano C., Jones C. B., Hanono A., Curtiss M., Babst M. Ist1 regulates vps4 localization and assembly. Mol. Biol. Cell. 2008;19:465–474. doi: 10.1091/mbc.E07-08-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durocher Y., Perret S., Kamen A. High-level and high-throughput recombinant protein production by transient transfection of suspension-growing human 293-EBNA1 cells. Nucleic Acids Res. 2002;30:E9. doi: 10.1093/nar/30.2.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii K., Hurley J. H., Freed E. O. Beyond Tsg 101, the role of Alix in ‘ESCRTing’ HIV-1. Nat. Rev. 2007;5:912–916. doi: 10.1038/nrmicro1790. [DOI] [PubMed] [Google Scholar]
- Garrus J. E., et al. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell. 2001;107:55–65. doi: 10.1016/s0092-8674(01)00506-2. [DOI] [PubMed] [Google Scholar]
- Gill D. J., Teo H., Sun J., Perisic O., Veprintsev D. B., Vallis Y., Emr S. D., Williams R. L. Structural studies of phosphoinositide 3-kinase-dependent traffic to multivesicular bodies. Biochem. Soc. Symp. 2007;74:47–57. doi: 10.1042/BSS0740047. [DOI] [PubMed] [Google Scholar]
- Hanson P. I., Roth R., Lin Y., Heuser J. E. Plasma membrane deformation by circular arrays of ESCRT-III protein filaments. J. Cell Biol. 2008;180:389–402. doi: 10.1083/jcb.200707031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann C., Chami M., Zachariae U., de Groot B. L., Engel A., Grutter M. G. Vacuolar protein sorting: two different functional states of the AAA-ATPase Vps4p. J. Mol. Biol. 2008;377:352–363. doi: 10.1016/j.jmb.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Howard T. L., Stauffer D. R., Degnin C. R., Hollenberg S. M. CHMP1 functions as a member of a newly defined family of vesicle trafficking proteins. J. Cell Sci. 2001;114:2395–2404. doi: 10.1242/jcs.114.13.2395. [DOI] [PubMed] [Google Scholar]
- Hurley J. H., Emr S. D. The ESCRT complexes: structure and mechanism of a membrane-trafficking network. Annu. Rev. Biophys. Biomol. Struct. 2006;35:277–298. doi: 10.1146/annurev.biophys.35.040405.102126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley J. H., Yang D. MIT domainia. Dev. Cell. 2008;14:6–8. doi: 10.1016/j.devcel.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Kieffer C., Skalicky J. J., Morita E., De Domenico I., Ward D. M., Kaplan J., Sundquist W. I. Two distinct modes of ESCRT-III recognition are required for VPS4 functions in lysosomal protein targeting and HIV-1 budding. Dev. Cell. 2008;15:62–73. doi: 10.1016/j.devcel.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Krogan N. J., et al. Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature. 2006;440:637–643. doi: 10.1038/nature04670. [DOI] [PubMed] [Google Scholar]
- Lata S., Roessle M., Solomons J., Jamin M., Gottlinger H. G., Svergun D. I., Weissenhorn W. Structural basis for auto-inhibition of ESCRT-III CHMP3. J. Mol. Biol. 2008;378:818–827. doi: 10.1016/j.jmb.2008.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Kimpler L. A., Naismith T. V., Lauer J. M., Hanson P. I. Interaction of the mammalian endosomal sorting complex required for transport (ESCRT) III protein hSnf7-1 with itself, membranes, and the AAA+ ATPase SKD1. J. Biol. Chem. 2005;280:12799–12809. doi: 10.1074/jbc.M413968200. [DOI] [PubMed] [Google Scholar]
- Lottridge J. M., Flannery A. R., Vincelli J. L., Stevens T. H. Vta1p and Vps46p regulate the membrane association and ATPase activity of Vps4p at the yeast multivesicular body. Proc. Natl. Acad. Sci. USA. 2006;103:6202–6207. doi: 10.1073/pnas.0601712103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita E., Sandrin V., Alam S. L., Eckert D. M., Gygi S. P., Sundquist W. I. Identification of human MVB12 proteins as ESCRT-I subunits that function in HIV budding. Cell Host Microbe. 2007a;2:41–53. doi: 10.1016/j.chom.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita E., Sandrin V., Chung H. Y., Morham S. G., Gygi S. P., Rodesch C. K., Sundquist W. I. Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. EMBO J. 2007b;26:4215–4227. doi: 10.1038/sj.emboj.7601850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muziol T., Pineda-Molina E., Ravelli R. B., Zamborlini A., Usami Y., Gottlinger H., Weissenhorn W. Structural basis for budding by the ESCRT-III factor CHMP3. Dev. Cell. 2006;10:821–830. doi: 10.1016/j.devcel.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Nickerson D. P., Russell M. R., Odorizzi G. A concentric circle model of multivesicular body cargo sorting. EMBO Rep. 2007;8:644–650. doi: 10.1038/sj.embor.7401004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson D. P., West M., Odorizzi G. Did2 coordinates Vps4-mediated dissociation of ESCRT-III from endosomes. J. Cell Biol. 2006;175:715–720. doi: 10.1083/jcb.200606113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obita T., Saksena S., Ghazi-Tabatabai S., Gill D. J., Perisic O., Emr S. D., Williams R. L. Structural basis for selective recognition of ESCRT-III by the AAA ATPase Vps4. Nature. 2007;449:735–739. doi: 10.1038/nature06171. [DOI] [PubMed] [Google Scholar]
- Rue S. M., Mattei S., Saksena S., Emr S. D. Novel ist1-did2 complex functions at a late step in multivesicular body sorting. Mol. Biol. Cell. 2008;19:475–484. doi: 10.1091/mbc.E07-07-0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A., Chung H. Y., Gonciarz-Swiatek M., Hill G. C., Whitby F. G., Gaspar J., Holton J. M., Viswanathan R., Ghaffarian S., Hill C. P., Sundquist W. I. Structural and mechanistic studies of VPS4 proteins. EMBO J. 2005a;24:3658–3669. doi: 10.1038/sj.emboj.7600818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A., Gaspar J., Stuchell-Brereton M. D., Alam S. L., Skalicky J. J., Sundquist W. I. Structure and ESCRT-III protein interactions of the MIT domain of human VPS4A. Proc. Natl. Acad. Sci. USA. 2005b;102:13813–13818. doi: 10.1073/pnas.0502165102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim S., Kimpler L. A., Hanson P. I. Structure/function analysis of four core ESCRT-III proteins reveals common regulatory role for extreme C-terminal domain. Traffic. 2007;8:1068–1079. doi: 10.1111/j.1600-0854.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- Shim S., Merrill S. A., Hanson P. I. Novel interactions of ESCRT-III with LIP5 and VPS4 and their implications for ESCRT-III disassembly. Mol. Biol. Cell. 2008;19:2661–2672. doi: 10.1091/mbc.E07-12-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuchell-Brereton M. D., Skalicky J. J., Kieffer C., Karren M. A., Ghaffarian S., Sundquist W. I. ESCRT-III recognition by VPS4 ATPases. Nature. 2007;449:740–744. doi: 10.1038/nature06172. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Protein production by auto-induction in high density shaking cultures. Protein Expr. Purif. 2005;41:207–234. doi: 10.1016/j.pep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Tsang H. T., Connell J. W., Brown S. E., Thompson A., Reid E., Sanderson C. M. A systematic analysis of human CHMP protein interactions: additional MIT domain-containing proteins bind to multiple components of the human ESCRT III complex. Genomics. 2006;88:333–346. doi: 10.1016/j.ygeno.2006.04.003. [DOI] [PubMed] [Google Scholar]
- von Schwedler U. K., et al. The protein network of HIV budding. Cell. 2003;114:701–713. doi: 10.1016/s0092-8674(03)00714-1. [DOI] [PubMed] [Google Scholar]
- Ward D. M., Vaughn M. B., Shiflett S. L., White P. L., Pollock A. L., Hill J., Schnegelberger R., Sundquist W. I., Kaplan J. The role of LIP5 and CHMP5 in multivesicular body formation and HIV-1 budding in mammalian cells. J. Biol. Chem. 2005;280:10548–10555. doi: 10.1074/jbc.M413734200. [DOI] [PubMed] [Google Scholar]
- Xiao J., Xia H., Zhou J., Azmi I. F., Davies B. A., Katzmann D. J., Xu Z. Structural basis of Vta1 function in the multivesicular body sorting pathway. Dev. Cell. 2008;14:37–49. doi: 10.1016/j.devcel.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z., Gonciarz M. D., Sundquist W. I., Hill C. P., Jensen G. J. Cryo-EM structure of dodecameric Vps4p and its 2, 1 complex with Vta1p. J. Mol. Biol. 2008;377:364–377. doi: 10.1016/j.jmb.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamborlini A., Usami Y., Radoshitzky S. R., Popova E., Palu G., Gottlinger H. Release of autoinhibition converts ESCRT-III components into potent inhibitors of HIV-1 budding. Proc. Natl. Acad. Sci. USA. 2006;103:19140–19145. doi: 10.1073/pnas.0603788103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.