Abstract
Previously, we identified cytoskeleton-associated protein 4 (CKAP4) as a major substrate of the palmitoyl acyltransferase, DHHC2, using a novel proteomic method called palmitoyl-cysteine identification, capture and analysis (PICA). CKAP4 is a reversibly palmitoylated and phosphorylated protein that links the ER to the cytoskeleton. It is also a high-affinity receptor for antiproliferative factor (APF), a small sialoglycopeptide secreted from bladder epithelial cells of patients with interstitial cystitis (IC). The role of DHHC2-mediated palmitoylation of CKAP4 in the antiproliferative response of HeLa and normal bladder epithelial cells to APF was investigated. Our data show that siRNA-mediated knockdown of DHHC2 and consequent suppression of CKAP4 palmitoylation inhibited the ability of APF to regulate cellular proliferation and blocked APF-induced changes in the expression of E-cadherin, vimentin, and ZO-1 (genes known to play a role in cellular proliferation and tumorigenesis). Immunocytochemistry revealed that CKAP4 palmitoylation by DHHC2 is required for its trafficking from the ER to the plasma membrane and for its nuclear localization. These data suggest an important role for DHHC2-mediated palmitoylation of CKAP4 in IC and in opposing cancer-related cellular behaviors and support the idea that DHHC2 is a tumor suppressor.
INTRODUCTION
Palmitoylation is the posttranslational addition of the 16-carbon palmitate group to specific cysteine residues of proteins (Smotrys and Linder, 2004) via a labile thioester bond. Unlike other forms of lipidation, such as myristoylation and prenylation, palmitoylation is reversible which allows for dynamic regulation of protein-membrane interactions, trafficking between membrane compartments (Wedegaertner and Bourne, 1994; Jones et al., 1997; Moran et al., 2001; Zacharias et al., 2002), and synaptic plasticity (el-Husseini Ael and Bredt, 2002). For many years it was believed that palmitoylation occurred primarily by autocatalytic mechanisms (Bizzozero et al., 1987; Bano et al., 1998); however, the recent discovery of a family of palmitoyl acyltransferase (PAT) enzymes that catalyze protein palmitoylation has reversed this notion, expanding the complexity of the mechanisms by which palmitoylation is regulated (Lobo et al., 2002; Roth et al., 2002; Fukata et al., 2004; Linder and Deschenes, 2007).
PATs are encoded by the ZDHHC gene family and are characterized by an Asp-His-His-Cys motif (DHHC) within a cysteine-rich domain (CRD). The DHHC and CRD domains are essential for palmitoyl acyltransferase activity (Roth et al., 2002; Fukata et al., 2004; Sharma et al., 2008). Twenty-three genes encoding proteins with DHHC–CRD domains have been identified in mouse and human databases (Fukata et al., 2004). Of these, seven have already been shown to be associated with human disease: DHHC8 with schizophrenia (Mukai et al., 2004); DHHC17/HIP14 with Huntington's disease (Yanai et al., 2006); DHHC15 and DHHC9 with X-linked mental retardation (Mansouri et al., 2005; Raymond et al., 2007); and DHHC2, DHHC9, DHHC17, and DHHC11 with cancer (Oyama et al., 2000; Ducker et al., 2004; Mansilla et al., 2007; Yamamoto et al., 2007). In several of these examples, the absence of PAT expression and subsequent failure to palmitoylate target substrates is the underlying problem.
Although now recognized as a PAT, DHHC2 was previously known as ream, an acronym for reduced expression associated with metastasis. As the name suggests, this gene was first identified because its expression level was consistently and significantly reduced in clonal murine colorectal adenocarcinoma cell lines with high metastatic potential, but not in clonal lines derived from the same tumor that did not metastasize (Tsuruo et al., 1983; Oyama et al., 2000). It was concluded that ream expression is inversely related to the metastatic potential of a cell, leading to speculation that this gene normally suppresses one or more of the processes by which cancer cells escape from blood vessels, invade into and proliferate in a target organ, and induce angiogenesis and form metastatic foci.
Human ZDHHC2 maps to a region of chromosome 8 (p21.3-22) that is frequently deleted in many types of cancer, including colorectal (Fujiwara et al., 1993, 1994; Ichii et al., 1993) hepatocellular carcinoma (Emi et al., 1993; Fujiwara et al., 1994), nonsmall cell lung (Fujiwara et al., 1993; Ohata et al., 1993), and cancers of the breast (Yaremko et al., 1996; Anbazhagan et al., 1998), urinary bladder (Knowles et al., 1993), and prostate (Bova et al., 1993). Loss of heterozygosity on chromosomal band 8p22 has been shown to be a common event in some epithelial tumors, pointing toward the likelihood that the region harbors potential tumor suppressor genes (Emi et al., 1993; Fujiwara et al., 1993; Ichii et al., 1993; Ohata et al., 1993).
Because DHHC2 has no other known signaling properties beyond palmitoylation, knowledge of its target substrates in a cancer cell line could yield significant clues about the role of DHHC2-mediated palmitoylation in metastasis and tumor suppression. In previous work, we used a novel, proteomic method called palmitoyl-cysteine identification, capture and analysis (PICA) to identify the target substrates of DHHC2 in HeLa cells, a cervical adenocarcinoma cell line. We determined that cytoskeleton-associated protein 4 (CKAP4, also known as p63, ERGIC-63, and CLIMP-63) is a principle, physiologically important substrate of DHHC2 (Zhang et al., 2008).
CKAP4 is a reversibly palmitoylated, type II transmembrane protein that has been shown to anchor rough endoplasmic reticulum (ER) to microtubules in epithelial cells (i.e., COS and HeLa; Schweizer et al., 1993a,b, 1994, 1995a; Vedrenne and Hauri, 2006). This function requires a direct interaction between the cytoplasmic N-terminal tail of the protein to microtubules and is regulated by phosphorylation of three critical serine residues (Klopfenstein et al., 1998). More recently, CKAP4 has been identified as a functional cell surface receptor for antiproliferative factor (APF; Conrads et al., 2006), a low-molecular-weight, Frizzled-8 protein–related sialoglycopeptide secreted from bladder epithelial cells in patients suffering from the chronic, painful bladder disorder, interstitial cystitis (IC; Keay et al., 2000, 2004a). APF profoundly inhibits normal bladder epithelial cell growth (Keay et al., 1996, 2000, 2004a). APF also inhibits the proliferation of bladder carcinoma cells and HeLa cells in vitro with an IC50 of ∼1 nM (Keay et al., 2004a, 2006; Conrads et al., 2006). CKAP4 has been shown to mediate the antiproliferative effects of APF, which is also known to alter transcription of at least 13 genes known to be involved in the regulation of proliferation and tumorigenesis (including E-cadherin, vimentin, cyclin D1, p53, and ZO-1; Keay et al., 2003; Conrads et al., 2006; Kim et al., 2007).
In this study, we examined the effects of reduced CKAP4 palmitoylation on APF-mediated signaling by silencing the expression of DHHC2 with targeted siRNA. Our data show that DHHC2-mediated palmitoylation of CKAP4 is a critical event regulating CKAP4 subcellular distribution and APF-stimulated changes in cellular proliferation and gene expression.
MATERIALS AND METHODS
DNA Constructs
A vector construct containing wild-type CKAP4 (WT CKAP4) fused in-frame to the N-terminus of the V5 and 6xHis epitope tags in the mammalian expression vector pcDNA3.1V5His-TOPO (Invitrogen, Carlsbad, CA) was generated by PCR using CKAP4 specific primers and cDNA from HeLa (ATCC CCL-2; American Type Culture Collection, Manassas, VA) cells. A palmitoylation-incompetent form of CKAP4 (CKAP4 C100S) was created by site-directed mutagenesis of the WT vector construct (Stratagene, La Jolla, CA) to alter the cysteine at position 100 to serine. DHHC2 was cloned in-frame to the C-terminus of mCFP in the mammalian expression vector pcDNA3.1 to generate an N-terminal CFP fusion protein. All constructs were verified by DNA sequencing.
Cell Culture and Transfections
HeLa (ATCC CCL-2; American Type Culture Collection, Manassas, VA) cells were maintained in DMEM containing 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100 μg/ml streptomycin, and 1 μg/ml fungizone (all from Invitrogen). Cells were transfected using FuGENE6 reagent (Roche, Basel, Switzerland) according to the manufacturer's instructions. To obtain stable clones, cells were diluted into 96-well plates (100 cells/well) 24 h after transfection and selected in the presence of 0.4 mg/ml Geneticin (G418; Invitrogen).
Normal primary bladder (NB) epithelial cells were isolated from patients as previously described (Keay et al., 1996, 2000, 2004a; Conrads et al., 2006) Cells were propagated in DMEM-F12 (Mediatech, Manassas, VA) with 10% heat-inactivated FBS, 1% antibiotic/antimycotic solution, 1% l-glutamine, 0.25 U/ml insulin (Sigma, St. Louis, MO), and 5 ng/ml human epidermal growth factor (R&D Systems, Minneapolis, MN) at 37°C in a 5% CO2 atmosphere and characterized by binding of AE-1/AE-3 pancytokeratin antibodies (Signet, Emeryville, CA) as previously described (Keay et al., 1996, 2004b).
Small Interfering RNA
Double-stranded small interfering RNA (siRNA; ON-TARGETplus) targeting ZDHHC2 and nonsense siRNA (ON-TARGETplus Control siRNA) were purchased from Dharmacon (Lafayette, CO). HeLa cells were trypsinized for 5 min at 37°C and centrifuged in DMEM growth medium, and the cell pellet was resuspended in serum-free medium at a density of 1 × 106 cells/ml. Two hundred microliters of the cell suspension was then transferred to a sterile 2-mm cuvette with 14 μg siRNA and electroporated at 160 V/500 μF capacitance using a Bio-Rad Gene Pulser Xcell (Hercules, CA). The cells were immediately transferred to 96-well plates for thymidine incorporation assay or to LabTek multiwell glass slides (Nalge Nunc, Rochester, NY) for immunocytochemistry. To determine the effectiveness of siRNA-mediated knockdown, we used quantitative real-time PCR (qRT-PCR), to measure the abundance of ZDHHC2 mRNA at times 0, 12, 24, 48, 72, and 96 h after transfection of the siRNA. These experiments were run in triplicate.
[3H]Thymidine Incorporation
Cell proliferation was measured by [3H]thymidine incorporation into the DNA of HeLa or NB epithelial cells. Briefly, synthetic APF or inactive control peptide (NeoMPS, San Diego, CA) was resuspended in acetonitrile/distilled water (1:1), diluted in serum-free DMEM, and applied to HeLa or NB cells; cell controls received acetonitrile/distilled water diluted in serum-free DMEM alone. Cells were then incubated at 37°C in a 5% CO2 atmosphere for 48 h. The cells were then labeled with 1 μCi/well [3H]thymidine for 4 h, trypsinized, insoluble cell contents harvested and methanol-fixed onto glass fiber filter paper, and the amount of radioactivity incorporated determined. Significant inhibition of [3H]thymidine incorporation was defined as a mean decrease in cpm of greater than 2 SDs from the mean of control cells for each plate.
Immunocytochemistry
HeLa cells stably transfected with WT CKAP4 or CKAP4 C100S were seeded at a density of 2 × 104 cells/well in eight-well LabTek chamber slides (Nalge Nunc) and grown to semiconfluence in DMEM medium containing 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 1 μg/ml fungizone, and 0.4 mg/ml G418 (all from Invitrogen). Cells were fixed for 20 min with 3% paraformaldehyde in phosphate-buffered saline (PBS), permeabilized with 0.1% Triton X-100 in PBS, and blocked in PBS/5% NGS (normal goat serum). Cells transfected with DHHC2 siRNA and treated with synthetic APF (Peptides International, Louisville, KY) were fixed using ethanol/acetone (1:1) for 15 min at room temperature and washed three times with 1× PBS before blocking in PBS/5% normal goat serum (NGS). The following primary antibodies were used: mouse mAb G1/296 against CKAP4 (“anti-CLIMP-63”, diluted 1:100, Alexis Biochemicals, San Diego, CA), rabbit pAb against calreticulin (diluted 1:1000, Abcam, Cambridge, MA), and fluorescein isothiocyanate (FITC)-conjugated mouse mAb against the V5 epitope (diluted 1:500, Invitrogen). Secondary antibodies were FITC-labeled goat anti-rabbit or goat anti-mouse (diluted 1:1000, Invitrogen) and tetramethyl rhodamine isothiocyanate (TRITC)-labeled goat anti-mouse (diluted 1:1000, Jackson ImmunoResearch Laboratories, West Grove, PA). Slides were mounted in SlowFade Antifade reagent (Invitrogen) and imaged using a Nikon TE2000 epifluorescence microscope (Melville, NY).
qRT-PCR
Total RNA was extracted from synthetic APF-treated, inactive control peptide-treated, or control untreated NB epithelial cells using the RNeasy Plus Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's protocol. RNA was assessed by visualization of the 28S/18S rRNA ratio on a 1% agarose gel, and total RNA concentration was determined by measuring the absorbance of each sample at 260 nm and 280 nm using a Gene Quant RNA/DNA Calculator (GE Healthcare, Piscataway, NJ). qRT-PCR for gene expression was performed using Quantitect Primers (Qiagen), SYBR Green RT-PCR kit reagents (Qiagen), and a Roche System II Light-Cycler (software version 3.5). Samples were tested in triplicate runs, and specific mRNA levels were quantified and compared with mRNA levels for β-actin using real-time PCR analysis software from Applied Biosystems (Foster City, CA).
Western Blot Analysis
Cells were lysed in ice-cold RIPA buffer containing protease inhibitors (Pierce, Rockford, IL), sonicated, and centrifuged for 15 min at 4°C. The supernatant protein concentration was measured using a Folin reagent-based protein assay kit (Bio-Rad). Proteins were separated by electrophoresis using 4–12% NuPAGE Novex Bis-Tris polyacrylamide gels in MOPS running buffer (Invitrogen) and then transferred to nitrocellulose. Membranes were blocked for 2 h at room temperature in TBST buffer (Tris-buffered saline, pH 7.4, with 0.1% Tween 20) containing 5% nonfat milk and incubated with specific antibodies against vimentin (diluted 1:2000; BD PharMingen, San Jose, CA) or ZO-1 (diluted 1:125; Zymed Laboratories, San Francisco, CA) overnight at 4°C. The membranes were subsequently washed with TBST, incubated for 1 h at room temperature in HRP-conjugated goat anti-mouse (diluted 1:40000; Santa Cruz Biotechnology, Santa Cruz, CA) or goat anti-rabbit (diluted 1:10000; Pierce) secondary antibodies, and developed by enhanced chemiluminescence (Pierce). To assess equal loading of protein, the membranes were stripped and reprobed for β-actin (diluted 1:5000; Sigma). The membranes were exposed to film (BioMax AR, Eastman Kodak, Rochester, NY), and the resulting images were scanned at 300 dpi. The protein bands of interest were quantified using ImageJ (http://rsb.info.nih.gov/ij/), and the integrated signal densities were normalized first to β-actin (the loading control) and subsequently expressed in terms of the fractional abundance relative to untreated control cells.
Immunoprecipitation
HeLa cells were transfected with mCFP:DHHC2 or mock-transfected using FuGENE6 reagent (Roche) according to the manufacturer's instructions. Forty-eight hours later, cells were washed in PBS, lysed in 500 μl ice-cold lysis buffer (25 mM HEPES, 150 mM NaCl, 5 mM MgCl2, 1% NP-40, and protease inhibitor cocktail), and incubated on ice for 90 min with vortexing. After normalizing for equal protein concentration, lysates were immunoprecipitated with a mAb against GFP (JL-8 BD Biosciences, San Jose, CA; also recognizes the GFP spectral mutant, CFP) overnight at 4°C with rocking. Protein G Sepharose 4B (Invitrogen) was added the following day, and the samples were incubated for an additional 18 h at 4°C with rocking. Proteins in the immunoprecipitation complex were washed four times in ice-cold lysis buffer and then heated in SDS sample buffer before separation by SDS-PAGE and transfer to nitrocellulose. Western blot analysis was performed as described above using a mAb against CKAP4 (G1/296, diluted 1:1000; Alexis Biochemicals).
RESULTS
Half-Life of ZDHHC2 mRNA
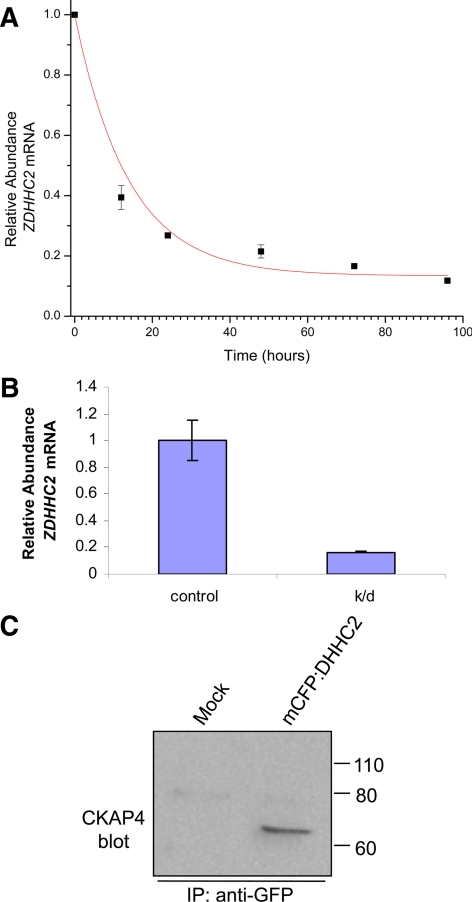
Previously, we identified CKAP4 as a substrate of the PAT, DHHC2, using a novel proteomic method called PICA. To investigate the effects of reduced CKAP4 palmitoylation on APF-mediated signaling, we silenced the expression of DHHC2 with targeted siRNA. The decrease in ZDHHC2 mRNA abundance was monitored at 0, 12, 24, 48, 72, and 96 h after ZDHHC2 siRNA transfection of HeLa cells by qRT-PCR (Figure 1A). The values at each time point were expressed as abundance relative to time zero and plotted versus time. The data were fit to a nonlinear, first-order exponential decay curve, and the half-life of the ZDHHC2 mRNA was calculated to be 13.87 h (R2 = 0.998, n = 3; error bars = SE). By 48 h, ZDHHC2 mRNA was reduced 79% relative to the non-siRNA–transfected control (Figure 1A), and after 96 h, ZDHHC2 mRNA was reduced 84% relative to the non-siRNA–transfected control (Figure 1, A and B). Thus, all experiments involving siRNA knockdown were performed 48-96 h after oligo transfection (all at 96 h except inhibition of thymidine incorporation, in which cells were treated with APF at 48 h and harvested at 96 h after transfection). It is important to note that in a previous study, we showed that siRNA-mediated ZDHHC2 knockdown effectively reduces [3H]palmitate labeling of CKAP4 in HeLa cells and does not change CKAP4 abundance (Zhang et al., 2008; Supplemental Figures S2 and S3).
Figure 1.
Half-life of DHHC2 mRNA. (A) The abundance of DHHC2 mRNA after siRNA-mediated DHHC2 knockdown was measured by qRT-PCR at 0, 12, 24, 48, 72, and 96 h after siRNA oligo transfection. These experiments were run in triplicate. The data were approximated most accurately using a nonlinear, first-order exponential decay. The half-life was calculated to be 13.87 h (R2 = 0.998, n = 3; error bars, SE). (B) The relative abundance of DHHC2 mRNA in siRNA-transfected cells versus mock-transfected control cells was determined after 96 h by qRT-PCR. DHHC2 expression was reduced ∼84% with siRNA knockdown. (C) CKAP4 interacts with CFP-DHHC2. CFP-tagged DHHC2 was immunoprecipitated from transiently-transfected HeLa cells using an anti-GFP mAb (see Materials and Methods). Western blot analysis with an anti-CKAP4 antibody detected a robust band at ∼63 kDa, characteristic of CKAP4 in lysates from cells transfected with CFP-DHHC2 but not in lysates from mock-transfected cells. Exposure time, 15 s.
Coimmunoprecipitation of DHHC2 with CKAP4
The transfer of palmitate from DHHC2 to CKAP4 should require at least a transient physical interaction between the two proteins. To determine if CKAP4 and DHHC2 interact, we immunoprecipitated cyan fluorescent protein (CFP)-tagged DHHC2 from transiently transfected HeLa cells using an anti-green fluorescent protein (GFP) mAb. Forty-eight hours after transfection, ∼80% of the cells were expressing CFP-DHHC2, as determined by epifluorescence microscopy. As seen in Figure 1C, Western blot analysis detected a ∼63-kDa band characteristic of CKAP4 in lysates from cells expressing CFP-DHHC2 but not in lysates from mock-transfected cells, indicating that under these conditions CKAP4 and CFP-DHHC2 coassociate. This result supports our previous findings that CKAP4 is a substrate of DHHC2.
Palmitoylation by DHHC2 Is Required for CKAP4 Trafficking from the ER to the Plasma Membrane
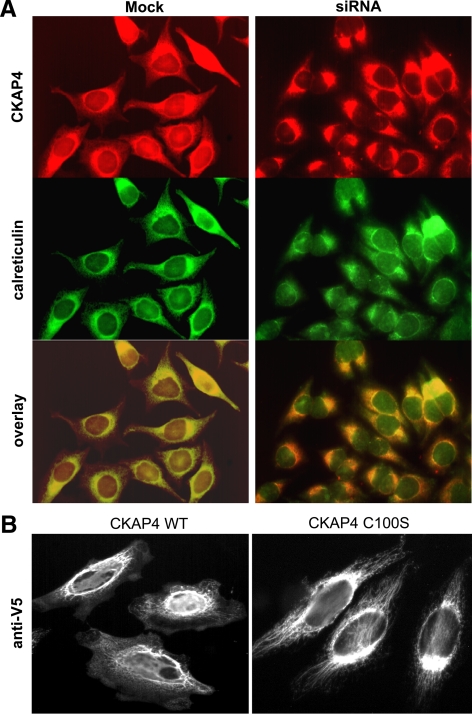
CKAP4 has been extensively characterized as an ER-resident protein (Schweizer et al., 1993a,b, 1994, 1995b; Klopfenstein et al., 2001). In a previous study, we showed that siRNA-mediated knockdown of DHHC2 expression blocks CKAP4 trafficking from the ER to the plasma membrane (PM; Zhang et al., 2008). To further examine the effect of palmitoylation on CKAP4 localization, we first labeled nonpermeabilized mock- or DHHC2 siRNA-transfected HeLa cells with an mAb against CKAP4 (Figure 2). In agreement with published reports, CKAP4 was localized on the cell surface of mock-transfected HeLa cells (Figure 2A) (Bates et al., 2008). In contrast, CKAP4 was not detected on the cell surface of HeLa cells transfected with DHHC2 siRNA (Figure 2B). Subsequently, we colabeled mock- or DHHC2 siRNA-transfected HeLa cells with antibodies against CKAP4 and calreticulin, an ER marker. As shown in Figure 3A, CKAP4 colocalizes with calreticulin in the ER but is also present on the PM and in the nucleus. However, when DHHC2 expression is knocked down, CKAP4 is no longer detected on the PM, which is consistent with our previous findings (see Figure 5; Zhang et al., 2008), and it is also no longer present in the nucleus (Figure 3A). We then generated HeLa cell lines stably expressing epitope-tagged WT CKAP4 or the palmitoylation-incompetent mutant, CKAP4 C100S, to examine the effect of complete depalmitoylation on CKAP4 subcellular distribution. Cells were fixed and incubated with an anti-V5-FITC–conjugated mAb, and the stably expressed proteins were visualized by epifluorescence microscopy. As shown in Figure 3B, WT CKAP4 is present on the plasma and perinuclear membranes and in the nucleus, similar to endogenous CKAP4 localization, whereas CKAP4 C100S is confined to the ER. Importantly, in cells stably expressing CKAP4 C100S, the ER retains its reticulated structure, radiating throughout the cell. However, when palmitoylation of endogenous CKAP4 is inhibited after DHHC2 knockdown, the ER contracts around the nucleus to a greater degree (Figure 4, D). These data corroborate our earlier findings indicating that palmitoylation is required for trafficking of CKAP4 from the ER to the PM (Zhang et al., 2008), and they suggest that palmitoylation may also be required for CKAP4 nuclear translocation.
Figure 2.
CKAP4 is expressed on the cell surface: immunolocalization in nonpermeabilized HeLa cells. Mock-transfected (A) or DHHC2 (B) siRNA-transfected HeLa cells were grown on multiwell glass slides, fixed in 4% buffered paraformaldehyde (not permeabilized), and incubated with a mAb G1/296 against CKAP4 (“anti-CLIMP-63”, diluted 1:100) followed by a TRITC-labeled, goat anti-mouse secondary antibody (diluted 1:1000). Controls included cells processed without primary and/or secondary antibodies. Images were acquired using a Nikon TE2000 epifluorescence microscope with NIS Elements software. (A) In mock-transfected, nonpermeabilized HeLa cells, CKAP4 immunostaining was localized on the cell surface. (B) However, CKAP4 was not detected on the cell surface of nonpermeabilized HeLa cells transfected with DHHC2 siRNA. (C) No staining was detected in mock-transfected, nonpermeabilized HeLa cells fixed and treated with the secondary, TRITC-conjugated antibody only.
Figure 3.
DHHC2-mediated palmitoylation of CKAP4 on cysteine 100 regulates its trafficking from the ER to the PM. (A) Mock-transfected or DHHC2 siRNA-transfected HeLa cells were grown on multiwell glass slides, fixed, and incubated with a mAb G1/296 against CKAP4 (anti-CLIMP-63, diluted 1:100) followed by a TRITC-labeled, goat anti-mouse secondary antibody (diluted 1:1000) or with a pAb against calreticulin (diluted 1:1000) followed by a FITC-labeled, goat anti-rabbit secondary antibody (diluted 1:1000). Controls included cells processed without primary and/or secondary antibodies. Images were acquired using a Nikon TE2000 epifluorescence microscope with NIS Elements software and overlayed using Adobe Photoshop software (San Jose, CA). In HeLa cells with reduced DHHC2 expression, CKAP4 localization is restricted to the ER. (B) HeLa cell lines stably expressing CKAP4 WT-V5 or the palmitoylation-incompetent mutant, CKAP4 C100S-V5, were grown on multiwell glass slides, fixed, and immunolabeled with a FITC-conjugated mAb antibody against the V5 epitope (1:500). CKAP4 WT was expressed on the plasma membrane and perinuclear membranes, whereas CKAP4 C100S expression was restricted to the ER. Epifluorescence images in B were made with a 100× 1.45 NA oil immersion objective.
Figure 5.
siRNA-mediated knockdown of DHHC2 blocks the antiproliferative response of HeLa and NB cells to APF. (A) HeLa cells were electroporated with DHHC2 double-stranded siRNA (●) or with nonsense siRNA (○) as a control for nonsequence-specific effects on day 1 and serum-starved on day 2, and varying concentrations of APF or control peptide (0.25-250 nM) were added to the medium on day 3; cells were then cultured for an additional 48 h under conditions of serum starvation. (B) NB epithelial cells were electroporated with DHHC2 double-stranded siRNA (●) or with nonsense siRNA (▲) as a control for nonsequence-specific effects on day 1 and serum-starved on day 2, and varying concentrations of APF or control peptide (0.25–250 nM) were added to the medium on day 3; cells were then cultured for an additional 48 h under conditions of serum starvation. For both cell types, cellular proliferation was assessed by inhibition of [3H]thymidine incorporation. Each data point represents the mean and SD of three independent experiments. Data are presented as percent inhibition of [3H]thymidine incorporation compared with controls. The ability of APF to block proliferation was inhibited in the presence of DHHC2 siRNA for all APF concentrations except 250 nM for HeLa cells or 25 and 250 nM for NB cells.
Figure 4.
siRNA-mediated knockdown of DHHC2 blocks APF-mediated translocation of CKAP4 to the nucleus. Mock-transfected or DHHC2 siRNA-transfected HeLa cells were treated with APF (20 nM) for 48 h. Cells were fixed and incubated with a mAb G1/296 against CKAP4 (anti-CLIMP-63) followed by a TRITC-labeled, goat anti-mouse secondary antibody). CKAP4 translocated to the nucleus in HeLa cells treated with APF (red arrows); however, this translocation was blocked in APF-treated HeLa cells transfected with DHHC2 siRNA. Epifluorescence images were made with a 60 × 1.45 NA oil immersion objective.
Palmitoylation of CKAP4 by DHHC2 Is Required for APF-stimulated Translocation of CKAP4 to the Nucleus
CKAP4 and APF have been shown to colocalize to the plasma membrane and to the perinuclear membrane of NB cells (Conrads et al., 2006). To examine the subcellular distribution of CKAP4 in HeLa cells treated with APF, we performed immunocytochemistry. As shown in Figure 4, CKAP4 is clearly localized to the PM and perinuclear membrane of HeLa cells (Figure 4A) and may also be present in the nucleus. However, APF treatment results in an obvious increase in nuclear translocation (Figure 4B). To determine if palmitoylation of CKAP4 by DHHC2 is required for its nuclear localization, we transfected HeLa cells with DHHC2 siRNA and then exposed the cells to APF for 48 h. In cells with reduced DHHC2 expression, CKAP4 is not detected in the nucleus after APF treatment (Figure 4C). These data indicate that CKAP4 must be palmitoylated by DHHC2 in order to translocate to the nucleus in response to APF-treatment as well as in response to other factors in untreated cells. These findings are consistent with the hypothesis that palmitoylation is required for translocation of CKAP4 to both the nucleus and the PM (where it can bind to APF).
CKAP4 Must Be Palmitoylated by DHHC2 for APF to Block HeLa and NB Cell Proliferation
Both HeLa and NB epithelial cells express endogenous CKAP4 and have been shown to be sensitive to the antiproliferative effects of APF (Conrads et al., 2006). To determine the effect of reduced CKAP4 palmitoylation on the proliferative response of HeLa and NB cells to APF, we knocked down the expression of DHHC2 using siRNA. Forty-eight hours after siRNA transfection, cells were incubated with varying concentrations of APF. As shown in Figure 5, A and B, DHHC2 knockdown profoundly inhibited the ability of both cell types to respond to APF. These results suggest that DHHC2-mediated palmitoylation of CKAP4 is necessary for APF-induced antiproliferative effects in HeLa and NB cells.
Palmitoylation of CKAP4 by DHHC2 Is Required for APF-induced Changes in Gene and Protein Expression
APF induces well-characterized, specific changes in the pattern of cellular gene expression, including decreased production of vimentin and tight junction proteins (zonula occludens-1 [ZO-1] and occludin) and increased production of E-cadherin, resulting in a more differentiated bladder epithelial cell phenotype (Keay et al., 2003; Zhang et al., 2005; Kim et al., 2007). To determine if palmitoylation of CKAP4 by DHHC2 is required for APF-induced changes in gene and protein expression, confluent NB epithelial cells were treated with APF or inactive peptide control for 48 h, and the mRNA and protein levels of vimentin, ZO-1, and E-cadherin were determined by qRT-PCR and Western blot analyses, respectively. As shown in Figure 6, A–C, APF-mediated decreased expression of ZO-1 and vimentin and increased expression of E-cadherin were inhibited by knocking down the expression of DHHC2 with siRNA. Western blot analyses confirmed that ZO-1 and vimentin protein levels were reduced after APF treatment of NB cells and that the ability of APF to induce these changes was blocked in cells transfected with DHHC2 siRNA (Figure 7A; although an increase in E-cadherin mRNA levels could be measured in NB cells after APF treatment, E-cadherin protein expression, in all conditions, remained below the threshold required for Western blot detection). These data demonstrate that palmitoylation of CKAP4 by DHHC2 is required for APF-induced changes in specific mRNA and protein expression in NB cells.
Figure 6.
APF-mediated changes in mRNA expression are dependent on palmitoylation of CKAP4 by DHHC2 in NB cells. Primary NB epithelial cells were electroporated with DHHC2 double-stranded siRNA or with nonsense siRNA on day 1 and serum-starved on day 2, and 2.5 nM APF or control peptide was added to the medium on day 3; cells were then cultured for an additional 48 h under conditions of serum starvation. Expression of ZO-1, vimentin, and E-cadherin mRNA was assessed by qRT-PCR as described in Materials and Methods. (A and B) APF alone or in the presence of nonsense siRNA reduced ZO-1 and vimentin mRNA levels by ∼93 and ∼97%, respectively. DHHC2 knockdown blocked this APF-stimulated reduction in ZO-1 and vimentin mRNA levels. (C) APF alone or in the presence of nonsense siRNA dramatically increased E-cadherin mRNA levels, an effect that was also blocked by DHHC2 knockdown. Please note: In contrast to the graphs in A and B, a logarithmic scale was required in C to demonstrate the full range of the increase in E-cadherin expression in response to APF as well as the degree to which this change is blocked by DHHC2 knockdown. ZO-1, vimentin, and E-cadherin mRNA levels were measured in triplicate runs and quantified by normalization to mRNA levels for β-actin using real-time PCR analysis software. The error in the normalized, relative abundance of each mRNA species was propagated forward from the SD of the mean Ct value for each of the experimental samples and the actin control.
Figure 7.
APF-mediated changes in protein expression are dependent on palmitoylation of CKAP4 by DHHC2 in NB and HeLa cells. (A) Primary NB epithelial cells were transfected with DHHC2 double-stranded siRNA or nonsense siRNA or were mock-transfected on day 1 and serum-starved on day 2, and 2.5 nM APF or control peptide was added to the medium on day 3; cells were then cultured for an additional 48 h under conditions of serum starvation. ZO-1 and vimentin protein expression was analyzed by SDS-PAGE followed by Western blotting with antibodies to ZO-1 (220 kDa) and vimentin (57 kDa) as described in Materials and Methods. To assess equal loading of protein, membranes were stripped and reprobed with a mAb to β-actin (1:5000). Proteins were visualized by enhanced chemiluminescence and subsequent exposure to film. (B) HeLa cells were transfected with DHHC2 double-stranded siRNA or nonsense siRNA or were mock-transfected and cultured for 48 h. Cells were then serum-starved, and the indicated cultures were incubated with APF (20 nM) for an additional 48 h. Expression of vimentin protein was analyzed by SDS-PAGE and Western blotting with a mAb antibody against vimentin (57 kDa) as described in Materials and Methods. To assess equal loading of protein, membranes were stripped and reprobed with a mAb to β-actin (1:5000; Sigma). Proteins were visualized by enhanced chemiluminescence and subsequent exposure to film.
Although APF has been shown to inhibit HeLa cell proliferation, APF-induced changes in gene expression have not been documented for this cell line. As shown in Figure 7B, APF treatment also reduced the expression of vimentin in HeLa cells. Changes in the expression of ZO-1 and E-cadherin could not be measured by qRT-PCR or by Western blot because their abundance in HeLa cells was too low. Importantly, as observed in NB cells, transfection of HeLa cells with DHHC2 siRNA inhibited the APF-mediated down-regulation of vimentin expression. These data demonstrate that DHHC2-mediated palmitoylation of CKAP4 is necessary for APF to induce changes in gene and protein expression in carcinoma (HeLa) cells as well as normal bladder cells.
DISCUSSION
Previously, we used three complementary methods to show that CKAP4 is one substrate of the PAT, DHHC2 (Zhang et al., 2008). In this report, we furthered those findings by demonstrating the physical interaction of endogenous CKAP4 with CFP-DHHC2 and by characterizing the functional significance of this interaction in HeLa and NB cells. We have shown that palmitoylation of CKAP4 by DHHC2 is a critical event required for APF-initiated signaling events, including 1) increased nuclear translocation of CKAP4, 2) inhibition of cellular proliferation, and 3) changes in gene and protein expression (i.e., vimentin, ZO-1, and E-cadherin; Keay et al., 2003; Zhang et al., 2005; see Figure 8 for a summary).
Figure 8.
Palmitoylation of CKAP4 by DHHC2 is required for APF-mediated signaling. (A) APF inhibits cellular proliferation and alters the expression of a specific set of genes by binding to its receptor, CKAP4 (Keay et al., 2003; Zhang et al., 2005; Kim et al., 2007). APF binds to CKAP4 with high affinity, and this binding has been shown to be necessary for mediation of APF-related signaling events (Conrads et al., 2006). CKAP4 is also one substrate (of an unknown number) of the palmitoyl acyltransferase, DHHC2 (Zhang et al., 2008). (B) siRNA-mediated knockdown of ZDHHC2 (the gene encoding the PAT, DHHC2) mRNA expression (see Figure 1, half-life or bar graph), prevents CKAP4 trafficking from the ER to the PM (see Figures 2–4) and in doing so, inhibits APF binding to CKAP4. Consequently, the inhibitory effect of APF on cellular proliferation (see Figure 5) and APF-mediated changes in gene transcription (see Figure 6 and 7) are blocked. Although other substrates of DHHC2 exist, APF-mediated changes in cellular proliferation and gene expression appear to be regulated by DHHC2-mediated palmitoylation of CKAP4.
APF profoundly inhibits cellular proliferation and induces well-characterized, specific changes in the expression of genes involved in cell migration and adhesion, with a concomitant change in the phenotype of the cells toward a more differentiated state (Keay et al., 2003; Zhang et al., 2005). These changes in cellular behavior are mediated through high-affinity binding of APF to CKAP4, as CKAP4 gene knockdown and immunodepletion abrogate APF signaling (Conrads et al., 2006). In addition, no other functional high-affinity receptor for APF other than CKAP4 was found. Our data show that palmitoylation of CKAP4 by DHHC2 is necessary for APF-mediated changes in proliferation, gene expression, and nuclear translocation of CKAP4. These results support the idea that the downstream effects of APF are mediated through CKAP4 and that palmitoylation of CKAP4 by DHHC2 is necessary, as loss of DHHC2 expression by siRNA-mediated knockdown is sufficient to inhibit these APF- and CKAP4-dependent signaling events. Importantly, the effects of reduced DHHC2 expression on APF-dependent signaling cannot be attributed to a reduction in CKAP4 abundance (as was the case with other DHHC2 substrates, CD9 and CD151; Sharma et al., 2008), because we have shown previously that CKAP4 expression is unaffected by DHHC2 knockdown in HeLa cells (Zhang et al., 2008).
Palmitoylation plays an important role in regulating the subcellular distribution and function of many proteins with key roles in diverse signaling networks (Resh, 2006; Greaves and Chamberlain, 2007; Linder and Deschenes, 2007; Nadolski and Linder, 2007). Taken together, our findings suggest that palmitoylation of CKAP4 by DHHC2 may facilitate trafficking of CKAP4 from the ER to the PM (where it can physically associate with APF). It is important to note that the general appearance of depalmitoylated CKAP4 was different when DHHC2 was knocked down versus when CKAP4 C100S was stably expressed: CKAP4 C100S was localized to an extended, tubular ER network held in place by palmitoylated, endogenous CKAP4, an organization that was lost after DHHC2 knockdown and depalmitoylation of endogenous CKAP4. Vedrenne and et al. (2005) reported a similar reorganization of the ER in cells expressing mutant versions of CKAP4 that were unable to bind to or bundle microtubules. One of these mutants replaced the three critical, phosphorylatable serines (residues 3, 17, and 19) with glutamic acids to mimic a state of phosphorylation. In cells expressing these phosphomimicking mutants, the ER retracted around the nucleus leaving the microtubular network intact. This suggests that stable anchoring of the ER to microtubules by CKAP4 is required to maintain its spatial distribution.
The markedly increased nuclear abundance of CKAP4 in HeLa cells in response to APF suggests that CKAP4 and/or APF could have a direct role in mediating gene and protein expression. We have not observed increased nuclear localization of CKAP4 in untreated HeLa cells, and nuclear translocation after binding of tPA or SPA was also not apparent in previous studies (Razzaq et al., 2003; Gupta et al., 2006). The C-terminus of CKAP4 is predicted to fold into an extensive coiled-coil domain containing a heptad repeat (residues 468-503) that is predicted to be a leucine zipper (http://www.predictprotein.org/). This portion of the protein is either outside of the cell if CKAP4 is on the PM or in the lumen of the ER if CKAP4 is a resident of the ER. The coiled-coil domain has been shown to be required for the formation of CKAP4 oligomers, as oligomerization is lost when this region is deleted (Klopfenstein et al., 2001). Leucine zipper-containing transcription factors bind to DNA as dimers (Lee, 1992; Sauve et al., 2004), a state likely to be adopted by CKAP4 in the cell. The sequence of residues that follows (503-602) is predicted by InterProScan (www.ebi.ac.uk/Tools/InterProScan/) to be helical as well. Interestingly, it is also relatively rich in the positively charged, basic amino acids histidine, arginine, and lysine. Examination of this sequence in helical wheel programs (e.g., http://cti.itc.virginia.edu/∼cmg/Demo/wheel/wheelApp.html) shows that the helix is amphipathic, suggesting that this region may also be involved in intermolecular interactions. The fact that CKAP4—an ER- and PM-localized, palmitoylated, transmembrane protein—translocates to the nucleus is remarkable in and of itself. To our knowledge, there is no other example of such a protein entering the nucleus. The mechanism by which this occurs is not obvious and requires further investigation. Furthermore, there have been no reported examples of palmitoylated proteins in the nucleus, suggesting that CKAP4 may be depalmitoylated and/or truncated before nuclear translocation.
Recently, DHHC2 has also been shown to palmitoylate the tetraspanins CD9 and CD151, promoting physical associations between them and protecting them from lysosomal degradation (Sharma et al., 2008). This discovery provides one plausible mechanism by which DHHC2 may function as a tumor suppressor. In this study by Sharma et al. (2008), a 70% reduction in ZDHHC2 mRNA expression (via siRNA-mediated knockdown) resulted in lysosomal targeting and rapid degradation of CD9 unless the lysosomal pH was increased by applying bafilomycin A1 (an inhibitor or vacuolar protein-translocating APTases) to the cells. This treatment spared CD9 from degradation and allowed Sharma and colleagues to determine that DHHC2 knockdown reduced palmitoylation of CD9 by 35–55%—a value similar to that observed for CKAP4 after DHHC2 knockdown (Zhang et al., 2008). However, an important question that remains for CD9 is which of its palmitoyl cysteines are palmitoylated by DHHC2; there is only one palmitoyl cysteine in CKAP4–Cysteine 100. It is possible that hypopalmitoylation of both CKAP4 and CD9 may increase tumor or metastatic behavior. In either case, the importance of maintaining DHHC2 expression to suppress metastatic cellular behavior is becoming clearer.
Although there is relatively little known about the 23 members of the mammalian PAT family in terms of their regulation and specificity for substrates, the remarkable number of known associations between disease and the genes that encode PATs demonstrates the importance of palmitoylation for human health. The significance of DHHC2-mediated palmitoylation of CKAP4 is likely to extend beyond IC and cancer. CKAP4 has also been identified as a functional, cell-surface receptor for tissue plasminogen activator (tPA) in vascular smooth muscle cells and for surfactant protein A (SP-A) in rat type II pneumocytes (Razzaq et al., 2003; Gupta et al., 2006). Like APF, tPA regulates cellular proliferation, migration, and invasion in the vasculature: behaviors that are also critically relevant to IC and cancer. tPA binding to CKAP4 on the plasma membrane regulates the response of vascular smooth muscle cells (VSMCs) to a variety of blood vessel injuries (Razzaq et al., 2003). After vascular injury, tPA stimulates VSMC migration and remodeling of the surrounding extracellular matrix, key features that promote vascular repair. SP-A levels are decreased in the lungs of patients with cystic fibrosis and respiratory distress syndrome, as well as chronic lung diseases (Heinrich et al., 2006), and it is thought that CKAP4 may play a role in SP-A recycling and SP-A signaling by mediating transport of SP-A from the ER to the plasma membrane and/or in SP-A binding at the PM and subsequent internalization (Gupta et al., 2006). Although it is not known whether CKAP4 palmitoylation is required for mediating the effects of tPA in smooth muscle cells or SP-A in type II pneumocytes, the functional significance of CKAP4 palmitoylation in cell membrane localization of CKAP4 and in APF-mediated signaling in IC illustrate the wide-ranging significance of palmitoylation.
ACKNOWLEDGMENTS
The authors thank Toby Chai (University of Maryland School of Medicine) for providing bladder biopsy specimens from which the explanted bladder epithelial cells were propagated and Kristopher Koch for assistance with the cell culture. We are also grateful to Nikon for the use of their White-Light TIRF microscope. This work was supported by funding from the Chris DeMarco, American Cancer Society Institutional Research Grant (Agency IRG-01-188-04) and the National Institutes of Health Grants MH071400-01, NSO53638-01, and National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1 DK52596. D.A.Z. is a Partner in Research with Nikon.
Abbreviations used:
- APF
antiproliferative factor
- CKAP4
cytoskeleton-associated protein 4/p63
- CRD
cysteine-rich domain
- DHHC
Asp-His-His-Cys
- ER
endoplasmic reticulum
- FITC
fluorescein isothiocyanate
- IC
interstitial cystitis
- LZ
leucine zipper
- MT
microtubule
- NB
normal bladder
- NGS
normal goat serum
- PAT
palmitoyl acyl transferase
- PICA
palmitoyl-cysteine isolation, capture and analysis
- PM
plasma membrane
- qRT-PCR
quantitative real-time PCR
- TRITC
tetramethyl rhodamine isothiocyanate
- VSMC
vascular smooth muscle cells.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-08-0849) on January 14, 2009.
REFERENCES
- Anbazhagan R., Fujii H., Gabrielson E. Allelic loss of chromosomal arm 8p in breast cancer progression. Am. J. Pathol. 1998;152:815–819. [PMC free article] [PubMed] [Google Scholar]
- Bano M. C., Jackson C. S., Magee A. I. Pseudo-enzymatic S-acylation of a myristoylated yes protein tyrosine kinase peptide in vitro may reflect non-enzymatic S-acylation in vivo. Biochem. J. 1998;330(Pt 2):723–731. doi: 10.1042/bj3300723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates S. R., Kazi A. S., Tao J. Q., Yu K. J., Gonder D. S., Feinstein S. I., Fisher A. B. Role of P63 (CKAP4) in binding of surfactant protein-A to type II pneumocytes. Am. J. Physiol. Lung Cell Mol. Physiol. 2008;295:L658–L669. doi: 10.1152/ajplung.90233.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzozero O. A., McGarry J. F., Lees M. B. Autoacylation of myelin proteolipid protein with acyl coenzyme A. J. Biol. Chem. 1987;262:13550–13557. [PubMed] [Google Scholar]
- Bova G. S., et al. Homozygous deletion and frequent allelic loss of chromosome 8p22 loci in human prostate cancer. Cancer Res. 1993;53:3869–3873. [PubMed] [Google Scholar]
- Conrads T. P., Tocci G. M., Hood B. L., Zhang C. O., Guo L., Koch K. R., Michejda C. J., Veenstra T. D., Keay S. K. CKAP4/p63 is a receptor for the frizzled-8 protein-related antiproliferative factor from interstitial cystitis patients. J. Biol. Chem. 2006;281:37836–37843. doi: 10.1074/jbc.M604581200. [DOI] [PubMed] [Google Scholar]
- Ducker C. E., Stettler E. M., French K. J., Upson J. J., Smith C. D. Huntingtin interacting protein 14 is an oncogenic human protein: palmitoyl acyltransferase. Oncogene. 2004;23:9230–9237. doi: 10.1038/sj.onc.1208171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Husseini Ael D., Bredt D. S. Protein palmitoylation: a regulator of neuronal development and function. Nat. Rev. Neurosci. 2002;3:791–802. doi: 10.1038/nrn940. [DOI] [PubMed] [Google Scholar]
- Emi M., Fujiwara Y., Ohata H., Tsuda H., Hirohashi S., Koike M., Miyaki M., Monden M., Nakamura Y. Allelic loss at chromosome band 8p21.3-p22 is associated with progression of hepatocellular carcinoma. Genes Chromosomes Cancer. 1993;7:152–157. doi: 10.1002/gcc.2870070307. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y., Emi M., Ohata H., Kato Y., Nakajima T., Mori T., Nakamura Y. Evidence for the presence of two tumor suppressor genes on chromosome 8p for colorectal carcinoma. Cancer Res. 1993;53:1172–1174. [PubMed] [Google Scholar]
- Fujiwara Y., Ohata H., Emi M., Okui K., Koyama K., Tsuchiya E., Nakajima T., Monden M., Mori T., Kurimasa A., Oshimura M., Nakamura Y. A 3-Mb physical map of the chromosome region 8p21.3-p22, including a 600-kb region commonly deleted in human hepatocellular carcinoma, colorectal cancer, and non-small cell lung cancer. Genes Chromosomes Cancer. 1994;10:7–14. doi: 10.1002/gcc.2870100103. [DOI] [PubMed] [Google Scholar]
- Fukata M., Fukata Y., Adesnik H., Nicoll R. A., Bredt D. S. Identification of PSD-95 palmitoylating enzymes. Neuron. 2004;44:987–996. doi: 10.1016/j.neuron.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Greaves J., Chamberlain L. H. Palmitoylation-dependent protein sorting. J. Cell Biol. 2007;176:249–254. doi: 10.1083/jcb.200610151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N., Manevich Y., Kazi A. S., Tao J. Q., Fisher A. B., Bates S. R. Identification and characterization of p63 (CKAP4/ERGIC-63/CLIMP-63), a surfactant protein A binding protein, on type II pneumocytes. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;291:L436–L446. doi: 10.1152/ajplung.00415.2005. [DOI] [PubMed] [Google Scholar]
- Heinrich S., Hartl D., Griese M. Surfactant protein A—from genes to human lung diseases. Curr. Med. Chem. 2006;13:3239–3252. doi: 10.2174/092986706778773112. [DOI] [PubMed] [Google Scholar]
- Ichii S., et al. Detailed analysis of genetic alterations in colorectal tumors from patients with and without familial adenomatous polyposis (FAP) Oncogene. 1993;8:2399–2405. [PubMed] [Google Scholar]
- Jones A., et al. Ligand-gated ion channel subunit partnerships: GABAA receptor alpha6 subunit gene inactivation inhibits delta subunit expression. J. Neurosci. 1997;17:1350–1362. doi: 10.1523/JNEUROSCI.17-04-01350.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keay S., Kleinberg M., Zhang C. O., Hise M. K., Warren J. W. Bladder epithelial cells from patients with interstitial cystitis produce an inhibitor of heparin-binding epidermal growth factor-like growth factor production. J. Urol. 2000;164:2112–2118. [PubMed] [Google Scholar]
- Keay S., Seillier-Moiseiwitsch F., Zhang C. O., Chai T. C., Zhang J. Changes in human bladder epithelial cell gene expression associated with interstitial cystitis or antiproliferative factor treatment. Physiol. Genom. 2003;14:107–115. doi: 10.1152/physiolgenomics.00055.2003. [DOI] [PubMed] [Google Scholar]
- Keay S., Tocci G., Koch K., Zhang C., Grkovic D., Michejda C. J. The frizzled 8-related antiproliferative factor from IC patients inhibits bladder and kidney carcinoma cell proliferation in vitro. Eur. J. Cancer Suppl. 2006;4:87–88. [Google Scholar]
- Keay S., Zhang C. O., Chai T., Warren J., Koch K., Grkovic D., Colville H., Alexander R. Antiproliferative factor, heparin-binding epidermal growth factor-like growth factor, and epidermal growth factor in men with interstitial cystitis versus chronic pelvic pain syndrome. Urology. 2004a;63:22–26. doi: 10.1016/j.urology.2003.08.024. [DOI] [PubMed] [Google Scholar]
- Keay S., Zhang C. O., Trifillis A. L., Hise M. K., Hebel J. R., Jacobs S. C., Warren J. W. Decreased 3H-thymidine incorporation by human bladder epithelial cells following exposure to urine from interstitial cystitis patients. J. Urol. 1996;156:2073–2078. [PubMed] [Google Scholar]
- Keay S. K., Szekely Z., Conrads T. P., Veenstra T. D., Barchi J. J., Jr, Zhang C. O., Koch K. R., Michejda C. J. An antiproliferative factor from interstitial cystitis patients is a frizzled 8 protein-related sialoglycopeptide. Proc. Natl. Acad. Sci. USA. 2004b;101:11803–11808. doi: 10.1073/pnas.0404509101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Keay S. K., Dimitrakov J. D., Freeman M. R. p53 mediates interstitial cystitis antiproliferative factor (APF)-induced growth inhibition of human urothelial cells. FEBS Lett. 2007;581:3795–3799. doi: 10.1016/j.febslet.2007.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfenstein D. R., Kappeler F., Hauri H. P. A novel direct interaction of endoplasmic reticulum with microtubules. EMBO J. 1998;17:6168–6177. doi: 10.1093/emboj/17.21.6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfenstein D. R., Klumperman J., Lustig A., Kammerer R. A., Oorschot V., Hauri H. P. Subdomain-specific localization of CLIMP-63 (p63) in the endoplasmic reticulum is mediated by its luminal alpha-helical segment. J. Cell Biol. 2001;153:1287–1300. doi: 10.1083/jcb.153.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles M. A., Shaw M. E., Proctor A. J. Deletion mapping of chromosome 8 in cancers of the urinary bladder using restriction fragment length polymorphisms and microsatellite polymorphisms. Oncogene. 1993;8:1357–1364. [PubMed] [Google Scholar]
- Lee K. A. Dimeric transcription factor families: it takes two to tango but who decides on partners and the venue? J. Cell Sci. 1992;103(Pt 1):9–14. doi: 10.1242/jcs.103.1.9. [DOI] [PubMed] [Google Scholar]
- Linder M. E., Deschenes R. J. Palmitoylation: policing protein stability and traffic. Nat. Rev. Mol. Cell Biol. 2007;8:74–84. doi: 10.1038/nrm2084. [DOI] [PubMed] [Google Scholar]
- Lobo S., Greentree W. K., Linder M. E., Deschenes R. J. Identification of a Ras palmitoyltransferase in Saccharomyces cerevisiae. J. Biol. Chem. 2002;277:41268–41273. doi: 10.1074/jbc.M206573200. [DOI] [PubMed] [Google Scholar]
- Mansilla F., Birkenkamp-Demtroder K., Kruhoffer M., Sorensen F. B., Andersen C. L., Laiho P., Aaltonen L. A., Verspaget H. W., Orntoft T. F. Differential expression of DHHC9 in microsatellite stable and instable human colorectal cancer subgroups. Br. J. Cancer. 2007;96:1896–1903. doi: 10.1038/sj.bjc.6603818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri M. R., Marklund L., Gustavsson P., Davey E., Carlsson B., Larsson C., White I., Gustavson K. H., Dahl N. Loss of ZDHHC15 expression in a woman with a balanced translocation t(X;15)(q13.3;cen) and severe mental retardation. Eur. J. Hum. Genet. 2005;13:970–977. doi: 10.1038/sj.ejhg.5201445. [DOI] [PubMed] [Google Scholar]
- Moran L. K., Gutteridge J. M., Quinlan G. J. Thiols in cellular redox signalling and control. Curr. Med. Chem. 2001;8:763–772. doi: 10.2174/0929867013372904. [DOI] [PubMed] [Google Scholar]
- Mukai J., Liu H., Burt R. A., Swor D. E., Lai W. S., Karayiorgou M., Gogos J. A. Evidence that the gene encoding ZDHHC8 contributes to the risk of schizophrenia. Nat. Genet. 2004;36:725–731. doi: 10.1038/ng1375. [DOI] [PubMed] [Google Scholar]
- Nadolski M. J., Linder M. E. Protein lipidation. FEBS J. 2007;274:5202–5210. doi: 10.1111/j.1742-4658.2007.06056.x. [DOI] [PubMed] [Google Scholar]
- Ohata H., Emi M., Fujiwara Y., Higashino K., Nakagawa K., Futagami R., Tsuchiya E., Nakamura Y. Deletion mapping of the short arm of chromosome 8 in non-small cell lung carcinoma. Genes Chromosomes Cancer. 1993;7:85–88. doi: 10.1002/gcc.2870070204. [DOI] [PubMed] [Google Scholar]
- Oyama T., Miyoshi Y., Koyama K., Nakagawa H., Yamori T., Ito T., Matsuda H., Arakawa H., Nakamura Y. Isolation of a novel gene on 8p21.3–22 whose expression is reduced significantly in human colorectal cancers with liver metastasis. Genes Chromosomes Cancer. 2000;29:9–15. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1001>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Raymond F. L., et al. Mutations in ZDHHC9, which encodes a palmitoyltransferase of NRAS and HRAS, cause X-linked mental retardation associated with a marfanoid habitus. Am. J. Hum. Genet. 2007;80:982–987. doi: 10.1086/513609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaq T. M., Bass R., Vines D. J., Werner F., Whawell S. A., Ellis V. Functional regulation of tissue plasminogen activator on the surface of vascular smooth muscle cells by the type-II transmembrane protein p63 (CKAP4) J. Biol. Chem. 2003;278:42679–42685. doi: 10.1074/jbc.M305695200. [DOI] [PubMed] [Google Scholar]
- Resh M. D. Palmitoylation of ligands, receptors, and intracellular signaling molecules. Sci. STKE. 2006;2006:re14. doi: 10.1126/stke.3592006re14. [DOI] [PubMed] [Google Scholar]
- Roth A. F., Feng Y., Chen L., Davis N. G. The yeast DHHC cysteine-rich domain protein Akr1p is a palmitoyl transferase. J. Cell Biol. 2002;159:23–28. doi: 10.1083/jcb.200206120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauve S., Tremblay L., Lavigne P. The NMR solution structure of a mutant of the Max b/HLH/LZ free of DNA: insights into the specific and reversible DNA binding mechanism of dimeric transcription factors. J. Mol. Biol. 2004;342:813–832. doi: 10.1016/j.jmb.2004.07.058. [DOI] [PubMed] [Google Scholar]
- Schweizer A., Ericsson M., Bachi T., Griffiths G., Hauri H. P. Characterization of a novel 63 kDa membrane protein. Implications for the organization of the ER-to-Golgi pathway. J. Cell Sci. 1993a;104(Pt 3):671–683. doi: 10.1242/jcs.104.3.671. [DOI] [PubMed] [Google Scholar]
- Schweizer A., Rohrer J., Hauri H. P., Kornfeld S. Retention of p63 in an ER-Golgi intermediate compartment depends on the presence of all three of its domains and on its ability to form oligomers. J. Cell Biol. 1994;126:25–39. doi: 10.1083/jcb.126.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer A., Rohrer J., Jeno P., DeMaio A., Buchman T. G., Hauri H. P. A reversibly palmitoylated resident protein (p63) of an ER-Golgi intermediate compartment is related to a circulatory shock resuscitation protein. J. Cell Sci. 1993b;104(Pt 3):685–694. doi: 10.1242/jcs.104.3.685. [DOI] [PubMed] [Google Scholar]
- Schweizer A., Rohrer J., Kornfeld S. Determination of the structural requirements for palmitoylation of p63. J. Biol. Chem. 1995a;270:9638–9644. doi: 10.1074/jbc.270.16.9638. [DOI] [PubMed] [Google Scholar]
- Schweizer A., Rohrer J., Slot J. W., Geuze H. J., Kornfeld S. Reassessment of the subcellular localization of p63. J. Cell Sci. 1995b;108(Pt 6):2477–2485. doi: 10.1242/jcs.108.6.2477. [DOI] [PubMed] [Google Scholar]
- Sharma C., Yang X. H., Hemler M. E. DHHC2 affects palmitoylation, stability, and functions of tetraspanins CD9 and CD151. Mol. Biol. Cell. 2008;19:3415–3425. doi: 10.1091/mbc.E07-11-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotrys J. E., Linder M. E. Palmitoylation of intracellular signaling proteins: regulation and function. Annu. Rev. Biochem. 2004;73:559–587. doi: 10.1146/annurev.biochem.73.011303.073954. [DOI] [PubMed] [Google Scholar]
- Tsuruo T., Yamori T., Naganuma K., Tsukagoshi S., Sakurai Y. Characterization of metastatic clones derived from a metastatic variant of mouse colon adenocarcinoma 26. Cancer Res. 1983;43:5437–5442. [PubMed] [Google Scholar]
- Vedrenne C., Hauri H. P. Morphogenesis of the endoplasmic reticulum: beyond active membrane expansion. Traffic. 2006;7:639–646. doi: 10.1111/j.1600-0854.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- Vedrenne C., Klopfenstein D. R., Hauri H. P. Phosphorylation controls CLIMP-63-mediated anchoring of the endoplasmic reticulum to microtubules. Mol. Biol. Cell. 2005;16:1928–1937. doi: 10.1091/mbc.E04-07-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedegaertner P. B., Bourne H. R. Activation and depalmitoylation of Gs alpha. Cell. 1994;77:1063–1070. doi: 10.1016/0092-8674(94)90445-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Chochi Y., Matsuyama H., Eguchi S., Kawauchi S., Furuya T., Oga A., Kang J. J., Naito K., Sasaki K. Gain of 5p15.33 is associated with progression of bladder cancer. Oncology. 2007;72:132–138. doi: 10.1159/000111132. [DOI] [PubMed] [Google Scholar]
- Yanai A., et al. Palmitoylation of huntingtin by HIP14 is essential for its trafficking and function. Nat. Neurosci. 2006;9:824–831. doi: 10.1038/nn1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaremko M. L., Kutza C., Lyzak J., Mick R., Recant W. M., Westbrook C. A. Loss of heterozygosity from the short arm of chromosome 8 is associated with invasive behavior in breast cancer. Genes Chromosomes Cancer. 1996;16:189–195. doi: 10.1002/(SICI)1098-2264(199607)16:3<189::AID-GCC6>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Zacharias D. A., Violin J. D., Newton A. C., Tsien R. Y. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science. 2002;296:913–916. doi: 10.1126/science.1068539. [DOI] [PubMed] [Google Scholar]
- Zhang C. O., Wang J. Y., Koch K. R., Keay S. Regulation of tight junction proteins and bladder epithelial paracellular permeability by an antiproliferative factor from patients with interstitial cystitis. J. Urol. 2005;174:2382–2387. doi: 10.1097/01.ju.0000180417.11976.99. [DOI] [PubMed] [Google Scholar]
- Zhang J., Planey S. L., Ceballos C., Stevens S. M., Jr, Keay S. K., Zacharias D. A. Identification of CKAP4/p63 as a major substrate of the palmitoyl acyltransferase DHHC2, a putative tumor suppressor, using a novel proteomics method. Mol. Cell Proteomics. 2008;7:1378–1388. doi: 10.1074/mcp.M800069-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]