Abstract
Intraneuronal β-amyloid (Aβi) accumulates early in Alzheimer's disease (AD) and inclusion body myositis. Several organelles, receptor molecules, homeostatic processes, and signal transduction components have been identified as sensitive to Aβ. Although prior studies implicate the insulin-PI3K-Akt signaling cascade, a specific step within this or any essential metabolic or survival pathway has not emerged as a molecular target. We tested the effect of Aβ42 on each component of this cascade. In AD brain, the association between PDK and Akt, phospho-Akt levels and its activity were all decreased relative to control. In cell culture, Aβi expression inhibited both insulin-induced Akt phosphorylation and activity. In vitro experiments identified that β-amyloid (Aβ), especially oligomer preparations, specifically interrupted the PDK-dependent activation of Akt. Aβi also blocked the association between PDK and Akt in cell-based and in vitro experiments. Importantly, Aβ did not interrupt Akt or PI3K activities (once stimulated) nor did it affect more proximal signal events. These results offer a novel therapeutic strategy to neutralize Aβ-induced energy failure and neuronal death.
INTRODUCTION
There is widening recognition that Alzheimer's disease (AD) is closely linked to a state of relative insulin resistance in the brain, so-called “type III diabetes” (de la Monte et al., 2006). Levels of insulin-like growth factor I (IGF-I), insulin, and/or cognate receptors are dysregulated in AD brain (Messier and Teutenberg, 2005; Moloney et al., 2008). In normal brain, IGF-I and insulin promote glucose utilization, energy metabolism, and neuronal survival (Hoyer, 2004), largely through PI3K/Akt/GSK-3β signaling (Bondy and Cheng, 2004). Insulin receptors (IRs) populate neuronal synapses and astrocytes in memory-processing brain regions (Lee et al., 2005). Acute insulin treatment increased memory function in rats on a passive-avoidance task (Park et al., 2000) and in small studies involving normal adults and AD patients (Craft and Watson, 2004), consistent with positive effects on synaptic plasticity (Horwood et al., 2006). How β-amyloid, a major culprit neurotoxin in AD, could cause central insulin resistance is unknown.
Intraneuronal β-amyloid (Aβi) in particular, is a significant factor in the early pathogenesis of AD (Gouras et al., 2005; LaFerla et al., 2007). Inclusion body myositis (IBM), another disorder associated with intracellular β-amyloid (Aβ) deposits, is a major cause of skeletal muscle inflammation and degeneration in the elderly. Cytoplasmic Aβ induces programmed cell death (apoptosis) in a number of experimental and transgenic models (Magrane et al., 2005; Oakley et al., 2006).
Interference with or alteration of the Akt signaling pathway has emerged as an important feature in several neurodegenerative diseases characterized by neuronal attrition including AD and schizophrenia (Emamian et al., 2004; Griffin et al., 2005). In previous studies of the effects of intracellular Aβ on this pathway using primary cortical neurons as well as Tg2576 mice that overexpress human Aβ precursor protein bearing the familial AD Swedish double mutation (KM-670/671-NL; Hsiao et al., 1996), we found that Aβi expression leads to a decrease in levels of p-Akt, an increase in the activation state of GSK-3β, and induction of apoptosis (Magrane et al., 2005). We hypothesize that extra- and intracellular Aβ have different targets in the insulin-signaling path and that the latter may well exert its effect distal to the immediate receptor and its adaptor protein.
The PI3K-Akt signaling pathway is responsive to trophic factors, metabolic signals, and environmental stress and regulates survival, growth, differentiation, and other homeostatic functions. This pathway is pivotally affected in the opposing processes of tumorigenesis and apoptosis (Martelli et al., 2006). Stimulated insulin- and IGF-receptor tyrosine kinases phosphorylate the insulin receptor substrate (IRS), an adaptor that initiates the PI3K-Akt signaling cascade (Asano et al., 2005). PI3K activation, in turn, catalyzes the 3′-phosphorylation of second messenger, membrane-bound signaling inositol lipids. The lipid phosphatidylinositol 3,4,5-trisphosphate [PtdIns(3,4,5)P3 or PIP3] brings together phosphoinositide-dependent kinase (PDK) and Akt in a submembrane complex through interaction with each of their pleckstrin homology (PH) domains (Andjelkovic et al., 1997). After two sequential activating phosphorylations, first on a regulatory hydrophobic domain and then by PDK on a T loop of the catalytic domain, p-Akt phosphorylates a number of cellular targets, regulating their function (Brunet et al., 2001; summarized in Figure 8). For instance, certain proapoptotic mediators, such as the transcription factor forkhead (FOXO), the tau kinase GSK-3β, and the Bcl2 antagonist BAD proteins, are inactivated by Akt (Cross et al., 1995; Datta et al., 1997; Zheng et al., 2000).
Figure 8.
Model of insulin-PI3K-Akt signaling in AD. Intracellular Aβ is depicted to interfere with Akt activation by PDK-1 via two possible mechanisms. This may occur at a possible hydrophobic interaction site between Akt and PDK or directly through inhibition of the ATP-dependent kinase's ability to phosphorylate T308 of Akt. PH, pleckstrin homology domain; RD, regulatory domain; KD, kinase domain; G, growth factor (insulin; IGF-I); IR, insulin receptor; IRS-1, insulin receptor substrate-1; PI3K, phosphoinositide 3′ kinase; PIP3, PI(3,4,5)P3 (phosphoinositide phosphate, see inset for details); mTOR, mammalian target of rapamycin; , phosphorylated residues; ●, phosphorylated inositide (see inset).
, phosphorylated residues; ●, phosphorylated inositide (see inset).
We embarked on a systematic study of the effect of Aβi on the complex series of steps leading to Akt activation. The following results were collected on human brain, and from cultures of neuronal and skeletal muscle cell lines and using in vitro assays. We conclude that Aβi specifically inhibits the PDK-dependent activation of Akt through a mechanism preventing their direct interaction. The inhibition of a defined step within this essential pathway suggests a target for the identification of a specific Aβ antagonist designed to reestablish Akt signaling.
MATERIALS AND METHODS
Human Brain Samples
Two sets of rapidly frozen control and Braak stage V and VI human brain samples from anterior frontal and temporal lobe, respectively, were generously provided by the Harvard Brain Tissue Resource Center, McLean Hospital (Belmont, MA; Supplemental Table 1). Human samples were extracted in lysis buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM Na3VO4, 1% Triton X-100, 10% glycerol, 1 mM Na4P2O7, 1 μg/ml leupeptin, 1 μg/ml pepstatin A, 1 μg/ml aprotinin, 0.1 mM phenylmethylsulfonyl fluoride; Sigma, St. Louis, MO, and protease inhibitor cocktail; Roche, Indianapolis, IN), incubated on ice for 10 min, and then centrifuged at 14,000 × g for 10 min. The supernatants were used directly for Western blot analysis or for immunoprecipitation (IP).
Cell Culture and Reagents
Mouse C2C12 cells (ATCC, Manassas, VA) were grown in DMEM, and 20% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA) and maintained for passage below 60% confluence. Cultures at or above 90% confluence were differentiated in DMEM, 2% adult horse serum (Differentiation Medium, DM). SH-SY5Y, and HEK-293, or NIH/3T3 cells were grown in DMEM, 10% FBS, at below 80% confluence. Recombinant PTEN (R&D Systems, Minneapolis, MN), PP2A (Upstate Biotechnology, Lake Placid, NY), protein kinase A (PKA; Promega, Madison, WI), and phospholipase A2 (PLA2; Sigma, St. Louis, MO) were stored at −20°C and used freshly. PKA inhibitor (PKI, BioSource Technologies, Camarillo, CA) and PI3K inhibitor, LY294002 (Cell Signaling, Beverly, MA) were diluted to 20 or 10 μM, respectively, in kinase buffer. The following antibodies were used: anti-cdk4, Akt, Actin, neuron-specific enolase (NSE), and IRα (Santa Cruz Biotechnology, Santa Cruz, CA); p-Akt (Ser473 and Thr308), p-GSK-3α/β (Ser21/9), GSK-3β, IRβ, p-Tyrosine, p-IR, PKA, and p-PDK (Ser241; Cell Signaling); IRS-1 and PDK (BD Biosciences, San Jose, CA); PI3K (p85) and SGK (Upstate Biotechnology); Rictor (Bethyl Laboratories, Montgomery, TX); 6E10 (Covance, Madison, WI); R1282 (gift from Dr. D. Selkoe, Harvard Medical School, Boston, MA). The lipids, dipalmitoyl-PIP3 (Matreya, State College, PA) and d-myo-PtdIns (PI; Echelon Biosciences, Salt Lake City, UT) were diluted into water or CHCl3:MeOH:H2O (1:2:0.8), aliquoted into 100-μg vials, and stored at −20°C. ATP (Cell Signaling), [γ-32P]ATP (300 μCi/ml; Perkin Elmer, Boston, MA) and thin-layer chromatography (TLC) silica gel plate (no. 105553, Merck, Rahway, NJ) coated with potassium oxalate (Sigma) were used in PI3K activity assays. The GSK-3 fusion peptide, a substrate for phosphorylations, was purchased from Cell Signaling.
Infection of C2C12 Myotubes with Adenoviruses
Adv Tet-On and TRE-Aβ42 viruses (Magrane et al., 2005) and Adv WT-Akt, expressing wild-type Akt (Fujio and Walsh, 1999) were described previously. C2C12 were switched to DM and on day 3 infected with Adv Aβ/TetOn (4:1 ratio, 24–36 h) before doxycycline induction (1 μg/ml) for an additional 24–36 h. Some cultures were infected with Adv WT-Akt for 48 h, before harvest. Cell extracts were prepared in lysis buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM Na3VO4, 1% NP-40, 10% glycerol, 1 mM Na4P2O7, 1 μg/ml leupeptin, 1 μg/ml pepstatin A, 1 μg/ml aprotinin, 0.1 mM phenylmethylsulfonyl fluoride, and protease inhibitor cocktail; Roche) and were stored at −80°C until use.
Cell Viability
C2C12 were grown in eight-chamber slides (Nalge Nunc, Rochester, NY) and infected/induced with Adv Aβ/TetOn. Cell profiles were captured and quantified on a Nikon TE200 microscope (10×; Melville, NY) coupled to a SPOT cooled CCD camera. Myotube thickness and length were measured in each of five unbiased, random fields. The cells were fixed using 4% paraformaldehyde in 0.1N Phosphate buffer, pH 7.4 and incubated with bis-benzimide (Hoechst 33258). Nuclear pyknosis was quantified by manual counting.
Aβ Peptides, Aβ-derived Diffusible Ligands, and Fibril Preparation
Aβ peptides were obtained from BioSource as dried trifluoroacetic acid salts. The scrambled Aβ42 sequence peptide was from rPeptide (Bogart, GA). Monomeric Aβ peptides were prepared by solubilization in 5% dimethyl sulfoxide (DMSO); 25 mM Tris-HCl, pH 7.4, and used fresh or flash-frozen. Aβ-derived diffusible ligands (ADDL) were prepared according to Lambert et al. (1998) and Klein (2002). Briefly, Aβ peptide was dissolved in 1,1,1,3,3,3-hexafluoro-2-propanol (Sigma) and evaporated on a Speedvac (Savant Instruments, Ramsey, MN). The Aβ film was resuspended in 100% anhydrous DMSO, diluted to 5 mM in F12 medium lacking phenol red (BioSource), and incubated at 4°C for 24–48 h. After incubation and centrifugation at 14,000 × g for 10 min at 4°C, the supernatant containing ADDL-enriched Aβ was transferred to a new tube. Fibrillar Aβ was made by diluting Aβ42 in DMSO to 100 μM in 10 mM HCl, immediate vortexing for 30 s, and incubation at 37°C for 24 h (Stine et al., 2003). All peptide preparations were flash-frozen and stored at −80°C until use.
Thioflavin T Fluorescence Assay
Fibril-enriched preparations of Aβ were verified according to Conway et al. (2000). Thioflavin T (100 μM; ThT; Sigma) was filtered through a 0.22-μm filter unit (Nalge Nunc). To construct a standard curve, 90 μl of each Aβ sample (0, 0.5, 1, 2, and 4 μg) was added to 100 μl glycine-NaOH (pH 8.5) solution, to which 10 μl ThT solution was added to a total volume of 200 μl per well in a C96 White Maxisorp plate (Nalge Nunc). After mixing, fluorescence was measured with a CytoFluor Multiwell plate reader (excitation at 450 nm, bandwidth 50; emission at 508 nm, bandwidth 20; PerSeptive Biosystems, Framingham, MA).
Immunoprecipitations and Western Blot Analysis
Cell lysates (see above) were incubated on ice and centrifuged at 14,000 × g for 10 min. IPs on 100∼300 μg of extract protein commenced with an overnight incubation at 4°C using 2–3 μg of primary antibody. Protein G-Sepharose was added for an additional 1.5 h. IPs were harvested at 5000 × g for 1 min at 4°C and washed two or three times in PBS containing 0.5% NP-40 and 0.1 mM Na3VO4. For kinase and phosphatase reactions, the samples were washed two more times. Whole cell extracts were used directly for Western blot analysis (20–30 μg for cell cultures or 60–80 μg for human samples). Extracts or IPs from either cultured cells or human brain samples, prepared in lysis buffer, were diluted into Laemmli sample buffer, heated at 95°C for 10 min, cleared by centrifugation, separated on SDS-PAGE, and transferred to PVDF membrane (Immobilon-P; Millipore, Bedford, MA). Membranes were blocked in TBS containing 0.3% Tween-20 and 5% (wt/vol) nonfat dry milk. After incubation with primary antibodies (18 h at 4°C in buffer containing 5% BSA and 0.05% NaN3), blots were washed and incubated in HRP-conjugated secondary antibodies (1:2000 dilution; Cell Signaling). Signals were detected using ECL reagents and quantified using a Kodak Image Station 4000R (Eastman Kodak, Rochester, NY).
Akt1 Activity Assay
Akt1 was immunoprecipitated overnight from 100 μg of human brain sample or extract from C2C12 myotubes infected with adenovirus encoding Aβ, using a 1:100 dilution of goat anti-Akt1 antibody. The following morning, 40 μl of 50% slurry of protein G-agarose (Roche) was added for 1.5 h. The collected beads were washed twice in buffer (1× PBS, 0.5% NP-40, and 0.1 mM Na3VO4) and twice in kinase buffer (25 mM Tris, pH 7.5, 2 mM DTT, 0.1 mM Na3VO4, 10 mM MgCl2, and 200 μM ATP). Finally GSK-3 fusion protein (1 μg/50 μl) was added in the presence of kinase buffer, and the reaction (50 μl) was incubated for 30 min at 30°C. The reaction was stopped by adding 40 μl of Laemmli buffer. Twenty microliters of sample was loaded onto a 10% polyacrylamide gel.
In Vitro p-Akt and Activity Levels
IPs of PDK and Akt1 were prepared from 100 μg of either SH-SY5Y, C2C12 myotubes or from insulin-treated cultures. ATP (200 μM), GSK-3 fusion protein (1 μg/50 μl), kinase buffer, and Aβ peptides were added (0.01, 0.1, 1, 5, 10, 20 μM; final). Akt activity was determined as above.
PDK and Akt1 Interaction Assay
For cell-based interaction studies, intracellular Aβ42 was induced in C2C12 myotubes exposed to Adv Aβ/TetOn. Cell extracts (100 μg) were used to IP endogenous Akt and determine the levels of associated PDK by Western blot. The in vitro interaction study involved first enriching for Akt through viral-mediated expression of WT-Akt. “Akt-enriched/PDK-depleted” extract was then prepared by removing the PDK through IP. Akt-depleted extracts were prepared from control extracts by pre-IP Akt1 and were relatively enriched with endogenous PDK. Enrichments and depletions were confirmed by Western blot. The interaction assay consisted of mixing 100 μg Akt-enriched and 100 μg control cell extract in the presence or absence of Aβ42 (up to 10 μM). These were incubated for 30 min at 30°C, followed by an overnight incubation at 4°C with goat anti-Akt1. Protein G beads were added for 1.5 h. PDK and Akt1 levels were determined by Western blot. In other experiments, 100 μg of Akt-enriched/PDK-depleted extract and 100 μg of a control extract depleted for Akt were mixed in the presence or absence of Aβ42 (up to 5 μM).
PI3K Activity Assay
IP PI3K from the NIH/3T3 cell lysates with rabbit anti-p85 subunit was used. The IPs were washed twice with cold PBS buffer containing 0.5% NP-40 and 0.1 mM Na3VO4 and then twice with TNE buffer (20 mM Tris.HCl, pH 7.4, 100 mM NaCl, and 0.5 mM EGTA). PI3K activity was determined by incubating the beads with kinase buffer (6.5 mM HEPES, pH 7.4, 10 mM MgCl2), 50 μM ATP, 2 μCi [γ-32P]ATP, 4 μg of 3-sn-phosphatidyl-l-serine and 2 μg of d-myo-PI for 10 min at 25°C. The reactions were stopped with 50 μl of 4N HCl. Phospholipids were extracted using 100 μl of CHCl3/methanol (1:1). Products were separated by TLC as described previously (Whitman et al., 1985). The conversion of PI to PI3P was quantified on a Storm Phosphorimager (GE Healthcare).
RESULTS
Akt Deactivation and PDK-Akt Dissociation in AD Brain
We first determined the levels of Akt phosphorylation, indicating activation, Akt activity and the extent to which PDK1 is physically associated with Akt in two sets of human AD brain. In AD frontal lobe, p-Akt (Thr308) levels were decreased relative to control (Figure 1A top). Akt1 activity was also decreased in immunoprecipitated AD brain samples relative to controls, judged by the reduced level of Ser 9 phosphorylation on a 30-kDa GSK-3β consensus peptide-fusion protein substrate (Figure 1A, middle). When combined with similar results using temporal lobe obtained from another set of patients (Supplemental Table S1), Akt activation and activity are significantly reduced in AD brain (bottom graphs). We next tested the interaction between PDK and Akt1 in pulldowns from brain extract. Both total Akt and p-Akt (Ser473) were probed in immunoprecipitates of PDK. Ser473 phosphorylation is required for full Akt activity and, similar to models of AGC kinase activation, precedes the phosphorylation of Thr308 by PDK-1 (Alessi and Cohen, 1998; Toker and Newton, 2000). Their interactions were decreased in the same AD brain samples (Figure 1B, top). The reverse pulldown experiment also showed less associated total PDK in the AD samples. IP samples were also tested for nonspecific and non-disease–related interactions. PKA (another AGC kinase) did not pull down with PDK or Akt1. Moreover, a known Akt-1–binding partner, HSP27 (Vandermoere et al., 2007), showed no changes in interaction level with disease state (Figure 1C, top).
Figure 1.
Akt activity and association with PDK: reductions in AD brain. Control brain: C1–3; AD brain: A1–3. (A) Top panels, p-Akt; levels of activated Akt (pThr308-Akt) are decreased in AD brain (cases A1–A3). Middle panels, Akt activity; a synthetic GSK-3β/substrate was less phosphorylated by IP Akt from AD brain samples (100 μg protein). Bottom graphs, quantitations of p-Akt and activity: Both p-Akt level and activity divided by total GSK were normalized over two AD brain sets (A1–A3 and A4–A6) using total Akt input. **p < 0.01 versus control; n = 6 (two-tailed Student's t test). (B) Akt and PDK interactions. Aβ coIPs with PDK and Akt1 in AD brain: top panel, IP PDK fractionated to detect association with Akt1 or pS473-Akt. Reverse IP shown below. Bottom panel, IPs of Akt, PDK and Aβ were probed with MAb 6E10 versus Aβ1-42. Synthetic Aβ peptide (50 ng) run as standard. (C) Control IPs of PKA with PDK and Akt1, and Aβ with PKA and NSE in AD brain are negative. IPs of PDK and Akt1 were probed with PKA Ab, and IPs of PKA and NSE were probed with MAb 6E10 versus Aβ1-42. Hsp27, a known Akt binding protein, is unchanged in AD samples. Synthetic Aβ peptide (50 ng) run as standard. NSE levels in whole tissue lysates demonstrate equal starting material.
One possibility for the down-regulation of Akt activation in AD brain is direct inhibition mediated by an increase in cellular Aβ. Aβ levels were found in IPs to be greatly increased in the AD brain samples (Figure 1B, bottom). More interestingly, the association of Aβ with each of Akt1 and PDK was increased in AD brain. There was no Aβ interaction with control neuronal proteins (NSE) or PKA, indicating that Aβ has specific affinity for PDK/Akt (Figure 1C, bottom). We investigated the proximal insulin/PI3K pathway for any changes in protein levels or interaction in the same AD samples, finding no significant differences (Supplemental Figure S1A). Another kinase with activity to regulate GSK, PKA, was not affected in the AD samples (Supplemental Figure S1B), confirming specificity. Because the AD samples had generally shorter postmortem intervals (PMI), we ruled out nonspecific changes in phosphoproteins due to either disease state or PMI. As expected (Wang et al., 2001), phosphorylation of tau and neurofilament proteins are elevated in our AD specimens (Supplemental Figure S1C). Most other tyrosine-phosphorylated proteins do not significantly change with respect to disease state or PMI (Supplemental Figure S1D), thus excluding nonspecific phosphatase effects on p-Akt in the AD cases. Finally, when cases with similar mean PMIs are subanalyzed (control, 17.7 h, n = 3; AD, 18.8 h, n = 3), the effect of AD to reduce Akt phosphorylation (p-Akt/total: control, 0.81; AD, 0.56; p < 0.05) and activity (arbitrary density units: control, 0.11; AD, 0.05, p < 0.05) remains consistent.
Aβ42 Toxicity and Akt1 Inhibition in Cultured Cells
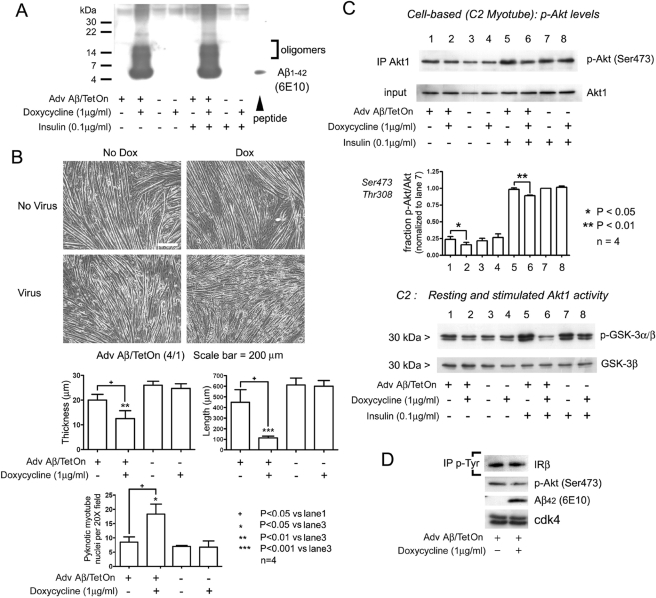
The specific inhibition of Akt activation in AD brain was explored mechanistically in neuronal (SH-SY5Y human neuroblastoma) and skeletal muscle cell line cultures. Previous studies have characterized infection of neurons with an inducible adenovirus construct encoding Aβ42 and signal peptide domain (Adv Aβ/TetOn). These show Aβ accumulation into intracellular membrane compartments that is contemporaneous with the exclusion of p-Akt immunocytolocalization (Magrane et al., 2005). After exposure to doxycycline, C2C12 cells expressing Aβ were treated for 30 min with insulin (0.1 μg/ml) or vehicle. Both monomers and oligomers of Aβ were inducibly expressed in this system (Figure 2A). Insulin treatment had no effect on expressed Aβ levels per se. Aβ-expressing myotubes exhibited significant reductions in both length and thickness (Figure 2B) and contained increased number of condensed nuclei (Figure 2B, graphs) consistent with apoptotic cell death.
Figure 2.
Intracellular Aβ (Aβi) in C2C12 myotubes: toxicity and inhibition of Akt activation. (A) Aβ1-42 expression. Myotube cultures were infected with Adenovirus Aβ42/TetOn and induced by doxycycline (Dox; 1 μg/ml). Cultures were treated with insulin (0.1 μg/ml) for 15 min before harvest. Monomers and oligomers are detected only in Dox-induced cultures. (B) Viability. Aβi expression decreases myotube length and caliber and increases pyknotic nuclei. Five random 20× fields were manually counted in each sample. + p < 0.05 versus lane 1; *p < 0.05 versus lane 3; **p < 0.01 versus lane 3; ***p < 0.001 versus lane 3; n = 4 (two-tailed Student's t test). (C) Aβ prevents Akt activation. C2C12 myotube cultures infected with AdvAβ42/TetOn were stimulated for 20 min with insulin, harvested, and used to IP Akt1. Phospho-Akt (Ser473) levels are reduced (top panels) in Aβ-expressing/insulin-stimulated cells (lane 6). Without insulin, a smaller but still significant decrease in Akt phosphorylation occurs (lane2). Quantitation of pSer473 and pThr308 Akt Western signals is shown below. *p < 0.05; **p < 0.01; n = 4 (two-tailed Student's t test). Lower panels, cell-based Akt kinase activity assay. Immunoblot of p-GSK-3α/β shows significantly inhibited phosphorylation in cultures treated with insulin. (D) Autophosphorylation of the insulin receptor is unchanged. Anti-phosphotyrosine IP from 100 μg myotube extracts infected with Adv Aβ/TetOn. Phosphorylation of IRβ was unaffected in Aβ-expressing cultures.
Myotube extracts were used for IP and Western blot to determine levels of p-Akt, Akt activity, and IR activation. Levels of Ser473 phosphorylation are inhibited to a modest degree in Aβ42-expressing cultures, more apparent under insulin-stimulated conditions (lane 6 vs. lane 5, p < 0.01; Figure 2C, top). As expected, insulin up-regulates p-Akt levels under all conditions. Testing in SH-SY5Y cells produced the same results (bar graph). More notable, the stimulated activity of Akt1 on the GSK substrate is markedly inhibited in the presence of intracellular Aβ42 (Figure 2C, bottom, lane 6). We tested for the effect of Aβ42 on the phosphotyrosine (p-Tyr) signature, indicating activation of the IRβ and found no change in IRβ phosphorylation in C2 (Figure 2D) or SH-SY5Y cells (not shown).
In Vitro Akt Activity Levels: Effects of Aβ Peptides
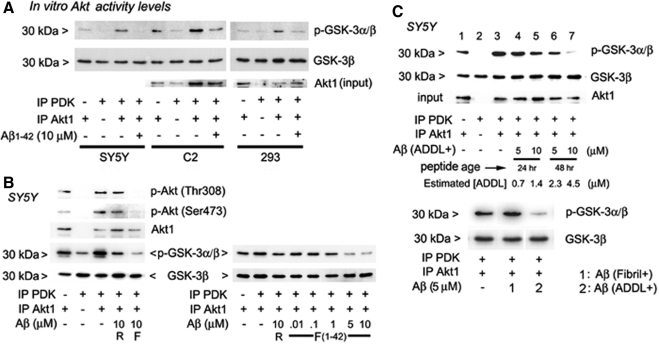
To confirm the down-regulation of both Akt activation and activity and test the amyloid species most accountable for the effect, we performed cell-free, in vitro reactions. These were done on precipitates from three cell lines, chosen for low, medium, and high levels of resting Akt phosphorylation. A mixture of immunopurified PDK and Akt1 is shown to induce maximal Akt phosphorylation and activity at 30 min, the time point chosen for all subsequent experiments (see Supplemental Figure S2A). When the effect of adding freshly prepared, synthetic Aβ42 was compared between the cells types, Aβ42 inhibited all PDK1-stimulated Akt activity levels (Figure 3A). Levels of activity in the presence of Aβ resemble basal levels corresponding to reactions in which only Akt precipitates are added. Thus, Aβ42 appears to interfere with the activation that is attributable to PDK1. The in vitro inhibition of Akt phosphorylation and activation is more specific to the forward peptide sequence (F) than to the reverse (R) and shows dose dependency (Figure 3B).
Figure 3.
In vitro–coupled assays and PDK-dependent phosphorylation and activation of Akt1. Effects of Aβ monomer, ADDL and fibril preparations. (A) Akt activity in three cell types. Three cell extracts (SH-SY5Y, HEK 293, and C2C12) were used to IP Akt and PDK for activity assays in the presence of synthetic, predominantly monomeric Aβ. In all cell types, PDK-dependent Akt activation was reversed by peptide. (B) Aβ specificity and dose dependence of inhibition in neuronal cells. IP PDK and IP Akt1 from SH-SY5Y cell extracts were mixed, and Akt activation was examined in vitro. pSer473 and pThr308 levels and activity to phosphorylate GSK-3α/β are affected by forward sequence (F) Aβ1-42 peptide (left panel), beginning at ∼1 μM (right panel). R, reverse Aβ (Aβ42-1). (C) Amyloid conformers have relative effects on PDK-dependent activation of Akt. Top panel, IP PDK and IP Akt1 from SH-SY5Y cells. Total amounts of Aβ in ADDL preparations are indicated. [ADDL] was estimated from the ratio of 12-mer to monomer in Supplemental Figure S2B. ADDLs that were aged 48 h more significantly prevented the activation of Akt. Bottom panel and Supplemental Figure S2C, fibril and ADDL-containing preparations, tested side-by-side.
Oligomeric species of Aβ have been most implicated in neuronal toxicity (Walsh et al., 2002). We freshly solubilized synthetic Aβ monomers and prepared Aβ oligomer—as ADDL- (Lambert et al., 1998) and fibril-containing aliquots (Supplemental Figure S2B). These crude preparations (ADDL+, aged 24 and 48 h and fibril+) were tested against Akt activity derived from SH-SY5Y cells (Figure 3C). In vitro assays of PDK1-stimulated Akt activity demonstrate that oligomer-containing preparations (ADDL+) are more potent inhibitors than either monomers or fibrillar Aβ. A near total inhibition of PDK-stimulated Akt1 activity occurred at 10 μM (conservatively estimated 4.5 μM oligomeric content; Figure 3C, top). In comparing monomer or ADDL- to fibril-containing preparations, the fibrillated ones appeared least active (Figure 3C, bottom, and Supplemental Figure S2C, respectively).
In Vitro p-Akt and Activity Levels: Roles of PIP3 and AGC Kinases
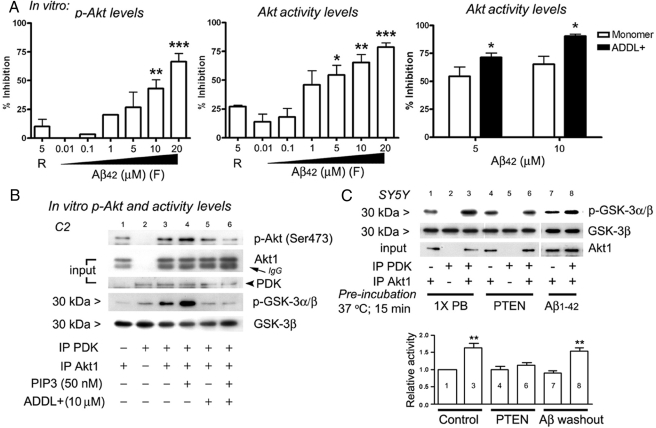
The dose-dependent, inhibitory effects of monomeric Aβ42 on both the phosphorylation and activation of Akt by PDK are summarized for the in vitro data in Figure 4A. Although 10 μM Aβ42 monomer produced up to 50% inhibition of Akt phosphorylation, only 1 μM was sufficient to inhibit Akt activation in the coupled assay to a similar degree (IC50 ≈ 1–5 μM). The reverse peptide (Aβ42-1, R) at 5 μM (or 10, not shown) had a significantly smaller yet measurable effect to inhibit Akt activation. Thus, the functional degree of Akt activation, as judged by enzyme activity level, is the more sensitive measure of impairment by Aβ. The ADDLs, compared with the same starting material of monomeric Aβ, are additively more injurious (Figure 4A, right; p < 0.05).
Figure 4.
Specific properties of Aβ-directed inhibition of PDK activity. (A) Quantification of PDK-dependent Akt1 phosphorylation and activation. Dose-dependent Aβ inhibition of Akt phosphorylation (left graph) and Akt activation (middle graph) expressed as percent of control reactions without added Aβ42. *p < 0.05; **p < 0.01; ***p < 0.001; two-tailed Student's t test vs. reverse peptide (R) results. ADDL preparations aged 48 h (2.3 and 4.5 μM; estimated oligomers) were significantly more active against PDK activity than monomers (right graph). Data represent the analysis of results in Figure 3 as well as experiments not shown (n = 6). (B) Role of second messenger, PIP3. IP PDK from insulin-treated (INS) C2C12 myotubes was added to IP Akt from SH-SY5Y cells. Akt1-beads were preincubated with PP2A. The mixing of PDK and Akt1 led to submaximal increases in p-Akt (Ser473) and Akt activity (lane 3), an effect further enhanced by the addition of PIP3 (lane 4). Inclusion of ADDL nearly eliminates PDK alone or PDK plus added PIP3-induced activations (lanes 5 and 6). (C) PIP3 tethered to Akt and PDK IPs is sufficient for activation. Aβ is not acting like a phosphatase. IP Akt1 and IP PDK preincubated in phosphatase buffer alone (1× PB) or with added PTEN (10 μg/ml) or Aβ. PDK addition increased Akt activity as expected in control coupled kinase reactions (lane 3). Combining PTEN-treated PDK and Akt1 beads eliminated stimulation of Akt activity (lane 6 vs. 4). Preincubation of the two IP kinases with Aβ, followed by washout, produced no inhibitory effect (lane 8).
PIP3 is a second messenger lipid that interacts with Akt1 and PDK, organizing their corecruitment to the submembrane for activation. If Aβ inhibits the action of PDK to activate Akt, one possible mechanism is by competition with PIP3. In Figure 3 in vitro experiments, it was not necessary to supplement PIP3 to obtain PDK-mediated activation of Akt. To assess the dynamic range of Akt activation from basal levels and whether PIP3 acts as a cofactor in our in vitro assay using the IP kinases, we dephosphorylated the Akt starting material from C2 cells with protein phosphatase 2A (PP2A). In Figure 4B, the large increase in PDK-stimulated p-Akt and activity levels (lane 3 vs. 1; see Figure 3A) is enhanced by adding PIP3 (50 nM, lane 4 vs. 3). The ADDL-containing Aβ42 peptide preparation suppressed PDK-dependent Akt1 phosphorylations and activations, with or without added PIP3. Thus, PIP3 addition does not overcome the Aβ42-mediated inhibition of Akt activation.
Another possibility is that Aβ acts like the lipid phosphatase and tensin homolog (PTEN) in dephosphorylating PIP3. In Figure 4C, we tested if preincubation of the kinase-bearing beads with PTEN, expected to remove endogenous coprecipitated PIP3, would eliminate PDK-stimulated Akt activity. Indeed, there is no activation in lane 6 versus 4, as there is in lane 3 versus 1. Moreover, preincubation with Aβ1-42, followed by washing of beads before setting up the activity reaction, had no effect on GSK phosphorylation (lane 8 vs. 7). The inference is that Aβ does not act like PTEN to remove 3′-phosphate from lipid cofactors or prevent their attachment.
Another AGC kinase, serum/glucocorticoid-regulated kinase (SGK), is activated by PDK. Similar to Akt, SGK has a PH domain and phosphorylates GSK-3α/β (Tessier and Woodgett, 2006). If Aβ inhibits the catalytic function of PDK to activate PH-containing kinase substrates in general, it is predicted to affect this reaction. Indeed, ADDL preparations suppressed PDK-stimulated SGK activity (Supplemental Figure S3A). We tested the role of PDK's PH domain by using another kinase that activates Akt but lacks a PH domain, Rictor (Hresko and Mueckler, 2005; Sarbassov et al., 2005). In Supplemental Figure S3B, we added IP Rictor to show that Akt activity is up-regulated. The enhanced activity was not, however, interrupted by Aβ (10 μM). Lastly, another AGC kinase that converges to phosphorylate GSK, PKA (Li et al., 2000), was tested to determine specificity. In Supplemental Figure S3B, c-AMP–dependent PKA (2U), although very active in phosphorylating the peptide substrate, was not inhibited by Aβ. Thus, the mechanism of Aβ inhibition of Akt activation is specific to PDK-like kinases and their substrate.
The Interaction of PDK with Akt1 Is Interrupted by Aβ42
If Aβ interferes with the action of PDK to activate Akt, we predict their physical interaction should be lessened, as shown in AD brain. In Figure 5A, the expression of Aβ42 in C2C12 myotube cells resulted in less pulldown of PDK with Akt. The direct association of PDK with Akt was similarly affected in the presence of synthetic Aβ in cell-free extracts (Figure 5B). We used extracts from C2C12 myotubes enriched for Akt after infection by Adv encoding WT-Akt1 and from control cell cultures, as the source of additional PDK, for starting material. In Figure 5B, the addition of Aβ42 to a mixture of Akt-enriched and control extracts resulted in the inhibited pulldown of Akt-PDK complexes (lane 4 vs. 3). Next, an Akt-enriched/PDK-depleted and a control/Akt-depleted extract were combined before Aβ treatment and IP. When tested for Akt pulldown after IP of PDK or in reverse, Aβ42 again inhibited the signals (Figure 5C, lane 2 vs. 1, left panel).
Figure 5.
Pulldown type PDK-Akt1 interaction assays. (A) Cell based. IP Akt1 from Aβ-expressing myotubes shows a decrease in coimmunoprecipitated PDK compared with control. Total Akt1 and PDK levels remain constant irrespective of Aβ (input). (B) Cell-free conditions. Akt-enriched cell extracts are prepared from myotube cultures infected with Adv WT-Akt. Extracts from unstimulated, control cultures are relatively abundant in PDK. Western blots of whole cell lysates (WCL) confirm expected levels of Akt and PDK (right panel). Cell extracts, 100 μg, each from Akt-enriched and control cultures, were mixed and incubated. Pulldown of PDK1 with Akt1 was significantly increased in the mixture of extracts (lane 3) but was abrogated by Aβ42 (lane 4). (C) In vitro interaction assay using PDK-modified cell extracts. Akt-enriched: PDK-depleted extracts are prepared by removing PDK through IP from Adv WT-Akt–expressing cell extracts. Control: Akt depleted lysates are prepared by removing Akt1 through IP from control cell extracts. Their respective levels are shown in lanes 3 and 4. These Akt1- and relative PDK-enriched fractions were mixed as before. The reverse IP of PDK pulled down less Akt1 and IP of Akt1 (as above) pulled down less PDK, in the presence of Aβ42.
Aβ Does Not Dephosphorylate or Inhibit Akt Action Once Established
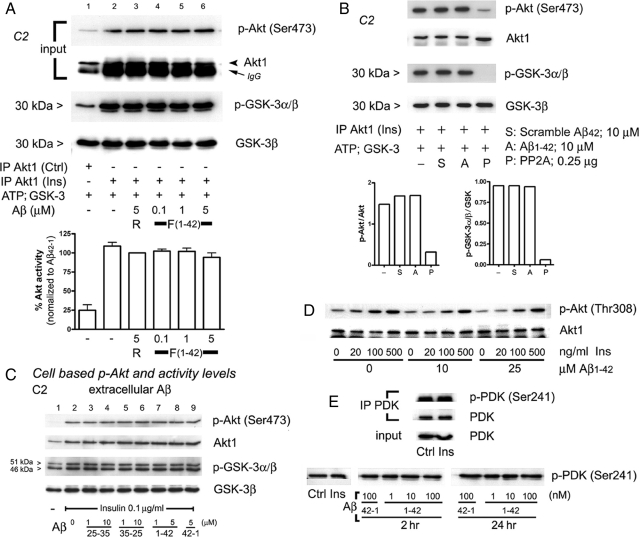
In Figure 3 in vitro experiments, it was observed that Aβ additions consistently reduced p-Akt levels and activity state. The degree of inhibition however sometimes exceeded the baseline activity in the absence of PDK. Thus, it is possible that Aβ may have an additional action to deactivate Akt. To test if Aβ additionally inhibits Akt phosphorylation and activation by dephosphorylating prestimulated Akt (acting as a phosphatase), we prepared control and insulin-treated cell extracts for the IP of preactivated Akt1. Insulin-stimulated Akt activity (Figure 6A, third row, lane 2 vs. 1) and p-Ser473 and p-Thr308 (not shown) levels (Figure 6A, top row), as expected. We show the lack of effect of Aβ42 on preactivated Akt1 levels and activity at various doses in Figure 6A, lanes 4–6.
Figure 6.
Negative effects to reverse established Akt activation by Aβi; extracellular applications. (A) Preactivated Akt is not inhibited by Aβ42. IP Akt1 from control and insulin-treated C2C12 myotubes in direct in vitro kinase assay. Insulin treatment before Akt1 IP showed expected increases in activity and phosphorylation, but was not inhibited by a range of Aβ42 doses (lanes 4–6). R, reverse peptide (42-1); F,Aβ1-42. (B) Preactivated Akt is dephosphorylated and inhibited by PP2A. Aβ42 and scrambled Aβ42 had no effect on insulin-conditioned Akt1 activity, whereas PP2A (0.25 μg/500 μl) did, quantified below. (C) Extracellular Aβ has no effect on Akt signaling. C2C12 myotubes were bath-exposed to Aβ25-35, reverse Aβ35-25, Aβ1-42, and reverse Aβ42-1 peptides for 24 h (or shorter times, not shown) before insulin (0.1 μg/ml, 15 min) treatments. Levels of endogenous p-Akt (Ser473), total Akt, p-GSK-3α/β, and total GSK-3β in whole cell extracts remain unchanged in the presence of extracellular Aβ. Insulin stimulation significantly increased levels of Akt activation (lane 2). (D) Insulin responsiveness unaffected by extracellular Aβ. C2C12 myotube cultures were exposed (15 min) to increasing doses of insulin at [Aβ1-42] = 0, 10, and 25 μM (2 h). Harvested cell extracts were fractionated by PAGE (10%) and developed for p-Akt (Thr308). (E) Phosphorylation status of PDK (pSer241). Insulin treatment did not further increase constitutively phosphorylated PDK (Ser241; top and bottom, lanes 1 and 2). Myotube cultures were exposed to insulin (500 ng/ml, 30 min) after either 2- or 24-h treatments of Aβ42 (1–100 nM).
We next tested if Aβ inhibits Akt activity or phosphorylation state (once established through the action of insulin) in side-to-side comparisons with a known Akt phosphatase, PP2A (Ugi et al., 2004). Neither scrambled nor forward Aβ peptide affected Akt p-Ser473 levels or activity (Figure 6B, lanes 2 and 3 vs. 1), whereas PP2A treatment decreased both as expected (lane 4 and graph below). Thus, Aβ does not have intrinsic phosphatase action.
The prior cell-based and in vitro experiments are based on intracellular Aβ effects. To determine this specificity, cell cultures were pretreated with extracellular Aβ peptides for 24 h. In the last 30 min before harvest, we added insulin (0.1 μg/ml). Although p-Akt (Ser473) and p-GSK-3α/β levels were increased by insulin treatment (Figure 6C, lane 2 vs. 1), they were not affected by extracellular Aβ treatments. Moreover, when the insulin response was measured in detail at varying doses, Aβ concentrations in the media up to 25 μM did not affect p-Akt (Thr308) levels (Figure 6D). Constitutive levels of PDK phosphorylated on S241 also remain unchanged by Aβ (Figure 6E, bottom lane). These experiments suggest that Aβi specifically interferes with the action of PDK to phosphorylate Akt.
In Vitro PI3K Activity Is Not Affected by the Addition of Aβ
Akt and PDK interactions are hosted by the second-messenger lipid PIP3. Results of Figure 4, B and C, suggest tethered and added PIP3 concentrations that although helpful to activation, are not limiting in the presence of Aβ. However, it remained possible that Aβ-induced interference with Akt activation involves a reduction in its critical availability. To test this, we grew NIH-3T3 cells to confluence and immunoprecipitated PI3K activity. PI was used as substrate in a PI3K activity assay (Figure 7A). We chose a 10-min incubation for subsequent experiments. Aβ reverse (42-1) and forward (1-42) peptides were tested in doses shown in Figure 7B. Aβ additions had no effect on PI3K activity, whereas the compound LY294002 completely abolished it (Figure 7B). Addition of PLA2, an enzyme that converts phospholipids (including PIP3 and PIP) into constituent fatty acids and other lipophilic substances, also showed the expected decrease in PI3P levels, whereas Aβ42 had no effect (Figure 7C). Recombinant PTEN blocked PI3K activity by 50%, whereas Aβ42 (10 μM) and scrambled Aβ (10 μM) did not (Figure 7D). We conclude that Aβ does not interfere with the activation or activity of PI3K to produce PI3P.
Figure 7.
Aβi expression and PI3K activity. (A) PI3K assay. IP PI3K was added to PI in the presence of [γ-32P]ATP. Phopholipids were extracted and separated by TLC. Below, results quantified over time. (B) PI3K activity maintained in the presence of Aβ. PI3K assay in the presence of the inhibitor LY294002 (LY, 100 μM), Aβ42-1 (5 μM), or increasing Aβ1-42 (0.05, 0.5, and 5 μM). (C) Dephosphorylation of PI3P by phospholipase A2 (PLA2) but not Aβ42. PLA2 (500 μg/ml) or Aβ42 (10 μM) were added just before extraction in CHCl3/methanol. (D) PTEN, but not Aβ, inhibits PI3K. PI3K activity was determined in the presence of PTEN (10 μg/ml) or Aβ42 (10 μM). The phosphatase inhibited PI3K activity ∼50%; Aβ42 (10 μM) had no effect. Scrambled peptide (Scr; 10 μM) is control.
DISCUSSION
Epidemiologic data that type II diabetes and peripheral insulin resistance are risk factors for AD implicate down-regulation of insulin signaling in AD. Decreased IR expression, IR desensitization, and/or tyrosine kinase inactivity are reported in AD brain (Hoyer, 2004; Steen et al., 2005). Defects in IGF-I receptor and an increase in the phospho-inactive form of IRS-1/2 in AD have recently been found, further indicating resistance to IGF-I signaling (Moloney et al., 2008). We however, did not find any change in levels of either IR or in protein interactions involving its adaptor-substrate, IRS-1, in whole brain tissue lysates. Apart from these differences, whether insulin deregulation is a causative factor in AD pathogenesis or a sideshow is debated (Gasparini et al., 2002), and the mechanism by which Aβ might cause this has received little attention.
Insulin and IGF-I signaling is transduced through the PI3K/Akt pathway and is vital to the metabolism and survival of neurons and muscle. Akt deactivation is a well-recognized mediator of oxidative and excitotoxic neuronal death (Luo et al., 2003). In a search for alternative mechanisms of insulin resistance to the defect in proximal components, we discovered that Akt activation itself is compromised. Furthermore, it is concluded here that this step is a target of intracellular Aβ.
With regard to the extracellular pool of Aβ, one study showed that application of soluble Aβ inhibits insulin-IR binding (Xie et al., 2002). In two other reports, extracellular Aβ oligomers (ADDL) caused losses in surface IR number and/or reductions in insulin-induced IR phosphorylation in neurons (Townsend et al., 2007; Zhao et al., 2008). These studies did not report on intracellular Aβ effects nor give evidence in brain as shown in Figure 1. Our results detail a novel mechanism through which intracellular Aβ disrupts insulin-Akt signaling in neurons and muscle. Notably, when we tested extracellular applied Aβ on insulin-stimulated myotubes in culture, it did not provoke any changes in Akt phosphorylation, Akt activity, or levels of activated PDK. The mechanisms by which applied Aβ induces apoptosis in cultured neuronal and nonneuronal cells are several including activation of cell death pathways (e.g., p53) and/or inhibition of other protective pathways, e.g., mitogen-activated protein kinase (Tong et al., 2004). One study overcame such toxicity with IGF-I treatments (Wei et al., 2002).
We first give evidence that p-Akt levels and activity are decreased in our AD brain specimens, consistent with the concept that Alzheimer's disease is a multifaceted insulin resistance state (Craft and Watson, 2004; de la Monte et al., 2006). Even on this point, there are studies indicating mixed or even opposite results. In AD temporal cortex, Griffin et al., 2005 showed that p-Akt (Ser473) protein levels were increased in particulate fractions and moderately decreased in cytosolic fractions, relative to total. Immunohistochemical levels of p-Akt were depleted in the cytosol of hippocampal neurons. Although Akt substrates were examined, no activity assay was reported. Rickle et al. (2004) did examine Akt enzyme activities and found them to be generally higher than control, but the amount of Akt precipitated was highly variable between cases. Finally, Pei et al. (2003) showed that p-Akt (Thr308) levels were increased in the frontal cortex of AD patients compared with normal and Huntington's disease controls, but total Akt levels were not given. Our results are most consistent with Steen et al. (2005), who also used detergent-soluble total brain lysates to show reductions in p-Akt and p-GSK-3 levels relative to total protein in AD brain. Differences in tissue handling and solubilization conditions, including detergent use, may thus contribute to the variable findings above. Additionally, we now show reductions in interaction between Akt and PDK (Figure 1B) to explain our insulin resistance results.
A search for other abnormalities more proximally found no major changes in the binding of IRS-1 to the IR α2β2 homodimer subunits or in the association of the SH2 domain–bearing PI3K p85 catalytic subunit with IRS-1. These end points reflect an unaltered IRS-1 activation state. Using a different endpoint, Moloney et al. (2008) found an increase in the inactive p-IRS form to total IRS ratio. However, this result does not prove that the transduction activity to PI3K is functionally lost (see Figure 8), and the activated phospho-IRS form was not reported. Phosphotyrosine modification of the IR also showed no major differences between AD and control in our hands, consistent with the expectation that changes in these proximal components should track together in the same direction if functionally linked (Steen et al., 2005).
To dissect the role of intracellular Aβ in the disruption of insulin signaling, we turned to experimental cell culture and in vitro conditions. Both skeletal muscle-like myotubes, because they are highly responsive to insulin, and SH-SY5Y cells were utilized. Aβ42 expression in C2C12 myotubes was cytotoxic (Figure 2B) and resulted in a modest inhibition of Akt phosphorylation, consistent with results in primary neurons (Magrané et al., 2005). The effect was more evident when Aβ expression preceded signal amplification with insulin (Figure 2C), suggesting interference with some forward step. Even more striking was the functional consequence, a severe inhibition of Akt activity. As in brain, no changes in phospho-tyrosine IR levels were produced in culture (Figure 2D).
Switching to an in vitro format and after validating the stimulation of Akt activity by PDK, we show a consistent reversal after additions of Aβ1-42. Oligomeric species of Aβ (ADDL) were more toxic to Akt activation than monomer predominant or fibril-containing preparations (summarized in Figure 4A). The IC50 for the monomer preparations of Aβ42 is 1–5 (∼2) μM in activity assays and ∼10 μM in Western blots of p-Akt levels. The more robust inhibition in the activity assay than when immunoblotting for phosphorylation levels was also shown in the cell-based experiments. This difference suggests that activity is steeply dependent on phosphorylation level. For the oligomer preparations, intracellular concentrations of Aβ42 in various AD models are difficult to come by; however, a range of 100 nM to a low micromolar concentration has been suggested, probably depending on the specific compartment (Gouras et al., 2000; Zhang et al., 2002; Magrane et al., 2005). Previous works on synaptic transmission and cell viability have detailed the heightened toxicity of oligomeric amyloid species (Lambert et al., 1998; Walsh et al., 2002; DeFelice et al., 2008). These studies used concentrations 0.5–5 μM, similar to those we estimate in our ADDL-based in vitro assays.
The lipid second-messenger PIP3 is necessary to the efficient activation of Akt by PDK. Importantly, Aβ addition greatly attenuated Akt activation in the presence or absence of added PIP3 (Figure 4, B and C), eliminating sequestration of/or competition with this lipid as a mechanism. To be clear on this, we tested oligomeric Aβ against the PDK-dependent, but PIP3-independent, stimulation of the SGK (Kobayashi and Cohen, 1999) and found a similar interruption (Supplemental Figure S3A). These together with control experiments using Rictor and PKA and the previously shown effect of myristoylated Akt to overcome Aβi (Magrane et al., 2005), all strongly suggest that Aβi inhibits the action of PDK to activate Akt independent of the lipid-PH domain modification.
Definitive evidence on the remaining possibility that Aβ inhibits p85-PI3K function to convert membrane phosphoinositides to 3′ phosphorylated second messengers was sought by directly measuring PI3K activity. Aβ additions had no effect on PIP3 generation, making it unlikely that Aβ interferes with PIP3 production or PH binding in vivo.
From the AD brain sample studies, it appeared highly plausible that Aβ interferes with the activation reaction by directly preventing the interaction between PDK and Akt. We confirmed the decrease in their association in a cell-based, Aβ expression assay, as well as when cell extracts were mixed in vitro in the presence of synthetic Aβ42. Importantly, we established that Aβ does not dephosphorylate or otherwise inactivate Akt once this has occurred.
In conclusion, our data point to the specific action of intracellular Aβ to interrupt insulin signaling by inhibiting PDK activity. The likely mechanism is via interference of binding with its target, Akt (Figure 8). Intracellular Aβ accumulation, particularly oligomeric forms (Takahashi et al., 2004) is an early marker of AD pathology (Gouras et al., 2005; Oakley et al., 2006), predicting from these results that insulin resistance could soon follow. Prevention of resistance to insulin signaling in AD and even strategies to up-regulate it may have the added beneficial effects to limit cellular Aβ production by reducing γ secretase action or promoting IDE activity and APP transport mechanisms (Phiel et al., 2003; Ho et al., 2004). Two recent trials of rosiglitazone, a PPARγ agonist and insulin sensitizer, showed positive trends in AD patients (Guo and Tabrizchi, 2006; Risner et al., 2006). Our findings suggest that a pharmacologic approach to reverse the blockade of Akt docking onto PDK or on PDK catalytic action may dramatically improve insulin signaling in AD brain.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Dr. Dennis Selkoe for R1282 and Dr. Peter Davies (Albert Einstein College of Medicine, New York, NY) for PHF-1 antibodies. We thank Dr. Lucia Rameh (BBRI) and Dr. Ji Luo and Tina Yuan of Dr. L. Cantley's laboratory (Beth Israel Hospital, Boston, MA) for technical advice on optimization of the PI3K activity assay. This work was supported in part by National Institutes of Health (NIH) Grant NINDS 41373 and by the Bennett Charitable Foundation. We thank the Harvard Brain Tissue Resource Center, supported in part by PHS Grant R24-MH068855, for rapid frozen adult human brain samples.
Abbreviations used:
- LY
LY294002
- PP2A
protein phosphatase 2A
- PLA2
phospholipase A2.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-07-0777) on January 14, 2009.
REFERENCES
- Alessi D. R., Cohen P. Mechanism of activation and function of protein kinase B. Curr. Opin. Genet. Dev. 1998;8:55–62. doi: 10.1016/s0959-437x(98)80062-2. [DOI] [PubMed] [Google Scholar]
- Andjelkovic M., Alessi D. R., Meier R., Fernandez A., Lamb N. J., Frech M., Cron P., Cohen P., Lucocq J. M., Hemmings B. A. Role of translocation in the activation and function of protein kinase B. J. Biol. Chem. 1997;272:31515–31524. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- Asano T., Yao Y., Shin S., McCubrey J., Abbruzzese J. L., Reddy S. A. Insulin receptor substrate is a mediator of phosphoinositide 3-kinase activation in quiescent pancreatic cancer cells. Cancer Res. 2005;65:9164–9168. doi: 10.1158/0008-5472.CAN-05-0779. [DOI] [PubMed] [Google Scholar]
- Bondy C. A., Cheng C. M. Signaling by insulin-like growth factor 1 in brain. Eur. J. Pharmacol. 2004;490:25–31. doi: 10.1016/j.ejphar.2004.02.042. [DOI] [PubMed] [Google Scholar]
- Brunet A., Datta S. R., Greenberg M. E. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr. Opin. Neurobiol. 2001;11:297–305. doi: 10.1016/s0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- Conway K. A., Lee S. J., Rochet J. C., Ding T. T., Williamson R. E., Lansbury P. T., Jr. Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson's disease: implications for pathogenesis and therapy. Proc. Natl. Acad. Sci. USA. 2000;97:571–576. doi: 10.1073/pnas.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft S., Watson G. S. Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol. 2004;3:169–178. doi: 10.1016/S1474-4422(04)00681-7. [DOI] [PubMed] [Google Scholar]
- Cross D. A., Alessi D. R., Cohen P., Andjelkovich M., Hemmings B. A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Datta S. R., Dudek H., Tao X., Masters S., Fu H., Gotoh Y., Greenberg M. E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- De Felice F. G., et al. Alzheimer's disease-type neuronal tau hyperphosphorylation induced by A beta oligomers. Neurobiol. Aging. 2008;29:1334–1347. doi: 10.1016/j.neurobiolaging.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte S. M., Tong M., Lester-Coll N., Plater M., Jr., Wands J. R. Therapeutic rescue of neurodegeneration in experimental type 3 diabetes: relevance to Alzheimer's disease. J. Alzheimers Dis. 2006;10:89–109. doi: 10.3233/jad-2006-10113. [DOI] [PubMed] [Google Scholar]
- Emamian E. S., Hall D., Birnbaum M. J., Karayiorgou M., Gogos J. A. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat. Genet. 2004;36:131–137. doi: 10.1038/ng1296. [DOI] [PubMed] [Google Scholar]
- Fujio Y., Walsh K. Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. J. Biol. Chem. 1999;274:16349–16354. doi: 10.1074/jbc.274.23.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini L., Netzer W. J., Greengard P., Xu H. Does insulin dysfunction play a role in Alzheimer's disease? Trends Pharmacol. Sci. 2002;23:288–293. doi: 10.1016/s0165-6147(02)02037-0. [DOI] [PubMed] [Google Scholar]
- Gouras G. K., Almeida C. G., Takahashi R. H. Intraneuronal Abeta accumulation and origin of plaques in Alzheimer's disease. Neurobiol. Aging. 2005;26:1235–1244. doi: 10.1016/j.neurobiolaging.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Gouras G. K., et al. Intraneuronal Abeta42 accumulation in human brain. Am. J. Pathol. 2000;156:15–20. doi: 10.1016/s0002-9440(10)64700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin R. J., Moloney A., Kelliher M., Johnston J. A., Ravid R., Dockery P., O'Connor R., O'Neill C. Activation of Akt/PKB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and PTEN are features of Alzheimer's disease pathology. J. Neurochem. 2005;93:105–117. doi: 10.1111/j.1471-4159.2004.02949.x. [DOI] [PubMed] [Google Scholar]
- Guo L., Tabrizchi R. Peroxisome proliferator-activated receptor gamma as a drug target in the pathogenesis of insulin resistance. Pharmacol. Ther. 2006;111:145–173. doi: 10.1016/j.pharmthera.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Ho L., et al. Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer's disease. FASEB J. 2004;18:902–904. doi: 10.1096/fj.03-0978fje. [DOI] [PubMed] [Google Scholar]
- Horwood J. M., Dufour F., Laroche S., Davis S. Signalling mechanisms mediated by the phosphoinositide 3-kinase/Akt cascade in synaptic plasticity and memory in the rat. Eur J. Neurosci. 2006;23:3375–3384. doi: 10.1111/j.1460-9568.2006.04859.x. [DOI] [PubMed] [Google Scholar]
- Hoyer S. Causes and consequences of disturbances of cerebral glucose metabolism in sporadic Alzheimer disease: therapeutic implications. Adv. Exp. Med. Biol. 2004;541:135–152. doi: 10.1007/978-1-4419-8969-7_8. [DOI] [PubMed] [Google Scholar]
- Hresko R. C., Mueckler M. mTOR.RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3–L1 adipocytes. J. Biol. Chem. 2005;280:40406–40416. doi: 10.1074/jbc.M508361200. [DOI] [PubMed] [Google Scholar]
- Hsiao K., Chapman P., Nilsen S., Eckman C., Harigaya Y., Younkin S., Yang F., Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Klein W. L. Abeta toxicity in Alzheimer's disease: globular oligomers (ADDLs) as new vaccine and drug targets. Neurochem. Int. 2002;41:345–352. doi: 10.1016/s0197-0186(02)00050-5. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Cohen P. Activation of serum- and glucocorticoid-regulated protein kinase by agonists that activate phosphatidylinositide 3-kinase is mediated by 3-phosphoinositide-dependent protein kinase-1 (PDK1) and PDK2. Biochem. J. 1999;339(Pt 2):319–328. [PMC free article] [PubMed] [Google Scholar]
- LaFerla F. M., Green K. N., Oddo S. Intracellular amyloid-beta in Alzheimer's disease. Nat. Rev. Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- Lambert M. P., et al. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. C., Huang C. C., Wu M. Y., Hsu K. S. Insulin stimulates postsynaptic density-95 protein translation via the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway. J. Biol. Chem. 2005;280:18543–18550. doi: 10.1074/jbc.M414112200. [DOI] [PubMed] [Google Scholar]
- Li M., Wang X., Meintzer M. K., Laessig T., Birnbaum M. J., Heidenreich K. A. Cyclic AMP promotes neuronal survival by phosphorylation of glycogen synthase kinase 3beta. Mol. Cell. Biol. 2000;20:9356–9363. doi: 10.1128/mcb.20.24.9356-9363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H. R., Hattori H., Hossain M. A., Hester L., Huang Y., Lee-Kwon W., Donowitz M., Nagata E., Snyder S. H. Akt as a mediator of cell death. Proc. Natl. Acad. Sci. USA. 2003;100:11712–11717. doi: 10.1073/pnas.1634990100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrane J., Rosen K. M., Smith R. C., Walsh K., Gouras G. K., Querfurth H. W. Intraneuronal beta-amyloid expression downregulates the Akt survival pathway and blunts the stress response. J. Neurosci. 2005;25:10960–10969. doi: 10.1523/JNEUROSCI.1723-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelli A. M., Faenza I., Billi A. M., Manzoli L., Evangelisti C., Fala F., Cocco L. Intranuclear 3′-phosphoinositide metabolism and Akt signaling: new mechanisms for tumorigenesis and protection against apoptosis? Cell Signal. 2006;18:1101–1107. doi: 10.1016/j.cellsig.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Messier C., Teutenberg K. The role of insulin, insulin growth factor, and insulin-degrading enzyme in brain aging and Alzheimer's disease. Neural Plast. 2005;12:311–328. doi: 10.1155/NP.2005.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney A. M., Griffin R. J., Timmons S., O'Connor R., Ravid R., O'Neill C. Defects in IGF-I receptor, insulin receptor and IRS-1/2 in Alzheimer's disease indicate possible resistance to IGF-I and insulin signalling. Neurobiol. Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.04.002. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Oakley H., et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J. Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C. R., Seeley R. J., Craft S., Woods S. C. Intracerebroventricular insulin enhances memory in a passive-avoidance task. Physiol. Behav. 2000;68:509–514. doi: 10.1016/s0031-9384(99)00220-6. [DOI] [PubMed] [Google Scholar]
- Pei J. J., et al. Role of protein kinase B in Alzheimer's neurofibrillary pathology. Acta Neuropathol. 2003;105:381–392. doi: 10.1007/s00401-002-0657-y. [DOI] [PubMed] [Google Scholar]
- Phiel C. J., Wilson C. A., Lee V. M., Klein P. S. GSK-3alpha regulates production of Alzheimer's disease amyloid-beta peptides. Nature. 2003;423:435–439. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- Rickle A., Bogdanovic N., Volkman I., Winblad B., Ravid R., Cowburn R. F. Akt activity in Alzheimer's disease and other neurodegenerative disorders. Neuroreport. 2004;15:955–959. doi: 10.1097/00001756-200404290-00005. [DOI] [PubMed] [Google Scholar]
- Risner M. E., Saunders A. M., Altman J. F., Ormandy G. C., Craft S., Foley I. M., Zvartau-Hind M. E., Hosford D. A., Roses A. D. Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer's disease. Pharmacogenom. J. 2006;6:246–254. doi: 10.1038/sj.tpj.6500369. [DOI] [PubMed] [Google Scholar]
- Sarbassov D. D., Guertin D. A., Ali S. M., Sabatini D. M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Steen E., Terry B. M., Rivera E. J., Cannon J. L., Neely T. R., Tavares R., Xu X. J., Wands J. R., de la Monte S. M. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease—is this type 3 diabetes? J. Alzheimers Dis. 2005;7:63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- Stine W. B., Jr., Dahlgren K. N., Krafft G. A., LaDu M. J. In vitro characterization of conditions for amyloid-beta peptide oligomerization and fibrillogenesis. J. Biol. Chem. 2003;278:11612–11622. doi: 10.1074/jbc.M210207200. [DOI] [PubMed] [Google Scholar]
- Takahashi R. H., Almeida C. G., Kearney P. F., Yu F., Lin M. T., Milner T. A., Gouras G. K. Oligomerization of Alzheimer's beta-amyloid within processes and synapses of cultured neurons and brain. J. Neurosci. 2004;24:3592–3599. doi: 10.1523/JNEUROSCI.5167-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier M., Woodgett J. R. Serum and glucocorticoid-regulated protein kinases: variations on a theme. J. Cell Biochem. 2006;98:1391–1407. doi: 10.1002/jcb.20894. [DOI] [PubMed] [Google Scholar]
- Toker A., Newton A. C. Akt/protein kinase B is regulated by autophosphorylation at the hypothetical PDK-2 site. J. Biol. Chem. 2000;275:8271–8274. doi: 10.1074/jbc.275.12.8271. [DOI] [PubMed] [Google Scholar]
- Tong L., Balazs R., Thornton P. L., Cotman C. W. Beta-amyloid peptide at sublethal concentrations downregulates brain-derived neurotrophic factor functions in cultured cortical neurons. J. Neurosci. 2004;24:6799–6809. doi: 10.1523/JNEUROSCI.5463-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend M., Mehta T., Selkoe D. J. Soluble Abeta inhibits specific signal transduction cascades common to the insulin receptor pathway. J. Biol. Chem. 2007;282:33305–33312. doi: 10.1074/jbc.M610390200. [DOI] [PubMed] [Google Scholar]
- Ugi S., Imamura T., Maegawa H., Egawa K., Yoshizaki T., Shi K., Obata T., Ebina Y., Kashiwagi A., Olefsky J. M. Protein phosphatase 2A negatively regulates insulin's metabolic signaling pathway by inhibiting Akt (protein kinase B) activity in 3T3–L1 adipocytes. Mol. Cell. Biol. 2004;24:8778–8789. doi: 10.1128/MCB.24.19.8778-8789.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandermoere F., El Yazidi-Belkoura I., Demont Y., Slomianny C., Antol J., Lemoine J., Hondermarck H. Proteomics exploration reveals that actin is a signaling target of the kinase Akt. Mol. Cell Proteomics. 2007;6:114–124. doi: 10.1074/mcp.M600335-MCP200. [DOI] [PubMed] [Google Scholar]
- Walsh D. M., Klyubin I., Fadeeva J. V., Rowan M. J., Selkoe D. J. Amyloid-beta oligomers: their production, toxicity and therapeutic inhibition. Biochem. Soc. Trans. 2002;30:552–557. doi: 10.1042/bst0300552. [DOI] [PubMed] [Google Scholar]
- Wang J., Tung Y. C., Wang Y., Li X. T., Iqbal K., Grundke-Iqbal I. Hyperphosphorylation and accumulation of neurofilament proteins in Alzheimer disease brain and in okadaic acid-treated SY5Y cells. FEBS Lett. 2001;507:81–87. doi: 10.1016/s0014-5793(01)02944-1. [DOI] [PubMed] [Google Scholar]
- Wei W., Wang X., Kusiak J. W. Signaling events in amyloid beta-peptide-induced neuronal death and insulin-like growth factor I protection. J. Biol. Chem. 2002;277:17649–17656. doi: 10.1074/jbc.M111704200. [DOI] [PubMed] [Google Scholar]
- Whitman M., Kaplan D. R., Schaffhausen B., Cantley L., Roberts T. M. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature. 1985;315:239–242. doi: 10.1038/315239a0. [DOI] [PubMed] [Google Scholar]
- Xie L., Helmerhorst E., Taddei K., Plewright B., Van Bronswijk W., Martins R. Alzheimer's beta-amyloid peptides compete for insulin binding to the insulin receptor. J. Neurosci. 2002;22:1–5. doi: 10.1523/JNEUROSCI.22-10-j0001.2002. RC221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., McLaughlin R., Goodyer C., LeBlanc A. Selective cytotoxicity of intracellular amyloid beta peptide1-42 through p53 and Bax in cultured primary human neurons. J. Cell Biol. 2002;156:519–529. doi: 10.1083/jcb.200110119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W. Q., De Felice F. G., Fernandez S., Chen H., Lambert M. P., Quon M. J., Krafft G. A., Klein W. L. Amyloid beta oligomers induce impairment of neuronal insulin receptors. FASEB J. 2008;22:246–260. doi: 10.1096/fj.06-7703com. [DOI] [PubMed] [Google Scholar]
- Zheng W. H., Kar S., Quirion R. Insulin-like growth factor-1-induced phosphorylation of the forkhead family transcription factor FKHRL1 is mediated by Akt kinase in PC12 cells. J. Biol. Chem. 2000;275:39152–39158. doi: 10.1074/jbc.M002417200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.