Abstract
Oculocutaneous albinism type 2 is caused by defects in the gene OCA2, encoding a pigment cell-specific, 12-transmembrane domain protein with homology to ion permeases. The function of the OCA2 protein remains unknown, and its subcellular localization is under debate. Here, we show that endogenous OCA2 in melanocytic cells rapidly exits the endoplasmic reticulum (ER) and thus does not behave as a resident ER protein. Consistently, exogenously expressed OCA2 localizes within melanocytes to melanosomes, and, like other melanosomal proteins, localizes to lysosomes when expressed in nonpigment cells. Mutagenized OCA2 transgenes stimulate melanin synthesis in OCA2-deficient cells when localized to melanosomes but not when specifically retained in the ER, contradicting a proposed primary function for OCA2 in the ER. Steady-state melanosomal localization requires a conserved consensus acidic dileucine-based sorting motif within the cytoplasmic N-terminal region of OCA2. A second dileucine signal within this region confers steady-state lysosomal localization in melanocytes, suggesting that OCA2 might traverse multiple sequential or parallel trafficking routes. The two dileucine signals physically interact in a differential manner with cytoplasmic adaptors known to function in trafficking other proteins to melanosomes. We conclude that OCA2 is targeted to and functions within melanosomes but that residence within melanosomes may be regulated by secondary or alternative targeting to lysosomes.
INTRODUCTION
Melanin pigments are synthesized by specialized cell types, including dermal and epidermal melanocytes and retinal pigment epithelial cells, within unique organelles known as melanosomes (Raposo and Marks, 2007). Melanosomes are members of a class of tissue-specific “lysosome-related organelles” characterized by an acidic lumenal pH and the presence of some lysosomal proteins (Dell'Angelica et al., 2000; Griffiths, 2002). Among lysosome-related organelles, melanosomes represent a subclass that coexists with bona fide lysosomes in their host cells (Raposo et al., 2007). Melanosomes are distinguished from lysosomes by the presence of cell–type-specific cargo proteins that confer unique functional and morphological properties. How these cargo proteins are diverted from traditional lysosomes and delivered to and maintained within melanosomes is incompletely understood, and the degree of cargo cross-talk between melanosomes and lysosomes is not known.
Melanosomes undergo a program of maturation within melanocytes by the ordered delivery of cargoes to nascent melanosomes via specialized trafficking pathways (Raposo and Marks, 2007). Some components of the melanosomal trafficking machinery are known, largely from analyses of the sorting of well-characterized cargo proteins, such as the melanogenic enzyme tyrosinase (Tyr) and tyrosinase-related protein 1 (Tyrp1), in both wild-type melanocytes and melanocytes derived from patients or mouse models of genetic hypopigmentary diseases such as Hermansky–Pudlak Syndrome (HPS) (Di Pietro and Dell'Angelica, 2005; Wei, 2006). Tyr and Tyrp1 contain cytoplasmic acidic dileucine motifs that are required for melanosomal sorting (Vijayasaradhi et al., 1995; Calvo et al., 1999; Simmen et al., 1999) and that are bound within endosomal intermediates by heterotetrameric adaptor proteins (APs) AP-1 or AP-3. AP-1 and AP-3 each bind the Tyr sorting signal (Honing et al., 1998; Theos et al., 2005), but they participate in distinct delivery pathways toward melanosomes (Theos et al., 2005). Consistently, Tyr is largely (but not completely) missorted in melanocytes derived from human HPS type 2 patients and HPS model pearl mice that bear mutations in the gene encoding the β3A subunit of AP-3 (Huizing et al., 2001; Theos et al., 2005). By contrast, the acidic dileucine-based sorting signal in Tyrp1 has been shown to bind AP-1 but not AP-3 (Theos et al., 2005), and accordingly Tyrp1 accumulates normally on melanosomes in AP-3–deficient melanocytes (Huizing et al., 2001; Setty et al., 2007) (although an unusually large cohort cycles through the plasma membrane; Di Pietro et al., 2006). These results corroborate the dependence on AP-1 and AP-3, respectively, for in vitro budding of Tyrp1 and tyrosinase from Golgi/endosomal membrane fractions (Chapuy et al., 2008). Interestingly, both lysosomal and melanosomal proteins depend on AP-1 and AP-3 for their trafficking, and it is not known how trafficking to these distinct organelles in melanocytes is distinguished. Additional components that influence Tyr and Tyrp1 trafficking to melanosomes include the biogenesis of lysosome-related organelles complex (BLOC)-1 and BLOC-2, subunits of which are defective in other forms of HPS, and the tissue-specific Rab proteins Rab32 and Rab38 (Richmond et al., 2005; Di Pietro et al., 2006; Wasmeier et al., 2006; Helip-Wooley et al., 2007; Setty et al., 2007). Tyrp1 and Tyr trafficking toward melanosomes or lysosomes is also regulated by lumenal interactions with glycosphingolipid-dependent membrane microdomains, presumably on endosomes (Sprong et al., 2001; Groux-Degroote et al., 2008).
The mechanisms regulating the delivery of other melanosomal proteins to melanosomes are less understood. Pmel17, a component of the fibrillar melanosome matrix on which melanins deposit, becomes incorporated into early stage melanosomes in a manner that seems to be unaffected in most, if not all, forms of HPS. Pmel17 is first delivered to endosomes by a dileucine-based internalization motif (Theos et al., 2006a) and is subsequently sorted to the internal membranes of multivesicular endosomes in a step that precedes and is required for fibril formation (Hoashi et al., 2006; Theos et al., 2006b). This latter step requires a lumenal determinant within Pmel17 but does not require components of the classical multivesicular body sorting machinery such as Hrs and endosomal sorting complex required for transport subunits (Theos et al., 2006b). The G protein-coupled receptor OA1 is targeted to melanosomes by a distinct class of sorting signal (Piccirillo et al., 2006), but the effectors that regulate its sorting are not known.
OCA2, also called pink-eyed dilution or P protein, is an enigmatic protein with a critical function in pigmentation. The OCA2 gene is mutated in oculocutaneous albinism (OCA) type 2, one of the most common forms of human albinism (Brilliant, 2001). In addition, nonpathological polymorphisms in both coding and noncoding regions of the OCA2 gene have been implicated as major determinants of skin color (Lao et al., 2007; Norton et al., 2007; Sulem et al., 2007), and variation in OCA2 expression through a polymorphism in an adjacent gene, HERC2, is thought to underlie blue eye color in humans (Eiberg et al., 2008; Sturm et al., 2008). OCA type 2 patients exhibit severe hypopigmentation of the skin, hair, and eyes. Defects in the corresponding murine gene (p) give rise to the pink-eyed dilution mouse (Rinchik et al., 1993), which has similar eye and coat hypopigmentation. Melanocytes from this model mouse contain melanosomes that are small, immature, and hypopigmented relative to normal melanosomes (Sidman et al., 1965; Rosemblat et al., 1998). Although these features clearly implicate OCA2 as being critical for pigmentation, the molecular function of OCA2 is still unclear. Its sequence predicts that OCA2 is a 12-transmembrane domain protein with homology to a superfamily of permeases (Rinchik et al., 1993; Lee et al., 1995). However, no transport substrate has yet been identified.
Not only is OCA2 function not known, but its subcellular site of action is also debated. OCA2 was originally thought to localize predominantly to mature melanized melanosomes based on subcellular fractionation of melanocytes (Rosemblat et al., 1994), poor extraction by detergent from melanized melanocytes relative to nonmelanized melanocytes (Donatien and Orlow, 1995), and interpretation of results from confocal immunofluorescence microscopy (IFM) analyses (Toyofuku et al., 2002). Tyrp1-containing compartments in melanocytes from OCA2-deficient mice are less acidic than in wild-type melanocytes, suggesting that OCA2 not only localizes to melanosomes but also modulates their pH (Puri et al., 2000), although this interpretation is disputed because Tyrp1 does not localize to melanosomes properly in OCA2-deficient melanocytes (Manga et al., 2001). The possibility that melanosomal pH might be affected by OCA2 deficiency is supported by an observed increase in melanin synthesis upon neutralization of organellar pH in otherwise hypopigmented OCA2-deficient melanocytes (Ancans et al., 2001). Other evidence, however, suggests that OCA2 localizes to and functions from the endoplasmic reticulum (ER). OCA2-immunoreactive subcellular fractions from melanocytes contain ER markers as well as melanin, and OCA2 colocalized extensively with ER markers by indirect IFM (Chen et al., 2002). Moreover, cells that lack OCA2 show a reduction in terminal glycosylation of Tyr (Chen et al., 2002; Toyofuku et al., 2002), suggesting that OCA2 activity might provide an optimal environment within the ER to facilitate Tyr folding. Thus far, this controversy has not been resolved.
To resolve this debate and extend our understanding of melanosomal protein trafficking, we attempted to clarify the subcellular localization and site of action of OCA2 and to define the determinants required for its localization. Our results demonstrate that human OCA2 is rapidly processed by Golgi enzymes, suggesting that it is not a resident ER protein. Consistently, ectopically expressed OCA2 localizes predominantly to melanosomes in a manner that depends on a cytoplasmic dileucine-based sorting signal, similar to those found in Tyr and Tyrp1. A second dileucine-based signal in human OCA2 facilitates steady-state lysosomal but not melanosomal localization. We further show that melanosomal localization of OCA2 correlates with its function in supporting melanin synthesis. These results strongly suggest that OCA2 is active in melanosomes and raise the possibility that OCA2 activity may be limited by additional sorting to lysosomes.
MATERIALS AND METHODS
Chemicals
All chemicals were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise specified.
Cell Culture and Transgene Expression
All culture reagents were purchased from Invitrogen (Carlsbad, CA) unless stated otherwise. All cells were grown in media that included 1% penicillin/streptomycin and incubated at 37°C and 10% CO2 unless stated otherwise. HeLa cells were grown in DMEM supplemented with 10% fetal bovine serum (HyClone Laboratories, Logan, UT). Immortalized mouse melanocyte cell lines melan-Ink4a (Sviderskaya et al., 2002) and melan-p1 (Sviderskaya et al., 1997) were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA) and 200 nM phorbol 12-myristate-13-acetate. MNT-1 human melanoma cells (Cuomo et al., 1991) were grown in DMEM supplemented with 15% fetal bovine serum, 10% AIM-V, 1% nonessential amino acids, 1% sodium pyruvate (Mediatech, Herndon, VA), and antibiotics. Chinese hamster ovary (CHO) cells subclone K1 were a gift from Dr. Monty Krieger (Massachusetts Institute of Technology, Cambridge, MA). Chinese hamster ovary (CHO) cells were grown in α-minimal essential medium with 5% fetal bovine serum (HyClone Laboratories) in 5% CO2. Transfections in HeLa, CHO, and mouse melanocytes were performed using FuGENE-6 (Roche Diagnostics, Indianapolis, IN) according to manufacturer's instructions with 1–2 μg of DNA. Transfections in MNT-1 cells were performed using Lipofectamine 2000 (Invitrogen) according to manufacturer's instructions, with 1.6 μg of DNA and Opti-MEM (Invitrogen).
Construction of OCA2 Expression Plasmids
To clone the full-length human OCA2, a human melanoma library in the Uni-Zap vector (Stratagene, La Jolla, CA) was screened using a 953-base pair probe derived from the central portion of OCA2 cDNA by reverse transcription-polymerase chain reaction (RT-PCR) (with Pfu DNA polymerase; Promega, Madison, WI) from total melanocyte cDNA synthesized with the SuperScript preamplification system kit (Invitrogen). Among several independent positive clones isolated, two contained full-length OCA2 cDNA with an internal deletion of 72 base pairs encompassing transmembrane domain 3 and corresponding to exon 10 of the OCA2 gene; the deleted region was reinserted by subcloning an encompassing unique AccI–HindIII fragment obtained by RT-PCR into AccI–HindIII-digested OCA2 cDNA. The entire cDNA was subcloned into the BamHI and XhoI sites of pCR3 (Invitrogen), and its sequence was confirmed (Bio Molecular Research, DNA sequencing service at the University of Padua, Italy; http://bmr.cribi.unipd.it/). Compared with the originally published sequence (Rinchik et al., 1993), the pCR3/OCA2 contains four silent mismatches and the previously reported nonpathologic polymorphism D257A (Oetting et al., 1998). In most experiments (except those in Figure 1), the entire 5′-untranslated region was replaced by a synthetic Kozak consensus sequence immediately upstream of the OCA2 translational start site. To generate a lumenally exposed epitope-tagged form of OCA2, three consecutive copies of an influenza hemagglutinin epitope tag (3xHA) were introduced into the first lumenal loop as follows. The codons for the unconserved R243P244 residues in this loop were subjected to site-directed mutagenesis by using two-step amplification (Higuchi et al., 1988) with AmpliTaq (Applied Biosystems, Foster City, CA) to generate a BsiWI restriction site (converting P244 to T). The 3xHA tag with a 5′ BsiWI site and a 3′ Acc65I site was amplified by thermal cycling with AmpliTaq from pCI-pallidin-HA (Moriyama and Bonifacino, 2002) and subcloned into the new BsiWI site on OCA2, creating OCA2-HA. To construct OCA2-HMGCR, we amplified a fragment beginning 5′ of the unique BstXI site, encoding the C terminus of human OCA2 fused to the last eight amino acids of human HMG CoA reductase (GACTKKTA), and followed by an XhoI site, and the product was subcloned in place of the BstXI–XhoI fragment of pCR3/OCA2-HA. Mutated forms of OCA2-HMGCR were constructed in a similar manner. To construct OCA2-ΔN91, an oligonucleotide encoding amino acids 92-102 of OCA2 and flanked by BamHI and EcoRI sites was subcloned to replace the BamHI–EcoRI fragment of pCR3/OCA2-HA, excising amino acids 2-91 of OCA2. MHRRR-OCA2-ΔN91 and MHAAA-OCA2-ΔN91 were constructed in the same way using oligonucleotides that encoded the sequences MHRRR (the first five amino acids of the p35 form of human Invariant chain; Schutze et al., 1994) or MHAAA (in which the tribasic ER retention signal is mutated) fused to amino acid 92 of OCA2. To alter the leucine residues within the N-terminal cytoplasmic domain of OCA2, the codons for the indicated pairs of leucine residues were replaced with those for two alanines by site-directed mutagenesis using two-step amplification. Expression vectors for chimeric OCA2-human transferrin receptor (hTfR) proteins were constructed from pCR3-OCA2 and pCDM8-hTfR (Marks et al., 1996) by site-directed mutagenesis using two-step amplification with primers that bridged the junction between the OCA2 N-terminal cytoplasmic domain and the transmembrane domain of human transferrin receptor. The final chimera contained amino acids 1-173 of human OCA2 and amino acids 62-761 of hTfR. To generate the GST fusion proteins, the 5′ end of the OCA2 cDNA, corresponding to the first 162 amino acids, was amplified from pCR3-OCA2 and cloned into the BamHI and SalI sites of the prokaryotic expression vector pGEX5X-1 (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom). Constructs containing the various combinations of mutations at the dileucine motifs were generated by amplification from the corresponding pCR3 vectors. Unless stated otherwise, all recombinant plasmids were verified by automated sequencing by the University of Pennsylvania Cell Center. Details of PCR primers, sequences, and conditions can be provided upon request.
Figure 1.
Human OCA2 is N-glycosylated and is not an ER resident protein. (a and b) Endogenous OCA2 in MNT-1 human melanoma cells (a) or transfected OCA2 in HeLa cells (b) was immunoprecipitated after metabolic labeling and indicated chase. Immunoprecipitates were digested with endoglycosidases, fractionated by SDS-PAGE, and analyzed by phosphorimaging. M, fully mature protein; P, precursor protein with incomplete glycosylation; D, deglycosylated protein; -, mock treatment; H, endoH treatment; F, PNGase F treatment; *, nonspecific band. All markers are indicated in kilodaltons.
Antibodies
Antibodies used were as follows: rat anti-HA 3F10 and mouse B3/25 anti-transferrin receptor were from Roche Diagnostics, TA99 (Mel-5) anti-Tyrp1 was from American Type Culture Collection (Manassas, VA), mouse H4A3 anti-human lysosome-associated membrane protein (LAMP)-1 and rat GL2A7 anti-mouse LAMP-2 were from Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA), rabbit anti-LAMP-1 was from Affinity Bioreagents (Golden, CO), mouse anti-HA 16B12 was from Covance Research Products (Princeton, NJ), mouse MAB3126 anti-calnexin was from Millipore (Billerica, MA), mouse anti-AP-3μ3A (anti-p47A) was from BD Biosciences Transduction Laboratories (Lexington, KY), mouse anti-Rab5 was from Synaptic Systems (Göttingen, Germany), and the mouse monoclonal antibodies 100/3 anti-AP-1γ, 100/2 anti-AP-2α, anti-α-tubulin, and anti-β-actin were from Sigma-Aldrich. The mouse anti-pallidin monoclonal antibody (mAb) 2G5 was described previously (Nazarian et al., 2008). Goat anti-immunoglobulin G (IgG) secondary antibodies conjugated to Alexa-488 and Alexa-594 were from Invitrogen. Donkey anti-IgG secondary antibodies conjugated to 7-amino-4-methylcoumarin-3-acetic acid were from Jackson ImmunoResearch Laboratories (West Grove, PA). The NOCA2 anti-OCA2 antibody was generated by conjugating the first thirteen amino acids of human OCA2 (MHLEGRDGRRYPG) with a C-terminal cysteine to keyhole limpet hemocyanin and immunizing rabbits from Genemed Biosynthesis (San Francisco, CA) with the conjugate. NOCA2 was further affinity purified against the antigenic peptide conjugated to SulfoLink coupling gel (Pierce Chemical, Rockford, IL) according to the manufacturer's instructions.
Glutathione Transferase (GST) Pull-Down Assays
The GST-OCA2 fusion constructs were expressed in Escherichia coli and purified as described previously (Starcevic and Dell'Angelica, 2004). Detergent extracts of MNT-1 or HeLa cells were prepared in MNT-1 buffer (0.1 M Tris-HCl, pH 7.5, 0.15 M NaCl, 1 MgCl2, 1 mM NaF, and 0.5% NP-40) or HeLa buffer (25 mM HEPES, pH 7.4, 0.15 M NaCl, 1 mM EDTA, 1 mM NaF, 0.5 mM MgCl2, and 0.5% Triton X-100), respectively, containing protease inhibitor mixture [1 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride, 10 μg/ml leupeptin, 5 μg/ml aprotinin, and 1 μg/l pepstatin A]. The MNT-1 extract was subsequently diluted with 1 volume of MNT-1 buffer lacking detergent and precleared by incubation with glutathione-Sepharose 4 Fast Flow beads (GE Healthcare) and centrifugation. Aliquots of the cleared detergent extracts were incubated for 1 h at 4°C with GST-fusion proteins (20 μg) that had been immobilized onto 15 μl of glutathione-Sepharose beads. After the incubation period, beads were collected by brief centrifugation and washed three times with MNT-1 buffer containing 0.1% NP-40 or HeLa buffer containing 0.1% Triton X-100, respectively, and one time with buffer lacking detergent. Proteins bound to the beads were fractionated by SDS-polyacrylamide gel electrophoresis (PAGE) and subjected to immunoblotting as described previously (d'Addio et al., 2000).
Metabolic Labeling and Endoglycosidase Assays
Metabolic labeling of MNT-1 cells or transiently transfected HeLa cells and immunoprecipitation from Triton X-100 cell lysates were done essentially as described previously (Berson et al., 2000) by using EXPRE35S35S 35S-cysteine/methionine mixture (PerkinElmer Life and Analytical Sciences, Boston, MA). Cells were labeled for 30 min at 37°C and chased for the indicated times. For endoglycosidase treatments, immunoprecipitates were denatured, split into equal aliquots, and mock treated or incubated with endoglycosidase H (endoH) or peptide N-glycosidase F (PNGase F) (both from New England Biolabs, Ipswich, MA), according to manufacturer's instructions, for 4 h with the addition of phenylmethylsulfonyl fluoride, leupeptin (Roche Diagnostics), and 1% NP-40 (Roche Diagnostics). Samples were fractionated by SDS-PAGE using 8% acrylamide gels, dried, and analyzed using a Storm 860 PhosphorImager and ImageQuest software (GE Healthcare) as described.
Transfections and Immunofluorescence Microscopy
For HeLa and CHO cells, cells were seeded onto glass coverslips in six-well dishes at 1.5 × 105 per well. Mouse melanocytes were seeded at 1 × 105 per well in six-well dishes or at 0.5 × 105 per well in 12-well dishes on coverslips coated with Matrigel (BD Biosciences, San Jose, CA) according to manufacturer's instructions. The next day, cells were transfected with 1–2 μg of the relevant OCA2 construct by using FuGENE-6 reagent. Two days later, cells were fixed for 30 min in 2% formaldehyde (Thermo Fisher Scientific, Waltham, MA), stained with primary and fluorochrome-conjugated secondary antibodies, and mounted onto glass slides as described previously (Calvo et al., 1999). In some experiments, transfected HeLa cells were incubated in fresh medium with or without 50 μg/ml cycloheximide for 3 h before fixation. For lysosomal inhibition, transfected CHO cells were incubated in fresh medium with or without 50 mM NH4Cl (Thermo Fisher Scientific) for 4 h before fixation. For surface staining, chilled cells were labeled with 1 μg/ml anti-HA antibody for 10–30 min on ice, and then unbound antibody was removed by rinsing in ice cold phosphate-buffered saline before fixation as described. Slides were analyzed on a DM IRBE microscope (Leica, Wetzlar, Germany) equipped with an Orca digital camera (Hamamatsu, Bridgewater, NJ). Images were captured and manipulated using OpenLab software (Improvision, Lexington, MA), with the volume deconvolution package. Final images were prepared using Adobe Photoshop (Adobe Systems, Mountain View, CA).
Statistical Analysis
Quantification of melanin synthesis rescue was performed as follows. Transfected cells were identified by positive labeling with anti-HA antibody by immunofluorescence microscopy and then assessed for the presence of pigmented melanosomes by bright field microscopy. Within each experiment, the percentage of transfected cells (>100 cells/experiment) that were positive for pigment rescue was calculated. Graphs represent rescue by each construct averaged over three independent experiments. In Figures 3 and 7, an initial repeated measures analysis of variance was performed on the matched sets, followed by Dunnett's multiple comparison test to compare rescue by each mutant to rescue by wild-type OCA2. In Figure 3, a two-tailed t test was used to compare rescue by OCA2-HMGCR and OCA2-HMGCR-AATA. All statistical analyses were performed using GraphPad Prism (GraphPad Software, San Diego, CA).
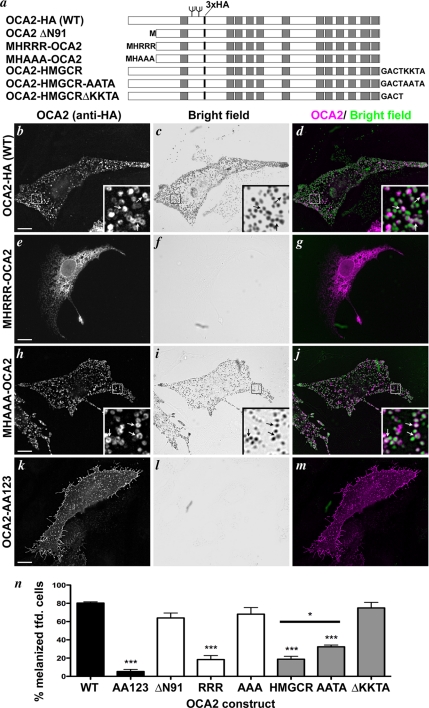
Figure 3.
OCA2 function correlates with localization to melanosomes. (a) Scheme of HA-tagged, chimeric, and truncated OCA2 proteins as described in the text. Added sequences in fusion proteins are indicated at the N or C termini. All constructs bear a triple HA epitope tag (3xHA, indicated by a black bar). Gray boxes indicate predicted transmembrane domains, and N-glycosylation sites are indicated by branching. (b–m) OCA2-deficient melan-p1 mouse melanocytes were transfected with human OCA2-HA (b–d), MHRRR-OCA2-ΔN91 (e–g), MHAAA-OCA2-ΔN91 (h–j), or OCA2-AA123 (k–m; see Figure 4a). Transfected cells were identified by anti-HA staining (b, e, h, and k) and visually inspected for the presence of pigmented melanosomes by bright field microscopy (c, f, i, and l). In the merged images (d, g, j, and m), the bright field image is inverted and colored green and anti-HA is colored magenta. Insets, 5X magnification of boxed regions. Arrows point to regions of overlap between OCA2-HA and melanosomes (b–d, h–j). Bar, 10 μm. (n) Bar graph of pigmentation rescue by each OCA2 construct. Shown is the percentage of transfected cells expressing each indicated construct that contained pigmented melanosomes (% pigmented cells). The degree of rescue induced by any construct bearing an ER targeting signal or lacking endogenous dileucine sorting signals was significantly different from rescue induced by wild-type OCA2-HA. *, p < 0.05; ***, p < 0.001.
Figure 7.
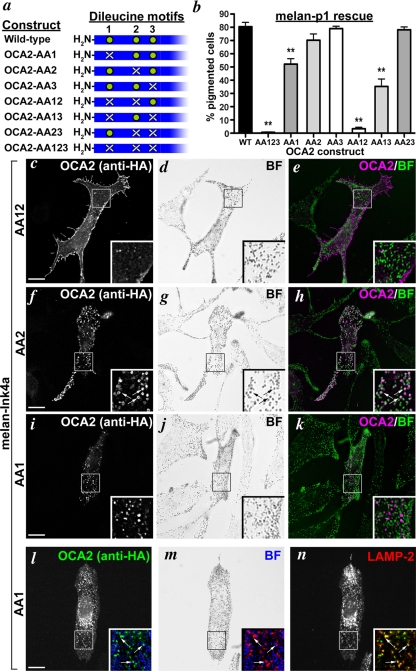
Localization and function of OCA2 dileucine mutants. (a) Schematic of the panel of human OCA2 dileucine mutants with the relevant N-terminal region of OCA2 highlighted. A green circle indicates the presence of LL1, LL2, or LL3, and a white X indicates its absence. (b) Dileucine mutants were expressed in melan-p1 cells, and transfected cells were visually inspected for the presence of pigmented melanosomes. Shown is the percentage of transfected cells expressing each indicated construct that contained pigmented melanosomes (% pigmented cells). Columns with asterisks were significantly different from rescue by OCA2-HA. **, p < 0.01. (c–n) IFM analysis of melan-Ink4a cells expressing selected mutant OCA2 variants. Melan-Ink4a melanocytes were transfected with OCA2-AA12 (c–e), OCA2-AA2 (f–h), or OCA2-AA1 (i–n). Transgenes were visualized with anti-HA antibodies (c, f, i, and l), melanosomes were visualized by bright field (BF) microscopy (d, g, j, and m), and late endosomes/lysosomes were visualized by labeling with anti-LAMP-2 antibody (n). e, h, and k are merged OCA2-HA (magenta) and inverted bright field (green) images. All insets show 3.5× magnified images of the boxed region, and insets in l–n show paired merged images of anti-HA (green), anti-LAMP-2 (red), and inverted bright field (blue). Arrows point to regions of overlap of OCA2 constructs with melanosomes (f–h) or with LAMP-2 (l–n). Bars, 10 μm.
RESULTS
OCA2 Transits to a Post-ER Compartment and Becomes Terminally Glycosylated
Analysis of the primary sequence of human OCA2 reveals three evolutionarily conserved consensus N-glycosylation sites within the first predicted lumenal loop (at Asn residues 214, 218, and 273) and additional sites elsewhere, but mouse OCA2 was suggested to be nonglycosylated based on lack of a mobility shift in tunicamycin-treated cells (Rosemblat et al., 1994). We thus tested whether human OCA2 was in fact N-glycosylated using endoglycosidase treatment in a metabolic pulse/chase and immunoprecipitation experiment. MNT-1 human melanoma cells that endogenously express OCA2 or HeLa cells transiently transfected with OCA2 were pulse-labeled with 35S-cysteine/methionine, chased for various times, and then lysed and subjected to immunoprecipitation with the NOCA2 anti-OCA2 antibody. Immunoprecipitates were mock treated or digested with endoH or PNGase F. Endogenous OCA2 from pulse-labeled MNT-1 cells is entirely sensitive to digestion by both endoH and PNGase F, indicating that it is modified by the addition of core N-linked oligosaccharides. By 1 h of chase, a cohort of OCA2 becomes resistant to cleavage by Endo H (Figure 1a, compare M and D, arrows), indicating modification by N-acetylglucosamine transferase in the medial Golgi. By 4 h of chase, all detectable OCA2 is resistant to endoH. As a control, PNGase F digestion increases the mobility of OCA2 at all time points. Similar results were obtained in transiently transfected HeLa cells expressing OCA2, although processing in the Golgi seemed to be faster (Figure 1b). Note that processed OCA2 disappeared rapidly from detergent lysates in melanocytes, consistent with earlier results, suggesting close association with melanin (Donatien and Orlow, 1995), but much less rapidly in transfected HeLa cells. The reduction in Mr resulting from PNGase F or endoH treatment of OCA2 was ∼7 kDa, consistent with loss of two N-linked oligosaccharide chains. Consistently, the Mr of OCA2 isolated from normal human melanocytes or OCA2-transfected COS-7 cells was reduced if cells were first treated with the N-glycosylation inhibitor tunicamycin or the mannosidase inhibitor deoxymannojirimycin (data not shown). Together, these results indicate that OCA2 is a glycoprotein and is not a resident of the ER but rather traffics to at least the medial Golgi.
OCA2 Localizes to Melanosomes in Melanocytes and Lysosomes in Nonmelanocytes
Because OCA2 is largely exported from the ER and thus does not behave like an ER resident protein, we next sought to determine the steady-state localization of OCA2 in melanocytes and nonmelanocytic HeLa cells. Endogenous OCA2 in human melanocytic cells could not be detected by IFM (data not shown), but a full-length human OCA2 transgene product, with or without a triple HA epitope tag inserted in-frame within the first lumenal loop of the protein (OCA2-HA; see Figure 4a) could be detected in several melanocytic cell lines after transient transfection. The HA tag did not affect OCA2 function (see Figure 3) and permitted detection of the OCA2-HA transgene with an anti-HA antibody. When expressed in pigmented melan-Ink4a mouse melanocytes, both OCA2 and OCA2-HA were distributed in either a reticular or vesicular pattern as revealed by labeling with NOCA2 or anti-HA antibodies and analysis by IFM and image deconvolution (Figure 2a; data not shown). The reticular pattern was similar to that observed with antibodies to ER markers (data not shown). Qualitative observations of transfected cells did not reveal an obvious correlation between transgene expression level and the subcellular distribution of the protein. In cells with vesicular labeling, the anti-OCA2 or anti-HA label overlaps almost entirely with that of the melanosomal marker Tyrp1 (data not shown), as well as with pigment granules visualized by bright field microscopy (Figure 2, b and c). These data suggest that although a cohort of exogenously expressed OCA2 accumulates in the ER, OCA2 that exits the ER is targeted to melanosomes.
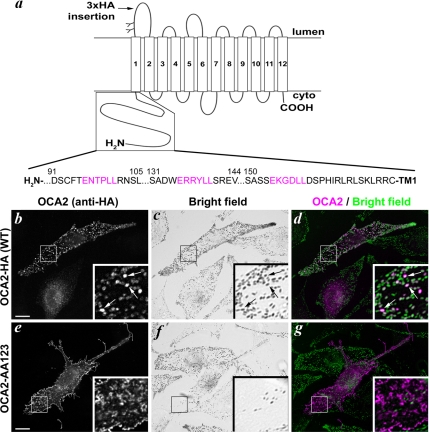
Figure 4.
OCA2 melanosomal sorting requires intact dileucine motifs.(a) Topology of human OCA2 and sequence of the cytoplasmic N terminus with putative acidic dileucine motifs indicated in magenta. (b–g) Wild-type OCA2-HA (b–d) or the triple dileucine mutant OCA2-AA123 (e–g) were expressed in pigmented melan-Ink4a melanocytes. Transfected cells are identified by anti-HA staining (b and e; colored magenta in merged images, d and g). Bright field images (c and f) were inverted and colored green in merged images (d and g). Insets, 4X magnification of boxed regions. Arrows point to regions of overlap between OCA2-HA and melanosomes (b–d). Bar, 10 μm.
Figure 2.
OCA2 localizes to melanosomes in melanocytes and to lysosomes in nonmelanocytes. (a–c) Melan-Ink4a melanocytes were transfected with human OCA2-HA and stained with anti-HA antibodies (a; magenta in the merge, c). The bright field image of pigmented melanosomes (b) is inverted and colored green in the merge (c). (d–f) HeLa cells transfected with human OCA2-HA were treated with 50 μg/ml cycloheximide for 3 h before fixation. Cells are stained with antibodies to HA (d; magenta in the merge, f) and the lysosomal marker LAMP-1 (e; green in the merge, f). Insets, 3X magnification of boxed regions. Arrows point to regions of overlap between OCA2-HA and melanosomes (a–c) or lysosomes (d–f). Asterisk indicates an overexpressing cell. Bar, 10 μm.
Most melanosomal proteins localize to late endosomes or lysosomes when expressed in nonmelanocytic cells (Bouchard et al., 1989; Vijayasaradhi et al., 1995; Calvo et al., 1999; Simmen et al., 1999; Berson et al., 2001). We therefore tested the localization of OCA2-HA expressed in nonmelanocytic HeLa cells. As with melanocytes, OCA2 or OCA2-HA was distributed either in a predominantly reticular pattern, characteristic of the ER, or a predominantly vesicular pattern (30.4 and 69.5% of transfected cells, respectively; Figure 2d; data not shown). The vesicles overlapped nearly completely with labeling for LAMP-1, a membrane protein that accumulates in late endosomes and lysosomes (Figure 2, e and f). Treatment of HeLa cells for 3 h with the protein synthesis inhibitor cycloheximide increased the percentage of cells displaying predominantly vesicular HA staining to 81.7%. Additionally, the fraction of cells exhibiting vesicular localization increased with increasing time after transfection (data not shown). A similar cycloheximide treatment in transfected melan-Ink4a melanocytes did not alter the ratio of cells with predominantly ER or vesicular staining, although it did significantly reduce the fraction of cells with any detectable labeling (data not shown). This is likely a consequence of the rapid degradation of OCA2 observed in melanocytic cells (Figure 1a), such that OCA2 fails to accumulate to detectable levels without continuous biosynthetic input. We therefore interpret these results to suggest that the ER-localized pool of OCA2 is transient and that OCA2 traffics to melanosomes in melanocytes and to lysosomes in nonmelanocytes. We cannot exclude the possibility that microheterogeneity in our cell culture system contributes to differential rates of ER exit or stability within melanosomes.
ER-localized OCA2 Cannot Rescue Pigmentation of OCA2-deficient Melanocytes
Because a significant cohort of exogenously expressed OCA2 resides in the ER at steady state, we tested whether OCA2 in the ER is functional. We took advantage of the fact that expression of human OCA2 in nonpigmented, OCA2-deficient mouse melan-p1 melanocytes can restore melanin synthesis and consequent pigmentation (Sviderskaya et al., 1997), and we asked whether purposeful retention of OCA2 in the ER is compatible with this function.
We first constructed two OCA2 mutants that are constitutively localized to the ER. OCA2-HMGCR was created by fusing the C-terminal cytoplasmic domain of human HMG CoA reductase (HMGCR), containing a canonical dilysine ER retrieval signal (Jackson et al., 1990), to the cytoplasmic C terminus of OCA2-HA (Figure 3a). OCA2-HMGCR was effectively retained in the ER when expressed in MNT-1 melanoma cells, as judged by colocalization with calnexin by IFM (Supplemental Figure S1, a–c). As controls, we constructed two mutants in which the lysine residues were mutagenized to alanine (OCA2-HMGCR-AATA) or in which the targeting signal was deleted (OCA2-HMGCR-ΔKKTA) (Figure 3a); these mutants were weakly and strongly localized to melanosomes, respectively (data not shown). Another OCA2 chimeric construct bore an N-terminal cytoplasmic ER-targeting arginine motif, MHRRR, derived from the p35 form of human Invariant chain (Schutze et al., 1994). This signal did not confer ER retention when fused to full-length OCA2 (data not shown). However, when appended to a deletion mutant that lacked the first 91 amino acids of OCA2 (OCA2-ΔΝ91) and that localized by IFM in pigmented MNT-1 melanoma cells to melanosomes like full-length OCA2-HA (Supplemental Figure S1, d–f), the resultant MHRRR-OCA2-ΔN91 fusion protein was effectively retained in the ER (Supplemental Figure S1, g–i); the Δ91 deletion presumably brought the N-terminal ER retention signal near enough to the membrane anchor to allow for interactions with membrane-proximal effectors. By contrast, when the triple arginine signal was mutagenized to a triple alanine (MHAAA-OCA2-ΔN91), melanosomal localization in melan-Ink4a was restored (data not shown, but see Figure 3).
To test whether ER-retained OCA2 is functional, each of these mutants was expressed in hypopigmented, OCA2-deficient mouse melan-p1 cells and restoration of pigmentation was scored by bright field microscopy. As expected, expression of human OCA2-HA was sufficient to rescue pigmentation in 80% of transfected melan-p1 cells (Figure 3, b–d, n), indicating that the addition of the lumenal triple HA tag does not negatively affect OCA2 function. Likewise, the level of rescue induced by OCA2-ΔN91, 69%, was not significantly different from wild-type, indicating that the first 91 amino acids of OCA2 are not essential for protein function (Figure 3n). Strikingly, expression of OCA2-HMGCR or MHRRR-OCA2-ΔN91, both of which localized in melan-p1 cells to a reticular pattern characteristic of ER as in MNT-1 cells (Figure 3, e–g; data not shown), rescued pigmentation in only 16 and 17% of transfected cells, respectively (Figure 3n). The corresponding fusion proteins with mutated ER targeting signals—MHAAA-OCA2-ΔN91 and OCA2-HMGCR-ΔKKTA—showed restored vesicular/melanosomal localization (Figure 3, h–j; data not shown) and induced pigmentation in a fraction of melan-p1 cells that was not significantly different from that induced by wild-type OCA2-HA (Figure 3n); OCA2-HMGCR-AATA rescued more efficiently than OCA2-HMGCR but not as efficiently as OCA2-HMGCR-ΔKKTA, consistent with its partial restoration of melanosome localization. This might reflect misfolding induced by the elongated C-terminal domain, as similar observations were made with other C-terminally extended OCA2 variants (data not shown). Overall, our results demonstrate that OCA2 is unable to promote melanin synthesis from the ER of melanocytes and therefore must function in a post-ER compartment.
OCA2 Localization Requires Cytoplasmic Dileucine Motifs
To begin to understand the mechanism by which OCA2 is sorted to melanosomes, we next investigated the cis-acting signals required for OCA2 localization. The cytoplasmic C-terminal domains of the melanosomal proteins Tyr and Tyrp1 contain sequences conforming to the [D/E]XXXL[L/I] consensus for acidic dileucine motifs that are necessary for sorting of these proteins to melanosomes (Vijayasaradhi et al., 1995; Calvo et al., 1999; Simmen et al., 1999). Mutagenesis of the two leucine residues within these motifs results in mislocalization to the cell surface. Three sequences conforming to the acidic dileucine consensus are present in the human OCA2 cytoplasmic N-terminal domain (Figure 4a). We tested whether these motifs are required for steady-state localization of OCA2-HA to melanosomes by simultaneously mutagenizing the leucine residues in all three motifs to alanine and examining the localization of the resultant mutant, expressed transiently in wild-type melan-Ink4a melanocytes, by IFM. Whereas wild-type human OCA2-HA localizes to melanosomes in these cells (Figure 4, b–d), the triple dileucine mutant (OCA2-AA123) localizes to the cell periphery (Figure 4, e–g). HA-positive structures within the cell perimeter did not colocalize with melanosomes (Figure 4g), but rather they seemed to correspond to surface projections. As with wild-type OCA2, the labeling pattern of OCA2-AA123 in transiently transfected melanocytes was heterogeneous, in that some cells showed a reticular pattern rather than peripheral protein localization. Similarly, whereas wild-type OCA2 localized to lysosomes in HeLa cells, OCA2-AA123 localized to the cell periphery in these cells as well (Supplemental Figure S3). To confirm localization to the cell surface, wild-type OCA2-HA or OCA2-AA123 was transiently expressed in pigmented melan-Ink4a melanocytes and incubated at 4°C with anti-HA antibodies to detect the lumenally exposed (extracellular) triple HA tag on the cell surface. Cells were then washed, fixed, permeabilized, and stained with NOCA2 to identify all transfected cells (NOCA2 binding is not affected by mutagenesis of the N-terminal dileucine motifs). Cells expressing OCA2-AA123 were labeled with the anti-HA antibody, whereas cells expressing wild-type OCA2-HA were not (Supplemental Figure S2, a–f). Together, these data show that the dileucine motifs in the N-terminal cytoplasmic domain of human OCA2 are necessary for melanosome/lysosome localization and that in their absence OCA2 localizes by default to the plasma membrane.
To determine whether sorting to melanosomes was required for OCA2 function, we tested whether the cell surface localized OCA2-AA123 could restore pigmentation upon expression in melan-p1 cells. As with cells expressing the ER-localized OCA2 fusion proteins, only 3% of melan-p1 cells expressing OCA2-AA123 were pigmented (Figure 3, k–n). This indicates that OCA2 cannot function on the cell surface and must function from an intracellular, post-ER compartment. Interestingly, lysosomes in HeLa cells that overexpressed OCA2-HA tended to be unusually clustered around the nucleus and swollen compared with untransfected cells (Supplemental Figure S3, compare insets 1 and 2); a similar phenomenon has been seen in HeLa cells expressing tyrosinase (Calvo et al., 1999). By contrast, lysosomes in HeLa cells expressing the surface-localized OCA2-AA123 seemed similar to those in untransfected cells (Supplemental Figure S3). These results suggest that overexpression of the intact OCA2 cytoplasmic sorting signals can alter late endosome/lysosome function or morphology, further supporting a functional role for these motifs in sorting to lysosome-like organelles.
OCA2 Dileucine Motifs Are Sufficient for Lysosomal Localization in Nonmelanocytes and Intracellular Targeting in Melanocytes
To determine whether the OCA2 dileucine motifs were sufficient to cause trafficking to lysosomes or melanosomes, we assessed their ability to direct the localization of a reporter molecule. We generated chimeric proteins in which the N-terminal cytoplasmic domain of either wild-type human OCA2 or the dileucine mutant, OCA2-AA123, was fused to the transmembrane and lumenal domains of human transferrin receptor (hTfR) to generate OCA2-TfR and AA123-TfR, respectively (Figure 5a). hTfR, which normally cycles between the plasma membrane and early endosomes, was chosen as a fusion partner because it is a type II integral membrane protein and thus allowed us to maintain the proper orientation of the OCA2 N-terminal domain. hTfR or the chimeric proteins were transiently expressed in CHO cells, which do not endogenously express human OCA2 or hTfR, and localization was determined by IFM. As expected, heterologously expressed hTfR localized to the cell surface and to peripheral intracellular puncta that did not overlap with the late endosome/lysosome marker LAMP-1 and that likely represent early endosomes (Figure 5, b–d). By contrast, OCA2-TfR localized exclusively to intracellular compartments that were predominantly accumulated in the perinuclear area (Figure 5, e–g). Mutagenesis of the three dileucine motifs in AA123-TfR restored cell surface localization (Figure 5, h–j). Surprisingly, the puncta to which OCA2-TfR localized did not appreciably overlap with LAMP-1. We reasoned that the hTfR lumenal domain, to which the antibody was directed, might be degraded within LAMP-1–containing late endosomes and lysosomes. To test this possibility, CHO cells that were cotransfected with both the OCA2-TfR chimera (Figure 5, l and o) and full-length OCA2-HA (Figure 5, k and n) were analyzed by IFM after treatment with 50 mM NH4Cl to neutralize lysosomes and interfere with proteolysis. Whereas neither protein colocalized with LAMP-1 in untreated cells (Figure 5, k–m), both OCA2-TfR and OCA2-HA redistributed to vesicular structures that colocalized with LAMP-1 in NH4Cl-treated cells (Figure 5, n–p). Consistently, OCA2-TfR localized to LAMP-1–positive compartments in HeLa cells even without lysosomal inhibition, and induced enlargement and perinuclear clustering like full-length OCA2-HA (Supplemental Figure S3). These results confirm that the OCA2 dileucine motifs are sufficient to direct trafficking to lysosomes in nonmelanocytes. Moreover, the enlargement and clustering of LAMP-1–positive compartments in HeLa cells induced by expression of OCA2-TfR indicates that these effects are a consequence of overexpression of the cytoplasmic sorting signals and not of OCA2 ion transport activity. Similar observations were made upon overexpression of a chimeric protein with the tyrosinase cytoplasmic domain (Calvo et al., 1999). It is unclear why this effect on lysosome morphology is not seen in transfected CHO cells, but it may reflect the apparently heightened sensitivity of lysosomally-localized OCA2 constructs to degradation in this cell type.
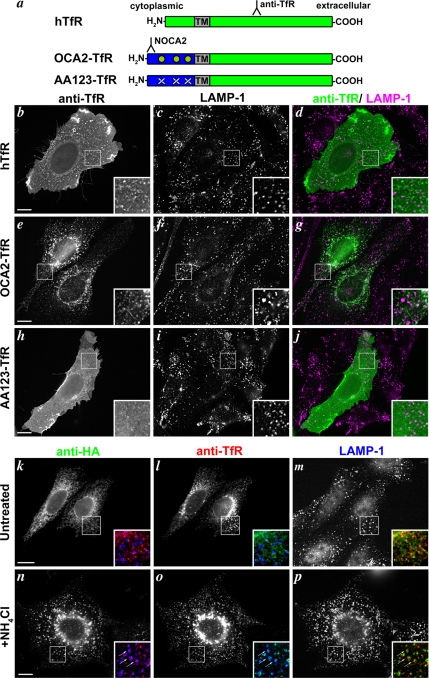
Figure 5.
OCA2 dileucine motifs are sufficient for lysosomal localization in nonmelanocytes. (a) Schematic of OCA2-transferrin receptor chimeras. Green, hTfR sequences; blue, cytoplasmic N terminus of human OCA2; TM, hTfR transmembrane domain; green circles, intact OCA2 dileucine motifs; white X's, disrupted dileucine motifs. Binding regions of relevant antibodies are indicated. The NOCA2 epitope does not overlap the dileucine motifs. (b–j) Chinese hamster ovary cells were transfected with hTfR (b–d), OCA2-TfR (e–g), or AA123-TfR (h–j), and the transgenic proteins were localized at steady-state by anti-hTfR staining (b, e, and h). The lysosomal marker LAMP-1 is stained in c, f, and i, and images are merged in d, g, and j with anti-hTfR in green and anti-LAMP-1 in magenta. Insets, 4X magnification of boxed regions. (k–p) Chinese hamster ovary cells were cotransfected with wild-type OCA2-HA (k and n) and OCA2-TfR (l and o) and incubated with (n–p) or without (k–m) 50 mM NH4Cl before fixation in order to inhibit lysosomal degradation. Lysosomes are marked by LAMP-1 staining (m and p). Insets show 3X magnifications of two-color merged images of OCA2-HA (green), OCA2-TfR (red), and LAMP-1 (blue). Arrows point to regions of overlap among the three proteins (n–p). Bar, 10 μm.
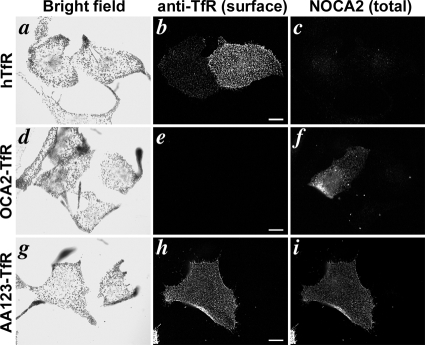
When expressed in melanocytic cells, OCA2-TfR was detected intracellularly on perinuclear vesicular structures that overlapped minimally with pigment granules and the melanosome marker Tyrp1 (Supplemental Figure S4). We were unable to alter its distribution by treatment with inhibitors of lysosomal proteases or deacidification reagents; however, similar results were obtained with other chimeras that target to lysosomes in other melanocytic cells (data not shown), suggesting that lysosomal proteolysis might be more difficult to disrupt in melanocytes than in other cell types. We thus could not directly determine whether the dileucine motifs were sufficient for localization to melanosomes. However, by labeling transfected melan-Ink4a cells with anti-hTfR antibodies without permeabilization at 4°C, we tested whether the dileucine motifs confer steady-state intracellular localization. Whereas intact cells expressing full-length hTfR (Figure 6, a–c) or AA123-TfR (Figure 6, g–i) were strongly labeled at the cell surface by anti-hTfR antibodies, cells expressing OCA2-TfR were not (Figure 6, d–f). These data indicate that the N-terminal OCA2 dileucine motifs are sufficient to confer intracellular localization in melanocytes, and they are consistent with the notion that they may confer melanosome localization.
Figure 6.
OCA2 dileucine motifs confer internal localization in melanocytes. Wild-type melan-Ink4a melanocytes were transfected with hTfR (a–c), OCA2-TfR (d–f), or AA123-TfR (g–i) and incubated at 4°C with antibodies to the extracellular region of hTfR (b, e, and h). After subsequent washing, fixation, and permeabilization, cells were labeled with NOCA2 antibody (c, f, and i) to identify transfected cells expressing chimeric proteins. Bright field images are shown in a, d, and g. Bar, 10 μm.
Individual OCA2 Dileucine Motifs Are Not Functionally Equivalent
To determine whether individual OCA2 dileucine motifs had distinct or redundant functions, we mutagenized each of the three motifs individually or in all possible combinations within the context of OCA2-HA (Figure 7a). The three motifs are designated LL1, LL2, and LL3, with LL1 being the most N-terminal and LL3 the most C-terminal. The corresponding OCA2 mutant proteins are designated OCA2-AA1, -AA2, and -AA3. Mutants were expressed in wild-type melan-Ink4a melanocytes and localization was determined by IFM. Disruption of LL3 (OCA2-AA3) did not affect steady-state localization of OCA2-HA to melanosomes (data not shown), whereas disruption of LL1 and LL2 together—leaving only LL3 intact (OCA2-AA12)—ablated localization to melanosomes and resulted in surface expression as seen with the triple mutant (Figure 7, c–e). Therefore, LL3 is dispensable for OCA2 trafficking. Strikingly, mutants in which LL2 alone (OCA2-AA2) or both LL2 and LL3 (OCA2-AA23) were mutagenized, leaving LL1 intact, were as efficiently localized at steady state to melanosomes as the wild-type protein (Figure 7, f–h; data not shown). This indicates that, within the context of full-length OCA2, LL1 is sufficient to confer melanosomal localization. Moreover, LL1 is necessary for steady-state melanosomal localization, because mutants in which LL2 was present but LL1 was disrupted (OCA2-AA1 and OCA2-AA13) did not colocalize with pigment granules (Figure 7, i–k; data not shown). Rather, these mutants localized at steady state to nonpigmented, cytoplasmic vesicles that were also labeled by antibodies to LAMP- 2 (Figure 7, l–n; data not shown), a lysosomal marker (Granger et al., 1990) that is largely excluded from pigment granules in melan-Ink4a cells. Together, these data indicate that LL1 is necessary and sufficient for melanosomal trafficking in the context of full-length OCA2, whereas LL2 confers steady-state localization to lysosomes, but not to melanosomes, in a manner that is masked by LL1 function. In HeLa cells, which do not have a separate melanosomal trafficking pathway, the presence of either LL1 or LL2 was sufficient to confer lysosomal localization of full-length OCA2 mutants, whereas the simultaneous loss of both motifs caused accumulation on the cell surface similar to that seen with the triple mutant (data not shown).
To determine whether melanosomal localization correlated with OCA2 function, we next tested how mutagenesis of distinct dileucine motifs affected the ability of OCA2 to rescue pigmentation when expressed in melan-p1 cells (Figure 7b). Transfected cells expressing a mutant in which LL1 was disrupted (AA1, AA12, and AA123) showed a significant reduction in the percentage of pigmentation, suggesting that melanosomal localization is needed for maximal OCA2 function in pigmentation. By contrast, disruption of either LL2 (AA2) or LL3 (AA3) had no significant effect, and a mutant in which only LL1 was intact (AA23) rescued pigmentation as efficiently as the wild-type protein. The surface-localized OCA2-AA12 mutant, lacking both LL1 and LL2, was as inefficient as the triple mutant in restoring pigmentation. These results corroborate the finding that of the three dileucine motifs in the N terminus of OCA2, LL1 is the most important for both localization to melanosomes and function. Interestingly, mutants lacking LL1 but retaining LL2 (OCA2-AA1 and -AA13) were able to partially restore pigmentation to melan-p1 cells. Because these mutants localize at steady state to lysosomes, one possible interpretation of this result is that a cohort of melanosome-localized OCA2 that is below the limit of detection is sufficient to rescue pigmentation in these cells. Alternatively, OCA2 might either transiently traverse melanosomes en route to late endosomes/lysosomes or function from late endosomal compartments.
OCA2 Binds Adaptors AP-1 and AP-3 in a Dileucine-dependent Manner
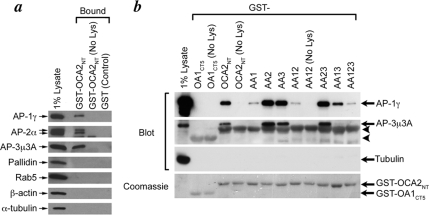
Previous studies have shown that acidic dileucine signals bind to members of the clathrin-associated heterotetrameric adaptor protein family (Bonifacino and Traub, 2003; Janvier et al., 2003; Chaudhuri et al., 2007; Doray et al., 2007). The acidic dileucine signal in Tyr mediates an interaction with both AP-3 and AP-1 (Honing et al., 1998; Theos et al., 2005), whereas the corresponding signal in Tyrp1 has been shown to interact with AP-1 only (Theos et al., 2005). We therefore used an affinity “pull-down” assay to test whether the dileucine motifs in the OCA2 cytoplasmic domain can be recognized by heterotetrameric adaptors. Recombinant fusion proteins consisting of GST fused to the cytoplasmic N terminus of wild-type (GST-OCA2NT) or mutagenized human OCA2 were purified, immobilized onto glutathione-Sepharose beads, and then incubated with MNT-1 or HeLa cell lysates. Bound proteins were analyzed by immunoblotting. As shown in Figure 8a, GST-OCA2NT bound the adaptor proteins AP-1, AP-2, and AP-3 but not the unrelated proteins β-actin, α-tubulin, Rab5, or the BLOC-1 subunit pallidin. We did not detect binding of AP-1 or AP-3 to GST fused to the C-terminal cytoplasmic domain of the melanosomal/lysosomal protein OA1 (GST-OA1CT5). This domain contains an unrelated sorting signal that does not conform to any canonical AP-binding consensus sequences (Figure 8b) (Piccirillo et al., 2006). These data suggest that the cytoplasmic domain of OCA2 interacts specifically with AP-1, AP-2, and AP-3.
Figure 8.
OCA2 cytoplasmic dileucine-based motifs differentially bind AP proteins. (a) GST alone (GST) or fused to the N-terminal cytoplasmic domain of human OCA2 (GST-OCA2NT) was bound to glutathione-Sepharose and then incubated without (No Lys) or with MNT-1 cell lysates. Bound proteins were fractionated by SDS-PAGE and immunoblotted with antibodies to the proteins indicated at left. The first lane includes lysate before incubation (1% of the total used for the pull-downs). (b) GST fused to wild-type (OCA2NT) or the indicated mutants of the N-terminal cytoplasmic region of human OCA2, or to the C-terminal cytoplasmic domain of OA1 as a negative control (OA1CT5), were bound to glutathione-Sepharose, incubated without (No Lys) or with HeLa cell lysate, and then analyzed by immunoblotting. Arrows point to the relevant bands in the immunoblot. Arrowheads point to cross-reactivity of the anti-AP-3μ3A antibody with GST-fusion proteins. Shown at bottom is a Coomassie-stained gel of identical reactions showing the GST-fusion proteins.
To determine whether adaptor binding was mediated by the dileucine-based motifs, the experiment was repeated using GST fused to OCA2 cytoplasmic domain mutants in which one or more dileucine motifs were disrupted by dileucine-to-dialanine mutations (Figure 8b). Whereas AP-2 binding to different mutants was detected inconsistently over multiple trials (data not shown), AP-3 was consistently bound to GST-OCA2NT fusions containing an intact LL1 (OCA2-AA2, -AA3, and -AA23) but not with fusions containing a disrupted LL1 (OCA2-AA1, -AA12, -AA13, and -AA123). Although low levels of bound AP-1 were observed even with a GST-OCA2NT fusion protein bearing mutations in all three dileucine motifs (OCA2-AA123), much higher levels of bound AP-1, as for AP-3, were observed with fusion proteins containing an intact LL1. Interestingly, intermediate binding to AP-1 was observed with a mutant bearing only an intact LL2 (OCA2-AA13). Detection of all AP complexes was dependent on incubation with cell lysates (Figure 8b, “No Lys”), and identical results were observed using lysates derived from MNT-1 cells (data not shown). These data suggest that LL1 mediates strong binding to AP-1 and AP-3, whereas LL2 mediates moderate binding to AP-1 and LL3 has no appreciable affinity for the adaptors we tested. Thus, strong binding to AP-1 and AP-3 correlates with steady-state localization to melanosomes and with OCA2 function, and modest binding to AP-1 correlates with steady-state lysosomal sorting. These data further corroborate the different roles of the dileucine motifs and implicate AP-1 and/or AP-3 in regulating the trafficking of OCA2.
DISCUSSION
OCA2-deficient melanocytes have clear defects in melanosome morphology and function, but previous reports have ascribed functions for OCA2 within different subcellular locations. Recent reports suggest that melanosome malformation in OCA2-deficient melanocytes results from misfolding of melanosomal proteins in the ER, and that OCA2 localizes to the ER (Chen et al., 2002). Our data indicate that endogenous OCA2 in melanocytic cells and OCA2 transgene products in nonmelanocytic cells reside only transiently within the ER and that spatial restriction of OCA2 to the ER or to the cell surface impairs the function of OCA2 in melanogenesis. IFM and deconvolution analyses show that exogenously expressed OCA2 localizes to pigmented structures in mouse melanocytes that bear markers of mature melanosomes. Moreover, like other melanosomal proteins, the targeting of OCA2 to melanosomes is dependent on cytoplasmic dileucine-based motifs that bind to AP-3 and AP-1 adaptors and mediate lysosomal sorting in nonmelanocytic cells. These data indicate that OCA2 traverses intracellular sorting pathways common to other melanosomal proteins. We conclude that OCA2 localizes to and functions primarily within melanosomes.
The molecular function of the OCA2 protein is not yet known, but it is predicted to be a transmembrane ion transporter based on sequence homology to the ArsB/NhaD family of permeases. Our results suggest that this proposed activity would be required to regulate the intralumenal ion concentration within melanosomes to promote melanin synthesis. The lack of melanin synthesis in OCA2-deficient cells is thus likely a direct consequence of the dysregulation of ion content. This model is consistent with in vitro data showing that tyrosinase activity is sensitive to pH and redox state (Townsend et al., 1984). Ion dysregulation could in turn have indirect consequences on other processes in OCA2-deficient cells. For example, alterations in intralumenal ion balance or pH can affect cellular membrane fusion events (Peters and Mayer, 1998; Ungermann et al., 1999; Pryor et al., 2000), perhaps explaining the accumulation of melanosomal cargo in vesicular structures in OCA2-deficient melanocytes (Manga et al., 2001). Moreover, disruption of OCA2 transport activity across the melanosomal membrane may indirectly alter ion concentrations or pH in the cytosol; for example, exogenous expression of OCA2 in Saccharomyces cerevisiae led to a depletion of cytoplasmic glutathione due to glutathione transport into the vacuole (Staleva et al., 2002). Alterations in substrate levels could in turn have downstream effects on ion transport between the cytosol and other compartments, such as the ER. Such effects might alter folding or disulfide bond formation of proteins such as tyrosinase in the ER, perhaps explaining the reduced rate of processing and ER exit of tyrosinase in OCA2-deficient melanocytes (Toyofuku et al., 2002). Tyrosinase contains 15 lumenal Cys residues and many intramolecular disulfide bonds (Wang and Hebert, 2006), so changes in the disulfide bonding capacity of the ER could have a significant effect on tyrosinase maturation. A similar indirect effect on cellular ion levels and consequent tyrosinase missorting has been hypothesized to underlie the pigmentation defects in cells bearing a mutated form of SLC24A5, a pigment cell-specific potassium-dependent sodium-calcium exchanger that was suggested to localize to the trans-Golgi network (Ginger et al., 2008).
OCA2 is unique among melanosomal proteins by virtue of having multiple functional dileucine-based trafficking signals. The three motifs we identified in the N terminus of OCA2 are the only cytoplasmic sequences in the protein that conform to the acidic dileucine consensus. Of these, only the first motif (LL1) has been specifically noted in the past (Lee et al., 1995). The cytoplasmic N terminus of OCA2 is poorly conserved across species, and LL1 is the only one of the three consensus dileucine signals in the human homologue that is conserved in the mouse. This raises the question of how human and murine OCA2 trafficking might differ. No posttranslational modifications of OCA2 (other than N-glycosylation) have been reported, but the N terminus contains many serine residues that could be potential sites of regulatory phosphorylation. It is also possible that the combined activities of both human dileucine signals are incorporated into the single mouse dileucine signal, which differs in specific sequence from human LL1. Consistently, the single dileucine signal of tyrosinase seems to be sufficient to target tyrosinase to either of two distinct pathways to the melanosome (Theos et al., 2005; Setty et al., 2007). Although dileucine-based signals clearly play crucial roles in melanosomal trafficking, it is worth noting that none of the documented pathological point mutations in human Tyr, Tyrp1, or OCA2 occur in the acidic dileucine motifs of the proteins. The ability of the dileucine motifs to direct lysosomal localization of the OCA2-TfR chimera in nonmelanocytes suggests that these motifs are likely sufficient for OCA2 trafficking, but the existence of additional and/or potentially interacting melanosomal trafficking determinants cannot be ruled out because we could not visualize OCA2-TfR on melanosomes in melanocytes.
The change in steady-state localization that arises from mutation of individual dileucine motifs raises the possibility that different motifs mediate different steps of the trafficking itinerary of human OCA2. IFM data suggest that LL2 confers steady-state localization to lysosomes but is dominated by LL1, which confers steady-state localization to melanosomes. The existence of two signals suggests either of two possible models for OCA2 trafficking within melanocytes. One model posits that distinct cohorts of OCA2 use LL1 and LL2 for melanosomal and lysosomal trafficking, respectively. Mutagenesis of either motif would thus render the mutant protein subject to the direction of the remaining signal. Because LL1 mutants localize at steady state to lysosomes and yet stimulate substantial melanin synthesis in melan-p1 cells, this model would require that OCA2 be able to support optimal melanin synthesis in the melanosome either from late endosomes/lysosomes but not from the ER or the cell surface, or via a cohort of melanosome-localized OCA2 that is below the level of detection by IFM. A second model posits that OCA2 traffics through both melanosomes and lysosomes sequentially by virtue of sequential utilization of LL1 and LL2. In this case, LL2 would function as a timer to limit the residence of OCA2 within melanosomes by targeting it to lysosomes for subsequent degradation. OCA2 mutants that lack the strong melanosomal targeting of LL1 but still stimulate significant melanin synthesis might be targeted inefficiently to melanosomes via the weak AP-1-binding activity of LL2 and subsequently travel more efficiently to lysosomes, which would constitute the predominant accumulation at steady state. This model would explain the short half-life of OCA2 in melanocytes and can potentially be vigorously tested by live cell imaging experiments. In either case, the differential localization of OCA2 mutants with only LL1 or LL2 intact indicates that cytoplasmic targeting signals, irrespective of potential integral membrane or lumenal targeting determinants, can be differentially decoded to confer steady-state localization to lysosomes or melanosomes. This contrasts with the targeting of Tyrp1, in which lumenal determinants seem to be required to distinguish these two fates (Groux-Degroote et al., 2008).
LL1 and LL2 have differing abilities to bind adaptor proteins, thus suggesting a potential mechanism behind the complex trafficking pathway of OCA2. Like that of tyrosinase, the OCA2 cytoplasmic domain binds both AP-1 and AP-3. This binding depends critically on the presence of LL1. LL2 binds AP-1 more weakly and does not bind significantly in our assay to AP-3. Thus, steady-state localization of OCA2 to melanosomes, seen with all constructs containing an intact LL1, correlates well with either AP-3 binding or high-affinity binding to either AP protein, similar to the situation for tyrosinase. By contrast, a weak affinity for AP-1 alone may be sufficient to drive targeting of constructs containing LL2 but lacking LL1 toward lysosomes, either directly from endosomes or secondarily from melanosomes. Interestingly, lysosomal enlargement is seen in nonmelanocytes that overexpress fusion proteins containing the wild-type dileucine motifs from OCA2 or Tyr, which possess both AP-1 and AP-3 binding ability; enlargement is not seen when the fusion protein contains the dileucine motif of Tyrp1, which binds only AP-1 (data not shown). Thus, in this case a functional alteration of the target organelle seems to correlate with the cargo protein's capacity for interaction with multiple AP proteins. How differences in affinity might distinguish melanosomal from lysosomal targeting in melanocytes remains to be elucidated. Moreover, whether the two adaptor proteins distinguish the dileucine motifs by virtue of differences in their sequence or in their position within the protein is not clear. Future experiments will more closely probe the sequence of events in the trafficking of OCA2 to define the relationship between melanosomal and lysosomal targeting and the role each plays in OCA2 function.
Supplementary Material
ACKNOWLEDGMENTS
We thank D. C. Bennett and E. V. Sviderskaya (St. George's, University of London, London, United Kingdom) for the generous gifts of the melan-p1 and melan-Ink4a cell lines and J. S. Bonifacino (National Institute of Child Health and Human Development, NIH, Bethesda, MD) for the pallidin-HA and Tac-E19 plasmids. This work was supported by National Institutes of Health grants EY-015625 (to M.S.M.) and EY-014540 (to M.V.S.) from the National Eye Institute and HL-068117 from the National Heart Lung and Blood Institute (to E.C.D.). A. S. was supported in part by the Training Program in Cell and Molecular Biology, T32 GM007229, from the National Institutes of Health.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-07-0710) on December 30, 2008.
REFERENCES
- Ancans J., Hoogduijn M. J., Thody A. J. Melanosomal pH, pink locus protein and their roles in melanogenesis. J. Invest. Dermatol. 2001;117:158–159. doi: 10.1046/j.0022-202x.2001.01397.x. [DOI] [PubMed] [Google Scholar]
- Berson J. F., Frank D. W., Calvo P. A., Bieler B. M., Marks M. S. A common temperature-sensitive allelic form of human tyrosinase is retained in the endoplasmic reticulum at the nonpermissive temperature. J. Biol. Chem. 2000;275:12281–12289. doi: 10.1074/jbc.275.16.12281. [DOI] [PubMed] [Google Scholar]
- Berson J. F., Harper D. C., Tenza D., Raposo G., Marks M. S. Pmel17 initiates premelanosome morphogenesis within multivesicular bodies. Mol. Biol. Cell. 2001;12:3451–3464. doi: 10.1091/mbc.12.11.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino J. S., Traub L. M. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- Bouchard B., Fuller B. B., Vijayasaradhi S., Houghton A. N. Induction of pigmentation in mouse fibroblasts by expression of human tyrosinase cDNA. J. Exp. Med. 1989;169:2029–2042. doi: 10.1084/jem.169.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilliant M. H. The mouse p (pink-eyed dilution) and human P genes, oculocutaneous albinism type 2 (OCA2), and melanosomal pH. Pigment Cell Res. 2001;14:86–93. doi: 10.1034/j.1600-0749.2001.140203.x. [DOI] [PubMed] [Google Scholar]
- Calvo P. A., Frank D. W., Bieler B. M., Berson J. F., Marks M. S. A cytoplasmic sequence in human tyrosinase defines a second class of di-leucine-based sorting signals for late endosomal and lysosomal delivery. J. Biol. Chem. 1999;274:12780–12789. doi: 10.1074/jbc.274.18.12780. [DOI] [PubMed] [Google Scholar]
- Chapuy B., Tikkanen R., Mülhausen C., Wenzel D., von Figura K., Höning S. AP-1 and AP-3 mediate sorting of melanosomal and lysosomal membrane proteins into distinct post-Golgi trafficking pathways. Traffic. 2008;9:1157–1172. doi: 10.1111/j.1600-0854.2008.00745.x. [DOI] [PubMed] [Google Scholar]
- Chaudhuri R., Lindwasser O. W., Smith W. J., Hurley J. H., Bonifacino J. S. Downregulation of CD4 by human immunodeficiency virus type 1 Nef is dependent on clathrin and involves direct interaction of Nef with the AP2 clathrin adaptor. J. Virol. 2007;81:3877–3890. doi: 10.1128/JVI.02725-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Manga P., Orlow S. J. Pink-eyed dilution protein controls the processing of tyrosinase. Mol. Biol. Cell. 2002;13:1953–1964. doi: 10.1091/mbc.02-02-0022.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuomo M., Nicotra M. R., Apollonj C., Fraioli R., Giacomini P., Natali P. G. Production and characterization of the murine monoclonal antibody 2G10 to a human T4-tyrosinase epitope. J. Invest. Dermatol. 1991;96:446–451. doi: 10.1111/1523-1747.ep12470092. [DOI] [PubMed] [Google Scholar]
- d'Addio M., Pizzigoni A., Bassi M. T., Baschirotto C., Valetti C., Incerti B., Clementi M., De Luca M., Ballabio A., Schiaffino M. V. Defective intracellular transport and processing of OA1 is a major cause of ocular albinism type 1. Hum. Mol. Genet. 2000;9:3011–3018. doi: 10.1093/hmg/9.20.3011. [DOI] [PubMed] [Google Scholar]
- Dell'Angelica E. C., Mullins C., Caplan S., Bonifacino J. S. Lysosome-related organelles. FASEB J. 2000;14:1265–1278. doi: 10.1096/fj.14.10.1265. [DOI] [PubMed] [Google Scholar]
- Di Pietro S. M., Dell'Angelica E. C. The cell biology of Hermansky-Pudlak syndrome: recent advances. Traffic. 2005;6:525–533. doi: 10.1111/j.1600-0854.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- Di Pietro S. M., Falcon-Perez J. M., Tenza D., Setty S. R., Marks M. S., Raposo G., Dell'Angelica E. C. BLOC-1 interacts with BLOC-2 and the AP-3 complex to facilitate protein trafficking on endosomes. Mol. Biol. Cell. 2006;17:4027–4038. doi: 10.1091/mbc.E06-05-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donatien P. D., Orlow S. J. Interaction of melanosomal proteins with melanin. Eur. J. Biochem. 1995;232:159–164. doi: 10.1111/j.1432-1033.1995.tb20794.x. [DOI] [PubMed] [Google Scholar]
- Doray B., Lee I., Knisely J., Bu G., Kornfeld S. The gamma/sigma1 and alpha/sigma2 hemicomplexes of clathrin adaptors AP-1 and AP-2 harbor the dileucine recognition site. Mol. Biol. Cell. 2007;18:1887–1896. doi: 10.1091/mbc.E07-01-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiberg H., Troelsen J., Nielsen M., Mikkelsen A., Mengel-From J., Kjaer K. W., Hansen L. Blue eye color in humans may be caused by a perfectly associated founder mutation in a regulatory element located within the HERC2 gene inhibiting OCA2 expression. Hum. Genet. 2008;123:177–187. doi: 10.1007/s00439-007-0460-x. [DOI] [PubMed] [Google Scholar]
- Ginger R. S., et al. SLC24A5 Encodes a trans-Golgi network protein with potassium-dependent sodium-calcium exchange activity that regulates human epidermal melanogenesis. J. Biol. Chem. 2008;283:5486–5495. doi: 10.1074/jbc.M707521200. [DOI] [PubMed] [Google Scholar]
- Granger B. L., Green S. A., Gabel C. A., Howe C. L., Mellman I., Helenius A. Characterization and cloning of lgp110, a lysosomal membrane glycoprotein from mouse and rat cells. J. Biol. Chem. 1990;265:12036–12043. [PubMed] [Google Scholar]
- Griffiths G. What's special about secretory lysosomes? Semin. Cell. Dev. Biol. 2002;13:279–284. doi: 10.1016/s1084-9521(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Groux-Degroote S., van Dijk S. M., Wolthoorn J., Neumann S., Theos A. C., De Maziere A. M., Klumperman J., van Meer G., Sprong H. Glycolipid-dependent sorting of melanosomal from lysosomal membrane proteins by lumenal determinants. Traffic. 2008;9:951–963. doi: 10.1111/j.1600-0854.2008.00740.x. [DOI] [PubMed] [Google Scholar]
- Helip-Wooley A., Westbroek W., Dorward H. M., Koshoffer A., Huizing M., Boissy R. E., Gahl W. A. Improper trafficking of melanocyte-specific proteins in Hermansky-Pudlak syndrome type-5. J. Invest. Dermatol. 2007;127:1471–1478. doi: 10.1038/sj.jid.5700737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi R., Krummel B., Saiki R. K. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988;16:7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoashi T., Muller J., Vieira W. D., Rouzaud F., Kikuchi K., Tamaki K., Hearing V. J. The repeat domain of the melanosomal matrix protein PMEL17/GP100 is required for the formation of organellar fibers. J. Biol. Chem. 2006;281:21198–21208. doi: 10.1074/jbc.M601643200. [DOI] [PubMed] [Google Scholar]
- Honing S., Sandoval I. V., von Figura K. A di-leucine-based motif in the cytoplasmic tail of LIMP-II and tyrosinase mediates selective binding of AP-3. EMBO J. 1998;17:1304–1314. doi: 10.1093/emboj/17.5.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizing M., Sarangarajan R., Strovel E., Zhao Y., Gahl W. A., Boissy R. E. AP-3 mediates tyrosinase but not TRP-1 trafficking in human melanocytes. Mol. Biol. Cell. 2001;12:2075–2085. doi: 10.1091/mbc.12.7.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. R., Nilsson T., Peterson P. A. Identification of a consensus motif for retention of transmembrane proteins in the endoplasmic reticulum. EMBO J. 1990;9:3153–3162. doi: 10.1002/j.1460-2075.1990.tb07513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janvier K., Kato Y., Boehm M., Rose J. R., Martina J. A., Kim B. Y., Venkatesan S., Bonifacino J. S. Recognition of dileucine-based sorting signals from HIV-1 Nef and LIMP-II by the AP-1 gamma-sigma1 and AP-3 delta-sigma3 hemicomplexes. J. Cell Biol. 2003;163:1281–1290. doi: 10.1083/jcb.200307157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao O., de Gruijter J. M., van Duijn K., Navarro A., Kayser M. Signatures of positive selection in genes associated with human skin pigmentation as revealed from analyses of single nucleotide polymorphisms. Ann. Hum. Genet. 2007;71:354–369. doi: 10.1111/j.1469-1809.2006.00341.x. [DOI] [PubMed] [Google Scholar]
- Lee S. T., Nicholls R. D., Jong M. T., Fukai K., Spritz R. A. Organization and sequence of the human P gene and identification of a new family of transport proteins. Genomics. 1995;26:354–363. doi: 10.1016/0888-7543(95)80220-g. [DOI] [PubMed] [Google Scholar]
- Manga P., Boissy R. E., Pifko-Hirst S., Zhou B.-K., Orlow S. J. Mislocalization of melanosomal proteins in melanocytes from mice with oculocutaneous albinism type 2. Exp. Eye Res. 2001;72:695–710. doi: 10.1006/exer.2001.1006. [DOI] [PubMed] [Google Scholar]
- Marks M. S., Woodruff L., Ohno H., Bonifacino J. S. Protein targeting by tyrosine- and di-leucine-based signals: evidence for distinct saturable components. J. Cell Biol. 1996;135:341–354. doi: 10.1083/jcb.135.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama K., Bonifacino J. S. Pallidin is a component of a multi-protein complex involved in the biogenesis of lysosome-related organelles. Traffic. 2002;3:666–677. doi: 10.1034/j.1600-0854.2002.30908.x. [DOI] [PubMed] [Google Scholar]
- Nazarian R., Huizing M., Helip-Wooley A., Starcevic M., Gahl W. A., Dell'Angelica E. C. An immunoblotting assay to facilitate the molecular diagnosis of Hermansky-Pudlak syndrome. Mol. Genet. Metab. 2008;93:134–144. doi: 10.1016/j.ymgme.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton H. L., Kittles R. A., Parra E., McKeigue P., Mao X., Cheng K., Canfield V. A., Bradley D. G., McEvoy B., Shriver M. D. Genetic evidence for the convergent evolution of light skin in Europeans and East Asians. Mol. Biol. Evol. 2007;24:710–722. doi: 10.1093/molbev/msl203. [DOI] [PubMed] [Google Scholar]
- Oetting W. S., Gardner J. M., Fryer J. P., Ching A., Durham-Pierre D., King R. A., Brilliant M. H. Mutations of the human P gene associated with type II oculocutaneous albinism (OCA2). Mutations in brief no. 205. Online. Hum. Mutat. 1998;12:434. doi: 10.1002/(SICI)1098-1004(1998)12:6<434::AID-HUMU16>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Peters C., Mayer A. Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature. 1998;396:575–580. doi: 10.1038/25133. [DOI] [PubMed] [Google Scholar]
- Piccirillo R., Palmisano I., Innamorati G., Bagnato P., Altimare D., Schiaffino M. V. An unconventional dileucine-based motif and a novel cytosolic motif are required for the lysosomal and melanosomal targeting of OA1. J. Cell Sci. 2006;119:2003–2014. doi: 10.1242/jcs.02930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor P. R., Mullock B. M., Bright N. A., Gray S. R., Luzio J. P. The role of intraorganellar Ca(2+) in late endosome-lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles. J. Cell Biol. 2000;149:1053–1062. doi: 10.1083/jcb.149.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri N., Gardner J. M., Brilliant M. H. Aberrant pH of melanosomes in pink-eyed dilution(p) mutant melanocytes. J. Invest. Dermatol. 2000;115:607–613. doi: 10.1046/j.1523-1747.2000.00108.x. [DOI] [PubMed] [Google Scholar]
- Raposo G., Marks M. S. Melanosomes–dark organelles enlighten endosomal membrane transport. Nat. Rev. Mol. Cell Biol. 2007;8:786–797. doi: 10.1038/nrm2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G., Marks M. S., Cutler D. F. Lysosome-related organelles: driving post-Golgi compartments into specialisation. Curr. Opin. Cell Biol. 2007;19:394–401. doi: 10.1016/j.ceb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond B., Huizing M., Knapp J., Koshoffer A., Zhao Y., Gahl W. A., Boissy R. E. Melanocytes derived from patients with Hermansky-Pudlak Syndrome types 1, 2, and 3 have distinct defects in cargo trafficking. J. Invest. Dermatol. 2005;124:420–427. doi: 10.1111/j.0022-202X.2004.23585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinchik E. M., Bultman S. J., Horsthemke B., Lee S. T., Strunk K. M., Spritz R. A., Avidano K. M., Jong M. T., Nicholls R. D. A gene for the mouse pink-eyed dilution locus and for human type II oculocutaneous albinism. Nature. 1993;361:72–76. doi: 10.1038/361072a0. [DOI] [PubMed] [Google Scholar]
- Rosemblat S., Durham-Pierre D., Gardner J. M., Nakatsu Y., Brilliant M. H., Orlow S. J. Identification of a melanosomal membrane protein encoded by the pink-eyed dilution (type II oculocutaneous albinism) gene. Proc. Natl. Acad. Sci. USA. 1994;91:12071–12075. doi: 10.1073/pnas.91.25.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosemblat S., Sviderskaya E. V., Easty D. J., Wilson A., Kwon B. S., Bennett D. C., Orlow S. J. Melanosomal defects in melanocytes from mice lacking expression of the pink-eyed dilution gene: correction by culture in the presence of excess tyrosine. Exp. Cell Res. 1998;239:344–352. doi: 10.1006/excr.1997.3901. [DOI] [PubMed] [Google Scholar]
- Schutze M. P., Peterson P. A., Jackson M. R. An N-terminal double-arginine motif maintains type II membrane proteins in the endoplasmic reticulum. EMBO J. 1994;13:1696–1705. doi: 10.1002/j.1460-2075.1994.tb06434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setty S. R., et al. BLOC-1 is required for cargo-specific sorting from vacuolar early endosomes toward lysosome-related organelles. Mol. Biol. Cell. 2007;18:768–780. doi: 10.1091/mbc.E06-12-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman R. L., Pearlstein R., Waymouth C. Pink-eyed dilution (p) gene in rodents: increased pigmentation in tissue culture. Dev. Biol. 1965;12:93–116. doi: 10.1016/0012-1606(65)90023-0. [DOI] [PubMed] [Google Scholar]
- Simmen T., Schmidt A., Hunziker W., Beermann F. The tyrosinase tail mediates sorting to the lysosomal compartment in MDCK cells via a di-leucine and a tyrosine-based signal. J. Cell Sci. 1999;112:45–53. doi: 10.1242/jcs.112.1.45. [DOI] [PubMed] [Google Scholar]
- Sprong H., Degroote S., Claessens T., van Drunen J., Oorschot V., Westerink B. H., Hirabayashi Y., Klumperman J., van der Sluijs P., van Meer G. Glycosphingolipids are required for sorting melanosomal proteins in the Golgi complex. J. Cell Biol. 2001;155:369–380. doi: 10.1083/jcb.200106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staleva L., Manga P., Orlow S. J. Pink-eyed dilution protein modulates arsenic sensitivity and intracellular glutathione metabolism. Mol. Biol. Cell. 2002;13:4206–4220. doi: 10.1091/mbc.E02-05-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starcevic M., Dell'Angelica E. C. Identification of snapin and three novel proteins (BLOS1, BLOS2, and BLOS3/reduced pigmentation) as subunits of biogenesis of lysosome-related organelles complex-1 (BLOC-1) J. Biol. Chem. 2004;279:28393–28401. doi: 10.1074/jbc.M402513200. [DOI] [PubMed] [Google Scholar]
- Sturm R. A., Duffy D. L., Zhao Z. Z., Leite F. P., Stark M. S., Hayward N. K., Martin N. G., Montgomery G. W. A single SNP in an evolutionary conserved region within intron 86 of the HERC2 gene determines human blue-brown eye color. Am. J. Hum. Genet. 2008;82:424–431. doi: 10.1016/j.ajhg.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulem P., et al. Genetic determinants of hair, eye and skin pigmentation in Europeans. Nat. Genet. 2007;39:1443–1452. doi: 10.1038/ng.2007.13. [DOI] [PubMed] [Google Scholar]
- Sviderskaya E. V., Bennett D. C., Ho L., Bailin T., Lee S. T., Spritz R. A. Complementation of hypopigmentation in p-mutant (pink-eyed dilution) mouse melanocytes by normal human P cDNA, and defective complementation by OCA2 mutant sequences. J. Invest. Dermatol. 1997;108:30–34. doi: 10.1111/1523-1747.ep12285621. [DOI] [PubMed] [Google Scholar]
- Sviderskaya E. V., Hill S. P., Evans-Whipp T. J., Chin L., Orlow S. J., Easty D. J., Cheong S. C., Beach D., DePinho R. A., Bennett D. C. p16(Ink4a) in melanocyte senescence and differentiation. J. Natl. Cancer Inst. 2002;94:446–454. doi: 10.1093/jnci/94.6.446. [DOI] [PubMed] [Google Scholar]
- Theos A. C., et al. Dual loss of ER export and endocytic signals with altered melanosome morphology in the silver mutation of Pmel17. Mol. Biol. Cell. 2006a;17:3598–3612. doi: 10.1091/mbc.E06-01-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theos A. C., et al. Functions of adaptor protein (AP)-3 and AP-1 in tyrosinase sorting from endosomes to melanosomes. Mol. Biol. Cell. 2005;16:5356–5372. doi: 10.1091/mbc.E05-07-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theos A. C., Truschel S. T., Tenza D., Hurbain I., Harper D. C., Berson J. F., Thomas P. C., Raposo G., Marks M. S. A lumenal domain-dependent pathway for sorting to intralumenal vesicles of multivesicular endosomes involved in organelle morphogenesis. Dev. Cell. 2006b;10:343–354. doi: 10.1016/j.devcel.2006.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend D., Guillery P., King R. A. Optimized assay for mammalian tyrosinase (polyhydroxyl phenyloxidase) Anal. Biochem. 1984;139:345–352. doi: 10.1016/0003-2697(84)90015-0. [DOI] [PubMed] [Google Scholar]
- Toyofuku K., Valencia J. C., Kushimoto T., Costin G. E., Virador V. M., Vieira W. D., Ferrans V. J., Hearing V. J. The etiology of oculocutaneous albinism (OCA) type II: the pink protein modulates the processing and transport of tyrosinase. Pigment Cell Res. 2002;15:217–224. doi: 10.1034/j.1600-0749.2002.02007.x. [DOI] [PubMed] [Google Scholar]
- Ungermann C., Wickner W., Xu Z. Vacuole acidification is required for trans-SNARE pairing, LMA1 release, and homotypic fusion. Proc. Natl. Acad. Sci. USA. 1999;96:11194–11199. doi: 10.1073/pnas.96.20.11194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayasaradhi S., Xu Y., Bouchard B., Houghton A. N. Intracellular sorting and targeting of melanosomal membrane proteins: identification of signals for sorting of the human brown locus protein, gp75. J. Cell Biol. 1995;130:807–820. doi: 10.1083/jcb.130.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N., Hebert D. N. Tyrosinase maturation through the mammalian secretory pathway: bringing color to life. Pigment Cell Res. 2006;19:3–18. doi: 10.1111/j.1600-0749.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- Wasmeier C., Romao M., Plowright L., Bennett D. C., Raposo G., Seabra M. C. Rab38 and Rab32 control post-Golgi trafficking of melanogenic enzymes. J. Cell Biol. 2006;175:271–281. doi: 10.1083/jcb.200606050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M. L. Hermansky-Pudlak syndrome: a disease of protein trafficking and organelle function. Pigment Cell Res. 2006;19:19–42. doi: 10.1111/j.1600-0749.2005.00289.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.