Abstract
Apoptotic cells (AC) are rapidly engulfed by professional phagocytes such as macrophages to avoid secondary necrosis and thus inflammation. Recognition of AC polarizes macrophages toward an anti-inflammatory phenotype, which shows homology to an alternatively activated M2 macrophage. However, mechanistic details provoking these phenotype alterations are incompletely understood. Here, we demonstrate a biphasic up-regulation of heme oxygenase-1 (HO-1), a protein that bears an antiapoptotic as well as an anti-inflammatory potential, in primary human macrophages, which were exposed to the supernatant of AC. Although the first phase of HO-1 induction at 6 h was accomplished by AC-derived sphingosine-1-phosphate (S1P) acting via S1P receptor 1, the second wave of HO-1 induction at 24 h was attributed to autocrine signaling of vascular endothelial growth factor A (VEGFA), whose expression and release were facilitated by S1P. Whereas VEGFA release from macrophages was signal transducer and activator of transcription (STAT) 1-dependent, vascular endothelial growth factor itself triggered STAT1/STAT3 heterodimer formation, which bound to and activated the HO-1 promoter. Knockdown of HO-1 proved its relevance in facilitating enhanced expression of the antiapoptotic proteins Bcl-2 and Bcl-XL, as well as the anti-inflammatory adenosine receptor A2A. These findings suggest that HO-1, which is induced by AC-derived S1P, is critically involved in macrophage polarization toward an M2 phenotype.
INTRODUCTION
Macrophages, as innate immune competent cells, participate in a multitude of physiological as well as pathophysiological settings, which is a result of their extreme functional plasticity. Distinct forms of macrophage activation provoke a continuum of functional responses that range from pro- toward anti-inflammatory outcomes. Macrophages are classically activated by microbial cell wall components and/or interferon-γ. The resulting phenotype is known as M1, which is characterized among others parameters by the production of proinflammatory mediators such as NO, superoxide, tumor necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6 (Gordon, 2003). Polarization toward the alternatively activated phenotype (M2 macrophage) is achieved by, e.g., glucocorticoids, IL-4, IL-13, or IL-10 (Mantovani et al., 2002; Gordon, 2003).
When cells enter the route of apoptotic cell death, phagocytosis of apoptotic debris by professional phagocytes such as macrophages avoids an inflammatory response (Rathmell and Thompson, 1999; Savill et al., 2002). During this process, the interaction of apoptotic cells (AC) with macrophages actively suppresses the release of proinflammatory mediators and provokes the formation of anti-inflammatory mediators. The mediator profile of these polarized macrophages resembles those of M2 cells, with the production of, e.g., IL-10, transforming growth factor-β1, or prostaglandin E2 (Voll et al., 1997; Fadok et al., 1998; Freire-de-Lima et al., 2000). Mechanisms attenuating proinflammatory signaling in macrophages by AC are at least in part attributed to defective lipopolysaccharide (LPS)-induced nuclear factor-κB activation, and thus inhibition of proinflammatory cytokine gene expression profiles (Cvetanovic and Ucker, 2004).
In contrast, intracellular pathways activating anti-inflammatory responses are widely elusive. Recently, we observed that AC released the bioactive lipid sphingosine-1-phosphate (S1P), which caused activation of survival pathways such as increased expression of the antiapoptotic proteins Bcl-2 and Bcl-XL in human macrophages (Weigert et al., 2006). Although pioneering studies identified S1P as a second messenger molecule mediating cell proliferation of Swiss 3T3 fibroblasts (Olivera and Spiegel, 1993), S1P was later identified as the ligand for a family of five different G protein-coupled receptors (Hla et al., 2001; Taha et al., 2004). Activation of S1P receptors exerts a powerful influence on a variety of immune cells and their responses (von Wenckstern et al., 2006). Moreover, S1P provoked M2 macrophage polarization either when added directly to cells (Hughes et al., 2008) or when being present in the supernatant of AC (Weigert et al., 2007).
An ideal candidate that would fulfill requirements of acting anti-inflammatory as well as antiapoptotic is heme oxygenase-1 (HO-1). HO-1 catalyzes the rate-limiting step in the oxidative degradation of heme to equimolar quantities of biliverdin, ferrous iron, and carbon monoxide (CO). In contrast to HO-2 and HO-3, which are constitutively expressed, HO-1 is inducible and is well known to protect from cell death, i.e., apoptosis, inflammation, and oxidative stress in vivo (Otterbein et al., 2000; Deshane et al., 2005; Kim et al., 2006). A connection between HO-1 induction and the S1P pathway was recently established in a model of hepatic ischemia-reperfusion injury, where the S1P receptor agonist FTY720 enhanced HO-1 expression in hepatocytes (Man et al., 2005).
MATERIALS AND METHODS
Cell Culture and Reagents
Jurkat T-cells were maintained in RPMI 1640 medium, supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% heat-inactivated fetal calf serum (FCS) (PAA Laboratories, Cölbe, Germany). SB203580 was obtained from Alexis (Lörrach, Germany); the Janus kinase (Jak) inhibitor I was purchased from Calbiochem (Darmstadt, Germany); S1P came from Avanti Polar Lipids (Alabaster, AL); fludarabine was obtained from Sigma-Aldrich (Steinheim, Germany); STA-21 was delivered by Biomol (Hamburg, Germany); and diethylenetriamine-NO (Deta-NO), bilirubin and tricarbonyldichloro ruthenium(II) dimer (CORM-2) were obtained from Sigma-Aldrich (Steinheim, Germany). CORM-2 was freshly dissolved in dimethyl sulfoxide for each experiment. As a negative control, CORM-2 was inactivated (iCORM-2) according to a previously described method (Sun et al., 2008). Trypan blue staining (Biochrom AG, Berlin, Germany) revealed that all used reagents were not toxic for macrophages.
Human Monocyte Isolation and Culture
Human monocytes were isolated as described previously (Weigert et al., 2006). In brief, using Ficoll-Hypaque gradients (PAA Laboratories) monocytes were isolated from buffy coats (DRK-Blutspendedienst Baden-Württemberg-Hessen, Institut für Transfusionsmedizin und Immunhämatologie Frankfurt am Main, Frankfurt/Main, Germany). Peripheral blood mononuclear cells were washed twice with phosphate-buffered saline (PBS) containing 2 mM EDTA and subsequently incubated for 1 h at 37°C in RPMI 1640 medium supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin to allow their adherence to culture dishes (Sarstedt, Nümbrecht, Germany). After removing nonadherent cells, monocytes were differentiated to macrophages with RPMI 1640 medium containing 10% AB-positive human plasma (DRK-Blutspendedienst Baden-Württemberg-Hessen, Institut für Transfusionsmedizin und Immunhämatologie Frankfurt am Main) for 7 d.
Generation of Conditioned Media (CM)
Apoptosis in Jurkat cells, cultured in RPMI 1640 medium without FCS, was induced with 0.5 μg/ml staurosporine (Sigma-Aldrich, Steinheim, Germany) for 3 h (Weigert et al., 2006). Necrotic Jurkat cells were generated by incubating cells for 30 min at 56°C in RPMI 1640 medium with FCS (Weigert et al., 2006). AC or necrotic cells (NC) were washed twice with PBS and incubated for another 3-h period in RPMI 1640 medium with 10% AB-positive human plasma. CM were harvested by centrifugation (1000 × g; 10 min) and filtration through 0.22-μm pore filters (Millipore, Schwalbach, Germany) to remove apoptotic bodies. The procedure to generate CM from viable cells (VC) was equivalent. Jurkat cells were incubated in RPMI 1640 medium with FCS, omitting a death stimulus. To obtain AC-CM without S1P, we used 20 μM dimethylsphingosine (DMS; Biomol), an inhibitor of sphingosine kinases. DMS was added simultaneously with staurosporine to Jurkat cells during initiation of apoptosis (Weigert et al., 2006). To generate macrophage CM (MΦ-CM), macrophages were incubated with AC-CM for 2 h, washed twice with PBS, and incubated for another 4-h period in RPMI 1640 medium with 10% AB-positive human plasma. MΦ-CM was harvested by centrifugation (1000 × g; 10 min). For protein degradation, MΦ-CM was incubated with 50 μg/ml proteinase K (Sigma-Aldrich, Steinheim, Germany) at 37°C for 1 h, followed by incubation at 100°C for 1 h. Cells were exposed to conditioned media for the times indicated. Inhibitors were preincubated for 1 h each. CM was generated using 1 × 107 Jurkat cells and later on added to 2 × 106 macrophages (ratio 5:1). MΦ-CM was directly transferred from generator to recipient cells.
Western Blot Analysis
Western blot analysis was performed as described previously (von Knethen et al., 2005). Polyclonal antibodies directed against HO-1 (Biomol), S1P1 (Orbigen, San Diego, CA), adenosine receptor A2A (Adora A2A) (Calbiochem, Darmstadt, Germany), Bcl-XL (BD Biosciences Transduction Laboratories, Lexington, KY), and Actin (Sigma-Aldrich) were used. Western blots were quantified using Odyssey infrared imaging system (Li-Cor Biosciences, Bad Homburg, Germany).
RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (PCR)
RNA from primary human macrophages was extracted using peqGold RNAPure (Peqlab Biotechnologie, Erlangen, Germany). Total RNA (1 μg) was transcribed with iScript cDNA synthesis kit (Bio-Rad Laboratories, München, Germany). Quantitative real-time PCR was performed using MyIQ real-time PCR system (Bio-Rad Laboratories) and Absolute Blue QPCR SYBR Green fluorescein mix (Thermo Scientific, Karlsruhe, Germany). The following primers (Biomers, Ulm, Germany) were used for quantitative real-time-PCR: human HO-1, 5′-GCC ACC AAG TTC AAG CAG CT-3′, 5′-CAG TGC CCA CGG TAA GGA AG-3′; human vascular endothelial growth factor (VEGF), 5′-TAC CTC CAC CAT GCC AAG TG-3′, 5′-AAG ATG TCC ACC AGG GTC TC-3′; and human actin, 5′-TGA CGG GGT CAC CCA CAC TGT GCC CAT CTA-3′, 5′-CTA GAA GCA TTT GCG GTG GAC GAT GGA GGG-3′. For Bcl-2, Bcl-XL, Adora A2A, indoleamine-2,3-dioxygenase (IDO), HLA-DMB, and 18S RNA, validated QuantiTect Primer Assays were purchased from QIAGEN (Hilden, Germany). Real-time PCR results were quantified using Gene Expression Macro (version 1.1) from Bio-Rad (München, Germany), with actin or 18S RNA expression as internal control.
Reporter Analysis
Reporter assays were performed with vector constructs containing 4000 base pairs, 2782 base pairs, or 1976 base pairs of the human HO-1 promoter fused to a firefly luciferase gene (Takahashi et al., 1999). The signal transducer and activator of transcription (STAT) 3 point mutation was created with the QuikChange II site-directed mutagenesis kit (Stratagene, La Jolla, CA). Experimentally, 10% of one buffy coat monocyte isolation preparation was seeded in one 12-well plate. After 7 d, macrophages were cotransfected with HO-1 promoter constructs and Renilla luciferase control vector pRL-CMV (Promega, Mannheim, Germany) by using Jet Pei transfection reagent (Polyplus transfection, Illkirch, France). After transfection, cells were incubated for 24 h, medium was changed, and cells were incubated for another 24 h followed by individual stimulation. Firefly luciferase activity normalized to Renilla luciferase activity was determined after 18-h incubations with MΦ-CM or after 24 h after incubations with 100 nM S1P.
Site-directed Mutagenesis
The online tool TFSearch (http://www.cbrc.jp/research/db/TFSEARCH.html) was used to identify potential STAT binding sites in the human HO-1 promoter (STAT response element [STATx]). QuikChange II XL site-directed mutagenesis kit (Stratagene) was used to introduce a point mutation of the putative STAT binding site at position −2361 to −2369 within the human HO-1 promoter, to impair STAT3 binding. The following primers (Biomers) were used to mutate the sequence from 5′-TTC CAG GAA-3′ to 5′-TTC CAG GCC-3′: 5′-CCA GGC ACT ATT CCA GGC CCT GGG AAT TTA CAA AGC-3′ and 5′-GCT TTG TAA ATT CCC AGG GCC TGG AAT AGT GCC TGG-3′. Elongation was performed at 68°C for 15 min. Site-directed mutagenesis was confirmed by sequencing (Agowa, Berlin, Germany).
Electrophoretic Mobility Shift Assay (EMSA)
Nuclear extracts were prepared as described previously (Von Knethen and Brune, 2001) and an established EMSA method (Weigert et al., 2007) was used. Briefly, 10 μg of nuclear protein was incubated for 30 min at room temperature with 2 μg of poly(dI-dC) from Amersham Biosciences (Freiburg, Germany), 2 μl of buffer D (20 mM HEPES/KOH, 20% glycerol, 100 mM KCl, 0.5 mM EDTA, 0.25% Nonidet P-40, 2 mM dithiothreitol [DTT], and 0.5 mM phenylmethylsulfonyl fluoride [PMSF], pH 7.9), 4 μl of buffer F (20% Ficoll-400, 100 mM HEPES/KOH, 300 mM KCl, 10 mM DTT, and 0.5 mM PMSF, pH 7.9), 250 fmol of 5′-IRD700-labeled oligonucleotide (Metabion, Planegg-Martinsried, Germany), and in the case of competitive EMSA in addition with 2500 or 25,000 fmol of unlabeled oligonucleotide in a final volume of 20 μl. Afterward samples were incubated on ice for 5 min. DNA–protein complexes were resolved on native 6% polyacrylamide gels and analyzed with Odyssey infrared imaging system (Li-Cor Biosciences). Oligonucleotides including the sequence of the putative STAT binding site at −2361 to −2369 of the human HO-1 promoter were used: 5′-IRD700-AGG CAC TAT TCC AGG AAC TGG GAA T-3′; 5′-IRD700-ATT CCC AGT TCC TGG AAT AGT GCC T-3′. For competitive EMSA, additional unlabeled oligonucleotides specific for STAT1 and STAT3 were used (Biomers): STAT1, 5′-CAT GTT ATG CAT ATT CCT GTA AGT-3′, 5′-ACT TAC AGG AAT ATG CAT AAC ATG-3′; and STAT3, 5′-GAT CCT TCT GGG AAT TCC TAG ATC-3′ 5′-GAT CTA GGA ATT CCC AGA AGG ATC-3′. Competitive EMSA was also performed with unlabeled oligonucleotides including the sequence of the putative STAT-binding site at −2361 to −2369, which included a point mutation to impair STAT3 binding (underlined): 5′-AGG CAC TAT TCC AGG CCC TGG GAA T-3′ and 5′-ATT CCC AGG GCC TGG AAT AGT GCC T-3′ (Metabion).
VEGF Quantitation in Cell Culture Supernatants
We incubated 5 × 105 cells with AC-CM for 2 h, washed cells twice with PBS, and incubated for another 16-h period. Supernatants were harvested by centrifugation (16,000 × g; 10 min). For the measurement of secreted VEGF, we used human VEGF Cytometric Bead Array Flex Sets (BD Biosciences, Heidelberg, Germany). Samples were analyzed with FACSCanto flow cytometer and quantitated using FCAP software (BD Biosciences).
Small Interfering RNA (siRNA) Transfections
siRNAs against HO-1 (Hs_HMOX1_5_HP_ Validated siRNA; QIAGEN), VEGF A (VEGFA) (Hs_VEGF_5_HP_ Validated siRNA; QIAGEN) or S1P1 (Ambion, Austin, TX) were nucleofected into 1.5 × 106 primary human macrophages using Nucleofector Technology (Amaxa, Köln, Germany). HO-1 and S1P1 knockdown in comparison with siControl nontargeting Duplex #1 (Dharmacon RNA Technologies, Lafayette, CO) was controlled by Western blot analysis. The knockdown of HO-1 as well as VEGF was routinely confirmed by quantitative real-time PCR.
Statistical Analysis
Each experiment was performed at least three times. The p values were calculated using the paired Student's t test and considered significant at *p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.001.
RESULTS
Apoptotic Cell Supernatants Provoke a Biphasic Up-Regulation of HO-1
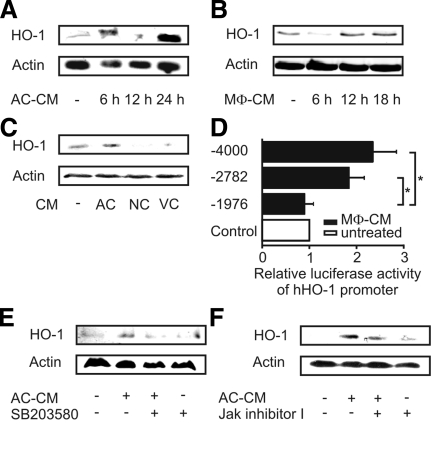
In a first set of experiments, we analyzed HO-1 protein expression in primary human macrophages after their exposure to AC-CM. HO-1 expression showed a biphasic response. A first peak was noticed after 6 h, whereas a second peak became detectable after 24-h lasting incubations (Figure 1A). To test whether the second peak of HO-1 expression was mediated by an autocrine factor, we harvested supernatants from macrophages (MΦ-CM), previously stimulated with AC-CM, and transferred MΦ-CM to fresh, resting macrophages. Indeed, not only AC-CM but also MΦ-CM caused HO-1 protein expression in primary human macrophages (Figure 1A and B). Pronounced HO-1 expression in response to MΦ-CM was observed after 12–18 h, which corresponded to the second peak of HO-1 expression in response to AC-CM, seen after 24 h. Importantly, HO-1 expression was only seen in response to AC-CM; it was not elicited by NC-CM or VC-CM (Figure 1C).
Figure 1.
Induction of HO-1 in primary human macrophages. (A and B) Western analysis of HO-1 expression after incubations of macrophages with AC-CM (A) or MΦ-CM (B) for times as indicated. (C) HO-1 expression in macrophages treated with CM of AC, NC, or VC cells for 24 h. (D) HO-1 promoter activity in primary human macrophages after transfection of individual reporter constructs and stimulation with MΦ-CM for 18 h. Firefly luciferase activity was normalized to Renilla luciferase activity. Data are means ± SEM of at least four independent experiments. Asterisks mark statistically significant differences (p ≤ 0.05). (E) HO-1 expression in primary human macrophages after incubation with AC-CM for 6 h in the presence or absence of 5 μM SB203580. (F). Treatment of macrophages with AC-CM for 24 h with 1 μM Jak inhibitor I being present. Western blots are representative for at least three individual experiments.
Induction of HO-1 by MΦ-CM after 18 h was further corroborated by reporter assays. Using the luciferase-coupled promoter constructs phHOLUC(-4000) as well as phHOLUC(-2782), we observed significant induction of luciferase activity after treatment with MΦ-CM for 18 h (Figure 1D). No activity was noticed with the shorter luciferase construct phHOLUC(-1976). Results so far indicate that apoptotic cell supernatants enhanced not only transcription of the HO-1 promoter but also caused protein expression, with the further notion that autocrine signaling was involved.
We then investigated signal transduction pathways contributing to HO-1 expression. Early expression of HO-1, seen at 6 h in response to AC-CM, was reduced by SB203580, an inhibitor of p38 MAPK (Figure 1E). Late phase HO-1 expression at 24 h was partially attenuated by inhibiting Janus kinase signaling (Figure 1F). Thus, the early HO-1 induction in response to an AC-CM–derived soluble factor was p38-mediated, whereas the late and second phase of HO-1 expression was facilitated by an autocrine factor, signaling via the Jak pathway.
S1P in AC-CM Is Crucial in Provoking HO-1 Induction
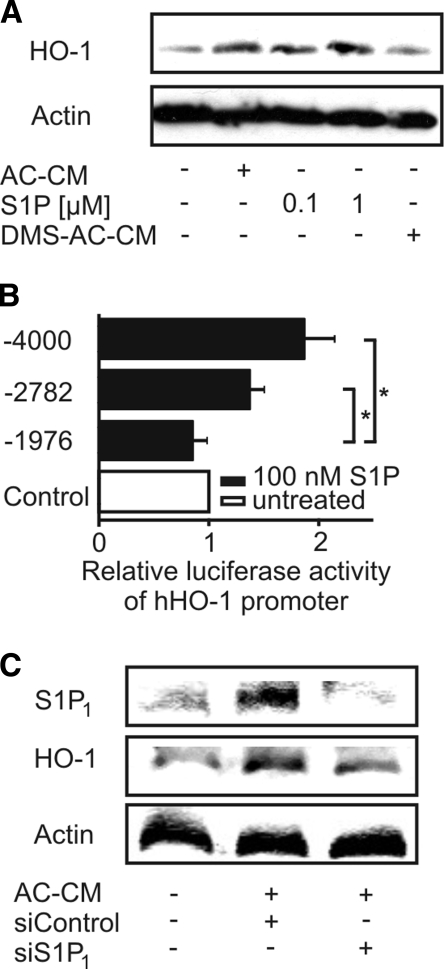
Considering that AC release S1P (Weigert et al., 2006; Gude et al., 2008) and the further notion that S1P potentially causes HO-1 expression (Man et al., 2005), we assumed that S1P in AC-CM was responsible for HO-1 expression in macrophages. To verify this hypothesis, we stimulated primary human macrophages with authentic S1P for 24 h (Figure 2A). S1P at 100 nM and 1 μM as well as AC-CM induced HO-1 protein expression. To validate the contribution of S1P in AC-CM, we used DMS, an inhibitor of sphingosine kinases to block their activity in Jurkat cells when generating AC-CM (Weigert et al., 2006). AC-CM generated in the presence of DMS failed in inducing HO-1 expression in macrophages (Figure 2A). Supporting data pointing to the contribution of S1P in HO-1 expression came from experiments when 100 nM S1P, supplied for 24 h, induced luciferase reporter activity. S1P induced phHOLUC(-4000) and phHOLUC(-2782) but not phHOLUC(-1976) HO-1 reporter activity (Figure 2B).
Figure 2.
Apoptotic cell-derived S1P mediates HO-1 induction. (A) Western analysis of HO-1 expression in primary human macrophages treated with AC-CM, S1P, or DMS-AC-CM for 24 h. DMS (20 μM) was used to block the release of S1P into the medium of apoptotic cells (DMS-AC-CM). (B) HO-1 promoter activity of corresponding reporter constructs in macrophages treated with 100 nM S1P for 24 h. Firefly luciferase activity normalized to Renilla luciferase activity is displayed. Data represent means ± SEM of at least three independent experiments. Asterisks mark statistically significant differences (p ≤ 0.05). (C) Macrophages were transfected with nontargeting siRNA or siRNA against S1P1. Western analysis of S1P1 and HO-1 was performed after 6-h treatments with AC-CM. Blots are representative for at least three individual experiments.
Recently, it was reported that activation of S1P1 limited the expression of proinflammatory cytokines (Hughes et al., 2008) and protected macrophages from apoptosis induced by the combination of TNF-α and cycloheximide (Weigert et al., 2006). Intrigued by these experiments, we knocked down S1P1 by using siRNA to assess its role in HO-1 induction by AC-CM. Although transfection of macrophages with nontargeting siRNA allowed HO-1 expression by AC-CM after 6 h, knockdown of S1P1 significantly reduced the HO-1 amount (Figure 2C). As a side effect, we noticed that AC-CM enhanced S1P1 expression in primary human macrophages, an effect suppressed by siRNA directed against S1P1 (Figure 2C).
STAT1 and STAT3 Provoke Transcription of the HO-1 Gene after AC-CM
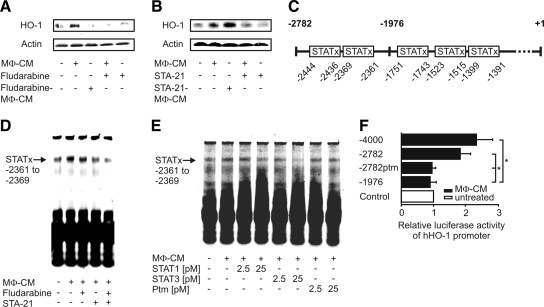
The induction of HO-1 after 24 h was Jak dependent (Figure 1F). Considering that HO-1 induction at 24 h was facilitated by an autocrine factor, we wanted to discern whether the release or the action of the putative autocrine factor would be Jak dependent. Therefore, we analyzed signaling downstream of Janus kinases, by using STAT1 and STAT3 inhibitors.
To attenuate the release of autocrine mediators, macrophages were preincubated for 1 h with either fludarabine, a specific inhibitor of STAT1 (Frank et al., 1999), or STA-21, a specific STAT3 inhibitor (Song et al., 2005), before addition of AC-CM. After a 2-h incubation period with AC-CM, macrophages were washed twice with PBS, followed by continuing incubations for 4 h in full medium, without the further addition of inhibitors. Thereafter, MΦ-CM was harvested from these cells and incubated with fresh, unstimulated macrophages. Inhibition of STAT1 during the production of MΦ-CM by fludarabine eliminated its potential to up-regulate HO-1, indicating that the production of the autocrine mediator was STAT1-dependent (Figure 3A, lane 3). In contrast, blocking STAT3 by STA-21 during the production of MΦ-CM did not reduce expression of HO-1 (Figure 3B, lane 3). Interestingly, inhibition of STAT1 or STAT3 attenuated HO-1 expression in response to MΦ-CM (Figure 3, A and B, lane 4), suggesting that the autocrine factor demands active STAT1 and STAT3 for signaling. With these initial data supporting a role of STAT1 and STAT3 in late phase (24 h) HO-1 expression by AC-CM, we screened the human HO-1 promoter for potential STAT binding sites (Figure 3C). Luciferase activity after treatment with MΦ-CM for 18 h was not induced when transfecting phHOLUC(-1976) into macrophages (Figure 1D), thus allowing to exclude three putative STAT binding sites located within this promoter construct as candidates involved in enhanced HO-1 transcription. Concerning the two remaining potentially critical STAT binding sites in the HO-1 promoter, only oligonucleotides resembling the STAT binding site at −2361 to −2369, but not the oligonucleotides containing the STAT binding site at −2436 to −2444 recruited transcription factors in EMSA analysis (Figure 3D). Supporting our observation that HO-1 expression by the autocrine factor present in MΦ-CM was inhibited by fludarabine and/or STA-21, EMSA analysis showed that transcription factor binding to the oligonucleotides spanning the STAT binding site at −2361 to −2369 was reduced when macrophages where incubated with fludarabine. Stronger inhibition was noticed when macrophages were treated with STA-21 before MΦ-CM stimulation, whereas the combined application of both STAT inhibitors reduced transcription factor binding most efficiently. These observations imply that STAT1/STAT3 heterodimer binding to the putative STAT binding site located at −2361 to −2369 at the human HO-1 promoter affects HO-1 induction after the treatment with MΦ-CM. To reinforce these results, we performed competitive EMSA analysis with unlabeled oligonucleotides specific for STAT1, STAT3, or with oligonucleotides for the putative STAT binding site at −2361 to −2369 containing a point mutation for STAT3 (Figure 3E). STAT binding to the oligonucleotides containing the STAT binding site at −2361 to −2369 was strongly reduced with increasing concentrations of the specific STAT1 and STAT3 oligonucleotides, which were added simultaneously. In contrast, STAT binding was not impaired when we used oligonucleotides with the STAT3 point mutation. In addition, we performed reporter assays in primary human macrophages with the construct phHOLUC(-2782ptm). This construct contained a point mutation within the STAT binding site at −2361 to −2369 to eliminate STAT3 binding (Figure 3F). Transfection of this construct into macrophages confirmed the results obtained by EMSA analysis, because luciferase activity elicited by MΦ-CM was significantly lower compared with transfection of the nonmutated phHOLUC(-2782).
Figure 3.
STAT1/STAT3 heterodimers mediate HO-1 promoter activation. Human primary macrophages were incubated for 6 h with AC-CM with or without the addition of 20 μM fludarabine (A) or 10 μM STA-21 (B). MΦ-CM was harvested and added to fresh macrophages for 18 h with or without the addition of fludarabine (A) or STA-21 (B). Western blots are representative for at least three individual experiments. (C) Putative STAT binding sites in the human HO-1 promoter are shown. (D) EMSA analysis using 250 fmol of the oligonucleotides resembling the putative STAT binding site at −2361 to −2369 of the human HO-1 promoter. (E) Competitive EMSA analysis using 2.5 or 25 pM of unlabeled oligonucleotides specific for STAT1, STAT3, or oligonucleotides for the putative STAT binding site at −2361 to −2369, which contained a STAT3 ptm, in addition. One representative EMSA out of three is displayed. (F) HO-1 promoter activity in macrophages after transfection of the corresponding promoter constructs and stimulation with MΦ-CM for 18 h. Histograms show firefly luciferase activity normalized to Renilla luciferase activity. Data represent means ± SEM of at least four independent experiments. Asterisks mark statistically significant differences (p ≤ 0.05).
Late-Phase HO-1 Induction in Macrophages Requires Autocrine VEGFA Signaling
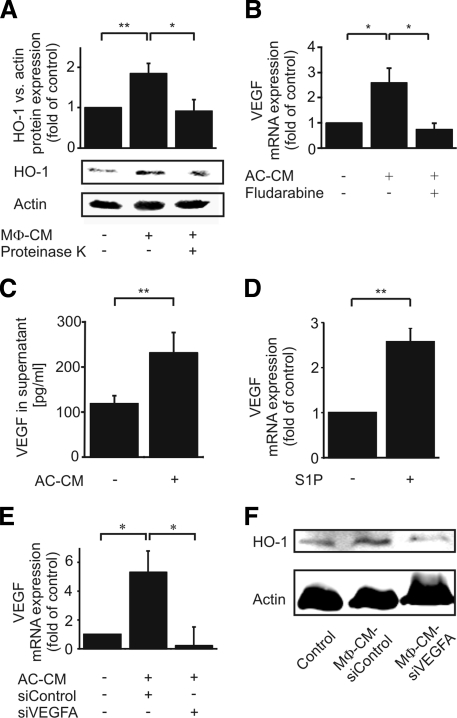
With the following experiments, we characterized the autocrine HO-1–inducing factor, released by macrophages, when treated with AC-CM. We degraded proteins in MΦ-CM with proteinase K digestion and subsequent denaturation. MΦ-CM, deprived by functional proteins, revealed a significantly lower ability to express HO-1 compared with untreated MΦ-CM (Figure 4A), implying that the autocrine factor might be a protein. As a candidate, we proposed VEGF. Our previous results (Figure 3A) suggested that the release of the autocrine factor mediating HO-1 induction was STAT dependent. This corresponds to the observations of Funamoto and colleagues, who identified VEGF as a STAT1 target gene in cardiac myocytes (Funamoto et al., 2000). Furthermore, VEGFA secretion from mouse mammary epithelial cells after phagocytosis of AC was demonstrated previously (Golpon et al., 2004) and signaling of VEGF in chronic lymphocytic leukemia B cells enhanced STAT1 and STAT3 actions (Lee et al., 2005). These observations match our data, showing that the autocrine protein factor in MΦ-CM–activated STAT1/STAT3 (Figure 3, A and B). Finally, VEGF induced HO-1 in a model of hyperoxic acute lung injury (Siner et al., 2007). With this background, we determined VEGF expression in macrophages in response to AC-CM. Indeed, VEGF mRNA was significantly elevated in macrophages stimulated with AC-CM for 1 h, compared with controls. Elevation of VEGF mRNA was blocked when macrophages were pretreated with fludarabine (Figure 4B). Additionally, we measured the release of VEGF into supernatants of AC-CM–treated macrophages by fluorescence-activated cell sorting analysis using human VEGF Cytometric Bead Array Flex Sets (Figure 4C). AC-CM–stimulated macrophages secreted significant amounts of VEGF protein. Accompanying experiments confirmed that 100 nM authentic S1P, incubated for 1 h, enhanced VEGF mRNA expression, supporting the notion that S1P in AC-CM may induce VEGF (Figure 4D). That HO-1 as well as VEGF expression was induced by S1P (Figures 2A and 4D) made the induction of HO-1 by autocrine VEGF signaling in primary human macrophages likely. To further scrutinize this hypothesis, we knocked down VEGF in primary human macrophages before their incubation with AC-CM (Figure 4E). Although VEGF mRNA was induced in macrophages after treatment with AC-CM, the response was abrogated by siRNA directed toward VEGFA (Figure 4E). Next, we analyzed the expression of HO-1 protein in macrophages, which were incubated with MΦ-CM generated from VEGF knockdown or siControl-transfected macrophages. When macrophages were transfected with nontargeting siRNA and incubated with AC-CM, MΦ-CM derived from these cells induced HO-1 in fresh, unstimulated cells. However, HO-1 was not induced with MΦ-CM from VEGFA knockdown macrophages (Figure 4F). These experiments suggest that VEGF mediated the autocrine induction of HO-1 after stimulation with AC-CM.
Figure 4.
HO-1 expression in human macrophages by autocrine VEGFA signaling. (A) Macrophages were controls or treated with either MΦ-CM or de-proteinated MΦ-CM (50 μg/ml proteinase K) for 18 h. One representative Western blot of seven is displayed. The graph shows the densitometric analysis. (B) VEGF mRNA expression after stimulation of macrophages with AC-CM or AC-CM together with 20 μM fludarabine for 1 h. (C) Quantitation of VEGF secretion by control or AC-CM-stimulated (18 h) macrophages. (D) VEGF mRNA expression in control or S1P-stimulated (100 nM; 1 h) macrophages. (E and F) Macrophages were transfected with nontargeting siRNA or siRNA against VEGFA. (E) VEGF mRNA expression in control or AC-CM-treated macrophages after 6 h. (F) Western analysis of HO-1 expression (18 h) after the treatment with MΦ-CM. One blot of three is displayed. Graphs display mean values ± SEM of at least four independent experiments, and asterisks indicate statistically significant differences *p ≤ 0.05 and **p ≤ 0.01.
HO-1 Affects Anti-Inflammatory and Antiapoptotic Pathways in Macrophages
HO-1 is known for its antiapoptotic and anti-inflammatory actions (Otterbein et al., 2000; Ryter and Otterbein, 2004; Deshane et al., 2005). Having recently shown that S1P from AC-CM induced Bcl-2 and Bcl-XL in macrophages (Weigert et al., 2006), we now asked whether induction of Bcl-2 and/or Bcl-XL required HO-1. Experimentally, we knocked down HO-1 in primary human macrophages by using siRNA technology. Transfection with siRNA directed against HO-1 efficiently blocked the mRNA increase of HO-1 after treatment with AC-CM for 9 h (Figure 5A). Although AC-CM up-regulated Bcl-2 and Bcl-XL mRNA in macrophages transfected with nontargeting siRNA, induction was significantly diminished when macrophages were transfected with siRNA against HO-1 (Figure 5B). This suggests a role of HO-1 in contributing to the antiapoptotic phenotype of macrophages elicited by AC-CM (Weigert et al., 2006).
Figure 5.
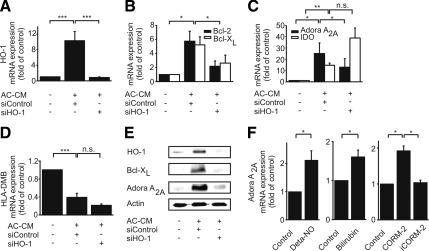
Expression regulation of Bcl-2, Bcl-XL, Adora A2A, IDO and HLA-DMB. (A–E) Macrophages were controls or transfected with siRNA against HO-1 or nontargeting siRNA. Graphs show mRNA expression of HO-1 (A), Bcl-2 and Bcl-XL (B), Adora A2A and IDO (C), and HLA-DMB in macrophages (D) after incubations with AC-CM for 9 h. Data show mean values ± SEM of at least five independent experiments. (E) Western analysis of HO-1, Bcl-XL, and Adora A2A expression in macrophages after stimulation with AC-CM for 16 h. One blot of three is shown. (F) Adora A2A mRNA expression after treatment with Deta-NO, bilirubin, or CORM-2. Primary human macrophages were incubated with 500 μM Deta-NO for 9 h, 10 μM bilirubin for 1 h, or 100 μM CORM-2 for 24 h. Graphs display mean values ± SEM of at least three independent experiments. Significant differences in mRNA expression are marked by *p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.001.
To determine whether increased HO-1 expression also conveyed anti-inflammatory properties in our system, we analyzed the expression of the markers Adora A2A, IDO, and HLA-DMB, which more generally are linked to anti-inflammatory responses in macrophages. Although the expression of Adora A2A was enhanced in control macrophages incubated with AC-CM, knockdown of HO-1 prevented this increase (Figure 5C). However, there was no correlation between the amount of HO-1 and expression of either IDO or HLA-DMB (Figure 5, C and D). Expression of IDO mRNA was enhanced in response to AC-CM in macrophages either transfected with nontargeting siRNA or with siRNA directed against HO-1 (Figure 5C). In contrast, HLA-DMB expression was decreased after stimulation with AC-CM, which was unaffected by knockdown of HO-1 (Figure 5D). In addition, we examined whether HO-1 affected protein expression of Bcl-XL and Adora A2A. Macrophages were transfected with nontargeting siRNA or HO-1–specific siRNA and incubated with AC-CM for 16 h. Both, Bcl-XL and Adora A2A were significantly induced by AC-CM in cells transfected with nontargeting siRNA, whereas their expression remained equivalent to controls in macrophages with siRNA-mediated knockdown of HO-1 (Figure 5E).
HO-1 is induced in inflammatory macrophages to initiate an anti-inflammatory negative feedback loop, dependent on LPS-induced NO production (Ashino et al., 2008). Thus, we investigated whether NO reproduced HO-1–dependent effects on gene expression in our system. Indeed, Deta-NO induced Adora A2A, although induction was less pronounced compared with AC-CM (Figures 5C and F). To examine which product of the HO-1–catalyzed reaction mediated Adora A2A induction, bilirubin and the CO-releasing molecule CORM-2 were used. Both agents significantly elevated Adora A2A mRNA expression (Figure 5F). However, expression was low compared with the impact of AC-CM. Interestingly, the inactivated, i.e., decomposed product of CORM-2 (iCORM-2) was without effect (Figure 5F). Considering that iron chelators showed no effect on Adora A2A mRNA expression (data not shown) excludes that ferrous iron, generated during heme degradation, is involved.
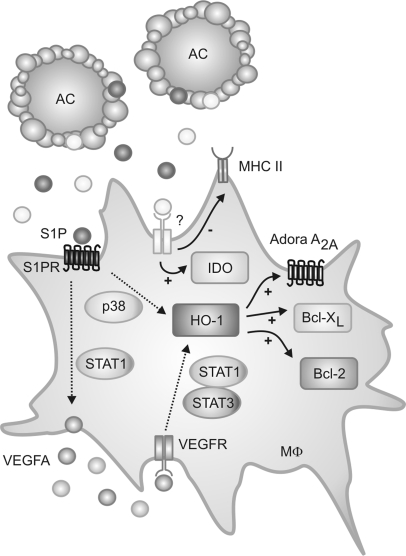
In conclusion, AC-CM caused expression of HO-1 in macrophages, which concomitantly evokes distinct antiapoptotic as well as anti-inflammatory responses in these cells (Figure 6).
Figure 6.
Cartoon, summarizing biphasic expression of HO-1 by AC-CM in macrophages. S1P, present in AC, activated S1P1. p38 MAPK activation and autocrine VEGF signaling induced HO-1. HO-1 contributed to alternative macrophage activation by regulating expression of Bcl-2, Bcl-XL as well as Adora A2A. AC-CM also modulated expression of IDO and HLA-DMB, independently of HO-1.
DISCUSSION
Our data suggest that AC-derived S1P induces HO-1 expression in primary human macrophages, which in turn increases the amount of antiapoptotic proteins such as Bcl-2 or Bcl-XL and Adora A2A. The notion that authentic or AC-derived S1P induces HO-1 is in line with a report by Man and coworkers, showing that FTY720 enhanced HO-1 expression (Man et al., 2005). Human macrophages express the S1P receptor subtypes 1–4 (Fueller et al., 2003). Considering that activation of S1P1 attenuated the expression of proinflammatory cytokines in mouse macrophages (Hughes et al., 2008) in conjunction with our previous observation that S1P1 signaling in macrophages protected from apoptosis (Weigert et al., 2006) fits well with the present study, demonstrating that S1P1 provoked an increase in HO-1 expression, which in turn triggered antiapoptotic as well as anti-inflammatory signals. Inhibitor studies revealed that in primary human macrophages p38 mitogen-activated protein kinase (MAPK), a pathway well known for HO-1 induction (Wijayanti et al., 2005; Kocanova et al., 2007), facilitated S1P-evoked HO-1 expression. However, other studies have shown that p38 MAPK was also activated downstream of S1P2 (Taha et al., 2004), which might explain that siRNA-mediated knockdown of S1P1 could not completely reduce HO-1 expression after stimulation with AC-CM, with the option that residual S1P2 signaling occurred.
Our study suggests a cross-talk between S1P-signaling and VEGFA secretion from human macrophages. A connection between S1P and VEGF signaling was recently also put forward for ML-1 thyroid follicular cancer as well as human FRO anaplastic thyroid cancer cells (Balthasar et al., 2008). Furthermore, VEGF secretion from epithelial cells after phagocytosis of AC was demonstrated previously (Golpon et al., 2004), although neither signal cross-talk between AC and epithelial cells nor signaling consequences have been fully understood. In our system there is evidence for a soluble factor generated by AC, rather than cell–cell contacts or phagocytosis being required for VEGF secretion. A connection between S1P and VEGF signaling is highlighted, especially for models of tumor angiogenesis, proposing that tumor-derived S1P stimulates VEGF formation in endothelial cells (Milstien and Spiegel, 2006; Sabbadini, 2006). Macrophages, as cells in the tumor microenvironment, are critical players stimulating angiogenesis in a variety of human tumors, where they exhibit a pronounced M2 anti-inflammatory and antiapoptotic phenotype. Our finding that VEGFA was not only secreted from macrophages in response to S1P but also caused autocrine signaling to further induce HO-1, implies an important role of S1P in macrophage polarization, with particular relevance in the tumor setting. Besides mechanisms such as tumor hypoxia, the interaction of tumor-associated macrophages (TAMs) with dying tumor cells could then promote VEGFA release to stimulate tumor angiogenesis. Our unexpected observation of elevated S1P1 expression after the treatment with AC-CM might also be attributed to autocrine VEGF, because S1P1 expression was increased in bovine aortic endothelial cells after their exposure to authentic VEGF (Igarashi et al., 2003).
VEGF expression was induced by S1P via STAT1. This finding is rather extraordinary because S1P receptor activation has not been linked to STAT signaling previously. However, Src kinase activation downstream of a G protein-coupled receptor such as S1P1 might be a missing communication link (Rivera and Olivera, 2007), although mechanisms of Src activation in response to G protein-coupled receptor agonists are not fully understood (Gutkind, 2000). Src kinase activation is upstream of STAT signaling in human monocytes (Norkina et al., 2007), and one could speculate that Src kinase links S1P receptor activation to STAT activation in our system. Strikingly, in rat aortic vascular smooth muscle cells, S1P-stimulated transactivation of STAT-coupled epidermal growth factor receptor and platelet-derived growth factor β receptor were Src-dependent (Tanimoto et al., 2004).
STAT1/STAT3 heterodimer formation was necessary for the autocrine induction of HO-1 by VEGF, which was observed previously in chronic lymphocytic leukemia B cells (Lee et al., 2005). Furthermore, STAT1 and STAT3 were involved in hyperoxia-induced gene transcription of HO-1 in RAW 264.7 macrophages (Lee et al., 2000). Activation of STATs presumably plays an important role in establishing the M2 macrophage phenotype in the tumor setting, because STAT1 is constitutively active in TAMs (Biswas et al., 2006) and its enhanced signaling properties mediate T cell deletion (Kusmartsev and Gabrilovich, 2005). Also, STAT3 and STAT6 are believed to contribute to M2 macrophage polarization (Sica and Bronte, 2007).
Our work reveals that HO-1 in macrophages, besides accomplishing antiapoptotic functions by enhancing the expression of survival promoting proteins such as Bcl-2 and Bcl-XL, also conveys an anti-inflammatory potential, exemplified by the expression of Adora A2A in response to AC-CM. The notion that Deta-NO induced Adora A2A less pronounced than AC-CM implies that HO-1–dependent anti-inflammatory effects were only partially mimicked by Deta-NO. This may point to induction of HO-1 by different signaling pathways and transcriptional regulators. Likely, Adora A2A is not only induced by HO-1, because we observed some expression also with a knockdown of HO-1. For NO, other regulators such as Nrf2 have been suggested. Thus, an anti-inflammatory response achieved with AC-CM is rather unique and differs from a situation with only a proinflammatory stimulus such as LPS or NO to induce HO-1 expression.
According to suggestions by Zhang et al. (2003), enhanced Bcl-2 and Bcl-XL expression by HO-1 could be attributed to CO, as shown in a murine model of ischemia-reperfusion. Generally, several antiapoptotic effects attributed to HO-1 are thought to be CO mediated (Ryter et al., 2002). This might also apply to Adora A2A, because overexpression of HO-1 as well as exposure of RAW264.7 macrophages to CO augmented Adora A2A mRNA as well as protein level (Haschemi et al., 2007). The rather marginal effect seen with CO and bilirubin in our experiments may open a further possibility that HO-1 translocates to the nucleus to bind to a transcription factor or a protein complex, resulting in enhanced transcription of Adora A2A (Lin et al., 2007). Adora A2A agonists are capable of not only blocking the inflammatory potential of human macrophages, such as pathogen-stimulated NO, TNF-α, or IL-12 production (Hasko et al., 2007) but also promoting wound healing in disease states such as diabetes (Montesinos et al., 1997).
Interestingly, expression of the anti-inflammatory marker IDO was, at least in human macrophages exposed to AC-CM, HO-1 independent. Nevertheless, the principle finding that AC-CM augments expression of IDO is exciting. IDO catalyzes the rate-limiting step of tryptophane degradation. Kynurenine, one of the products of this reaction, affects proliferation as well as differentiation of helper T cells (Brusko et al., 2005; Munn and Mellor, 2007). Likely, in an inflammatory environment the presence of AC might help to promote healing, once an inflammatory stimulus is eliminated, by modulating adaptive immune responses. This phenotype pattern is further corroborated by our finding that macrophages down-regulate HLA-DMB in response to AC. Although it was reported that bilirubin, a degradation product of biliverdin, suppressed MHC II expression in endothelial cells (Wu et al., 2005), we could not confirm that HLA-DMB expression was HO-1 dependent in macrophages. Despite these cell type differences, reduced expression of HLA-DMB and increased abundance of IDO favor an attenuated response of TH1 cells, which is important to progress from inflammation toward healing.
Together, apoptotic cell supernatants provoked alternative activation in human macrophages, characterized by up-regulation of Bcl-2, Bcl-XL, Adora A2A, and IDO, but down-regulation of MHC II expression. The establishment of this anti-inflammatory phenotype was in part dependent on the induction of HO-1 by AC-derived S1P (Figure 6). Thus, targeting HO-1 and/or its downstream effectors could be a therapeutic approach to treat patients suffering from diseases linked to anti-inflammatory macrophage polarization during, e.g., the late immunosuppressive phase of sepsis (Hasko and Pacher, 2008) or in cancer (Mantovani et al., 2002), because this would influence M2 macrophage viability as well their polarization.
ACKNOWLEDGMENTS
We thank Dr. Shigeru Takahashi for providing the HO-1 promoter luciferase constructs. The work was supported by grants from Deutsche Forschungsgemeinschaft (Br 999, FOG 784, Excellence Cluster Cardiopulmonary System), Deutsche Krebshilfe, Sander Foundation, LOEWE program, and European Community (PROLIGEN).
Abbreviations used:
- AC
apoptotic cells
- Adora A2A
adenosine receptor A2A
- CM
conditioned medium/media
- CORM-2
tricarbonyldichloro ruthenium (II) dimer
- Deta-NO
diethylenetriamine-NO
- DMS
dimethyl-sphingosine
- EMSA
electrophoretic mobility shift assay
- HO-1
heme oxygenase-1
- IDO
indoleamine-2,3-dioxygenase
- Jak
Janus kinase
- MΦ-CM
macrophage CM
- NC
necrotic cells
- ptm
point mutation
- S1P
sphingosine-1-phosphate
- S1P1
S1P-receptor 1
- STATx
STAT response element
- TAM
tumor-associated macrophage
- VC
viable cells
- VEGFA
vascular endothelial growth factor A.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-10-1005) on January 7, 2009.
REFERENCES
- Ashino T., Yamanaka R., Yamamoto M., Shimokawa H., Sekikawa K., Iwakura Y., Shioda S., Numazawa S., Yoshida T. Negative feedback regulation of lipopolysaccharide-induced inducible nitric oxide synthase gene expression by heme oxygenase-1 induction in macrophages. Mol. Immunol. 2008;45:2106–2115. doi: 10.1016/j.molimm.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Balthasar S., Bergelin N., Lof C., Vainio M., Andersson S., Tornquist K. Interactions between sphingosine-1-phosphate and vascular endothelial growth factor signalling in ML-1 follicular thyroid carcinoma cells. Endocr. Relat. Cancer. 2008;15:521–534. doi: 10.1677/ERC-07-0253. [DOI] [PubMed] [Google Scholar]
- Biswas S. K., et al. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation) Blood. 2006;107:2112–2122. doi: 10.1182/blood-2005-01-0428. [DOI] [PubMed] [Google Scholar]
- Brusko T. M., Wasserfall C. H., Agarwal A., Kapturczak M. H., Atkinson M. A. An integral role for heme oxygenase-1 and carbon monoxide in maintaining peripheral tolerance by CD4+CD25+ regulatory T cells. J. Immunol. 2005;174:5181–5186. doi: 10.4049/jimmunol.174.9.5181. [DOI] [PubMed] [Google Scholar]
- Cvetanovic M., Ucker D. S. Innate immune discrimination of apoptotic cells: repression of proinflammatory macrophage transcription is coupled directly to specific recognition. J. Immunol. 2004;172:880–889. doi: 10.4049/jimmunol.172.2.880. [DOI] [PubMed] [Google Scholar]
- Deshane J., Wright M., Agarwal A. Heme oxygenase-1 expression in disease states. Acta Biochim. Pol. 2005;52:273–284. [PubMed] [Google Scholar]
- Fadok V. A., Bratton D. L., Konowal A., Freed P. W., Westcott J. Y., Henson P. M. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D. A., Mahajan S., Ritz J. Fludarabine-induced immunosuppression is associated with inhibition of STAT1 signaling. Nat. Med. 1999;5:444–447. doi: 10.1038/7445. [DOI] [PubMed] [Google Scholar]
- Freire-de-Lima C. G., Nascimento D. O., Soares M. B., Bozza P. T., Castro-Faria-Neto H. C., de Mello F. G., DosReis G. A., Lopes M. F. Uptake of apoptotic cells drives the growth of a pathogenic trypanosome in macrophages. Nature. 2000;403:199–203. doi: 10.1038/35003208. [DOI] [PubMed] [Google Scholar]
- Fueller M., Wang D. A., Tigyi G., Siess W. Activation of human monocytic cells by lysophosphatidic acid and sphingosine-1-phosphate. Cell Signal. 2003;15:367–375. doi: 10.1016/s0898-6568(02)00117-1. [DOI] [PubMed] [Google Scholar]
- Funamoto M., et al. Signal transducer and activator of transcription 3 is required for glycoprotein 130-mediated induction of vascular endothelial growth factor in cardiac myocytes. J. Biol. Chem. 2000;275:10561–10566. doi: 10.1074/jbc.275.14.10561. [DOI] [PubMed] [Google Scholar]
- Golpon H. A., Fadok V. A., Taraseviciene-Stewart L., Scerbavicius R., Sauer C., Welte T., Henson P. M., Voelkel N. F. Life after corpse engulfment: phagocytosis of apoptotic cells leads to VEGF secretion and cell growth. FASEB J. 2004;18:1716–1718. doi: 10.1096/fj.04-1853fje. [DOI] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Gude D. R., Alvarez S. E., Paugh S. W., Mitra P., Yu J., Griffiths R., Barbour S. E., Milstien S., Spiegel S. Apoptosis induces expression of sphingosine kinase 1 to release sphingosine-1-phosphate as a “come-and-get-me” signal. FASEB J. 2008;22:2629–2638. doi: 10.1096/fj.08-107169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutkind J. S. Regulation of mitogen-activated protein kinase signaling networks by G protein-coupled receptors. Sci. STKE. 2000;2000:RE1. doi: 10.1126/stke.2000.40.re1. [DOI] [PubMed] [Google Scholar]
- Haschemi A., Wagner O., Marculescu R., Wegiel B., Robson S. C., Gagliani N., Gallo D., Chen J. F., Bach F. H., Otterbein L. E. Cross-regulation of carbon monoxide and the adenosine A2a receptor in macrophages. J. Immunol. 2007;178:5921–5929. doi: 10.4049/jimmunol.178.9.5921. [DOI] [PubMed] [Google Scholar]
- Hasko G., Pacher P. A2A receptors in inflammation and injury: lessons learned from transgenic animals. J. Leukoc. Biol. 2008;83:447–455. doi: 10.1189/jlb.0607359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasko G., Pacher P., Deitch E. A., Vizi E. S. Shaping of monocyte and macrophage function by adenosine receptors. Pharmacol. Ther. 2007;113:264–275. doi: 10.1016/j.pharmthera.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hla T., Lee M. J., Ancellin N., Paik J. H., Kluk M. J. Lysophospholipids–receptor revelations. Science. 2001;294:1875–1878. doi: 10.1126/science.1065323. [DOI] [PubMed] [Google Scholar]
- Hughes J. E., Srinivasan S., Lynch K. R., Proia R. L., Ferdek P., Hedrick C. C. Sphingosine-1-phosphate induces an antiinflammatory phenotype in macrophages. Circ. Res. 2008;102:950–958. doi: 10.1161/CIRCRESAHA.107.170779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi J., Erwin P. A., Dantas A. P., Chen H., Michel T. VEGF induces S1P1 receptors in endothelial cells: implications for cross-talk between sphingolipid and growth factor receptors. Proc. Natl. Acad. Sci. USA. 2003;100:10664–10669. doi: 10.1073/pnas.1934494100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. P., Ryter S. W., Choi A. M. Co as a cellular signaling molecule. Annu. Rev. Pharmacol. Toxicol. 2006;46:411–449. doi: 10.1146/annurev.pharmtox.46.120604.141053. [DOI] [PubMed] [Google Scholar]
- Kocanova S., Buytaert E., Matroule J. Y., Piette J., Golab J., de Witte P., Agostinis P. Induction of heme-oxygenase 1 requires the p38MAPK and PI3K pathways and suppresses apoptotic cell death following hypericin-mediated photodynamic therapy. Apoptosis. 2007;12:731–741. doi: 10.1007/s10495-006-0016-x. [DOI] [PubMed] [Google Scholar]
- Kusmartsev S., Gabrilovich D. I. STAT1 signaling regulates tumor-associated macrophage-mediated T cell deletion. J. Immunol. 2005;174:4880–4891. doi: 10.4049/jimmunol.174.8.4880. [DOI] [PubMed] [Google Scholar]
- Lee P. J., Camhi S. L., Chin B. Y., Alam J., Choi A. M. AP-1 and STAT mediate hyperoxia-induced gene transcription of heme oxygenase-1. Am. J. Physiol. Lung Cell Mol. Physiol. 2000;279:L175–L182. doi: 10.1152/ajplung.2000.279.1.L175. [DOI] [PubMed] [Google Scholar]
- Lee Y. K., Shanafelt T. D., Bone N. D., Strege A. K., Jelinek D. F., Kay N. E. VEGF receptors on chronic lymphocytic leukemia (CLL) B cells interact with STAT 1 and 3, implication for apoptosis resistance. Leukemia. 2005;19:513–523. doi: 10.1038/sj.leu.2403667. [DOI] [PubMed] [Google Scholar]
- Lin Q., et al. Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. J. Biol. Chem. 2007;282:20621–20633. doi: 10.1074/jbc.M607954200. [DOI] [PubMed] [Google Scholar]
- Man K., Ng K. T., Lee T. K., Lo C. M., Sun C. K., Li X. L., Zhao Y., Ho J. W., Fan S. T. FTY720 attenuates hepatic ischemia-reperfusion injury in normal and cirrhotic livers. Am. J. Transplant. 2005;5:40–49. doi: 10.1111/j.1600-6143.2004.00642.x. [DOI] [PubMed] [Google Scholar]
- Mantovani A., Sozzani S., Locati M., Allavena P., Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- Milstien S., Spiegel S. Targeting sphingosine-1-phosphate: a novel avenue for cancer therapeutics. Cancer Cell. 2006;9:148–150. doi: 10.1016/j.ccr.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Montesinos M. C., et al. Wound healing is accelerated by agonists of adenosine A2 (G alpha s-linked) receptors. J. Exp. Med. 1997;186:1615–1620. doi: 10.1084/jem.186.9.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn D. H., Mellor A. L. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J. Clin. Invest. 2007;117:1147–1154. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norkina O., Dolganiuc A., Shapiro T., Kodys K., Mandrekar P., Szabo G. Acute alcohol activates STAT3, AP-1, and Sp-1 transcription factors via the family of Src kinases to promote IL-10 production in human monocytes. J. Leukoc. Biol. 2007;82:752–762. doi: 10.1189/jlb.0207099. [DOI] [PubMed] [Google Scholar]
- Olivera A., Spiegel S. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 1993;365:557–560. doi: 10.1038/365557a0. [DOI] [PubMed] [Google Scholar]
- Otterbein L. E., Bach F. H., Alam J., Soares M., Tao Lu H., Wysk M., Davis R. J., Flavell R. A., Choi A. M. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- Rathmell J. C., Thompson C. B. The central effectors of cell death in the immune system. Annu. Rev. Immunol. 1999;17:781–828. doi: 10.1146/annurev.immunol.17.1.781. [DOI] [PubMed] [Google Scholar]
- Rivera J., Olivera A. Src family kinases and lipid mediators in control of allergic inflammation. Immunol. Rev. 2007;217:255–268. doi: 10.1111/j.1600-065X.2007.00505.x. [DOI] [PubMed] [Google Scholar]
- Ryter S. W., Otterbein L. E. Carbon monoxide in biology and medicine. Bioessays. 2004;26:270–280. doi: 10.1002/bies.20005. [DOI] [PubMed] [Google Scholar]
- Ryter S. W., Otterbein L. E., Morse D., Choi A. M. Heme oxygenase/carbon monoxide signaling pathways: regulation and functional significance. Mol. Cell Biochem. 2002;234–235:249–263. doi: 10.1023/A:1015957026924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbadini R. A. Targeting sphingosine-1-phosphate for cancer therapy. Br. J. Cancer. 2006;95:1131–1135. doi: 10.1038/sj.bjc.6603400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J., Dransfield I., Gregory C., Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat. Rev. Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- Sica A., Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J. Clin. Invest. 2007;117:1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siner J. M., Jiang G., Cohen Z. I., Shan P., Zhang X., Lee C. G., Elias J. A., Lee P. J. VEGF-induced heme oxygenase-1 confers cytoprotection from lethal hyperoxia in vivo. FASEB J. 2007;21:1422–1432. doi: 10.1096/fj.06-6661com. [DOI] [PubMed] [Google Scholar]
- Song H., Wang R., Wang S., Lin J. A low-molecular-weight compound discovered through virtual database screening inhibits Stat3 function in breast cancer cells. Proc. Natl. Acad. Sci. USA. 2005;102:4700–4705. doi: 10.1073/pnas.0409894102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B., Sun Z., Jin Q., Chen X. CO-releasing molecules (CORM-2)-liberated CO attenuates leukocytes infiltration in the renal tissue of thermally injured mice. Int. J. Biol. Sci. 2008;4:176–183. doi: 10.7150/ijbs.4.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha T. A., Argraves K. M., Obeid L. M. Sphingosine-1-phosphate receptors: receptor specificity versus functional redundancy. Biochim. Biophys. Acta. 2004;1682:48–55. doi: 10.1016/j.bbalip.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Takahashi Y., Ito K., Nagano T., Shibahara S., Miura T. Positive and negative regulation of the human heme oxygenase-1 gene expression in cultured cells. Biochim. Biophys. Acta. 1999;1447:231–235. doi: 10.1016/s0167-4781(99)00156-6. [DOI] [PubMed] [Google Scholar]
- Tanimoto T., Lungu A. O., Berk B. C. Sphingosine 1-phosphate transactivates the platelet-derived growth factor beta receptor and epidermal growth factor receptor in vascular smooth muscle cells. Circ. Res. 2004;94:1050–1058. doi: 10.1161/01.RES.0000126404.41421.BE. [DOI] [PubMed] [Google Scholar]
- Voll R. E., Herrmann M., Roth E. A., Stach C., Kalden J. R., Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- von Knethen A., Tautenhahn A., Link H., Lindemann D., Brune B. Activation-induced depletion of protein kinase C alpha provokes desensitization of monocytes/macrophages in sepsis. J. Immunol. 2005;174:4960–4965. doi: 10.4049/jimmunol.174.8.4960. [DOI] [PubMed] [Google Scholar]
- Von Knethen A. A., Brune B. Delayed activation of PPARgamma by LPS and IFN-gamma attenuates the oxidative burst in macrophages. FASEB J. 2001;15:535–544. doi: 10.1096/fj.00-0187com. [DOI] [PubMed] [Google Scholar]
- von Wenckstern H., Zimmermann K., Kleuser B. The role of the lysophospholipid sphingosine 1-phosphate in immune cell biology. Arch. Immunol. Ther. Exp. 2006;54:239–251. doi: 10.1007/s00005-006-0028-9. [DOI] [PubMed] [Google Scholar]
- Weigert A., Johann A. M., von Knethen A., Schmidt H., Geisslinger G., Brune B. Apoptotic cells promote macrophage survival by releasing the antiapoptotic mediator sphingosine-1-phosphate. Blood. 2006;108:1635–1642. doi: 10.1182/blood-2006-04-014852. [DOI] [PubMed] [Google Scholar]
- Weigert A., Tzieply N., von Knethen A., Johann A. M., Schmidt H., Geisslinger G., Brune B. Tumor cell apoptosis polarizes macrophages role of sphingosine-1-phosphate. Mol. Biol. Cell. 2007;18:3810–3819. doi: 10.1091/mbc.E06-12-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijayanti N., Kietzmann T., Immenschuh S. Heme oxygenase-1 gene activation by the NAD(P)H oxidase inhibitor 4-(2-aminoethyl) benzenesulfonyl fluoride via a protein kinase B, p38-dependent signaling pathway in monocytes. J. Biol. Chem. 2005;280:21820–21829. doi: 10.1074/jbc.M502943200. [DOI] [PubMed] [Google Scholar]
- Wu J., Ma J., Fan S. T., Schlitt H. J., Tsui T. Y. Bilirubin derived from heme degradation suppresses MHC class II expression in endothelial cells. Biochem. Biophys. Res. Commun. 2005;338:890–896. doi: 10.1016/j.bbrc.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Zhang X., Shan P., Alam J., Davis R. J., Flavell R. A., Lee P. J. Carbon monoxide modulates Fas/Fas ligand, caspases, and Bcl-2 family proteins via the p38alpha mitogen-activated protein kinase pathway during ischemia-reperfusion lung injury. J. Biol. Chem. 2003;278:22061–22070. doi: 10.1074/jbc.M301858200. [DOI] [PubMed] [Google Scholar]