Abstract
Neuronal lysosomes and their biogenesis mechanisms are primarily thought to clear metabolites and proteins whose abnormal accumulation leads to neurodegenerative disease pathology. However, it remains unknown whether lysosomal sorting mechanisms regulate the levels of membrane proteins within synaptic vesicles. Using high-resolution deconvolution microscopy, we identified early endosomal compartments where both selected synaptic vesicle and lysosomal membrane proteins coexist with the adaptor protein complex 3 (AP-3) in neuronal cells. From these early endosomes, both synaptic vesicle membrane proteins and characteristic AP-3 lysosomal cargoes can be similarly sorted to brain synaptic vesicles and PC12 synaptic-like microvesicles. Mouse knockouts for two Hermansky–Pudlak complexes involved in lysosomal biogenesis from early endosomes, the ubiquitous isoform of AP-3 (Ap3b1−/−) and muted, defective in the biogenesis of lysosome-related organelles complex 1 (BLOC-1), increased the content of characteristic synaptic vesicle proteins and known AP-3 lysosomal proteins in isolated synaptic vesicle fractions. These phenotypes contrast with those of the mouse knockout for the neuronal AP-3 isoform involved in synaptic vesicle biogenesis (Ap3b2−/−), in which the content of select proteins was reduced in synaptic vesicles. Our results demonstrate that lysosomal and lysosome-related organelle biogenesis mechanisms regulate steady-state synaptic vesicle protein composition from shared early endosomes.
INTRODUCTION
Endosomes are sorting hubs that receive proteins and lipids from the cell surface or the trans-Golgi network and further deliver them to either downstream endocytic compartments, the exocytic pathway, or back to the cell surface. In addition to these functions, neurons possess endosomes that participate in specialized sorting mechanisms, such as retrograde delivery of neurotrophic signals to the cell body through signaling endosomes (Howe and Mobley, 2005); or in the biogenesis of synaptic vesicles (Murthy and De Camilli, 2003). Signaling endosomes and synaptic vesicles originate at the nerve terminal, a cell domain lacking ultrastructurally identifiable lysosomes. These lytic organelles and their components are thought to be restricted to neuronal cell bodies (Parton et al., 1992). Because of this spatial segregation, the biogenesis of signaling endosomes and synaptic vesicles is thought to be independent from traditional lysosome biogenesis. However, recent evidence indicates that diverse lysosomal proteins, either those resident in lysosomes or involved in traffic to lysosomes, are also found in signaling endosomes, nerve terminals, and synaptic vesicles (Overly and Hollenbeck, 1996; Stobrawa et al., 2001; Salazar et al., 2005, 2006; Saxena et al., 2005; Arantes and Andrews, 2006; Deinhardt et al., 2006; Karten et al., 2006; Scheuber et al., 2006; Takamori et al., 2006; Talbot et al., 2006; Burre and Volknandt, 2007). The mechanism to account for the presence of lysosomal proteins in organelles generated at the nerve terminal remains unknown. The presence of lysosomal proteins in synaptic vesicles challenges a conventional view where neuronal early endosomes function in a pathway for sorting synaptic vesicle proteins and lysosomal cargoes away from each other into different vesicles bound to the cell surface or lysosomal compartments, respectively. Here, we propose that sorting machineries specialized in lysosome biogenesis in nonneuronal cells contribute to the generation of presynaptic compartments by regulating the targeting of synaptic vesicle and lysosomal membrane proteins to synaptic vesicles. We tested this hypothesis by using genetic deficiencies of Hermansky–Pudlak lysosomal sorting complexes that localize to early endosomes. These deficiencies include Ap3b1−/−, a mouse lacking the ubiquitous adaptor protein complex 3 (AP-3) and mutedmu/mu, a mouse defective in the biogenesis of lysosome-related organelles complex 1 (BLOC-1).
AP-3 and BLOC-1 possess well-established roles in the sorting of membrane proteins into vesicles bound to lysosomes, lysosome-related organelles, and synaptic vesicle fates (for reviews, see Di Pietro and Dell'Angelica, 2005; Ohno, 2006; Danglot and Galli, 2007; Newell-Litwa et al., 2007). AP-3 exists as two isoforms defined by their subunit composition. The subunits δ and ς3 are common to all AP-3 isoforms, yet β3 and μ3 subunits exist as two differentially expressed gene products each (A and B). The expression of β3B and μ3B subunits is restricted to neuronal cells (Nakatsu et al., 2004; Seong et al., 2005). These subunits are believed to assemble into the neuronal isoform complex together with δ and ς3. In contrast, β3A, μ3A, δ, and ς3 subunits generate the ubiquitous AP-3 adaptor present in all cells. Mice deficient for both AP-3 complexes due to a mutation in δ adaptin (mocha), shared by both AP-3 isoforms, are characterized by defective targeting of proteins to synaptic vesicles, lysosomes, and lysosome-related organelles (Kantheti et al., 1998). These phenotypes are recapitulated in part by isoform specific AP-3 deficiencies. Thus, β3A loss-of-function alleles, such as Ap3b1−/−, which abrogate ubiquitous AP-3 complex expression, possess defective assembly of lysosomes and lysosome-related organelles (Feng et al., 1999; Zhen et al., 1999; Yang et al., 2000; Seong et al., 2005). These phenotypes are also found in mice lacking any one of the eight subunits found in the BLOC-1 complex, a complex that genetically and biochemically interacts at least with ubiquitous AP-3. Mutations in genes encoding individual BLOC-1 subunits, such as mutedmu/mu, lead to down-regulation of the whole BLOC-1 complex (Di Pietro and Dell'Angelica, 2005; Di Pietro et al., 2006; Gautam et al., 2006; Salazar et al., 2009). In humans, genetic deficiencies in loci encoding for proteins of either the ubiquitous AP-3 or BLOC-1 complex trigger a disease known as Hermansky–Pudlak syndrome (OMIM: 203300) (Di Pietro and Dell'Angelica, 2005; Raposo and Marks, 2007). In contrast, β3B alleles (Ap3b2−/−) abolish the expression of neuronal AP-3. Neuronal AP-3 mutants recapitulate synaptic vesicle targeting and neurological phenotypes found in the mocha allele but lack their systemic lysosomal phenotypes (Nakatsu et al., 2004; Seong et al., 2005). These findings have led to the notion that AP-3 isoforms perform segregated sorting functions in mammals. However, this view fails to consider that both AP-3 adaptor isoforms (Seong et al., 2005; Salazar et al., 2006), as well as BLOC-1, are present in neuronal cell bodies and that AP-3 adaptor isoforms share their ability to recognize similar sorting motifs in membrane proteins (Bonifacino and Traub, 2003). This raises the question of whether membrane proteins targeted to the synapse could be regulated by both AP-3 adaptor isoforms.
Based on this evidence, we hypothesize that divergent endocytic sorting routes, synaptic vesicle biogenesis mediated by the neuronal AP-3 isoform and lysosomal transport mediated by the ubiquitously expressed AP-3 isoform and BLOC-1 complex, regulate synaptic vesicle composition by sorting similar cargo from shared early endosomes. This mechanism would operate as a “seesaw” from a common early endosome. From this endosome, AP-3 adaptor isoforms would behave as branches of a seesaw. Thus, neuronal AP-3 would sort both synaptic vesicle and lysosomal AP-3 cargoes into synaptic vesicles, whereas ubiquitous AP-3 would compete for both types of cargoes and deliver them to lysosomal compartments. In the absence of ubiquitous AP-3, cargoes that normally would have been sorted to lysosomes would instead be routed to synaptic vesicles and vice versa. The seesaw model is consistent with the increased targeting of zinc transporter 3 (ZnT3) to ubiquitous AP-3-null brain synaptic vesicles (Seong et al., 2005). The seesaw mechanism leads to the following predictions: 1) Donor early endosomes in neurons will contain both lysosomal and synaptic vesicle cargoes so that these cargoes can be subsequently targeted to either a lysosomal or synaptic vesicle destination. 2) Both synaptic vesicle and lysosomal AP-3 cargoes are sorted to a common synaptic-like microvesicle in PC12 cells and synaptic vesicle in neurons. 3) Finally, a balance between lysosomal and synaptic vesicle targeting branches of the seesaw will define synaptic vesicle composition. In the absence of either targeting mechanism, cargoes will be more available to enter the alternate route, thereby disrupting the balance of synaptic vesicle cargoes. Therefore, genetic deficiencies of Hermansky–Pudlak complexes involved in the biogenesis of lysosomes or lysosome-related organelles (ubiquitous AP-3/Ap3b1−/− and BLOC-1/mutedmu/mu) would increase synaptic vesicle content of synaptic vesicle and lysosomal AP-3 cargoes, whereas genetic deficiencies in synaptic vesicle sorting machinery (neuronal AP-3/Ap3b2−/−) would reduce the levels of lysosomal and synaptic vesicle cargo proteins in synaptic vesicles. The following experiments address these predictions and provide evidence supporting our hypothesis that lysosomal and synaptic vesicle targeting mechanisms recognize similar proteins and deliver them to alternate destinations, either synaptic vesicles or lysosomes. Ultimately, our findings demonstrate a novel role for lysosomal sorting machineries in regulating the fate of synaptic vesicle membrane proteins and composition of synaptic vesicle fractions.
MATERIALS AND METHODS
Antibodies
The following antibodies were used in this study: Monoclonal antibodies against GM130, TGN38, CD63, Vti1B, Syntaxin 8, and clathrin heavy chain were from BD Biosciences (Franklin Lakes, NJ); monoclonal antibodies (mAbs) against VGlut1, vesicular GABA transporter (VGAT), and vesicle-associated membrane protein (VAMP) 2 (69.1) and polyclonal antibodies against green fluorescent protein (GFP) and VAMP2 were from Synaptic Systems (Goettingen, Germany); polyclonal antibody against pallidin was from Proteintech Group (Chicago, IL); mAb against synaptophysin (SY38) and polyclonal antibody against microtubule-associated protein 2 were from Millipore Bioscience Research Reagents (Billerica, MA); mAb against transferrin receptor (H68.4) was from Zymed Laboratories/Invitrogen (Carlsbad, CA); mAb against tubulin was from Sigma-Aldrich (St. Louis, MO); monoclonal antibodies against AP3-δ (SA4), synaptic vesicle (SV) 2 (10H4), mouse lysosomal membrane protein (LAMP) 1 (1D4B), and human LAMP1 (H4A3) were from Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA); and polyclonal antibody against myc were from Bethyl Laboratories (Montgomery, TX). A mAb against lysobisphosphatidic acid (LBPA) (6CY) was the kind gift of Dr. Jean Gruenberg (University of Geneva, Geneva, Switzerland; Kobayashi et al., 1998), whereas polyclonal antibodies against Syntaxin 13 and Sialin were the gifts of Drs. A. Peden (University of Cambridge, Cambridge, United Kingdom) and R. Reimer (Stanford University, Stanford, CA) respectively. The mAb against VAMP7-TI has been described in Advani et al. (1999) and is further characterized in Supplemental Figure 1. The polyclonal antibody against phosphatidylinositol-4-kinase type IIα (PI4KIIα) has been described in Guo et al. (2003). KF4 mAb against AP-3δ was developed by Dr. A. Peden and is described in Craige et al. (2008). Polyclonal antibodies against AP-3 ς3 and ZnT3 have been described in Faundez et al. (1998) and Salazar et al. (2004b), respectively.
DNA Constructs
VAMP2N49A-glutathione transferase (GST) is described in Salem et al. (1998). VAMP7-GST was a gift of Dr. A. Peden. Recombinant proteins were prepared as described previously (Roos and Kelly, 1998). Rab5Q79L-GFP plasmid and PC12 cell transfections were described previously (Craige et al., 2008).
Oligonucleotide-mediated Small Interfering RNA (siRNA)
All siRNA constructs were obtained from Dharmacon RNA Technologies/Thermo Fisher Scientific (Lafayette, CO). The following oligonucleotide sequences were used to silence expression of human VAMP7-TI (Dharmacon siGENOME SMARTpool reagent M-020864-00-0005; human SYBL1, NM_005638): sense sequence (1): G.G.A.G.A.A.A.G.A.U.U.G.G.A.A.U.U.A.U.U.U and antisense sequence (1): 5′-P.A.U.A.A.U.U.C.C.A.A.U.C.U.U.U.C.U.C.C.U.U; sense sequence (2): G.U.A.C.U.C.A.C.A.U.G.G.C.A.A.U.U.A.U.U.U and antisense sequence (2): 5′-P.A.U.A.A.U.U.G.C.C.A.U.G.U.G.A.G.U.A.C.U.U; sense sequence (3): A.A.G.A.A.G.A.G.G.U.U.C.C.A.G.A.C.U.A.U.U and antisense sequence (3): 5′-P.U.A.G.U.C.U.G.G.A.A.C.C.U.C.U.U.C.U.U.U.U; and sense sequence (4): G.C.U.A.A.G.A.U.A.C.C.U.U.C.U.G.A.A.A.U.U and antisense sequence (4): 5′-P.U.U.U.C.A.G.A.A.G.G.U.A.U.C.U.U.A.G.C.U.U. As a control, we used Dharmacon siCONTROL NonTargeting siRNA Pool #1 D-001206-13-05.
Human embryonic kidney (HEK) or HeLa ATCC cells plated in six-well plates were grown to ∼85% confluence and treated with a final concentration of 25 nM double-stranded VAMP7-TI along with 5 μl of Lipofectamine 2000 (Invitrogen) and 1 ml of Opti-MEM (Invitrogen). Control cells were treated in parallel with the same concentration of control siRNA. After 4-h incubation at 37°C and 10%CO2, 1 ml of 10% fetal bovine serum (FBS) DMEM was added to the cells. The following day, the cells were either left alone or grown in regular 10% FBS DMEM. After 24 h, the same transfection procedure was repeated again. The next day, cells were once again split into maintenance media, and after 24 h they were used for experimentation.
Animals
The Ap3b1−/− mouse strain was obtained from Dr. S. Mansour (University of Utah, Salt Lake City, UT) (Yang et al., 2000). The Ap3b2−/− mouse strain was generated as described in Seong et al. (2005). Ap3b1−/−, Ap3b2−/−, and wild-type C57BL mice were all bred in-house at Emory University. Mutedmu/mu (Zhang et al., 2002) mice were obtained from Dr. R. Swank (Roswell Park Cancer Institute, Buffalo, NY) and bred in-house.
Cell Culture
PC12 cells were cultured in DMEM containing 10% horse serum, 5% fetal bovine serum, and 100 U/ml penicillin and 100 μg/ml streptomycin at 10% CO2 and 37°C. PC12 cells stably expressing ZnT3 were maintained with the addition of 0.2 mg/ml G418 (Salazar et al., 2004b). HeLa ATCC cells were grown in DMEM containing 10% fetal bovine serum and 100 U/ml penicillin and 100 μg/ml streptomycin at 10% CO2 and 37°C (Craige et al., 2008).
Mouse neocortical neurons were harvested from E18.5 embryos and prepared similarly to (Tippens and Lee, 2007). Briefly, neocortical tissue was dissociated by incubation with papain (Worthington Biochemicals, Lakewood, NJ) for 1 h at 37°C and 5% CO2, followed by tituration in 10% FBS minimal essential medium (MEM) inactivation solution. Cells were plated at an average density of 75,000–100,000 cells/12-mm coverslip precoated with polylysine HBr (Sigma-Aldrich) in growth media containing 1 mM pyruvate, 0.6% dextrose, 5% fetal bovine serum, B-27 supplement (Invitrogen), 0.5 mM penicillin/streptomycin/glutamine, and 0.001% mito-serum extender (BD Biosciences) and incubated at 5% CO2 and 37°C. After 72 h, 5 μM 1-β-d-arabinofuranosylcytosine was added. After 2 wk (days in vitro [DIV] = 14), cells were used for immunofluorescence.
Microscopy
Immunofluorescence was preformed as described in Salazar et al. (2004b) and Craige et al. (2008). Briefly, PC12 cells were plated on Matrigel (BD Biosciences)-coated coverslips, whereas primary neurons were cultured on poly-lysine HBr (Sigma-Aldrich)-coated coverslips. Images were acquired with a scientific-grade cooled charge-coupled device (CoolSNAP HQ with ORCA-ERchip) on a multiwavelength, wide-field, three-dimensional microscopy system (Intelligent Imaging Innovations, Denver, CO), based on a 200M inverted microscope using a 63× numerical aperture 1.4 lens (Carl Zeiss, Thornwood, NY). Immunofluorescent samples were imaged at room temperature using a Sedat filter set (Chroma Technology, Rockingham, UT), in successive 0.20-μm focal planes. Out-of-focus light was removed with a constrained iterative deconvolution algorithm (Swedlow et al., 1997). Images were processed and analyzed using MetaMorph software version 3.0 (Molecular Devices, Sunnyvale, CA). Three consecutive z-series were analyzed per image by thresholding to similar levels and determining colocalization as the percentage of pixel area overlap for the respective channels.
Labeled Epidermal Growth Factor (EGF) Internalization
PC12 cells plated on Matrigel coated-coverslips were serum deprived overnight with 0.1% FBS DMEM. Cells were labeled on ice with 100 nM fluorescein-conjugated EGF (E-3478; Invitrogen). After labeling, cells were washed with cold phosphate-buffered saline (PBS). EGF fluorescein was internalized by adding warm DMEM for 5, 15, or 30 min, and incubating the cells at 37°C. After internalization, cells were fixed, immunostained, and imaged as described in the microscopy section.
Subcellular Fractionation
Brains from 12 C57B, eight Ap3b2−/−, nine Ap3b1−/−, eight Mutedmu/mu, and eight Muted+/mu mice aged 7–12 wk were fractionated according to Craige et al. (2004) and Salazar et al. (2004a). Synaptic vesicle fractions were resolved by 5–25% glycerol gradient velocity sedimentation. All brains from the same genotype were processed together. Purified rat brain synaptic vesicles were prepared as described previously (Clift-O'Grady et al., 1990; Craige et al., 2004).
Subcellular Fractionation of PC12 cells and PC12 cells expressing ZnT3 (Salazar et al., 2004b) was performed according to the Clift–O'Grady method as described in Clift-O'Grady et al. (1990), Craige et al. (2004), and Salazar et al. (2004a). Synaptic-like microvesicle (SLMV) fractions were resolved by 5–25% glycerol gradient velocity sedimentation. Immunomagnetic vesicular isolation of PC12 vesicles and mouse brain synaptic vesicles was performed as detailed in Craige et al. (2004) and Salazar et al. (2004a). Quantification of immunoreactive bands on glycerol gradient Western blots was done using NIH Image 1.63f (Grote et al., 1995b; Salazar et al., 2004b).
Dithiobis(succinimidyl Propionate) (DSP) Cross-Linking of AP-3 Immunocomplexes
DSP Cross-linking of AP-3 immunocomplexes and their isolation has been described in Craige et al. (2008) and Salazar et al. (2009).
Statistics
All data are expressed as average ± SE. Experimental conditions were compared with the one-way analysis of variance followed by Student–Newman–Keuls multiple comparison as a post hoc test by using KaleidaGraph version 3.6.2 (Synergy, Reading, PA). Kolmogorov–Smirnov test was performed using the engine http://www.physics.csbsju.edu/stats/KS-test.n.plot_form.html.
RESULTS
AP-3–sorted Lysosomal Cargoes and Synaptic Vesicle Membrane Proteins Colocalize in Early Endosomes
Purified PC12 cell synaptic-like microvesicles and rat brain synaptic vesicles copurify with proteins either targeted to or involved in the biogenesis of lysosomes. These include AP-3 and BLOC-1 subunits as well as AP-3 cargo membrane proteins such as PI4KIIα, the lysosomal vesicle-(R)-soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) VAMP7-TI, LAMP-1, and chloride channel-3 (Salazar et al., 2005; Takamori et al., 2006). A conventional view of the organization of endosomes in neurons would predict that synaptic vesicle proteins would recycle back to the cell surface. Thus, synaptic vesicle proteins would be segregated from lysosomal cargoes, which are bound to late endosome and lysosome compartments. In contrast, our seesaw model predicts that synaptic vesicle proteins and lysosomal AP-3 cargoes will coexist in the same donor endosomes as well as in synaptic vesicles. To discriminate between these models, we explored whether 1) lysosomal and synaptic vesicle cargoes are sorted from shared donor endocytic compartments, and 2) whether they are targeted into the same synaptic vesicle.
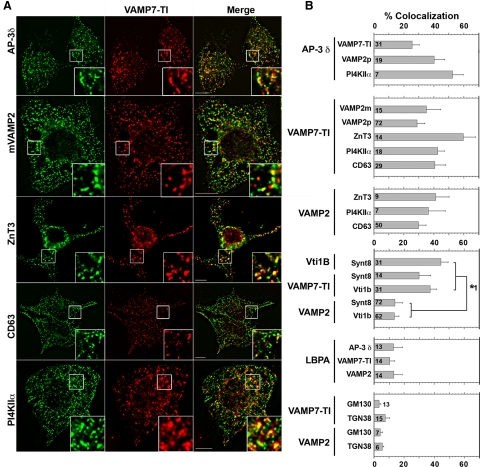
As a first step, we determined whether synaptic vesicle and AP-3–sorted lysosomal membrane proteins colocalize at steady state in PC12 cells and cultured mouse primary neurons by immunomicroscopy. This approach does not distinguish between whether this colocalization occurs in either a donor early endosome, synaptic vesicles, or both types of organelle. We used quantitative wide-field deconvolution immunofluorescence microscopy of cells labeled with combinations of antibodies against AP-3–sorted lysosomal membrane proteins (PI4KIIα, cd63, LAMP1, and VAMP7-TI), bona fide synaptic vesicle membrane proteins (the SNARE synaptobrevin 2/VAMP2 and ZnT3), and/or late endosomal markers (syntaxin 8, Vti1b, and LBPA) (Baumert et al., 1989; Le Borgne et al., 1998; Cole et al., 1999; Dell'Angelica et al., 1999; Rous et al., 2002; Martinez-Arca et al., 2003; Peden et al., 2004; Salazar et al., 2004b, 2005, 2006; Craige et al., 2008). If synaptic vesicle proteins are segregated from lysosomal cargoes, a basic prediction is that colocalization of either ZnT3 or VAMP2 with AP-3 lysosomal cargoes should be negligible at steady state. In contrast, if SV and lysosomal cargoes are sorted from shared early endosomes into synaptic vesicles then the colocalization between synaptic vesicle proteins and AP-3 lysosomal cargo will exceed the colocalization between synaptic vesicle proteins and late endosomal markers. However, this does not exclude that AP-3 lysosomal cargoes, like VAMP7-TI, will significantly colocalize with traditional late endosomal-lysosomal markers. We tested this latter prediction by using the late endosomal SNAREs syntaxin 8 and Vti1b, which form cognate SNARE pairs with VAMP7-TI, but not VAMP2 (Antonin et al., 2000; Wade et al., 2001; Bogdanovic et al., 2002; Pryor et al., 2004). Predictably, ∼30% of late endosomes immunoreactive for vti1b or syntaxin 8 also possessed VAMP7-TI (Figure 1B and Supplemental Figure 3). Yet, VAMP2 was present in only ∼10% of vti1b- or syntaxin 8-positive late endosomes (Figure 1B, and Supplemental Figure 3). This colocalization value is similar to a background colocalization of 3–7% defined by the overlap of either VAMP2 or VAMP7-TI with Golgi markers (Figure 1B and Supplemental Figure 4, GM130 and TGN38). These results, obtained in PC12 cells, indicate that synaptic vesicle SNARE VAMP2 is below detection level in late endosome compartments at steady state.
Figure 1.
AP-3 synaptic vesicle and lysosomal cargoes selectively colocalize in PC12 Cells. (A) Fixed PC12 cells immunostained for VAMP7-TI and indicated protein. Cells were imaged by wide-field deconvolution microscopy. Bar, 5 μm. (B) Quantification of colocalization between AP-3 and synaptic vesicle (VAMP2) or lysosomal cargo (VAMP7-TI and PI4KIIα); VAMP7-TI and synaptic vesicle (VAMP2 and ZnT3) or lysosomal cargo (PI4KIIα and CD63); VAMP2 and synaptic vesicle or lysosomal cargo. Cognate SNARE interactions and late endosomal marker LBPA illustrate the minimal contribution of late endosomes to the observed synaptic vesicle/lysosomal cargo colocalization. Golgi markers GM130 and TGN38 were used as negative controls to assess background levels of colocalization. Number of analyzed cells is indicated at the bottom of each bar graph. Asterisk represents p values for comparison of colocalization between either Vti1b or VAMP7-TI and either Syntaxin 8 or Vti1b, with colocalization between VAMP2 and Syntaxin 8 or Vti1b. All p < 0.0001. One-way analysis of variance (ANOVA) Student–Newman–Keuls multiple comparison. Representative images of these quantifications are presented in Supplemental Figures 2–4.
In contrast with a conventional model where synaptic vesicle and lysosomal AP-3 cargoes are segregated into distinct organelles, our model predicts that AP-3–sorted lysosomal membrane proteins should colocalize with synaptic vesicle membrane markers in endosomes and synaptic vesicles. Although synaptobrevin/VAMP2 is not detectable in late endosomes, 36.6 ± 5% of VAMP2 colocalized with VAMP7-TI (Figure 1, A and B, and Supplemental Figure 2). Antibodies against VAMP2 and VAMP7-TI did not cross-react (Supplemental Figure 1). Similarly, VAMP7-TI colocalized extensively with the synaptic vesicle protein ZnT3 (Figure 1, A and B). The colocalization between the synaptic vesicle SNARE VAMP2 and the AP-3 lysosomal cargo VAMP-7-TI was also observed with other AP-3 lysosomal cargoes. In fact, ∼30–40% of VAMP2-positive compartments were also positive for the lysosomal AP-3 cargoes PI4KIIα and CD63 (Figure 1, A and B, and Supplemental Figure 2). The colocalization of VAMP2 with AP-3 lysosomal cargoes (cd63, PI4KIIα, and VAMP7-TI) exceeds by approximately two- to threefold VAMP2 colocalization with diverse late endosomal markers (syntaxin 8, vti1b, and LBPA) (Kobayashi et al., 1999; Lebrand et al., 2002) (Figure 1B and Supplemental Figures 3 and 4). These results suggest that the synaptic vesicle SNARE VAMP2 and AP-3 lysosomal cargoes coexist in an endosome upstream of late endosomal compartments.
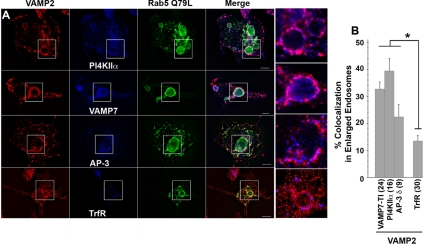
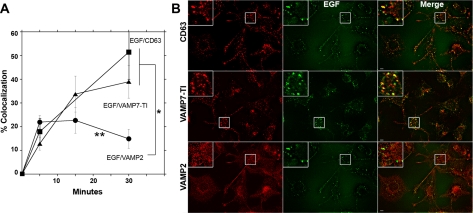
We used three strategies to define whether synaptic vesicle proteins and AP-3 lysosomal cargoes occupy the same early endosome donor compartment. First, we assessed whether VAMP2 and the AP-3 lysosomal cargoes VAMP7-TI and PI4KIIα were present in endosomes positive for the adaptor complex AP-3. This adaptor complex has been localized to early endosomes in diverse cell types, including PC12 cells (Dell'Angelica et al., 1997; Peden et al., 2004; Theos et al., 2005; Craige et al., 2008). AP-3 was present in 25–50% of those structures immunoreactive for the synaptic vesicle membrane protein VAMP2 or AP-3 lysosomal cargoes (VAMP7-TI and PI4KIIα, Figure 1B and Supplemental Figure 2). Second, we transfected PC12 cells with rab5Q79L-GFP to enlarge early endosomes. The limiting membrane of these enlarged endosomes is highlighted by the GFP signal, allowing the identification of membrane proteins present in these endosomes (Raiborg et al., 2002, 2006; Craige et al., 2004). VAMP2 colocalized with AP-3 lysosomal cargoes (VAMP7-TI and PI4KIIα) and AP-3 in the limiting membrane of enlarged early endosomes (Figure 2A). This colocalization was above the level observed between VAMP2 and transferrin receptor, a receptor not trafficked by AP-3 (Figure 2B) (Dell'Angelica et al., 1999). Additional controls revealed that PC12 rab5Q79L-enlarged endosomes are almost devoid of the adaptor complex AP-1 (Craige et al., 2008). Third, we labeled endosomes with surface internalized fluorescently labeled-EGF (Figure 3). Fluorescein-EGF was bound at 4°C, and unbound ligand was washed away. EGF internalization was resumed at 37°C for either 5–15 min to label early endosomes or 30 min to label late endosomes (de Wit et al., 1999). EGF reached compartments positive for VAMP2 and the lysosome AP-3 cargoes (VAMP7-TI and CD63) between 5 and 15 min (Figure 3A). However, EGF moved away from VAMP2-positive endosomes after 30 min, yet progressively accumulated in late endosomal compartments also positive for VAMP7-TI and CD63 (Figure 3, A and B). This is consistent with the steady-state colocalization analysis of PC12 cells, in which VAMP7-TI, but not VAMP2, significantly colocalizes with late-endosomal markers. These combined results from steady-state colocalizations, rab5Q79L-enlarged early endosomes, and pulse-chase with fluorescently labeled internalized EGF demonstrate that synaptic vesicle proteins and AP-3 lysosomal cargoes coexist in early endosome donor compartments but not late endosomes.
Figure 2.
Synaptic Vesicle and AP-3 lysosomal cargoes are present in rab5Q79L early endosomes. (A) PC12 cells were transfected with the GFP-tagged rab5 GTP mutant Q79L. Fixed cells were double labeled for VAMP2 with either PI4KIIα, VAMP7-TI, AP-3 δ, or transferrin receptor (TfrR). Cells were imaged by wide-field deconvolution microscopy. (B) Colocalization quantification at the limiting membrane of enlarged endosomes. All p < 0.003. One-way ANOVA Student–Newman–Keuls multiple comparison. Number in parentheses represent the number of enlarged endosomes analyzed.
Figure 3.
Internalized EGF labels early endosomes positive for VAMP2 and AP-3 lysosomal cargoes. PC12 cells endosomes were labeled with surface-bound fluorescein-EGF. Ligand was bound at 4°C, and excess ligand washed away. EGF internalization was resumed at 37°C for different times from 0 to 30 min. Cells were fixed and stained with antibodies against the synaptic vesicle protein VAMP2 and the AP-3 lysosomal cargoes VAMP7-TI and/or CD63. Cells were imaged by wide-field deconvolution microscopy and the extent of colocalization determined using MetaMorph software (A). In A, EGF reached compartments positive for VAMP2 and the AP-3 lysosomal cargoes (VAMP7-TI and CD63) between 5 and 15 min, but at 30 min, only AP-3 lysosomal cargoes localize to EGF-labeled late endosomes. Asterisk, p < 0.001. Double asterisk compares the colocalization extent of EGF and VAMP2 at 15 and 30 min, p < 0.005. One-way ANOVA Student–Newman–Keuls multiple comparison. Thirty to 145 cells collected per point from three to four independent experiments were analyzed. (B) Depicts images of cells after 30 min of EGF internalization. Cells were double or triple labeled with EGF and either CD63, VAMP2, or VAMP7-TI antibodies. Bar, 5 μm.
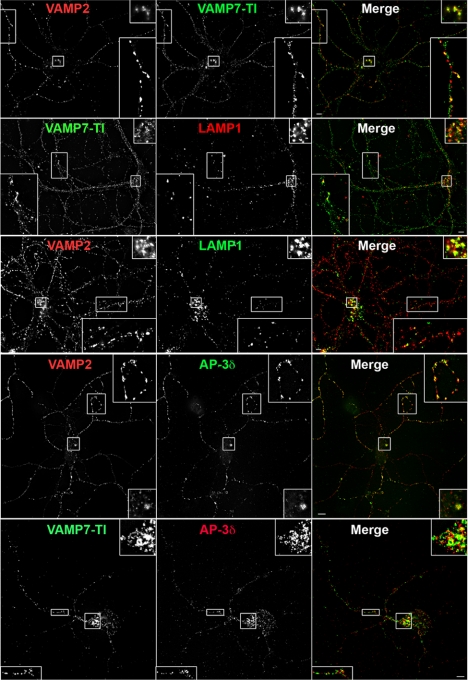
We further explored the extent of overlap between synaptic vesicle membrane proteins and lysosomal AP-3 cargoes in mouse primary neocortical neurons. Mouse primary neurons recapitulate our central findings in PC12 cells. For example, within the cell body of mouse primary neurons VAMP2 colocalizes with AP-3–sorted lysosomal proteins VAMP7-TI and LAMP1 to the same extent that these two lysosomal proteins colocalize together (Figure 4 and Supplemental Figure 5). Immunoreactivity for VAMP2 or VAMP7-TI is present in cell body compartments also positive for AP-3 (Figure 4 and Supplemental Figure 5). Furthermore, the signal overlap between VAMP2 and lysosomal AP-3 cargoes in neuronal cell bodies was significantly above the colocalization of either VAMP2 or VAMP7-TI with the late endosomal marker LBPA (Supplemental Figure 5). The low colocalization of VAMP7-TI with LBPA in primary cultures of neurons and PC12 cells may be due to the fact that LBPA labels a limited set of late endosomes in other cell types (White et al., 2006). In contrast with cell bodies, although we were still able to resolve distinct punctae in which synaptic vesicle proteins and lysosomal AP-3 cargoes colocalize, the overlap between VAMP2 and VAMP7-TI in neuronal processes was similar to the overlap of either one of these markers with LBPA (Supplemental Figure 5). This finding was the same regardless of whether processes where further classified as dendrites or axons (Supplemental Figure 5). The constrained architecture of slender cellular processes likely contributes to the overlap of VAMP2 and VAMP7-TI with LBPA. However, the entry of VAMP2 and VAMP7 into a shared synaptic vesicle compartment was also addressed by biochemical vesicular isolation (see below).
Figure 4.
Synaptic vesicle and lysosomal AP-3 cargoes colocalization in mouse primary neocortical neurons. Fixed E18.5 mouse primary neurons (DIV 14) from wild-type C57B or heterozygous controls immunostained for indicated proteins: VAMP2, VAMP7-TI, LAMP1, and AP-3 δ. Enlarged insets allow for comparison of colocalization in cell body and processes. Bar, 5 μm.
Collectively, our results in PC12 cells and primary cultured neurons favor a model in which at steady-state synaptic vesicle proteins and AP-3 lysosomal cargoes reside in shared early endosomes. From this donor compartment, synaptic vesicle and AP-3 lysosomal cargoes may be sorted to a common synaptic vesicle if they interact with synaptic vesicle biogenesis machinery on early endosomes, which includes the neuronal AP-3 complex.
Lysosomal AP-3 Cargoes and Synaptic Vesicle Membrane Proteins Are Sorted to Common Synaptic-like Microvesicles and Synaptic Vesicles
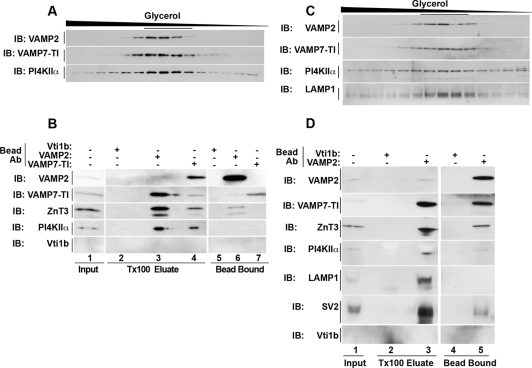
We tested whether synaptic vesicle proteins and AP-3 lysosomal cargoes are sorted into a common vesicle by analyzing the composition of synaptic-like microvesicles and brain synaptic vesicles. PC12 synaptic-like microvesicles are generated from early endosomes (Faundez et al., 1998; Lichtenstein et al., 1998; de Wit et al., 1999). PC12 synaptic-like microvesicles were isolated by differential centrifugation and size fractionated in glycerol gradients (Figure 5A). VAMP2 and AP-3–sorted lysosomal proteins (PI4KIIα and VAMP7-TI) comigrated in similarly sized vesicles (∼40 nm; Schmidt et al., 1997; Salazar et al., 2005). To determine whether these proteins reside on the same vesicle, glycerol gradient peak fractions (Figure 5A, underlined fractions) were used for immunomagnetic vesicle isolation. Synaptic-like microvesicles were immunoisolated using monoclonal antibodies that recognize cytosolic epitopes in the synaptic vesicle protein VAMP2 or the AP-3 lysosomal cargo VAMP7-TI (Figure 5B, lanes 3 and 4 and 6 and 7). Antibodies against late endosome SNARE Vti1b were used as controls (Figure 5B, lanes 2 and 5). Late endosomes are virtually absent from synaptic-like microvesicles and brain synaptic vesicle fractions (Supplemental Figure 6). Thus, no bound vesicles were expected with vti1b-beads, equating this control to a nonspecific antibody control. Synaptic-like microvesicles bound by antibodies against either VAMP2 or VAMP7-TI contained AP-3–sorted lysosomal proteins PI4KIIα and VAMP7-TI in addition to known synaptic vesicle proteins VAMP2 and ZnT3 (Salazar et al., 2004b). No vesicle binding was observed with magnetic beads decorated with Vti1b antibodies (Figure 5B, lanes 2 and 5). Similarly, VAMP2 and AP-3–sorted lysosomal proteins (VAMP7-TI, PI4KIIα, and LAMP1) comigrated in mouse brain synaptic vesicles resolved by glycerol sedimentation (Figure 5C). Brain synaptic vesicles immunoisolated with monoclonal antibodies against VAMP2 contained AP-3–sorted lysosomal proteins (VAMP7-TI, PI4KIIα, and LAMP1; Figure 5D, lanes 3 and 5) as well as known synaptic vesicle proteins (ZnT3 and SV2). Synaptic vesicle binding to control beads was negligible (Figure 5D, lanes 2 and 4). Similar results were obtained when synaptic vesicles were purified from rat brain by a different procedure (data not shown; Clift-O'Grady et al., 1990). Thus, biochemical characterization of both PC12 cell synaptic-like microvesicles and mouse brain synaptic vesicles demonstrates that synaptic vesicle proteins and lysosomal AP-3 cargoes are sorted into a common organelle.
Figure 5.
Synaptic vesicles and synaptic-like microvesicles contain AP-3 lysosomal cargoes. Glycerol gradient-sedimented synaptic-like microvesicles from PC12 cells (A) or mouse brain (C) were resolved by SDS-PAGE. Glycerol gradient fractions (A and C) were probed for VAMP2, VAMP7-TI, and PI4KIIα, as well as LAMP1 in mouse brain (C only). Synaptic-like microvesicles and synaptic vesicles peak in the middle of the gradient. (B and D) Immunomagnetic vesicular isolation from PC12 SLMVs and mouse brain SVs. Peak fractions (fractions 7–12, underlined) of above-mentioned glycerol gradients (A and C) were combined. Pooled vesicles were isolated with monoclonal antibodies to either the late endosome SNARE Vti1b, which serves as a negative control (B and D), the synaptic vesicle protein VAMP2 (B and D), or the AP-3 lysosomal cargo VAMP7-TI (B). Bound vesicles were eluted in Triton X-100 buffer, and both eluate and the remaining bead-bound material were resolved by SDS-PAGE. Contents were analyzed by Western blotting for synaptic vesicle proteins (VAMP2, ZnT3, and SV2) or AP-3 lysosomal cargoes (LAMP1, PI4KIIα, and VAMP7-TI). Inputs represent 10%. Data are representative of three independent experiments.
Hermansky–Pudlak Gene Products Involved in Lysosome and Lysosome-related Organelle Biogenesis Control the Composition of Synaptic Vesicle Fractions
Synaptic vesicle and lysosomal membrane proteins are present in a common donor early endosome that is positive for the adaptor complex AP-3. This raises the question as to which adaptor(s) sort(s) lysosomal proteins into synaptic vesicle fractions. We analyzed the content of synaptic vesicle and lysosomal AP-3 cargoes in synaptic vesicle fractions isolated from control, Ap3b1−/− and mutedmu/mu mouse brains. These two mutants disrupt subunits of the ubiquitous AP-3 and BLOC-1 complexes, which are affected in Hermansky–Pudlak syndrome, a disorder that affects the biogenesis of lysosomes and lysosome-related organelles (Di Pietro and Dell'Angelica, 2005; Raposo and Marks, 2007). We contrasted the effects of these mutations with Ap3b2−/−, a mutant that affects the neuronal AP-3 complex and is involved in synaptic vesicle biogenesis (Nakatsu et al., 2004; Seong et al., 2005).
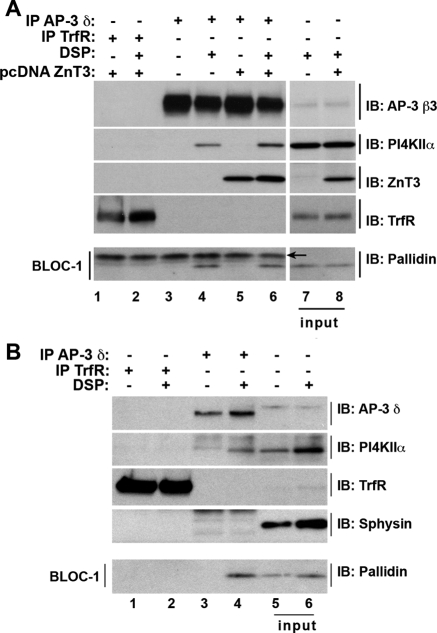
We first determined whether the interaction between AP-3 and BLOC-1 complexes observed in nonneuronal cells (Di Pietro et al., 2006; Salazar et al., 2009) is recapitulated in PC12 cells and mouse primary cultured neurons (Figure 6, A and B, respectively). We used in vivo DSP cross-linking to stabilize AP-3 interactors (Craige et al., 2008). Cross-linked AP-3 complexes from PC12 cells and neurons were immunoprecipitated with antibodies against the AP-3 δ subunit and immunocomplexes analyzed by Western blot. We identified the following cargoes: PI4KIIα in AP-3 precipitates from cross-linked PC12 and mouse primary neurons (Figure 6A, compare lanes 3 and 4; Figure 6B, compare lanes 3 and 4) and PI4KIIα and ZnT3 in AP-3 precipitates from cross-linked PC12-ZnT3 cells (Figure 6A, compare lanes 5 and 6). Importantly, cross-linked AP-3 complexes from all these cells contained BLOC-1 detected with antibodies against the BLOC-1 subunit pallidin (Falcon-Perez et al., 2002; Ciciotte et al., 2003). The specificity of these interactions was confirmed in immunoprecipitations with transferrin receptor antibodies, which were free of AP-3 subunits, pallidin, PI4KIIα, and ZnT3 (Figure 6, A and B, lanes 1 and 2). Moreover, transferrin receptor and synaptophysin, two proteins whose targeting is not affected by AP-3 deficiencies were absent from AP-3 immunocomplexes (Figure 6, A and B) (Dell'Angelica et al., 1999; Salazar et al., 2004b). The presence of PI4KIIα and BLOC-1 in cross-linked AP-3 complexes predicts that the targeting of PI4KIIα to synaptic vesicle fractions should be perturbed in AP-3 and BLOC-1 deficiencies. In contrast, synaptophysin, which is absent from cross-linked complexes, should remain unaffected.
Figure 6.
AP-3 and BLOC-1 form a complex in PC12 cell and mouse primary neurons. PC12 cells or PC12-ZnT3 cells (A) and E18.5 wild-type C57 mouse primary neurons (DIV 14) (B) were cross-linked with DSP. Cross-linked AP-3 complexes were immunoprecipitated with either AP-3 δ antibodies or transferrin receptor antibodies (TrfR). Immunocomplexes were resolved by SDS-PAGE and analyzed by immunoblot with antibodies against: the AP-3 subunits δ and β 3, transferrin receptor, synaptophysin (Sphysin), ZnT3, PI4KIIα, and pallidin, a BLOC-1 subunit. Input A, 1.4% and B, 3.33%. Arrow A indicates IgG light chains.
To assess sorting of synaptic vesicle proteins and lysosomal AP-3 cargoes to synaptic vesicles, synaptic vesicle fractions were isolated by glycerol sedimentation of high-speed brain supernatants. In these gradients, synaptic vesicles migrate in the middle of the gradient as a symmetric peak. Defective sorting of a defined protein is assessed either by changes in its distribution and/or content in the gradient (Grote et al., 1995a; Schmidt et al., 1997; Clift-O'Grady et al., 1998; Thiele et al., 2000; Craige et al., 2004; Salazar et al., 2004b). Synaptic vesicle membrane protein contents were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) and Western blotting for specific synaptic vesicle proteins (ZnT3, VGAT, synaptophysin, VAMP2, Vglut1, and SV2; Takamori et al., 2006) and AP-3 lysosomal cargoes (PI4KIIα and VAMP7-TI). For lysosomal cargo, we focused on PI4KIIα and VAMP7-TI because these proteins are reliably detected in synaptic vesicles fractions (Salazar et al., 2005, 2006). Moreover, PI4KIIα and VAMP7-TI accumulate in the cell body and are depleted from nerve terminals of neurons lacking both AP-3 isoforms (mocha) (Salazar et al., 2005; Scheuber et al., 2006).
Consistent with the presence of PI4KIIα and BLOC-1 in cross-linked AP-3 complexes (Figure 6), the distribution of PI4KIIα in glycerol gradients was modified in all AP-3 and BLOC-1 deficiencies tested (Figures 7 and 8). In contrast, neither the glycerol gradient distribution nor content of synaptophysin, a protein absent from cross-linked AP-3 complexes, was altered in AP-3 or BLOC-1-null brain synaptic vesicle fractions (Figures 7 and 8). These results indicate that glycerol velocity sedimentation of vesicles isolated from wild-type and mutant brains discriminates the sorting phenotypes of membrane proteins predicted from AP-3 cross-linked complexes (Figure 6).
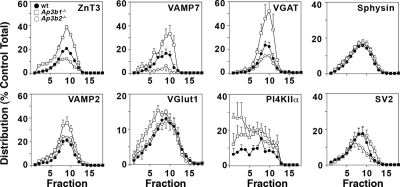
Figure 7.
Ubiquitous and neuronal AP-3 isoforms regulate synaptic vesicle protein content. Glycerol gradient velocity sedimentation of S2 fractions from wild type C57B (closed circles), Ap3b1−/− (open squares), and Ap3b2−/− (open circles) mouse brains. Synaptic vesicle fractions were resolved by SDS-PAGE and analyzed by Western blot for the indicated protein. Protein levels were quantified using NIH Imager and were standardized to control C57B levels. In the absence of ubiquitous AP3 (Ap3b1−/−), peak synaptic vesicle levels of ZnT3 (p < 0.003), VAMP7-TI (p < 0.0008), VGAT (p < 0.004), and VAMP2 (p < 0.04) increase, whereas VGLUT1 (p < 0.0005) and PI4KIIα (p < 0.03) show increases in larger vesicle fractions; synaptophysin and SV2 remain unaffected. In the absence of neuronal AP-3 (Ap3b2−/−), peak synaptic vesicle levels of ZnT3 (p < 0.0001), VAMP7 (p < 0.003), VGAT (p < 0.02), and SV2 decrease (p < 0.05), whereas the levels of PI4KIIα increase in larger vesicle fractions (p < 0.0004); synaptophysin (Sphysin), VAMP2, and VGlut1 remain unaffected. ZnT3, n = 7, 7; VAMP7-TI, n = 4, 4; VGAT, n = 4, 5; Sphysin, n = 5, 10; PI4KIIα, n = 3, 3; SV2, n = 4, 4; VAMP2, n = 4, 4; VGlut1, n = 3, 3 (number of independent fractionations, number of independent Western blot analyses). One-way ANOVA Student–Newman–Keuls multiple comparison. Representative blots for these experiments can be found in Supplemental Figure 7.
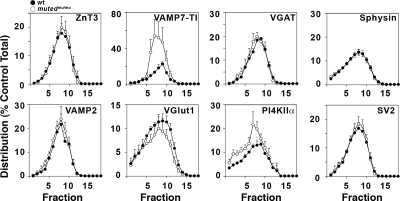
Figure 8.
BLOC-1 selectively regulates synaptic vesicle levels of PI4KIIα and VAMP7-TI. Glycerol gradient velocity sedimentation of S2 fractions from BLOC-1-deficient mutedmu/mu (open circles) and control Muted+/mu (closed circles) mouse brains. Synaptic vesicle fractions were resolved by SDS-PAGE and analyzed by Western blot for the indicated protein. Protein levels were quantified using NIH Imager and were standardized to control muted+/mu levels. Synaptic vesicle levels of ZnT3, VGAT, Sphysin, VAMP2, VGLUT1, and SV2 are unaffected by the loss of muted. However, the peak synaptic vesicle levels of both VAMP7-TI (p < 0.0005) and PI4KIIα (p < 0.01) increase in Mutedmu/mu. ZnT3, n = 3, 3; VAMP7-TI, n = 4, 4; VGAT, n = 4, 4; Sphysin, n = 4, 9; PI4KIIα, n = 4, 4; SV2, n = 3, 3; VAMP2, n = 3, 3; and VGlut1, n = 4, 4 (number of independent fractionations, number of independent Western blot analyses). Wilcoxon–Mann–Whitney test. Representative blots for these experiments can be found in Supplemental Figure 8.
We explored two seesaw model predictions by using this experimental paradigm. First, we asked whether the targeting of characteristic synaptic vesicle proteins to synaptic vesicle fractions was altered by deficiencies in transport to lysosomes and/or lysosome-related organelles (Ap3b1−/−, mutedmu/mu). The seesaw model predicts that in the absence of ubiquitous AP-3 (Ap3b1−/−) or BLOC-1 (mutedmu/mu), AP-3 cargoes that normally would have been sorted to lysosomes would instead be routed to synaptic vesicles. The content of the synaptic vesicle proteins ZnT3, VGAT, VAMP2, and the lysosomal AP-3 cargo VAMP7-TI was increased in Ap3b1−/− synaptic vesicle fractions. In addition, the synaptic vesicle proteins Vglut1 and the lysosomal AP-3 cargo PI4KIIα sedimented faster in fractions isolated from Ap3b1−/− (Figure 7). Vglut1 and PI4KIIα sedimentation in Ap3b1−/− is consistent with a potential mistargeting into larger vesicles. In contrast with the pleiotropic effects of Ap3b1−/− in synaptic vesicle composition, mutedmu/mu only affected the targeting of VAMP7 and PI4KIIα (Figure 8). Much like Ap3b1−/−, the content of VAMP7-TI was increased in synaptic vesicle fractions, whereas PI4KIIα sedimented faster in synaptic vesicle fractions isolated from mutedmu/mu brains (Figure 8). These changes in the targeting of synaptic vesicle proteins and AP-3 lysosomal cargoes observed in Ap3b1−/− and mutedmu/mu were not due to the presence of contaminant recycling or late endosome markers comigrating with synaptic vesicles (Supplemental Figure 6, Sagne and Gasnier, 2008). Furthermore, these changes in vesicle composition were not attributable to overall changes in the expression of these proteins in mutant brains (Supplemental Figure 9). Nor are our results attributable to global changes in synaptic vesicle protein fractionation, as indicated by the normal content and distribution of synaptophysin and SV2 in Ap3b1−/− vesicle fractions, or ZnT3, VGAT, synaptophysin, VAMP2, Vglut1, and SV2 in vesicles isolated from mutedmu/mu brains (Figures 7 and 8). Second, we asked whether neuronal AP-3 directs the incorporation of AP-3 lysosomal cargo into synaptic vesicle fractions by examining synaptic vesicles isolated from the neuronal AP-3–deficient, Ap3b2−/−, mouse brain. The seesaw model predicts that in the absence of neuronal AP-3, lysosomal AP-3 cargoes that normally would have been targeted to synaptic vesicles would be reduced in synaptic vesicles because of their increased routing to lysosome compartments. The content of the lysosomal cargo VAMP7-TI was decreased in synaptic vesicle fractions from Ap3b2−/− (Figure 7). In addition, PI4KIIα glycerol sedimentation pattern was altered by the absence of neuronal AP-3 (Figure 7). This altered distribution could reflect the dual role for PI4KIIα as both an AP-3 recruitment factor and AP-3 cargo (Craige et al., 2008). As reported previously and consistent with neuronal AP-3's role in synaptic vesicle biogenesis, ZnT3 and VGAT targeting to synaptic vesicle fractions was decreased in Ap3b2−/− mouse brain vesicles (Figure 7) (Nakatsu et al., 2004; Seong et al., 2005). These results indicate that lysosomal (ubiquitous AP-3 and BLOC-1) and synaptic vesicle targeting mechanisms (neuronal AP-3) control the delivery of characteristic synaptic vesicle and AP-3–sorted lysosomal membrane proteins to a common synaptic vesicle. Moreover, our data indicate that cargoes sorted by neuronal AP-3, ubiquitous AP-3, or BLOC-1 are partially overlapping.
DISCUSSION
Synapses depend on vesicle biogenesis mechanisms to deliver membrane proteins from the cell body to nerve terminals. Current models that account for membrane protein delivery to synaptic compartments do not consider the contribution of lysosome biogenesis mechanisms. In fact, lysosomal sorting and synaptic vesicle biogenesis machinery are thought to regulate mutually exclusive cargo proteins. However, our work challenges this model by presenting evidence for a mechanism where both lysosomal sorting and synaptic vesicle biogenesis machinery jointly regulate both synaptic vesicle and lysosomal cargo proteins from shared early endosome donor compartments. We have referred to this mechanism as the seesaw model, because both sorting machineries are necessary for a properly balanced synaptic vesicle composition. First, we provide high-resolution microscopy evidence, indicating that lysosomal and synaptic vesicle membrane proteins coexist with AP-3 in early endosomes. From these endosomes, synaptic vesicle and AP-3 lysosomal cargoes are sorted into synaptic-like microvesicles of PC12 cells and synaptic vesicles of mouse brain. Second, we demonstrate that deficiencies in an AP-3 adaptor isoform involved in lysosome biogenesis (ubiquitous AP-3, Ap3b1−/−) lead to changes in the targeting of characteristic synaptic vesicle membrane proteins (ZnT3, VGAT, VAMP2, and Vglut1) to synaptic vesicle fractions. Third, we demonstrate that mutant mice lacking an adaptor involved in the sorting of synaptic vesicle proteins (neuronal AP-3, Ap3b2−/−) exhibit decreased targeting of several membrane proteins to synaptic vesicle fractions (Figure 7). Predictably, a group of synaptic vesicle membrane proteins is affected in Ap3b2−/− brains (ZnT3, VGAT, and SV2) (Figure 7). However, a more significant finding is the perturbed targeting of lysosomal proteins (VAMP7-TI and PI4KIIα) by a deficiency in a synaptic vesicle-sorting mechanism (Figure 7). Fourth, we show that mouse models defective in protein complexes involved in the biogenesis of lysosomes and lysosome-related organelles (ubiquitous AP-3, Ap3b1−/−, and BLOC-1, mutedmu/mu) possess increased levels of membrane proteins found in lysosomes (VAMP7-TI and PI4KIIα) in their synaptic vesicle fractions.
How do ubiquitous and neuronal AP-3 regulate membrane protein content in synaptic vesicle fractions? A model ought to account for both the opposite effects that deficiencies in these adaptor complexes have in the targeting of ZnT3, VAMP7-TI, or VGAT to synaptic vesicle fractions. Although ubiquitous AP-3 deficiency in Ap3b1−/− mice increases the content of these proteins in synaptic vesicle fractions (Figure 7), in contrast, the absence of neuronal AP-3 in Ap3b2−/− mice decreases their content in synaptic vesicle fractions (Figure 7). The simplest explanation is that ubiquitous and neuronal AP-3 complexes compete for these cargoes to deliver them to two alternative fates. Neuronal AP-3 would deliver these proteins into vesicles bound to nerve terminals and away from the lysosomal route controlled by the ubiquitous AP-3 complex (Dell'Angelica et al., 1999; Robinson, 2004; Newell-Litwa et al., 2007). Thus, both adaptors would behave as branches of a seesaw, such that in the absence of one AP-3 isoform the other sorting route would experience an increased availability of membrane proteins for delivery. For example, in the absence of ubiquitous AP-3, membrane proteins that otherwise would have been destined for late endosomes-lysosomes are rerouted to synaptic vesicle fractions by neuronal AP-3.
This seesaw mechanism would define the composition of synaptic vesicle precursors leaving the cell body. This model, which integrates divergent AP-3 sorting routes at the level of cell body endosomes, provides a conceptual framework to understand: 1) the coexistence of both AP-3 isoforms in neuronal cell bodies (Seong et al., 2005); 2) the convergence of AP-3, synaptic vesicle, and lysosomal membrane proteins in early endocytic compartments in PC12 and primary cultured neurons (Figures 1–4); 3) the observation that the absence of both AP-3 isoforms in mocha (Ap3dmh/mh) leads to a depletion of ZnT3, VAMP7-TI and PI4KIIα from nerve terminals concomitantly with their accumulation in cell bodies (Kantheti et al., 1998; Salazar et al., 2004b, 2005; Scheuber et al., 2006); 4) the previously puzzling identification of diverse late endosomal/lysosomal proteins, such as Npc1, dysbindin, vps33b, rab7, ClC-3, VAMP7-TI, LAMP-1, and PI4KIIα, in either developing axonal projections, nerve terminals, synaptic-like microvesicles, and/or synaptic vesicles (Overly and Hollenbeck, 1996; Stobrawa et al., 2001; Guo et al., 2003; Talbot et al., 2004; Salazar et al., 2005, 2006; Saxena et al., 2005; Arantes and Andrews, 2006; Deinhardt et al., 2006; Karten et al., 2006; Takamori et al., 2006; Talbot et al., 2006). Consistent with a role for AP-3–dependent targeting of lysosomal proteins to a synaptic vesicle fate, all of these lysosomal proteins found in axonal-synaptic compartments are either present in AP-3–derived vesicles or they genetically and/or biochemically interact with AP-3 (Stepp et al., 1997; Le Borgne et al., 1998; Dell'Angelica et al., 1999; Martinez-Arca et al., 2003; Salazar et al., 2004a, 2005; Newell-Litwa et al., 2007). Despite the fact that competitive AP-3 sorting would be limited to the cell body, in which both AP-3 isoforms are present, the seesaw model does not preclude an individualized role for neuronal AP-3 directly in presynaptic terminals.
How do our results illuminate the sorting mechanisms controlled by AP-3 isoforms and the BLOC-1 complex? Membrane transport pathways are typically explored by analyzing a single cargo reporter. However, key concepts in membrane protein sorting have emerged from a collective view of the behavior of multiple cargoes in a trafficking route (Motley et al., 2003; Traub, 2003). Therefore, to comprehensively assess the role of AP-3 isoforms and BLOC-1 in membrane protein sorting, we simultaneously analyzed the targeting of multiple synaptic vesicle and/or lysosomal membrane proteins. We took advantage of the defined composition of synaptic vesicles, the availability of multiple AP-3 cargoes, as well as mouse deficiencies in AP-3 isoforms and BLOC-1. The picture that emerges from our studies is that deficiencies in neuronal AP-3, ubiquitous AP-3 and BLOC-1 affect the targeting of a core group of membrane proteins constituted at least by VAMP7-TI and PI4KIIα. PI4KIIα is found in a complex with AP-3 and BLOC-1 subunits in neurons and the inclusion of PI4KIIα into cross-linked AP-3 complexes is decreased in BLOC-1–deficient fibroblasts (Figure 6; Salazar et al., 2009). These results could be interpreted as BLOC-1 acting as a factor that facilitates AP-3 recognition of cargoes such as VAMP7-TI and PI4KIIα (Di Pietro et al., 2006; Salazar et al., 2006). However, how can we understand the difference in affected cargoes between synaptic vesicle fractions from ubiquitous AP-3 deficient (Ap3b1−/−) BLOC-1 deficient (mutedmu/mu) mouse brain? On one hand, BLOC-1 could serve as a cargo-specific adaptor, similar to β-arrestin, epsins, or autosomal recessive hypercholesterolemia protein, which bind selected membrane proteins and bring them into nascent AP-2–derived clathrin-coated vesicles (Traub, 2003). Our observations resemble these findings in that AP-3 and BLOC-1 are found in a complex (Figure 6), and BLOC-1 affects the targeting of a restricted subset of AP-3 cargoes (Figure 8). Alternatively, BLOC-1 could participate in a sorting mechanism independent of AP-3 isoforms, as is the case for ATP7A and Tyrp1, whose sorting to melanosomes, a lysosome related organelle, is independent of AP-3 (Setty et al., 2007, 2008).
In addition to PI4KIIα and VAMP7-TI, neuronal and ubiquitous AP-3 deficiencies also regulate synaptic vesicle targeting of membrane proteins not affected by BLOC-1 (ZnT3 and VGAT) (compare gradients in Figures 7 and 8). Yet, other synaptic vesicle membrane proteins (VAMP2, Vglut1, and SV2) are affected by a deficiency in just one of the AP-3 isoforms (Figure 7). These results support the notion that both AP-3 isoforms recognize distinct, as well as overlapping, membrane protein cargoes independently of BLOC-1. Furthermore, our results indicate that not all synaptic vesicle proteins reach synaptic vesicle pools through an AP-3–BLOC-1 route. A clear example is synaptophysin. This protein does not interact with AP-3; rather, it associates with the AP-1 adaptor or components of the AP-2 vesicle biogenesis machinery (Figure 6) (Daly and Ziff, 2002; Horikawa et al., 2002). Predictably, neither AP-3 nor BLOC-1 deficiencies explored here, nor Ap3dmh/mh and Ap3m2−/− deficiencies, affect synaptophysin targeting to nerve terminals/synaptic vesicle fractions (Figures 7 and 8) (Nakatsu et al., 2004; Salazar et al., 2004b).
Our research reveals significant alterations in the content of critical neurotransmitter and ionic transporters within synaptic vesicle fractions of mice deficient for AP-3 and BLOC-1 endocytic transport (Figures 7 and 8). Whereas neuronal AP-3 deficiencies selectively affect the inhibitory neurotransmitter transporter VGAT, ubiquitous AP-3 deficiencies modulate the synaptic vesicle levels of VGAT as well as the excitatory neurotransmitter transporter, VGlut1 (Figure 7). In Ap3m2−/− mouse brain, just a partial reduction of VGAT in synaptic vesicles directly correlates with decreased inhibitory neurotransmission (Ohno et al., 1999). Therefore, the up-regulation of both VGlut1 and VGAT could affect both inhibitory and excitatory neurotransmission, especially with increasing evidence that presynaptic mechanisms, such as the content of neurotransmitter transporters in synaptic vesicles, modulate quantal size (Edwards, 2007). Diverting neurotransmitter transporters to synaptic vesicles by a seesaw mechanism could change the luminal content of neurotransmitters. Consistent with this idea, brefeldin A inhibition of AP-3–dependent sorting restores not only VGlut1 recycling but also excitatory neurotransmission, under high-frequency stimulation (Voglmaier et al., 2006). Similarly, increased levels of ZnT3 in ubiquitous AP-3 null (Ap3b1−/−) brain synaptic vesicles correlate with enhanced histochemically reactive zinc in brain tissue (Seong et al., 2005). Thus, a seesaw mechanism defining synaptic vesicle composition could regulate quantal size independent of transcriptional regulation. Finally, our model may provide a complementary view to understand the association of deficiencies affecting lysosomes or lysosome trafficking mechanisms with synaptic defects. This association is illustrated in Drosophila, Caenorhabditis elegans, as well as neurodegenerative disorders in humans (Narayanan et al., 2000; Nixon et al., 2000; Bahr and Bendiske, 2002; Skibinski et al., 2005; Ramirez et al., 2006; Rubinsztein, 2006; Sweeney et al., 2006; Grill et al., 2007). In these abnormal states, perturbed synaptic function follows cell and/or synapse loss due to product buildup. Thus, late endosomes and lysosomes are thought to just dispose of unwanted molecules preventing product amassment. However, our findings offer a mechanism that expands the function of lysosome trafficking by linking lysosomes with synaptic vesicle composition.
Supplementary Material
ACKNOWLEDGMENTS
We are indebted to the Faundez laboratory members, Dr. Erica Werner, and anonymous reviewer 2 for helpful comments. This work was supported by National Institutes of Health grants NS-42599 and GM-077569 (to V. F.) and F31NS058163 (to K.N.-L.). K.N.-L. was supported by a grant-in-aid of research from the National Academy of Sciences, administered by Sigma Xi, The Scientific Research Society.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-05-0456) on January 14, 2009.
REFERENCES
- Advani R. J., Yang B., Prekeris R., Lee K. C., Klumperman J., Scheller R. H. VAMP-7 mediates vesicular transport from endosomes to lysosomes. J. Cell Biol. 1999;146:765–776. doi: 10.1083/jcb.146.4.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonin W., Holroyd C., Fasshauer D., Pabst S., Von Mollard G. F., Jahn R. A SNARE complex mediating fusion of late endosomes defines conserved properties of SNARE structure and function. EMBO J. 2000;19:6453–6464. doi: 10.1093/emboj/19.23.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arantes R. M., Andrews N. W. A role for synaptotagmin VII-regulated exocytosis of lysosomes in neurite outgrowth from primary sympathetic neurons. J. Neurosci. 2006;26:4630–4637. doi: 10.1523/JNEUROSCI.0009-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr B. A., Bendiske J. The neuropathogenic contributions of lysosomal dysfunction. J. Neurochem. 2002;83:481–489. doi: 10.1046/j.1471-4159.2002.01192.x. [DOI] [PubMed] [Google Scholar]
- Baumert M., Maycox P. R., Navone F., De Camilli P., Jahn R. Synaptobrevin: an integral membrane protein of 18,000 daltons present in small synaptic vesicles of rat brain. EMBO J. 1989;8:379–384. doi: 10.1002/j.1460-2075.1989.tb03388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanovic A., Bennett N., Kieffer S., Louwagie M., Morio T., Garin J., Satre M., Bruckert F. Syntaxin 7, syntaxin 8, Vti1 and VAMP7 (vesicle-associated membrane protein 7) form an active SNARE complex for early macropinocytic compartment fusion in Dictyostelium discoideum. Biochem. J. 2002;368:29–39. doi: 10.1042/BJ20020845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino J. S., Traub L. M. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- Burre J., Volknandt W. The synaptic vesicle proteome. J. Neurochem. 2007;101:1448–1462. doi: 10.1111/j.1471-4159.2007.04453.x. [DOI] [PubMed] [Google Scholar]
- Ciciotte S. L., Gwynn B., Moriyama K., Huizing M., Gahl W. A., Bonifacino J. S., Peters L. L. Cappuccino, a mouse model of Hermansky-Pudlak syndrome, encodes a novel protein that is part of the pallidin-muted complex (BLOC-1) Blood. 2003;101:4402–4407. doi: 10.1182/blood-2003-01-0020. [DOI] [PubMed] [Google Scholar]
- Clift-O'Grady L., Desnos C., Lichtenstein Y., Faundez V., Horng J. T., Kelly R. B. Reconstitution of synaptic vesicle biogenesis from PC12 cell membranes. Methods. 1998;16:150–159. doi: 10.1006/meth.1998.0662. [DOI] [PubMed] [Google Scholar]
- Clift-O'Grady L., Linstedt A. D., Lowe A. W., Grote E., Kelly R. B. Biogenesis of synaptic vesicle-like structures in a pheochromocytoma cell line PC-12. J. Cell Biol. 1990;110:1693–1703. doi: 10.1083/jcb.110.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole T. B., Wenzel H. J., Kafer K. E., Schwartzkroin P. A., Palmiter R. D. Elimination of zinc from synaptic vesicles in the intact mouse brain by disruption of the ZnT3 gene. Proc. Natl. Acad. Sci. USA. 1999;96:1716–1721. doi: 10.1073/pnas.96.4.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craige B., Salazar G., Faundez V. Current Protocols in Cell Biology. New York: John Wiley & Sons; 2004. Isolation of synaptic vesicles. Chapter 3, Unit 3.12. [DOI] [PubMed] [Google Scholar]
- Craige B., Salazar G., Faundez V. Phosphatidylinositol-4-kinase type II alpha contains an AP-3-sorting motif and a kinase domain that are both required for endosome traffic. Mol. Biol. Cell. 2008;19:1415–1426. doi: 10.1091/mbc.E07-12-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly C., Ziff E. B. Ca2+-dependent formation of a dynamin-synaptophysin complex: potential role in synaptic vesicle endocytosis. J. Biol. Chem. 2002;277:9010–9015. doi: 10.1074/jbc.M110815200. [DOI] [PubMed] [Google Scholar]
- Danglot L., Galli T. What is the function of neuronal AP-3? Biol. Cell. 2007;99:349–361. doi: 10.1042/BC20070029. [DOI] [PubMed] [Google Scholar]
- de Wit H., Lichtenstein Y., Geuze H., Kelly R. B., van der Sluijs P., Klumperman J. Synaptic vesicles form by budding from tubular extensions of sorting endosomes in PC12 cells. Mol. Biol. Cell. 1999;10:4163–4176. doi: 10.1091/mbc.10.12.4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deinhardt K., Salinas S., Verastegui C., Watson R., Worth D., Hanrahan S., Bucci C., Schiavo G. Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron. 2006;52:293–305. doi: 10.1016/j.neuron.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Dell'Angelica E. C., Ohno H., Ooi C. E., Rabinovich E., Roche K. W., Bonifacino J. S. AP-3, an adaptor-like protein complex with ubiquitous expression. EMBO J. 1997;16:917–928. doi: 10.1093/emboj/16.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Angelica E. C., Shotelersuk V., Aguilar R. C., Gahl W. A., Bonifacino J. S. Altered trafficking of lysosomal proteins in Hermansky-Pudlak syndrome due to mutations in the β3A subunit of the AP-3 adaptor. Mol. Cell. 1999;3:11–21. doi: 10.1016/s1097-2765(00)80170-7. [DOI] [PubMed] [Google Scholar]
- Di Pietro S. M., Dell'Angelica E. C. The cell biology of Hermansky-Pudlak syndrome: recent advances. Traffic. 2005;6:525–533. doi: 10.1111/j.1600-0854.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- Di Pietro S. M., Falcon-Perez J. M., Tenza D., Setty S.R.G., Marks M. S., Raposo G., Dell'Angelica E. C. BLOC-1 interacts with BLOC-2 and the AP-3 complex to facilitate protein trafficking on endosomes. Mol. Biol. Cell. 2006;17:4027–4038. doi: 10.1091/mbc.E06-05-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R. H. The neurotransmitter cycle and quantal size. Neuron. 2007;55:835–858. doi: 10.1016/j.neuron.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Falcon-Perez J. M., Starcevic M., Gautam R., Dell'Angelica E. C. BLOC-1, a novel complex containing the pallidin and muted proteins involved in the biogenesis of melanosomes and platelet-dense granules. J. Biol. Chem. 2002;277:28191–28199. doi: 10.1074/jbc.M204011200. [DOI] [PubMed] [Google Scholar]
- Faundez V., Horng J. -T., Kelly R. B. A function for the AP3 coat complex in synaptic vesicle formation from endosomes. Cell. 1998;93:423–432. doi: 10.1016/s0092-8674(00)81170-8. [DOI] [PubMed] [Google Scholar]
- Feng L., et al. The beta3A subunit gene (Ap3b1) of the AP-3 adaptor complex is altered in the mouse hypopigmentation mutant pearl, a model for Hermansky–Pudlak syndrome and night blindness. Hum. Mol. Genet. 1999;8:323–330. doi: 10.1093/hmg/8.2.323. [DOI] [PubMed] [Google Scholar]
- Gautam R., Novak E. K., Tan J., Wakamatsu K., Ito S., Swank R. T. Interaction of Hermansky-Pudlak syndrome genes in the regulation of lysosome-related organelles. Traffic. 2006;7:779–792. doi: 10.1111/j.1600-0854.2006.00431.x. [DOI] [PubMed] [Google Scholar]
- Grill B., Bienvenut W. V., Brown H. M., Ackley B. D., Quadroni M., Jin Y. C. elegans RPM-1 regulates axon termination and synaptogenesis through the Rab GEF GLO-4 and the Rab GTPase GLO-1. Neuron. 2007;55:587–601. doi: 10.1016/j.neuron.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Grote E., Hao J. C., Bennett M. K., Kelly R. B. A targeting signal in VAMP regulating transport to synaptic vesicles. Cell. 1995a;81:581–589. doi: 10.1016/0092-8674(95)90079-9. [DOI] [PubMed] [Google Scholar]
- Grote E., Hao J. C., Bennett M. K., Kelly R. B. A targeting signal in VAMP regulating transport to synaptic vesicles. Cell. 1995b;81:581–589. doi: 10.1016/0092-8674(95)90079-9. [DOI] [PubMed] [Google Scholar]
- Guo J., Wenk M. R., Pellegrini L., Onofri F., Benfenati F., De Camilli P. Phosphatidylinositol 4-kinase type IIalpha is responsible for the phosphatidylinositol 4-kinase activity associated with synaptic vesicles. Proc. Natl. Acad. Sci. USA. 2003;100:3995–4000. doi: 10.1073/pnas.0230488100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa H. P., Kneussel M., El Far O., Betz H. Interaction of synaptophysin with the AP-1 adaptor protein gamma-adaptin. Mol. Cell. Neurosci. 2002;21:454–462. doi: 10.1006/mcne.2002.1191. [DOI] [PubMed] [Google Scholar]
- Howe C. L., Mobley W. C. Long-distance retrograde neurotrophic signaling. Curr. Opin. Neurobiol. 2005;15:40–48. doi: 10.1016/j.conb.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Kantheti P., et al. Mutation in AP-3 Δ in the mocha mouse links endosomal transport to storage deficiency in platelets, melanosomes, and synaptic vesicles. Neuron. 1998;21:111–122. doi: 10.1016/s0896-6273(00)80519-x. [DOI] [PubMed] [Google Scholar]
- Karten B., Campenot R. B., Vance D. E., Vance J. E. The Niemann-Pick C1 protein in recycling endosomes of presynaptic nerve terminals. J. Lipid Res. 2006;47:504–514. doi: 10.1194/jlr.M500482-JLR200. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Beuchat M. H., Lindsay M., Frias S., Palmiter R. D., Sakuraba H., Parton R. G., Gruenberg J. Late endosomal membranes rich in lysobisphosphatidic acid regulate cholesterol transport. Nat. Cell Biol. 1999;1:113–118. doi: 10.1038/10084. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Stang E., Fang K. S., de Moerloose P., Parton R. G., Gruenberg J. A lipid associated with the antiphospholipid syndrome regulates endosome structure and function. Nature. 1998;392:193–197. doi: 10.1038/32440. [DOI] [PubMed] [Google Scholar]
- Le Borgne R., Alconada A., Bauer U., Hoflack B. The mammalian AP-3 adaptor-like complex mediates the intracellular transport of lysosomal membrane glycoproteins. J. Biol. Chem. 1998;273:29451–29461. doi: 10.1074/jbc.273.45.29451. [DOI] [PubMed] [Google Scholar]
- Lebrand C., Corti M., Goodson H., Cosson P., Cavalli V., Mayran N., Faure J., Gruenberg J. Late endosome motility depends on lipids via the small GTPase Rab7. EMBO J. 2002;21:1289–1300. doi: 10.1093/emboj/21.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein Y., Desnos C., Faundez V., Kelly R. B., Clift-O'Grady L. Vesiculation and sorting from PC12-derived endosomes in vitro. Proc. Natl. Acad. Sci. USA. 1998;95:11223–11228. doi: 10.1073/pnas.95.19.11223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Arca S., et al. A dual mechanism controlling the localization and function of exocytic v-SNAREs. Proc. Natl. Acad. Sci. USA. 2003;100:9011–9016. doi: 10.1073/pnas.1431910100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motley A., Bright N. A., Seaman M. N., Robinson M. S. Clathrin-mediated endocytosis in AP-2-depleted cells. J. Cell Biol. 2003;162:909–918. doi: 10.1083/jcb.200305145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy V. N., De Camilli P. Cell biology of the presynaptic terminal. Annu. Rev. Neurosci. 2003;26:701–728. doi: 10.1146/annurev.neuro.26.041002.131445. [DOI] [PubMed] [Google Scholar]
- Nakatsu F., et al. Defective function of GABA-containing synaptic vesicles in mice lacking the AP-3B clathrin adaptor. J. Cell Biol. 2004;167:293–302. doi: 10.1083/jcb.200405032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan R., Kramer H., Ramaswami M. Drosophila endosomal proteins hook and deep orange regulate synapse size but not synaptic vesicle recycling. J. Neurobiol. 2000;45:105–119. doi: 10.1002/1097-4695(20001105)45:2<105::aid-neu5>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Newell-Litwa K., Seong E., Burmeister M., Faundez V. Neuronal and non-neuronal functions of the AP-3 sorting machinery. J. Cell Sci. 2007;120:531–541. doi: 10.1242/jcs.03365. [DOI] [PubMed] [Google Scholar]
- Nixon R. A., Cataldo A. M., Mathews P. M. The endosomal-lysosomal system of neurons in Alzheimer's disease pathogenesis: a review. Neurochem. Res. 2000;25:1161–1172. doi: 10.1023/a:1007675508413. [DOI] [PubMed] [Google Scholar]
- Ohno H. Physiological roles of clathrin adaptor AP complexes: lessons from mutant animals. J. Biochem. 2006;139:943–948. doi: 10.1093/jb/mvj120. [DOI] [PubMed] [Google Scholar]
- Ohno H., Tomemori T., Nakatsu F., Okazaki Y., Aguilar R. C., Foelsch H., Mellman I., Saito T., Shirasawa T., Bonifacino J. S. μ1B, a novel adaptor medium chain expressed in polarized epithelial cells. FEBS Lett. 1999;449:215–220. doi: 10.1016/s0014-5793(99)00432-9. [DOI] [PubMed] [Google Scholar]
- Overly C. C., Hollenbeck P. J. Dynamic organization of endocytic pathways in axons of cultured sympathetic neurons. J. Neurosci. 1996;16:6056–6064. doi: 10.1523/JNEUROSCI.16-19-06056.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton R. G., Simons K., Dotti C. G. Axonal and dendritic endocytic pathways in cultured neurons. J. Cell Biol. 1992;119:123–137. doi: 10.1083/jcb.119.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden A. A., Oorschot V., Hesser B. A., Austin C. D., Scheller R. H., Klumperman J. Localization of the AP-3 adaptor complex defines a novel endosomal exit site for lysosomal membrane proteins. J. Cell Biol. 2004;164:1065–1076. doi: 10.1083/jcb.200311064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor P. R., Mullock B. M., Bright N. A., Lindsay M. R., Gray S. R., Richardson S. C., Stewart A., James D. E., Piper R. C., Luzio J. P. Combinatorial SNARE complexes with VAMP7 or VAMP8 define different late endocytic fusion events. EMBO Rep. 2004;5:590–595. doi: 10.1038/sj.embor.7400150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiborg C., Bache K. G., Gillooly D. J., Madshus I. H., Stang E., Stenmark H. Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat. Cell Biol. 2002;4:394–398. doi: 10.1038/ncb791. [DOI] [PubMed] [Google Scholar]
- Raiborg C., Wesche J., Malerod L., Stenmark H. Flat clathrin coats on endosomes mediate degradative protein sorting by scaffolding Hrs in dynamic microdomains. J. Cell Sci. 2006;119:2414–2424. doi: 10.1242/jcs.02978. [DOI] [PubMed] [Google Scholar]
- Ramirez A., et al. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat. Genet. 2006;38:1184–1191. doi: 10.1038/ng1884. [DOI] [PubMed] [Google Scholar]
- Raposo G., Marks M. S. Melanosomes–dark organelles enlighten endosomal membrane transport. Nat. Rev. 2007;8:786–797. doi: 10.1038/nrm2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M. S. Adaptable adaptors for coated vesicles. Trends Cell Biol. 2004;14:167–174. doi: 10.1016/j.tcb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Roos J., Kelly R. B. Dap160, a neural-specific Eps15 homology and multiple SH3 domain-containing protein that interacts with Drosophila dynamin. J. Biol. Chem. 1998;273:19108–19119. doi: 10.1074/jbc.273.30.19108. [DOI] [PubMed] [Google Scholar]
- Rous B. A., Reaves B. J., Ihrke G., Briggs J.A.G., Gray S. R., Stephens D. J., Banting G., Luzio J. P. Role of adaptor complex AP-3 in targeting wild-type and mutated CD63 to lysosomes. Mol. Biol. Cell. 2002;13:1071–1082. doi: 10.1091/mbc.01-08-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein D. C. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006;443:780–786. doi: 10.1038/nature05291. [DOI] [PubMed] [Google Scholar]
- Sagne C., Gasnier B. Molecular physiology and pathophysiology of lysosomal membrane transporters. J. Inherited Metab. Dis. 2008;31:258–266. doi: 10.1007/s10545-008-0879-9. [DOI] [PubMed] [Google Scholar]
- Salazar G., et al. BLOC-1 complex deficiency alters the targeting of adaptor protein complex-3 Cargoes. Mol. Biol. Cell. 2006;17:4014–4026. doi: 10.1091/mbc.E06-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar G., Craige B., Wainer B. H., Guo J., De Camilli P., Faundez V. Phosphatidylinositol-4-kinase type II {alpha} is a component of adaptor protein-3-derived vesicles. Mol. Biol. Cell. 2005;16:3692–3704. doi: 10.1091/mbc.E05-01-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar G., Love R., Styers M. L., Werner E., Peden A., Rodriguez S., Gearing M., Wainer B. H., Faundez V. AP-3-dependent mechanisms control the targeting of a chloride channel (ClC-3) in neuronal and non-neuronal cells. J. Biol. Chem. 2004a;279:25430–25439. doi: 10.1074/jbc.M402331200. [DOI] [PubMed] [Google Scholar]
- Salazar G., Love R., Werner E., Doucette M. M., Cheng S., Levey A., Faundez V. The zinc transporter ZnT3 interacts with AP-3 and it is preferentially targeted to a distinct synaptic vesicle subpopulation. Mol. Biol. Cell. 2004b;15:575–587. doi: 10.1091/mbc.E03-06-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar G., Zlatic S., Craige B., Peden A. A., Pohl J., Faundez V. Hermansky-Pudlak syndrome protein complexes associate with phosphatidylinositol-4-kinase type II alpha in neuronal and non-neuronal cells. J. Biol. Chem. 2009;284:1790–1802. doi: 10.1074/jbc.M805991200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem N., Faundez V., Horng J. -T., Kelly R. B. A v-SNARE participates in synaptic vesicle formation mediated by the AP3 adaptor complex. Nat. Neurosci. 1998;1:551–556. doi: 10.1038/2787. [DOI] [PubMed] [Google Scholar]
- Saxena S., Bucci C., Weis J., Kruttgen A. The small GTPase Rab7 controls the endosomal trafficking and neuritogenic signaling of the nerve growth factor receptor TrkA. J. Neurosci. 2005;25:10930–10940. doi: 10.1523/JNEUROSCI.2029-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuber A., Rudge R., Danglot L., Raposo G., Binz T., Poncer J. C., Galli T. Loss of AP-3 function affects spontaneous and evoked release at hippocampal mossy fiber synapses. Proc. Natl. Acad. Sci. USA. 2006;103:16562–16567. doi: 10.1073/pnas.0603511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Hannah M. J., Huttner W. B. Synaptic-like microvesicles of neuroendocrine cells originate from a novel compartment that is continuous with the plasma membrane and devoid of transferrin receptor [published erratum appears in J. Cell Biol. 1997 Jun 2;137(5):1197] J. Cell Biol. 1997;137:445–458. doi: 10.1083/jcb.137.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong E., Wainer B. H., Hughes E. D., Saunders T. L., Burmeister M., Faundez V. Genetic analysis of the neuronal and ubiquitous AP-3 adaptor complexes reveals divergent functions in brain. Mol. Biol. Cell. 2005;16:128–140. doi: 10.1091/mbc.E04-10-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setty S. R., Tenza D., Sviderskaya E. V., Bennett D. C., Raposo G., Marks M. S. Cell-specific ATP7A transport sustains copper-dependent tyrosinase activity in melanosomes. Nature. 2008;454:1142–1146. doi: 10.1038/nature07163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setty S.R.G., et al. BLOC-1 is required for cargo-specific sorting from vacuolar early endosomes toward lysosome-related organelles. Mol. Biol. Cell. 2007;18:768–780. doi: 10.1091/mbc.E06-12-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibinski G., et al. Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat. Genet. 2005;37:806–808. doi: 10.1038/ng1609. [DOI] [PubMed] [Google Scholar]
- Stepp J. D., Huang K., Lemmon S. K. The yeast adaptor protein complex, AP-3, is essential for the efficient delivery of alkaline phosphatase by the alternate pathway to the vacuole. J. Cell Biol. 1997;139:1761–1774. doi: 10.1083/jcb.139.7.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobrawa S. M., et al. Disruption of ClC-3, a chloride channel expressed on synaptic vesicles, leads to a loss of the hippocampus. Neuron. 2001;29:185–196. doi: 10.1016/s0896-6273(01)00189-1. [DOI] [PubMed] [Google Scholar]
- Swedlow J. R., Sedat J. W., Agard D. A. Deconvolution in optical microscopy. In: Jansson P. A., editor. Deconvolution of Images and Spectra. San Diego, CA: Academic Press; 1997. pp. 284–307. [Google Scholar]
- Sweeney N. T., Brenman J. E., Jan Y. N., Gao F. -B. The coiled-coil protein shrub controls neuronal morphogenesis in Drosophila. Curr. Biol. 2006;16:1006–1011. doi: 10.1016/j.cub.2006.03.067. [DOI] [PubMed] [Google Scholar]
- Takamori S., et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Talbot K., Cho D. S., Ong W. Y., Benson M. A., Han L. Y., Kazi H. A., Kamins J., Hahn C. G., Blake D. J., Arnold S. E. Dysbindin-1 is a synaptic and microtubular protein that binds brain snapin. Hum. Mol. Genet. 2006;15:3041–3054. doi: 10.1093/hmg/ddl246. [DOI] [PubMed] [Google Scholar]
- Talbot K., et al. Dysbindin-1 is reduced in intrinsic, glutamatergic terminals of the hippocampal formation in schizophrenia. J. Clin. Invest. 2004;113:1353–1363. doi: 10.1172/JCI20425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theos A. C., et al. Functions of adaptor protein (AP)-3 and AP-1 in tyrosinase sorting from endosomes to melanosomes. Mol. Biol. Cell. 2005;16:5356–5372. doi: 10.1091/mbc.E05-07-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele C., Hannah M. J., Fahrenholz F., Huttner W. B. Cholesterol binds to synaptophysin and is required for biogenesis of synaptic vesicles. Nat. Cell Biol. 2000;2:42–49. doi: 10.1038/71366. [DOI] [PubMed] [Google Scholar]
- Tippens A. L., Lee A. Caldendrin, a neuron-specific modulator of Cav/1.2 (L-type) Ca2+ channels. J. Biol. Chem. 2007;282:8464–8473. doi: 10.1074/jbc.M611384200. [DOI] [PubMed] [Google Scholar]
- Traub L. M. Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection. J. Cell Biol. 2003;163:203–208. doi: 10.1083/jcb.200309175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmaier S. M., Kam K., Yang H., Fortin D. L., Hua Z., Nicoll R. A., Edwards R. H. Distinct endocytic pathways control the rate and extent of synaptic vesicle protein recycling. Neuron. 2006;51:71–84. doi: 10.1016/j.neuron.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Wade N., Bryant N. J., Connolly L. M., Simpson R. J., Luzio J. P., Piper R. C., James D. E. Syntaxin 7 complexes with mouse Vps10p tail interactor 1b, syntaxin 6, vesicle-associated membrane protein (VAMP)8, and VAMP7 in b16 melanoma cells. J. Biol. Chem. 2001;276:19820–19827. doi: 10.1074/jbc.M010838200. [DOI] [PubMed] [Google Scholar]
- White I. J., Bailey L. M., Aghakhani M. R., Moss S. E., Futter C. E. EGF stimulates annexin 1-dependent inward vesiculation in a multivesicular endosome subpopulation. EMBO J. 2006;25:1–12. doi: 10.1038/sj.emboj.7600759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Li C., Ward D. M., Kaplan J., Mansour S. L. Defective organellar membrane protein trafficking in Ap3b1-deficient cells. J. Cell Sci. 2000;113:4077–4086. doi: 10.1242/jcs.113.22.4077. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Li W., Novak E. K., Karim A., Mishra V. S., Kingsmore S. F., Roe B. A., Suzuki T., Swank R. T. The gene for the muted (mu) mouse, a model for Hermansky-Pudlak syndrome, defines a novel protein which regulates vesicle trafficking. Hum. Mol. Genet. 2002;11:697–706. doi: 10.1093/hmg/11.6.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen L., Jiang S., Feng L., Bright N. A., Peden A. A., Seymour A. B., Novak E. K., Elliott R., Gorin M. B., Robinson M. S., Swank R. T. Abnormal expression and subcellular distribution of subunit proteins of the AP-3 adaptor complex lead to platelet storage pool deficiency in the pearl mouse. Blood. 1999;94:146–155. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.