Abstract
Cells normally undergo physiological turnover through the induction of apoptosis and phagocytic removal, partly through exposure of cell surface phosphatidylserine (PS). In contrast, neutrophils appear to possess apoptosis-independent mechanisms of removal. Here we show that Galectin-1 (Gal-1) induces PS exposure independent of alterations in mitochondrial potential, caspase activation, or cell death. Furthermore, Gal-1–induced PS exposure reverts after Gal-1 removal without altering cell viability. Gal-1–induced PS exposure is uniquely microdomain restricted, yet cells exposing PS do not display evident alterations in membrane morphology nor do they exhibit bleb formation, typically seen in apoptotic cells. Long-term exposure to Gal-1 prolongs PS exposure with no alteration in cell cycle progression or cell growth. These results demonstrate that Gal-1–induced PS exposure and subsequent phagocytic removal of living cells represents a new paradigm in cellular turnover.
INTRODUCTION
Cellular turnover represents one of the most fundamental homeostatic processes of multicellular organisms. Although many tissues experience cellular division and removal, cells of the immune system possess a unique capacity to rapidly proliferate in response to pathogenic challenge (Kaech and Ahmed, 2001). Significant expansion of leukocytes involved in both innate and adaptive immunity ultimately results in neutralization and removal of invading pathogens (Nathan, 2006). However, for effective immunological homeostasis to be maintained, efficient contraction of activated leukocyte populations must occur (Antia et al., 2005). Failure to appropriately eliminate activated leukocytes not only enhances the probability of cellular transformation, but also results in leukocyte-mediated damage of viable tissue and can eventually result in autoimmunity (Strasser et al., 2000; Danial and Korsmeyer, 2004).
Many factors regulate leukocyte turnover, including members of the tumor necrosis factor (TNF) and galectin families (Liu and Rabinovich, 2005; Toscano et al., 2007). TNF family members, including Fas, TRAIL, and TNF-α effect leukocyte contraction through the induction of apoptotic cell death. Similarly, several galectin family members, including galectin-3 and -9 (Fukumori et al., 2003; Zhu et al., 2005; Gal-3 and -9), also induce leukocyte removal through apoptosis (Strasser, 2005; Zhu et al., 2005; Stowell et al., 2008c). Cells undergoing apoptotic cell death typically express phosphatidylserine (PS), a phospholipid normally confined to the inner leaflet of the plasma membrane, which serves as a ligand for receptor-mediated phagocytosis (Fadok et al., 2000). Apoptotic cell death also results in DNA degradation and eventual cellular fragmentation (Jacobson et al., 1997; Stroh and Schulze-Osthoff, 1998). Importantly, apoptosis occurs in a coordinated manner, ultimately resulting in homeostatic cellular removal without inciting the deleterious consequences of an inflammatory response (Jacobson et al., 1997).
In contrast to Gal-3, Gal-9, and members of the TNF family, several studies suggest that galectin-1 (Gal-1) may induce PS exposure in leukocytes independent of apoptosis. For example, Gal-1 induces PS exposure in leukocytes in the absence of detectable DNA fragmentation (Dias-Baruffi et al., 2003). Externalization of PS exposure in viable cells may not be limited to Gal-1, as activation of some leukocytes, in particular T-cells and mast cells, can also result in apoptosis-independent PS exposure (Frasch et al., 2004; Elliott et al., 2005; Fischer et al., 2006; Smrz et al., 2007, 2008). Activation-induced PS exposure in viable cells appears to regulate diverse cellular processes ranging from cellular trafficking to proper formation of the immunological synapse (Elliott et al., 2005; Fischer et al., 2006). Taken together, these previous studies strongly suggest that cellular pathways exist whereby PS exposure may be induced in viable cells with diverse consequences.
Although Gal-1–induced PS exposure may occur in viable cells and therefore alter cellular processes as described previously (Frasch et al., 2004; Elliott et al., 2005; Fischer et al., 2006; Smrz et al., 2007, 2008), Gal-1–induced PS exposure sensitizes cells to phagocytic removal (Dias-Baruffi et al., 2003), in a manner similar to cells undergoing apoptotic cell death (Fadok et al., 2001). Such results suggest that Gal-1–induced PS exposure may accompany apoptotic cell death, consistent with several other studies suggesting that Gal-1 actually induces apoptotic cell death in leukocytes (Perillo et al., 1995; Pace et al., 2003). However, Gal-1 is uniquely sensitive to oxidative inactivation. As a result, studies showing Gal-1–induced apoptosis included dithiothreitol (DTT) in cell treatments (Perillo et al., 1997; Pace et al., 2003; Toscano et al., 2007). Indeed, utilization of DTT appears to render cells susceptible to Gal-1–induced cell death (Carlow et al., 2003; Stowell et al., 2007, 2008c). However, the treatment of living cells with DTT can induce the unfolded protein response and can directly induce apoptotic cell death (Braakman et al., 1992; Tartier et al., 2000; Murray et al., 2004), making interpretations about the effects of Gal-1 on leukocyte viability in the presence of DTT difficult.
Although the inclusion of DTT may complicate assays on cellular viability, the ability of Gal-1 to induce PS externalization and prime cells for phagocytic removal, both of which represent characteristic features of cells undergoing apoptotic cell death (Fadok et al., 2000; Dias-Baruffi et al., 2003), suggests that Gal-1 could induce apoptotic cell death, even in the absence of DTT. To help resolve these conflicting interpretations, we have more thoroughly examined whether Gal-1–induced PS exposure occurs in the presence or absence of cell death. In this study we demonstrate that Gal-1–induced PS exposure occurs in the absence of cell death, which suggest a new paradigm in cellular removal.
MATERIALS AND METHODS
Preparation and Derivatization of Gal-1
The expression and purification of human Gal-1 was described previously (Stowell et al., 2004). Gal-1 was alkylated with iodoacetamide followed by removal of free iodoacetamide using a PD-10 column (Amersham Pharmacia Biotech, Piscataway, NJ) equilibrated in PBS, as outlined previously (Stowell et al., 2008a). Gal-1 was labeled using C5 maleimide or carboxylic acid, succinimidyl ester, and dilithium salt reactive dyes (Molecular Probes, Eugene, OR). Before derivatization, β-mercaptoethanol and lactose were removed from Gal-1 using a PD-10 column equilibrated in PBS, followed by incubation with the Alexa-488 reactive dye with 100 mM lactose for 1 h at RT. Free dye was removed by subjection of the reaction mixture to PD-10 column gel filtration.
Cell Culture and Phagocytosis Assay
The isolation of neutrophils was in accordance with a protocol approved by the Emory Institutional Review Board and was accomplished as outlined previously (Stowell et al., 2007). HL60 cells were obtained from ATCC (Manassas, VA) and maintained as outlined previously (Dias-Baruffi et al., 2003). After treatment with the indicated concentrations of galectin, anti-Fas, DTT, lactose, or camptothecin (CAMP), cells were incubated in 50 mM lactose to disengage galectin-treated cells. Cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) as outlined previously (Kaech and Ahmed, 2001). Cell phagocytosis was measured as described previous (Dias-Baruffi et al., 2003).
Flow Cytometric Analysis
For annexin-V (An-V) staining after disengagement, cells were stained in a 100 μl final volume in HEPES buffer (10 mM HEPES, 140 mM NaCl, 5 mM CaCl2, pH 7.4) with a mixture of FITC-conjugated An-V (An-V-FITC; Roche Applied Science, Indianapolis, IN; 2 μl of conjugate per 100 μl of resuspended cells, as described by the manufacturer) and propidium iodide (PI; Molecular Probes; 1 μg/ml final concentration) at 4°C for 15 min. For DNA fragmentation, cells were assessed using the TUNEL reaction (In Situ Cell Death Detection Kit, Roche Applied Science) or hypodiploid analysis as outlined previously (Stowell et al., 2007). Analysis of mitochondrial potential loss using rhodamine-123 was also accomplished as outlined previously (Koya et al., 2000). All samples were assayed using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) with a minimum of 10,000 counts/sample. Data were analyzed using Cell Quest software (BD Biosciences).
Cell Sorting
Cells were stained with An-V-FITC, passed through a 70-μM mesh filter to remove any cell aggregates, mounted in a water-cooled jacket to maintain a temperature of 4°C, and analyzed utilizing a MoStar cell sorter (MoFlo, Dako, Denmark, and FacStar Plus, BD Biosciences, at the University of Oklahoma Health Sciences Center). Cells were then physically sorted into An-V–positive and –negative populations based on gates obtained from untreated stained cells. Cells were then washed in Ca2+-free buffer to remove An-V-FITC, examined by flow cytometry, and shown to display no An-V positivity regardless of fraction. Cells were then either restained with An-V-FITC and examined by flow cytometry to determine the integrity of the sorting process or resuspended in complete RMPI and allowed to incubate for the time indicated.
Western Blot Analysis
Cells were treated as indicated followed by SDS-PAGE and transfer to an Immobilon-P membrane (Millipore, Bedford, MA) using a semidry Transblot apparatus (Bio-Rad, Hercules, CA). After transfer, poly(ADP-ribose) polymerase (PARP) was detected using anti-PARP mAb (Novus Biologicals, Littleton, CO).
Confocal Analysis
Cells were incubated with An-V-biotin as outlined above for 1 h at 4°C. After washing, cells were incubated with streptavidin Alexa-488 (Molecular Probes) for 1 h at 4°C. Cells were plated on coverslips pretreated with poly-l-Lysine (Sigma, St. Louis, MO) and allowed to adhere for 30 min at 4°C. Cells were fixed with 2% paraformaldehyde buffered in PBS at 4°C for 2 h as outlined previously (Stowell et al., 2008c). Cells were analyzed using a Leica TCS NT confocal microscope and Leica TCS software (Deerfield, IL). Brightness and contrast adjustment was applied equally to the entire image.
Scanning Electronic Micrograph Analysis
For scanning electronic micrograph (SEM) analysis, neutrophils were treated with Gal-1, PBS, or anti-Fas for 8 h followed by removal of galectin in lactose and fixation in 2% glutaraldehyde in Hanks' balanced salt solution. After fixation, cells were dehydrated by sequential incubation with increasing concentrations of methanol solutions and then dried in liquid carbon dioxide by using a critical point dryer (Autosamdri 814). These fixed dehydrated cells were then mounted on bulk specimen holders (JEOL, Peabody, MA), coated with 60:40 gold/palladium utilizing a Hummer VI sputter coater, and viewed with a JEOL JSM880 SEM under 15-kV accelerating voltage (Oklahoma University, Norman, OK).
RESULTS
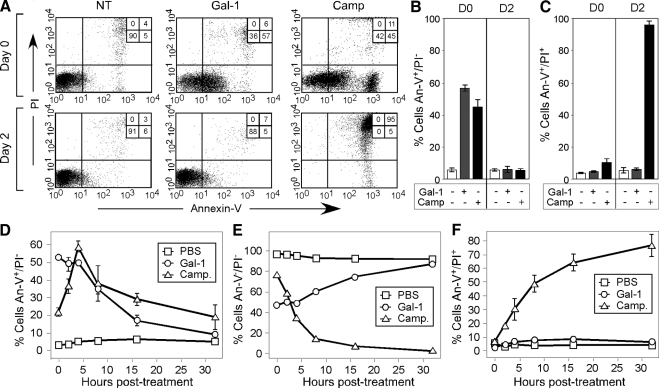
To determine whether Gal-1 induces PS exposure in the presence or absence of cell death, we first sought to examine whether Gal-1–induced PS exposure exhibits reversibility after Gal-1 removal, because PS exposure represents a terminal process in cells undergoing apoptosis (Fadok et al., 2000; Hoffmann et al., 2001). Consistent with previous results, Gal-1 induced robust PS exposure in leukocytes (Figure 1, A and B) as measured by An-V staining after prolonged incubation, similar to cells undergoing apoptotic cell death. However, Gal-1–treated cells displayed complete reversion of PS exposure after Gal-1 removal (Figure 1, A and B), with a cell surface half-life of ∼10 h (Figure 1, D–F). By contrast, cells induced to undergo apoptosis by Camp treatment displayed near complete conversion from a PS-positive/PI-negative state to a PS-positive/PI-positive state over the same period of time (Figure 1, A, C, and F).
Figure 1.
Gal-1 induces reversible PS exposure. (A) HL60 cells were treated with PBS (NT), 10 μM Gal-1, or 10 μM camptothecin (Camp) for 12 h followed by immediate detection for PS exposure using annexin-V (An-V)-FITC, propidium iodide (PI), and subsequent flow cytometric analysis (0 h) or were washed in lactose and reincubated in complete RPMI for 2 d followed by detection for PS exposure using An-V-FITC, PI, and subsequent flow cytometric analysis. (B and C) As outlined in A, quantification of PS exposure (B) by An-V staining or cell death (C) indicated by the PI positivity of cells evaluated immediately after treatment for 12 h (D0) or after removal of Gal-1 and incubation for 2 d (D2). (D–F) HL60 cells were treated with PBS (NT), 10 μM Gal-1, or 10 μM camptothecin (Camp) for 12 h followed by Gal-1 removal and detection for PS exposure using An-V-FITC, PI, and subsequent flow cytometric analysis at the indicated times after Gal-1 removal. (D) Cells that only stain with An-V-FITC; (E) Cells staining with neither An-V-FITC or PI; and (F) Cells staining with both An-V-FITC and PI.
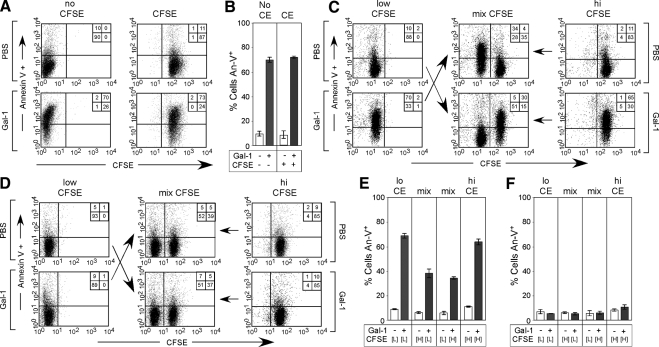
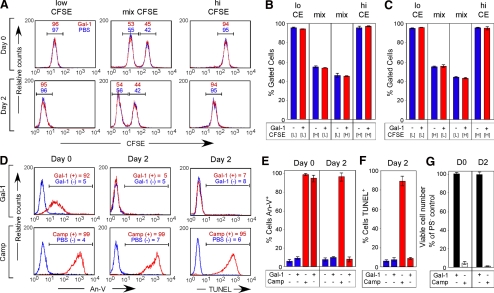
These results strongly suggest that Gal-1–induced PS exposure may be reversible after Gal-1 removal. PS externalization sensitizes neutrophils for phagocytic removal by surrounding macrophages (Dias-Baruffi et al., 2003). Although neutrophils do not normally phagocytose other neutrophils undergoing apoptotic cell death (Nathan, 2006), we considered the possibility that Gal-1–induced PS exposure may potentially sensitize cells for phagocytic removal by PS-negative cells, similar to recent reports showing that transformed epithelial cells can cannibalize neighboring cells (Overholtzer et al., 2007). In addition, because previous studies suggested that Gal-1 may be mitogenic in some cell populations (Levi and Teichberg, 1983; Adams et al., 1996), we determined whether PS reversion might reflect outgrowth of PS-negative cells. To differentiate between these possibilities, we labeled cells with the intracellular dye CFSE to enable tracking of two distinct cell populations within a mixture. To ensure that CFSE did not alter cellular sensitivity to Gal-1–induced PS exposure, cells were labeled with or without CFSE, followed by incubation with Gal-1. Importantly, CFSE did not alter cellular sensitivity to Gal-1 (Figure 2, A and B), and CFSE-labeled cells could be easily distinguished after Gal-1 treatment within a mixture of cells (Figure 2C). Gal-1–treated PS-positive cells experienced full reversion with no alteration in the percent of cells within each population (Figure 2, D–F). This demonstrates that there was no removal or loss of cells (Barber et al., 2003). In addition, the Gal-1–treated cell population, compared with nontreated cells, displayed nearly identical CFSE dilution (Figure 3, A–C), which is a mark of cell proliferation (Kaech and Ahmed, 2001). These results demonstrate that PS reversion does not represent overgrowth or engulfment by a subpopulation of PS-negative cells and that cells undergoing reversion of PS experience normal cell growth kinetics. Furthermore, physically sorted PS-positive cells after initial Gal-1 treatment reverted PS upon removal of Gal-1, similar to cells mixed with untreated cells (Figure 3, A–E). This reversion occurred without alterations in DNA degradation or cell growth (Figure 3, D, F, and G), which further demonstrated that Gal-1–induced PS exposure autonomously reverts after Gal-1 removal.
Figure 2.
PS reversion does not represent PS-positive cellular removal. (A) Cells were incubated with or without CFSE followed by incubation with Gal-1 and detection for PS exposure using An-V-FITC. (B) Quantification of PS exposure of cells treated with or without CFSE (CE). (C) Cells were differentially stained with CFSE followed by incubation with PBS or 10 μM Gal-1 for 4 h. After the 4-h incubation, cells were incubated with 50 mM lactose to remove Gal-1. Cells were then either incubated alone or mixed, followed by immediate examination of PS exposure using An-V-FITC (C) or were resuspended in complete RPMI and allowed to incubate alone or mixed as outlined for 2 d, followed by detection for PS using An-V-FITC (D). (E) Quantification of PS exposure of cells treated in C. (F) Quantification of PS exposure of cells treated in D.
Figure 3.
PS-positive cells display unaltered cell division during PS reversion. (A) Cells were differentially stained with CFSE followed by incubation with PBS or 10 μM Gal-1 for 4 h. After the 4-h incubation, cells were incubated with 50 mM lactose to remove Gal-1. Cells were then either not mixed or mixed followed either by immediate enumeration of cell percentages in each population (Day 0) or resuspended in complete RPMI and allowed to incubate alone or mixed as outlined for 2 d, followed by enumeration of cell percentages in each population (Day 2), with red representing those cells treated with Gal-1 and blue representing those cells treated with PBS. (B and C) Quantification of the percent of cells in each population at Day 0 (B) and Day 2 (C), with red representing those cells treated with Gal-1 and blue representing those cells treated with PBS. (D) Top panels, cells were sorted into PS-positive (red, Gal-1–treated PS-positive) or PS negative (blue, Gal-1–treated PS negative) fractions followed by either restaining for PS using An-V-FITC or reincubating in complete RPMI for 2 d to allow PS reversion. After the 2-d incubation, cells were stained for PS using An-V-FITC or analyzed for DNA degradation using TUNEL. Bottom, cells were treated with PBS or 10 μM Camp followed by sorting into PS-positive (red, Camp-treated PS-positive) or PS-negative (blue, PBS-treated PS negative) fractions, followed by either restaining for PS using An-V-FITC or reincubating in complete RPMI for 2 d to allow PS reversion. After the 2-d incubation, cells were stained for PS using An-V-FITC or analyzed for DNA degradation using TUNEL. (E and F) Quantification of cells treated as outlined in D for either PS exposure (E) or DNA fragmentation using TUNEL (F). (G) PS-positive cells sorted after treatment with Gal-1 or Camp as outlined in D were resuspended in RPMI for 2 d, followed by enumeration of viable cell number using a hemocytometer and trypan blue exclusion. Cell numbers are reported as the percent of the PBS-treated PS-negative control.
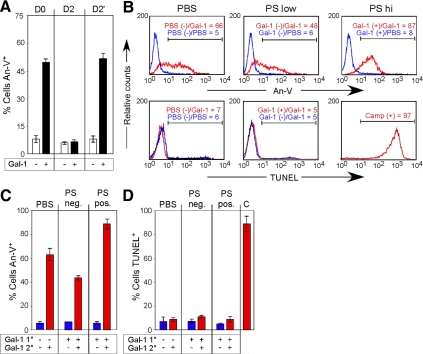
We next determined whether cells previously positive for PS remain sensitive to Gal-1 signaling after PS reversion. Cells retreated with Gal-1 displayed virtually identical sensitivity to Gal-1 in terms of reexpression of PS (Figure 4A), thus demonstrating that cells remain sensitive to Gal-1 after PS reversion. However, PS reexpression may represent PS exposure in cells previously PS negative after primary incubation with Gal-1. To address this possibility, we physically sorted cells after initial Gal-1 treatment into PS-positive and -negative populations (Figure 3D, Gal-1 Day 0). After reversion of PS exposure in PS-positive cells (Figure 4B), we incubated previously PS-positive or -negative cells with Gal-1. PS-positive cells not only remained sensitive to Gal-1 retreatment after PS reversion, but displayed enhanced sensitivity to Gal-1 when compared with PS-negative cells (Figure 4, B and C), with no detectable DNA fragmentation after reincubation with Gal-1 (Figure 4, B and D).
Figure 4.
Cells previously positive for PS remain sensitive to restimulation by Gal-1. (A) Cells were incubated with 10 μM Gal-1 for 4 h followed by removal of Gal-1 with 50 mM lactose and either detection of PS exposure by An-V-FITC and PI staining or resuspension in complete RPMI. Resuspended cells were allowed to incubate for 2 d (Day 2) to revert PS, followed by incubation with 10 μM Gal-1 for 4 h and detection for PS (Day 2′). (B) Top, PS-positive or -negative cells previously incubated with Gal-1 or PS-negative cells treated with PBS were retreated with 10 μM Gal-1 or PBS for 4 h (Primary treatment/Secondary treatment) followed by detection of PS with An-V-FITC. Gate values are shown. Bottom, PS-positive or -negative cells previously incubated with Gal-1 or PS-negative cells treated with PBS were retreated with 10 μM Gal-1 or PBS for 10 h (Primary treatment/Secondary treatment) followed by analysis for DNA fragmentation. Gate values are shown. (C and D) Quantification of cells treated as outlined in B for either PS exposure (C) or DNA fragmentation using TUNEL (D).
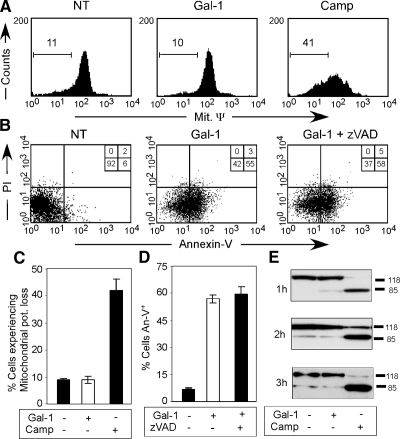
The reversible nature of Gal-1–induced PS exposure suggests that Gal-1 does not engage irreversible cellular pathways that normally accompany apoptotic cell death, such as mitochondrial potential depolarization and caspase activation (Lakhani et al., 2006). Consistent with this, Gal-1 failed to alter mitochondrial potential, although cells undergoing apoptotic cell death demonstrated significant depolarization (Figure 5, A and C). Furthermore, treatment of cells with the pan-caspase inhibitor, zVAD-fmk, did not inhibit Gal-1–induced PS exposure (Figure 5, B and D), although it inhibited PS exposure in cells undergoing apoptotic cell death (data not shown). In addition, cells treated with Gal-1 failed to display cleavage of the common caspase substrate PARP (Lazebnik et al., 1994); by contrast, cells undergoing apoptotic cell death displayed significant PARP cleavage (Figure 5E). These results show that Gal-1 induces PS exposure independent of irreversible processes, such as mitochondrial potential loss or caspase activation, consistent with the ability of PS to revert in its surface exposure after Gal-1 treatment and withdrawal and strongly suggesting that Gal-1–induced PS exposure occurs independent of cell death.
Figure 5.
Gal-1 induces PS exposure through a caspase-independent process. (A) Cells were incubated with PBS (NT), 10 μM Gal-1, or 10 μM Camp for 8 h as indicated, followed by examination for alterations in mitochondrial potential change. (B) Cells were incubated with or without zVAD-fmk, followed by incubation with 10 μM Gal-1 for 4 h and analyzed for PS exposure by staining with An-V-FITC. (C) Quantification of cells treated as outlined in A. (D) Quantification of cells treated as outlined in B. (E) Cells were incubated with 10 μM Gal-1 or 10 μM Camp for the indicated times, followed by cell lysis and examination of PARP cleavage by SDS-PAGE and Western blot analysis.
In contrast to Gal-1–induced PS exposure, apoptotic cells generated by Camp treatment not only converted to a double PS/PI-positive state over time, which indicated conversion to late apoptosis, but also displayed a fraction of cells that displayed a greater magnitude of PS positivity during the single PS-positive state (Figure 1A). By contrast, significant numbers of apoptotic cells also exhibited similar PS positivity to Gal-1–treated cells during early apoptosis (Figure 1A). These differences may reflect the amount and distribution of PS on the cell surface, as previous studies demonstrated that cells undergoing apoptosis often display punctate PS exposure during early phases of apoptosis that progresses to encompass the entire cell surface over time (Godard et al., 1999). Previous results suggested that Gal-1–induced PS exposure may exhibit some asymmetric distribution on the plasma membrane, similar to early phases of apoptotic cell death (Godard et al., 1999), suggesting that cells incubated with Gal-1 may fail to transition to later phases of apoptosis where greater PS positivity occurs. To examine this in more detail, we analyzed cells by confocal analysis for localization of PS expression after incubation with Gal-1 at later time points. In contrast to cells undergoing apoptotic cell death, Gal-1–treated cells retained punctate PS exposure, whereas cells undergoing apoptosis progressed to nearly uniform PS exposure (Figure 6A).
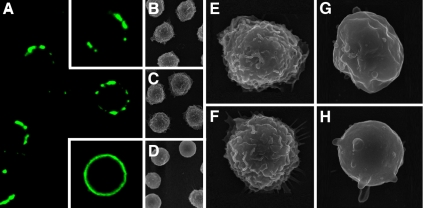
Figure 6.
Gal-1–induced PS exposure resides in punctate microdomains. (A) Cells were incubated with 10 μM Gal-1 for 12 h followed by staining with An-V. Top inset, representative cell treated with 10 μM Gal-1. Bottom inset, representative cell treated with 10 μM Camp. (B–H) SEM analysis of cells treated with PBS (B and E), 10 μM Gal-1 (C and F), or anti-Fas (D, G, and H).
In addition to alterations in phospholipid asymmetry, cells undergoing apoptosis experience gross morphological change in membrane architecture, including surface flattening of microvilli and the formation of apoptotic blebs (Scott et al., 2001). We examined whether cell surface alterations that normally accompany apoptosis might also occur during Gal-1–induced PS exposure. Consistent with previous results (Scott et al., 2001), SEM analysis of apoptotic cells showed significant retraction of microvilli and cell surface flattening with prominent formation of apoptotic blebs (Figure 6, D, G, and H). By contrast, cells exposed to Gal-1 displayed morphological features indistinguishable from nonapoptotic controls (Figure 6, B, C, E, and F). These results support the conclusion that Gal-1 induces PS exposure independent of cell death and without changes in cellular morphology.
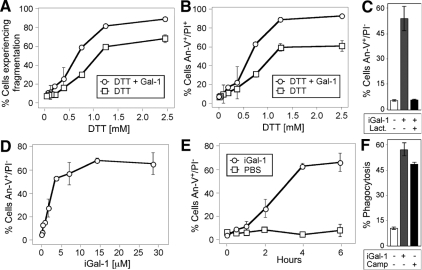
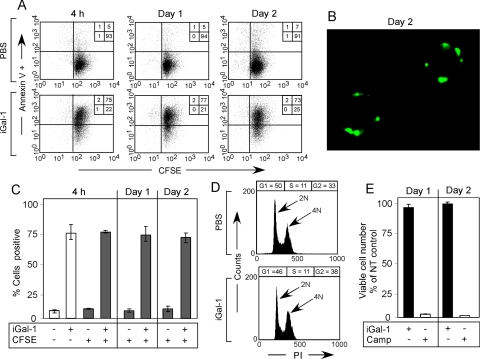
Although PS exposure induced by Gal-1 clearly reverts after Gal-1 removal, we sought to define whether continuous Gal-1 incubation could induce sustained PS exposure over prolonged incubation periods. However, Gal-1 loses significant activity after prolonged incubation periods (days; Hirabayashi and Kasai, 1991; Cho and Cummings, 1995a). Previous studies utilized DTT in cell treatments to prevent Gal-1 oxidation (Perillo et al., 1995; Pace et al., 2003; Toscano et al., 2007). However, DTT alone can induce significant alterations in cellular viability and artificially sensitize cells to Gal-1–induced apoptosis (Figure 7, A and B; Tartier et al., 2000; Stowell et al., 2007, 2008c). As a result, we stabilized Gal-1 by derivatization with iodoacetamide (iGal-1), as outlined previously (Whitney et al., 1986; Stowell et al., 2008c). Similar to the unmodified protein, iGal-1 induced significant PS exposure in cells (Figure 7C). iGal-1–induced PS exposure required binding to cell surface carbohydrates, because lactose, a general inhibitor of galectin-ligand interactions (Stowell et al., 2008b), completely blocked iGal-1–induced PS exposure (Figure 7C). Furthermore, iGal-1–induced PS exposure displayed equivalent kinetics, occurred over a similar dose response, and also sensitized cells to phagocytosis at levels comparable to cells undergoing apoptotic cell death as observed previously (Figure 7, D–F; Dias-Baruffi et al., 2003).
Figure 7.
DTT sensitizes cells to Gal-1–induced apoptosis. (A) Cells were incubated with PBS, 10 μM Gal-1, or the indicated concentration of DTT for 9 h, followed by detection for cellular fragmentation as indicated by changes in forward (FSC) and side scatter (SSC) profiles of cells. (B) Cells were incubated with PBS, 10 μM Gal-1, or the indicated concentration of DTT for 9 h, followed by detection for cell death by PS exposure and membrane integrity loss by An-V-FITC and PI staining. (C) Cells were incubated with PBS, 10 μM iGal-1, or 10 μM iGal-1 with 20 mM lactose, followed by detection for PS exposure by An-V-FITC staining and PI exclusion. (D) Cells were incubated with PBS or the indicated concentration of iGal-1 for 8h followed by detection for PS exposure by An-V-FITC staining and PI exclusion. (E) Cells were incubated with PBS or 10 μM iGal-1 for the indicated time followed by detection for PS exposure by An-V-FITC staining and PI exclusion. (F) Cells were incubated with PBS, 10 μM iGal-1, or 10 μM Camp for 8 h, followed by incubation of peritoneal macrophages for 1 h and microscopic examination of phagocytosis.
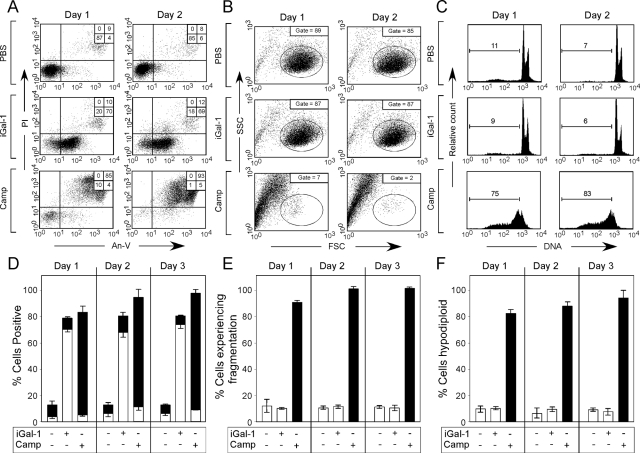
Because iGal-1 had activity similar to Gal-1 in short-term treatments, we examined the effects of iGal-1 on cell viability and PS exposure over prolonged time in the absence of DTT (Tartier et al., 2000; Stowell et al., 2007, 2008c). Cells incubated with iGal-1 in the absence of DTT maintained continual PS exposure over 3 d (Figure 8, A and D). Importantly, although iGal-1–induced sustained PS exposure, no detectable changes in PI staining, cellular fragmentation, or DNA degradation occurred (Figure 8, A–F). By contrast, cells undergoing apoptosis displayed significant increases in PI staining, cellular fragmentation, and DNA degradation over the same time period (Figure 8, A–F).
Figure 8.
iGal-1 induces continuous PS exposure. (A) Cells were incubated with PBS, 10 μM iGal-1, or 10 μM Camp for 1 or 2 d as indicated, followed by detection for PS exposure by An-V-FITC staining and PI exclusion. (B) Cells were incubated with PBS, 10 μM iGal-1, or 10 μM Camp for 1 or 2 d as indicated, followed by examination for cellular fragmentation as indicated by changes in forward (FSC) and side scatter (SSC) profiles of cells. Gate = % of cells experiencing no fragmentation. (C) Cells were incubated with PBS, 10 μM iGal-1, or 10 μM Camp for 1 or 2 d as indicated, followed by measuring DNA fragmentation by hypodiploid analysis. Gate = % of cells experiencing DNA fragmentation. (D) Quantification of cells treated in A. White bars = % An-V+, black bars = % PI+. (E) Quantification of cells treated in B. (F) Quantification of cells treated in (C).
The ability of iGal-1 to induce unaltered PS exposure over a prolonged period may represent iGal-1–induced cell cycle arrest, despite normal cell division after Gal-1 removal during PS reversion (Figure 3A). To determine whether cells incubated with iGal-1 continued to divide or whether iGal-1–induced cell cycle arrest in the presence of sustained PS exposure, we labeled cells with CFSE (Kaech and Ahmed, 2001). Cells treated with iGal-1 continued to experience CFSE dilution while maintaining uniform PS exposure over time (Figure 9, A and C). PS exposure also remained punctate over 2 d of continuous iGal-1 incubation (Figure 9B) with no alterations in cell cycle progression or viable cell number (Figure 9, D and E). Taken together, these results demonstrate that iGal-1 induces sustained PS exposure without altering cell growth or viability.
Figure 9.
iGal-1 induces sustained PS exposure without altering cell division. (A) Cells were labeled with CFSE, followed by incubation with 10 μM iGal-1 for 4 h, 1 d, or 2 d as indicated, followed by detection for PS exposure by An-V-FITC. (B) Cells were incubated with 10 μM iGal-1 for 2 d followed by confocal analysis for PS exposure by An-V. (C) Quantification of data in A. (D) Cells were incubated with 10 μM iGal-1 for 24 h, followed by cell cycle analysis. (E) Cells were incubated with PBS, 10 μM iGal-1 or 10 μM Camp for 1 or 2 d, followed by enumerating viable cell number using trypan blue exclusion.
DISCUSSION
The ability of Gal-1 to induce sustained PS exposure with no detectable alterations in DNA degradation, cell size, membrane integrity, or cell division demonstrates that Gal-1–induced PS exposure occurs in the absence of cell death. The reversion of PS exposure after Gal-1 removal demonstrates that unlike apoptotic PS exposure, Gal-1–induced PS exposure does not represent a terminal event and further demonstrates that Gal-1 induces PS exposure in viable cells. Given the ability of Gal-1 to sensitize cells to phagocytic removal (Dias-Baruffi et al., 2003; Figure 7, D–F), these results demonstrate that Gal-1 induces phagocytic removal of living cells, as opposed to apoptotic cells, suggesting a new paradigm in cellular turnover.
Although many studies suggested that Gal-1 affects leukocyte turnover and function (van Kooyk and Rabinovich, 2008), whether Gal-1 directly alters leukocyte viability remained enigmatic. Many previous studies utilized the reducing agent DTT when evaluating the effect of Gal-1 on leukocyte viability (Perillo et al., 1995; Perillo et al., 1997; Pace et al., 2003; Toscano et al., 2007). However, DTT induces the unfolded protein response, directly induces apoptosis, and sensitizes cells to Gal-1–induced cell death (Braakman et al., 1992; Tartier et al., 2000; Murray et al., 2004; Stowell et al., 2007, 2008c). Because the extracellular environment is largely oxidative and DTT artificially penetrates cell membranes (Kemp et al., 2008), the physiological relevance of DTT inclusion remains unclear.
Other studies demonstrate that Gal-1 induces PS exposure and sensitizes cells to phagocytic removal in the absence of DTT (Dias-Baruffi et al., 2003), suggesting that Gal-1 may alter cell viability regardless of DTT inclusion. However, these studies failed to detect DNA fragmentation or alterations in membrane integrity after Gal-1 incubation (Dias-Baruffi et al., 2003; Stowell et al., 2007, 2008c), suggesting that either definitive signs of Gal-1–induced cell death required prolonged Gal-1 incubation or that Gal-1 induces phagocytic removal of living cells. To clarify the effect of Gal-1 on cell viability we utilized iGal-1, which retains previously defined activities of nonderivatized Gal-1, yet remains resistant to oxidation in the absence of DTT (Whitney et al., 1986; Dias-Baruffi et al., 2003; He et al., 2003; Stowell et al., 2008c). Cells treated with iGal-1 maintained PS exposure for 3 continuous days without inducing changes in membrane integrity, DNA degradation, or cell size. Furthermore, cells continued to growth normally as indicated by absolute viable cell numbers, CFSE dilution, and cell cycle analysis. These results not only demonstrate that Gal-1 fails to alter cell viability while maintaining prolonged PS externalization, but also suggest that PS asymmetry is not a strict requirement for cell division.
The ability of Gal-1 to induce PS exposure despite failing to alter cellular viability presented the unusual possibility that cells incubated with Gal-1 may reverse PS exposure after Gal-1 removal. This was important to definitively evaluate, as PS externalization is commonly thought to be a terminal event of, and directly correlated with, cells undergoing apoptotic cell death (Fadok et al., 2000; Hoffmann et al., 2001). Indeed, removal of Gal-1 resulted in PS reversion without detectable alterations in cell viability, which further demonstrated the nonapoptotic nature of Gal-1–induced PS exposure. Importantly, although several studies suggest that Gal-1 may be mitogenic at low concentrations (Levi and Teichberg, 1983; Adams et al., 1996), reversion did not represent mitogenic outgrowth of a PS negative population, as differentially labeled cells and physically sorted cells autonomously reverted PS. The ability of PS-positive cells to autonomously revert PS also ruled out the possibility that PS reversion reflected cannibalism by bystander cells, as recently reported to occur in transformed epithelial cells (Overholtzer et al., 2007). The ability of Gal-1 to induce PS exposure despite failing to engage irreversible pathways commonly associated with apoptotic cell death, such as caspase activation and loss of mitochondrial potential (Hengartner, 2000), also corroborates the reversible nature of Gal-1–induced PS exposure. Equally important, although some studies demonstrate that caspases may directly induce PS exposure (Martin et al., 1996; Vanags et al., 1996), caspase activation is not a prerequisite or necessary for PS exposure to occur.
In addition to inducing PS exposure in the absence of apoptosis, Gal-1–induced PS exposure morphologically differed from PS exposure accompanying cell death. Gal-1–induced PS exposure remained punctate regardless of the duration of Gal-1 incubation, whereas cells undergoing apoptotic cell death displayed uniform PS exposure before transition to late apoptosis and loss of membrane integrity. Previous results demonstrate that apoptotic cells also exhibit punctate PS exposure during early phases of apoptosis, with gradual progression over the entire cell surface with time (Godard et al., 1999). Although the exact players responsible for PS mobilization remain uncertain (Schlegel and Williamson, 2007), PS asymmetry does require an ATP driven aminophospholipid translocase (APT) activity that translocates any external PS back to the inner leaflet of the plasma membrane (Dolis et al., 1997). By contrast, putative phospholipid scramblases, which are thought to facilitate PS externalization, often occur in membrane microdomains (Damek-Poprawa et al., 2006). Failure of cells incubated with Gal-1 to undergo cell death may prevent full inactivation of ATP-driven APT (Seigneuret and Devaux, 1984; Gleiss et al., 2002), suggesting that Gal-1 may activate PS exposure in areas near lipid rafts, followed by reinternalization by incompletely inactivated APT, resulting in the appearance of punctate PS. Although Gal-1–induced PS exposure occurs independent of caspase activation, cells undergoing apoptosis activate putative scramblases through caspase-mediated activation of protein kinase C (PKC) that results in scramblase phosphorylation and activation (Frasch et al., 2000). The ability of Gal-1 to activate PKC (Karmakar et al., 2005) suggests that Gal-1 may be able to induce PS exposure independent of caspase activation by directly activating scramblase.
Gal-1 displays unique sensitivity to oxidative inactivation. Thus, increases in environmental oxidation associated with failure to remove pathogens may trigger reversal of PS exposure in leukocytes originally targeted for removal to allow reengagement in host defense (Hirabayashi and Kasai, 1991; Cho and Cummings, 1995b; Nathan, 2006; Kemp et al., 2008). Such normally oxidative environments may be especially important for granulocytes, which do not possess antigen specific immunity and therefore have limited capacity to directly detect pathogen levels (Nathan, 2006). Failure to remove pathogens results in tissue damage and injury, which causes additional leukocyte recruitment and enhanced oxidation of the extracellular environment. Enhanced oxidation leads to Gal-1 inactivation (Inagaki et al., 2000), allowing cells originally targeted for removal to revert PS and become reengaged in host defense. Consistent with this, continual incubation of cells with nonstabilized Gal-1 results in spontaneous Gal-1 oxidation and PS reversion (Stowell and Cummings, unpublished observations). Thus, by such mechanisms Gal-1 may be uniquely suited to sense oxidative environments and alter leukocyte turnover and accumulation.
Although apoptosis generally prevents inflammation during cellular turnover, exuberant apoptosis, as can occur during inflammation, can actually be proinflammatory (Misawa et al., 2001). Consistent with this, several studies suggest that granulocytes have a unique apoptosis-independent pathway(s) of removal. Granulocytes transgenically overexpressing bcl-2 fail to undergo apoptotic cell death, yet display normal sensitivity to phagocytic removal in vivo and in vitro (Lagasse and Weissman, 1994). Furthermore, Kupffer cells phagocytose nonapoptotic neutrophils in vivo (Shi et al., 2001). In addition, Fas and FasL null mice, which display significant defects in lymphocyte turnover, display no alterations in homeostatic granulocyte turnover, both in resting and inflammatory conditions (Fecho and Cohen, 1998). In contrast to the reversible nature of Gal-1–induced PS exposure, the ability of Gal-1 to sustain PS exposure may reflect successful pathogen removal, inflammation resolution, and therefore reduced Gal-1 oxidation. In this way, Gal-1–induced turnover of viable cells allows granulocytes to actively maintain membrane integrity until successfully phagocytosed.
In addition to regulating leukocyte turnover, recent studies suggest that Gal-1–induced PS exposure in viable cells may also regulate other fundamental cellular processes. For example, PS exposure during T-cell activation appears to alter ion transport, cellular trafficking, and immunological synapse formation (Elliott et al., 2005; Fischer et al., 2006), although the extent to which PS directly modulates these processes remains uncertain (Martinez et al., 2006). In addition to T-cells, several studies suggest that B cells may also externalize PS independent of apoptosis (Hammill et al., 1999; Dillon et al., 2000, 2001; Elliott et al., 2006). Brief PS externalization may also facilitate the release of preformed inflammatory mediators, as macrophages and mast cells display transient PS exposure after activation (Martin et al., 2000; MacKenzie et al., 2001). By contrast, recent studies demonstrated that ligation of cell surface GPI (glycosylphosphotidylinositol)-anchored proteins on mast cells can induce sustained PS exposure independent of cell death (Smrz et al., 2007, 2008). Similar to Gal-1–induced PS exposure, sustained PS exposure in mast cells may contribute to the removal of viable cells harboring pathogens during infection (Smrz et al., 2007).
Before the discovery of alterations in PS asymmetry in cells undergoing apoptosis (Schlegel and Williamson, 2001), many studies documented a key role for PS asymmetry on activated platelets in thrombosis (Monroe et al., 2002). Similar to platelets, PS exposure on cells can also induce thrombosis (Tracy et al., 1985; Williamson et al., 1992), which suggests that in addition to regulating neutrophil turnover, Gal-1–induced PS exposure may also contribute to fibrin deposition. Consistent with this, improper resolution of inflammatory responses often results in significant peripheral fibrin deposition (Loeuille et al., 2005), suggesting a role for PS-positive neutrophils awaiting removal in this process.
ACKNOWLEDGMENTS
We thank Jamie Heimburg-Molinaro for critical reading of the manuscript. This work was supported by National Institutes of Health Grant HL085607 to R.P.M. and R.D.C.
Abbreviations used:
- An-V
annexin V
- Camp
camptothecin
- CFSE
carboxyfluorescein diacetate, succinimidyl ester
- DTT
dithiothreitol
- Gal-1
galectin-1
- Gal-3
galectin-3
- Gal-9
galectin-9
- PARP
poly(ADP-ribose) polymerase
- PI
propidium iodide
- PS
phosphatidylserine
- SEM
scanning electron microscopy
- TNF
tumor necrosis factor.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-07-0786) on December 30, 2008.
REFERENCES
- Adams L., Scott G. K., Weinberg C. S. Biphasic modulation of cell growth by recombinant human galectin-1. Biochim. Biophys. Acta. 1996;1312:137–144. doi: 10.1016/0167-4889(96)00031-6. [DOI] [PubMed] [Google Scholar]
- Antia R., Ganusov V. V., Ahmed R. The role of models in understanding CD8+ T-cell memory. Nat. Rev. Immunol. 2005;5:101–111. doi: 10.1038/nri1550. [DOI] [PubMed] [Google Scholar]
- Barber D. L., Wherry E. J., Ahmed R. Cutting edge: rapid in vivo killing by memory CD8 T cells. J. Immunol. 2003;171:27–31. doi: 10.4049/jimmunol.171.1.27. [DOI] [PubMed] [Google Scholar]
- Braakman I., Helenius J., Helenius A. Manipulating disulfide bond formation and protein folding in the endoplasmic reticulum. EMBO J. 1992;11:1717–1722. doi: 10.1002/j.1460-2075.1992.tb05223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlow D. A., Williams M. J., Ziltener H. J. Modulation of O-glycans and N-glycans on murine CD8 T cells fails to alter annexin V ligand induction by galectin 1. J. Immunol. 2003;171:5100–5106. doi: 10.4049/jimmunol.171.10.5100. [DOI] [PubMed] [Google Scholar]
- Cho M., Cummings R. D. Galectin-1, a beta-galactoside-binding lectin in Chinese hamster ovary cells. I. Physical and chemical characterization. J. Biol. Chem. 1995a;270:5198–5206. doi: 10.1074/jbc.270.10.5198. [DOI] [PubMed] [Google Scholar]
- Cho M., Cummings R. D. Galectin-1, a beta-galactoside-binding lectin in Chinese hamster ovary cells. II. Localization and biosynthesis. J. Biol. Chem. 1995b;270:5207–5212. doi: 10.1074/jbc.270.10.5207. [DOI] [PubMed] [Google Scholar]
- Damek-Poprawa M., Golub E., Otis L., Harrison G., Phillips C., Boesze-Battaglia K. Chondrocytes utilize a cholesterol-dependent lipid translocator to externalize phosphatidylserine. Biochemistry. 2006;45:3325–3336. doi: 10.1021/bi0515927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danial N. N., Korsmeyer S. J. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- Dias-Baruffi M., Zhu H., Cho M., Karmakar S., McEver R. P., Cummings R. D. Dimeric galectin-1 induces surface exposure of phosphatidylserine and phagocytic recognition of leukocytes without inducing apoptosis. J. Biol. Chem. 2003;278:41282–41293. doi: 10.1074/jbc.M306624200. [DOI] [PubMed] [Google Scholar]
- Dillon S. R., Constantinescu A., Schlissel M. S. Annexin V binds to positively selected B cells. J. Immunol. 2001;166:58–71. doi: 10.4049/jimmunol.166.1.58. [DOI] [PubMed] [Google Scholar]
- Dillon S. R., Mancini M., Rosen A., Schlissel M. S. Annexin V binds to viable B cells and colocalizes with a marker of lipid rafts upon B cell receptor activation. J. Immunol. 2000;164:1322–1332. doi: 10.4049/jimmunol.164.3.1322. [DOI] [PubMed] [Google Scholar]
- Dolis D., Moreau C., Zachowski A., Devaux P. F. Aminophospholipid translocase and proteins involved in transmembrane phospholipid traffic. Biophys. Chem. 1997;68:221–231. doi: 10.1016/s0301-4622(97)00048-3. [DOI] [PubMed] [Google Scholar]
- Elliott J. I., Sardini A., Cooper J. C., Alexander D. R., Davanture S., Chimini G., Higgins C. F. Phosphatidylserine exposure in B lymphocytes: a role for lipid packing. Blood. 2006;108:1611–1617. doi: 10.1182/blood-2005-11-012328. [DOI] [PubMed] [Google Scholar]
- Elliott J. I., Surprenant A., Marelli-Berg F. M., Cooper J. C., Cassady-Cain R. L., Wooding C., Linton K., Alexander D. R., Higgins C. F. Membrane phosphatidylserine distribution as a non-apoptotic signalling mechanism in lymphocytes. Nat. Cell Biol. 2005;7:808–816. doi: 10.1038/ncb1279. [DOI] [PubMed] [Google Scholar]
- Fadok V. A., Bratton D. L., Rose D. M., Pearson A., Ezekewitz R. A., Henson P. M. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- Fadok V. A., de Cathelineau A., Daleke D. L., Henson P. M., Bratton D. L. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J. Biol. Chem. 2001;276:1071–1077. doi: 10.1074/jbc.M003649200. [DOI] [PubMed] [Google Scholar]
- Fecho K., Cohen P. L. Fas ligand (gld)- and Fas (lpr)-deficient mice do not show alterations in the extravasation or apoptosis of inflammatory neutrophils. J. Leukoc. Biol. 1998;64:373–383. doi: 10.1002/jlb.64.3.373. [DOI] [PubMed] [Google Scholar]
- Fischer K., Voelkl S., Berger J., Andreesen R., Pomorski T., Mackensen A. Antigen recognition induces phosphatidylserine exposure on the cell surface of human CD8+ T cells. Blood. 2006;108:4094–4101. doi: 10.1182/blood-2006-03-011742. [DOI] [PubMed] [Google Scholar]
- Frasch S. C., Henson P. M., Kailey J. M., Richter D. A., Janes M. S., Fadok V. A., Bratton D. L. Regulation of phospholipid scramblase activity during apoptosis and cell activation by protein kinase Cdelta. J. Biol. Chem. 2000;275:23065–23073. doi: 10.1074/jbc.M003116200. [DOI] [PubMed] [Google Scholar]
- Frasch S. C., Henson P. M., Nagaosa K., Fessler M. B., Borregaard N., Bratton D. L. Phospholipid flip-flop and phospholipid scramblase 1 (PLSCR1) co-localize to uropod rafts in formylated Met-Leu-Phe-stimulated neutrophils. J. Biol. Chem. 2004;279:17625–17633. doi: 10.1074/jbc.M313414200. [DOI] [PubMed] [Google Scholar]
- Fukumori T., Takenaka Y., Yoshii T., Kim H. R., Hogan V., Inohara H., Kagawa S., Raz A. CD29 and CD7 mediate galectin-3-induced type II T-cell apoptosis. Cancer Res. 2003;63:8302–8311. [PubMed] [Google Scholar]
- Gleiss B., Gogvadze V., Orrenius S., Fadeel B. Fas-triggered phosphatidylserine exposure is modulated by intracellular ATP. FEBS Lett. 2002;519:153–158. doi: 10.1016/s0014-5793(02)02743-6. [DOI] [PubMed] [Google Scholar]
- Godard T., Deslandes E., Lebailly P., Vigreux C., Sichel F., Poul J. M., Gauduchon P. Early detection of staurosporine-induced apoptosis by comet and annexin V assays. Histochem. Cell Biol. 1999;112:155–161. doi: 10.1007/s004180050402. [DOI] [PubMed] [Google Scholar]
- Hammill A. K., Uhr J. W., Scheuermann R. H. Annexin V staining due to loss of membrane asymmetry can be reversible and precede commitment to apoptotic death. Exp. Cell Res. 1999;251:16–21. doi: 10.1006/excr.1999.4581. [DOI] [PubMed] [Google Scholar]
- He L., Andre S., Siebert H. C., Helmholz H., Niemeyer B., Gabius H. J. Detection of ligand- and solvent-induced shape alterations of cell-growth-regulatory human lectin galectin-1 in solution by small angle neutron and x-ray scattering. Biophys. J. 2003;85:511–524. doi: 10.1016/S0006-3495(03)74496-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengartner M. O. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- Hirabayashi J., Kasai K. Effect of amino acid substitution by sited-directed mutagenesis on the carbohydrate recognition and stability of human 14-kDa beta-galactoside-binding lectin. J. Biol. Chem. 1991;266:23648–23653. [PubMed] [Google Scholar]
- Hoffmann P. R., deCathelineau A. M., Ogden C. A., Leverrier Y., Bratton D. L., Daleke D. L., Ridley A. J., Fadok V. A., Henson P. M. Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J. Cell Biol. 2001;155:649–659. doi: 10.1083/jcb.200108080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki Y., Sohma Y., Horie H., Nozawa R., Kadoya T. Oxidized galectin-1 promotes axonal regeneration in peripheral nerves but does not possess lectin properties. Eur. J. Biochem. 2000;267:2955–2964. doi: 10.1046/j.1432-1033.2000.01311.x. [DOI] [PubMed] [Google Scholar]
- Jacobson M. D., Weil M., Raff M. C. Programmed cell death in animal development. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- Kaech S. M., Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat. Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmakar S., Cummings R. D., McEver R. P. Contributions of Ca2+ to galectin-1–induced exposure of phosphatidylserine on activated neutrophils. J. Biol. Chem. 2005;280:28623–28631. doi: 10.1074/jbc.M414140200. [DOI] [PubMed] [Google Scholar]
- Kemp M., Go Y. M., Jones D. P. Nonequilibrium thermodynamics of thiol/disulfide redox systems: a perspective on redox systems biology. Free Radic. Biol. Med. 2008;44:921–937. doi: 10.1016/j.freeradbiomed.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya R. C., Fujita H., Shimizu S., Ohtsu M., Takimoto M., Tsujimoto Y., Kuzumaki N. Gelsolin inhibits apoptosis by blocking mitochondrial membrane potential loss and cytochrome c release. J. Biol. Chem. 2000;275:15343–15349. doi: 10.1074/jbc.275.20.15343. [DOI] [PubMed] [Google Scholar]
- Lagasse E., Weissman I. L. bcl-2 inhibits apoptosis of neutrophils but not their engulfment by macrophages. J. Exp. Med. 1994;179:1047–1052. doi: 10.1084/jem.179.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhani S. A., Masud A., Kuida K., Porter G. A., Jr., Booth C. J., Mehal W. Z., Inayat I., Flavell R. A. Caspases 3 and 7, key mediators of mitochondrial events of apoptosis. Science. 2006;311:847–851. doi: 10.1126/science.1115035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazebnik Y. A., Kaufmann S. H., Desnoyers S., Poirier G. G., Earnshaw W. C. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature. 1994;371:346–347. doi: 10.1038/371346a0. [DOI] [PubMed] [Google Scholar]
- Levi G., Teichberg V. I. Selective interactions of electrolectins from eel electric organ and mouse thymus with mouse immature thymocytes. Immunol. Lett. 1983;7:35–39. doi: 10.1016/0165-2478(83)90052-4. [DOI] [PubMed] [Google Scholar]
- Liu F. T., Rabinovich G. A. Galectins as modulators of tumour progression. Nat. Rev. Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- Loeuille D., et al. Macroscopic and microscopic features of synovial membrane inflammation in the osteoarthritic knee: correlating magnetic resonance imaging findings with disease severity. Arthritis Rheum. 2005;52:3492–3501. doi: 10.1002/art.21373. [DOI] [PubMed] [Google Scholar]
- MacKenzie A., Wilson H. L., Kiss-Toth E., Dower S. K., North R. A., Surprenant A. Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity. 2001;15:825–835. doi: 10.1016/s1074-7613(01)00229-1. [DOI] [PubMed] [Google Scholar]
- Martin S., Pombo I., Poncet P., David B., Arock M., Blank U. Immunologic stimulation of mast cells leads to the reversible exposure of phosphatidylserine in the absence of apoptosis. Int. Arch. Allergy Immunol. 2000;123:249–258. doi: 10.1159/000024451. [DOI] [PubMed] [Google Scholar]
- Martin S. J., Finucane D. M., Amarante-Mendes G. P., O'Brien G. A., Green D. R. Phosphatidylserine externalization during CD95-induced apoptosis of cells and cytoplasts requires ICE/CED-3 protease activity. J. Biol. Chem. 1996;271:28753–28756. doi: 10.1074/jbc.271.46.28753. [DOI] [PubMed] [Google Scholar]
- Martinez M. C., Kunzelmann C., Freyssinet J. M. Phosphatidylserine and signal transduction: who needs whom? Sci. STKE. 2006;2006:pe3. doi: 10.1126/stke.3182006pe3. [DOI] [PubMed] [Google Scholar]
- Misawa R., Kawagishi C., Watanabe N., Kobayashi Y. Infiltration of neutrophils following injection of apoptotic cells into the peritoneal cavity. Apoptosis. 2001;6:411–417. doi: 10.1023/a:1012406121993. [DOI] [PubMed] [Google Scholar]
- Monroe D. M., Hoffman M., Roberts H. R. Platelets and thrombin generation. Arterioscler. Thromb. Vasc. Biol. 2002;22:1381–1389. doi: 10.1161/01.atv.0000031340.68494.34. [DOI] [PubMed] [Google Scholar]
- Murray J. I., Whitfield M. L., Trinklein N. D., Myers R. M., Brown P. O., Botstein D. Diverse and specific gene expression responses to stresses in cultured human cells. Mol. Biol. Cell. 2004;15:2361–2374. doi: 10.1091/mbc.E03-11-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- Overholtzer M., Mailleux A. A., Mouneimne G., Normand G., Schnitt S. J., King R. W., Cibas E. S., Brugge J. S. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell. 2007;131:966–979. doi: 10.1016/j.cell.2007.10.040. [DOI] [PubMed] [Google Scholar]
- Pace K. E., Hahn H. P., Baum L. G. Preparation of recombinant human galectin-1 and use in T-cell death assays. Methods Enzymol. 2003;363:499–518. doi: 10.1016/S0076-6879(03)01075-9. [DOI] [PubMed] [Google Scholar]
- Perillo N. L., Pace K. E., Seilhamer J. J., Baum L. G. Apoptosis of T cells mediated by galectin-1. Nature. 1995;378:736–739. doi: 10.1038/378736a0. [DOI] [PubMed] [Google Scholar]
- Perillo N. L., Uittenbogaart C. H., Nguyen J. T., Baum L. G. Galectin-1, an endogenous lectin produced by thymic epithelial cells, induces apoptosis of human thymocytes. J. Exp. Med. 1997;185:1851–1858. doi: 10.1084/jem.185.10.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel R. A., Williamson P. Phosphatidylserine, a death knell. Cell Death Differ. 2001;8:551–563. doi: 10.1038/sj.cdd.4400817. [DOI] [PubMed] [Google Scholar]
- Schlegel R. A., Williamson P. P.S. to PS (phosphatidylserine)—pertinent proteins in apoptotic cell clearance. Sci. STKE. 2007;2007:pe57. doi: 10.1126/stke.4082007pe57. [DOI] [PubMed] [Google Scholar]
- Scott R. S., McMahon E. J., Pop S. M., Reap E. A., Caricchio R., Cohen P. L., Earp H. S., Matsushima G. K. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- Seigneuret M., Devaux P. F. ATP-dependent asymmetric distribution of spin-labeled phospholipids in the erythrocyte membrane: relation to shape changes. Proc. Natl. Acad. Sci. USA. 1984;81:3751–3755. doi: 10.1073/pnas.81.12.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Gilbert G. E., Kokubo Y., Ohashi T. Role of the liver in regulating numbers of circulating neutrophils. Blood. 2001;98:1226–1230. doi: 10.1182/blood.v98.4.1226. [DOI] [PubMed] [Google Scholar]
- Smrz D., Draberova L., Draber P. Non-apoptotic phosphatidylserine externalization induced by engagement of glycosylphosphatidylinositol-anchored proteins. J. Biol. Chem. 2007;282:10487–10497. doi: 10.1074/jbc.M611090200. [DOI] [PubMed] [Google Scholar]
- Smrz D., Lebduska P., Draberova L., Korb J., Draber P. Engagement of phospholipid scramblase 1 in activated cells: implication for phosphatidylserine externalization and exocytosis. J. Biol. Chem. 2008;283:10904–10918. doi: 10.1074/jbc.M710386200. [DOI] [PubMed] [Google Scholar]
- Stowell S. R., Arthur C. M., Mehta P., Slanina K. A., Blixt O., Leffler H., Smith D. F., Cummings R. D. Galectin-1, -2, and -3 exhibit differential recognition of sialylated glycans and blood group antigens. J. Biol. Chem. 2008a;283:10109–10123. doi: 10.1074/jbc.M709545200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell S. R., Arthur C. M., Slanina K. A., Horton J. R., Smith D. F., Cummings R. D. Dimeric Galectin-8 induces phosphatidylserine exposure in leukocytes through polylactosamine recognition by the C-terminal domain. J. Biol. Chem. 2008b;283:20547–20559. doi: 10.1074/jbc.M802495200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell S. R., Dias-Baruffi M., Penttila L., Renkonen O., Nyame A. K., Cummings R. D. Human galectin-1 recognition of poly-N-acetyllactosamine and chimeric polysaccharides. Glycobiology. 2004;14:157–167. doi: 10.1093/glycob/cwh018. [DOI] [PubMed] [Google Scholar]
- Stowell S. R., Karmakar S., Stowell C. J., Dias-Baruffi M., McEver R. P., Cummings R. D. Human galectin-1, -2, and -4 induce surface exposure of phosphatidylserine in activated human neutrophils but not in activated T cells. Blood. 2007;109:219–227. doi: 10.1182/blood-2006-03-007153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell S. R., Qian Y., Karmakar S., Koyama N. S., Dias-Baruffi M., Leffler H., McEver R. P., Cummings R. D. Differential roles of galectin-1 and galectin-3 in regulating leukocyte viability and cytokine secretion. J. Immunol. 2008c;180:3091–3102. doi: 10.4049/jimmunol.180.5.3091. [DOI] [PubMed] [Google Scholar]
- Strasser A. The role of BH3-only proteins in the immune system. Nat. Rev. Immunol. 2005;5:189–200. doi: 10.1038/nri1568. [DOI] [PubMed] [Google Scholar]
- Strasser A., O'Connor L., Dixit V. M. Apoptosis signaling. Annu. Rev. Biochem. 2000;69:217–245. doi: 10.1146/annurev.biochem.69.1.217. [DOI] [PubMed] [Google Scholar]
- Stroh C., Schulze-Osthoff K. Death by a thousand cuts: an ever increasing list of caspase substrates. Cell Death Differ. 1998;5:997–1000. doi: 10.1038/sj.cdd.4400451. [DOI] [PubMed] [Google Scholar]
- Tartier L., McCarey Y. L., Biaglow J. E., Kochevar I. E., Held K. D. Apoptosis induced by dithiothreitol in HL-60 cells shows early activation of caspase 3 and is independent of mitochondria. Cell Death Differ. 2000;7:1002–1010. doi: 10.1038/sj.cdd.4400726. [DOI] [PubMed] [Google Scholar]
- Toscano M. A., et al. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat. Immunol. 2007;8:825–834. doi: 10.1038/ni1482. [DOI] [PubMed] [Google Scholar]
- Tracy P. B., Eide L. L., Mann K. G. Human prothrombinase complex assembly and function on isolated peripheral blood cell populations. J. Biol. Chem. 1985;260:2119–2124. [PubMed] [Google Scholar]
- van Kooyk Y., Rabinovich G. A. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat. Immunol. 2008;9:593–601. doi: 10.1038/ni.f.203. [DOI] [PubMed] [Google Scholar]
- Vanags D. M., Porn-Ares M. I., Coppola S., Burgess D. H., Orrenius S. Protease involvement in fodrin cleavage and phosphatidylserine exposure in apoptosis. J. Biol. Chem. 1996;271:31075–31085. doi: 10.1074/jbc.271.49.31075. [DOI] [PubMed] [Google Scholar]
- Whitney P. L., Powell J. T., Sanford G. L. Oxidation and chemical modification of lung beta-galactoside-specific lectin. Biochem. J. 1986;238:683–689. doi: 10.1042/bj2380683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson P., Kulick A., Zachowski A., Schlegel R. A., Devaux P. F. Ca2+ induces transbilayer redistribution of all major phospholipids in human erythrocytes. Biochemistry. 1992;31:6355–6360. doi: 10.1021/bi00142a027. [DOI] [PubMed] [Google Scholar]
- Zhu C., Anderson A. C., Schubart A., Xiong H., Imitola J., Khoury S. J., Zheng X. X., Strom T. B., Kuchroo V. K. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]