Abstract
There is substantial evidence which shows that constraints in the early life environment is an important determinant of risk of metabolic and cardiovascular disease. There is emerging evidence that higher birth weight, which reflects a more abundant prenatal environment, is associated with increased risk of cancer, in particular breast cancer and childhood leukaemia. Using specific examples from epidemiology and experimental studies, this review discusses the hypothesis that increased susceptibility to cardiovascular, metabolic disease and cancer have a common origin in developmental changes induced in the developing fetus by aspects of the intra uterine environment including nutrition which involve stable changes to the epigenetic regulation of specific genes. However, the induction of specific disease risk is dependent upon the nature of the environmental challenge and interactions between the susceptibility set by the altered epigenome and the environment throughout the life course.
Introduction
Cancer is the result of derangement of cellular processes which control cell division and apoptosis(1). There is a large body of information which shows that environmental exposures, including nutrition, play a significant role in the aetiology of the disease. Such exposures usually precede the appearance of clinical disease by a prolonged period of time, often several decades(1). While gene mutation has a role in the aetiology of cancer, there is increasing evidence which shows that epigenetic processes such as DNA methylation and covalent modifications to histones are also involved(2). Such epigenetic changes represent potential for altered gene activity and hence cellular dysregulation, but these may only be manifest when the gene is exposed to an appropriate environmental signal which is enhanced or diminished as a consequence of the epigenetic change compared to normal cells. This suggestion is consistent with a life-course perspective on cancer risk. The early life environment has been shown to be an important factor in determining risk for some types of cancer. Measures of growth in early life show statistical associations with risk of specific cancers. In this context, there appear to be parallels between causal processes in cancer with metabolic and cardiovascular disease in which the early life environment and epigenetic processes lead to a susceptibility which increases the risk to later specific environmental exposures(3). The purpose of this review is to discuss the hypothesis that the early life environment, in particular nutrition, induces changes in the development of the offspring which lead to increased risk of cancer, or metabolic and cardiovascular disease. The extent to which altered epigenetic processes established during development represent a common mechanism in cancer and metabolic disease, diseases which are generally considered as resulting from widely different pathological processes, will also be discussed.
Early life environment and risk of metabolic disease
Studies in humans
Epidemiological studies show that the environment experienced before birth and shortly afterwards has long-term effects on the subsequent risk of metabolic diseases including cardiovascular disease, type 2 diabetes mellitus and obesity. Barker and colleagues showed that adults who were small at birth had a higher risk of developing metabolic and cardiovascular disease, and that increasing birth weight was associated with a graded decrease in risk(4,5). Such induction of increased risk as a result of intra-uterine constraint has been termed ‘fetal programming’. Importantly, these effects occur over the usual range of birth weights rather than being the result of pathological states such as intra-uterine growth retardation or, at the opposite end of the spectrum, macrosomy(4). These findings have been replicated in a substantial number of studies in different populations world wide, although there are important variations in specific outcome measures (see(6) for examples). Size at birth reflects the growth of the fetus and small size reflects a developmental constraint imposed by the intra-uterine environment. Nutrition, together with hormones and oxygen supply, is an important determinant of fetal growth and has been studied extensively with respect to its role in the induction of risk of metabolic disease. Perhaps the most persuasive example of a specific role for prenatal nutrition in determining subsequence risk of metabolic disease comes from studies of adults who were in utero during the Dutch Hunger Winter which occurred during a discrete period of 1944 - 45(7). These studies show that compared to adults born before or conceived after the famine, adults who were fetuses during the famine showed increased risk of hypertension, obesity and insulin resistance. Furthermore, the severity of such effects was graded according to the stage of development of the fetus at the time of famine(7). This suggests that different organ systems are susceptible to nutritional constraint during specific periods of development.
The paradigm that environmental constraint in early life determines subsequent risk on non-communicable diseases has been extended by Gluckman and Hanson(8). They suggest that this association is an example of a wider spectrum of developmental responses to environmental stimuli which through developmental plasticity induce adaptations to the phenotype of the fetus which predict the postnatal environment. If correct, such predictions confer a survival advantage. This hypothesis has been supported by recent observations between fatness at birth in women, and their subsequent reproductive capacity(9). It is postulated that a mismatch between the predicted environment and that which the offspring experiences after birth results in a disadvantage that in humans leads to increased risk of disease(8). The extent and nature of the mismatch remains to be determined.
There is emerging data that the effects of prenatal nutrition on health in adulthood can be transmitted to more than one subsequent generation(10). For example, examination of historical records the population in Overkalix, Sweden showed that mortality from diabetes increased in men if the paternal grandfather was exposed to abundant nutrition during their pre-pubertal period of slow growth(11). The children born to women who were in their first trimester during the Dutch Hunger Winter were heavier and those born to women in their third trimester were lighter than the children of women born before the famine(12). This suggests the effects of the famine during the grandmother’s pregnancy was transmitted to their grandchildren.
Studies in animal models
The findings of epidemiological studies have been largely supported by the results of studies of the effects of specific nutritional interventions during pregnancy and / or lactation in laboratory animals, mainly rats and mice (for detailed reviews see(13,14)), although studies have also been conducted in other species. The nutritional interventions include feeding a diet with reduced protein content within the physiological range for rodents(15), global reduction in food intake(16), or a high fat diet(17). In general terms, feeding these diets to pregnant rodents induced in the offspring aspects of metabolic and cardiovascular disease which in humans have been associated with a poor prenatal environment. The offspring of these animal models show, albeit to different degrees, impaired control of blood pressure (hypertension and endothelial dysfunction), insulin resistance, dyslipidaemia, obesity, altered locomotor behaviour. In contrast to humans, birthweight in rodents is generally unaffected by nutritional constraint during pregnancy, possibly because of the relative immaturity of the pups at birth and the difference in the timing of fat deposition during development. This emphasises that birthweight is a proxy marker for the effects of the intra-uterine environment on the development of the fetus rather than necessarily being a causal component of the mechanism which links environmental exposure to later risk of disease (also consider reference 7).
Animal models also provide a means of testing the effects of interventions to prevent or reverse the effects of the prenatal environment on the development of the fetus. Two interventions have so far been shown to prevent fetal programming. First, administration of leptin during the neonatal period in rats prevented excessive weight gain in response to a high fat diet in the offspring of dams which received a 70% reduction in global nutrition during pregnancy(16). Secondly, increasing the availability of metabolites involved in 1-carbon metabolism, specifically glycine and folic acid, to pregnant rats consuming a protein-restricted (PR) diet prevented induction of hypertension and vascular dysfunction in the offspring(18-20). These findings suggest that 1-carbon metabolism plays a central role in the induction of an altered phenotype.
Prevention of hypertension by increasing the glycine(18) or impaired lipid and glucose homeostasis by increasing the folic acid by a similar amount to the daily intake of pregnant women using folic acid supplements(21) content of the maternal PR diet decreased the growth of the offspring, which suggests normalisation of vascular function and metabolism may occur at the expense of growth and so represent a developmental trade-off. Although increasing the folic acid content of the maternal PR diet prevented hypertension in the offspring, increasing the folic acid content of the protein-sufficient control diet by the same amount increased blood pressure in the offspring(21,22). The relative protein and folic acid content of the diet fed to pregnant rats induces in the offspring opposing changes in fasting blood lipid and glucose concentrations(22). However, the magnitude of such effects also depends upon the amount of fat fed to the offspring after weaning(22). Transmission between generations of obesity associated with the epigenetically-regulated agouti phenotype in mice was also prevented by dietary supplementation with methyl donors(23). Thus the physiological phenotype of the offspring is dependent upon interactions between prenatal nutrition and diet in later life. One implication of these findings is that altered susceptibility to disease may reflect the early life environment, but risk may be modified by environmental exposures throughout the life course.
Growth before birth and risk of cancer
Information regarding the effect of the prenatal environment on later risk of cancer is rather more limited than that on the early life origins of cardiovascular and metabolic disease. With the exception of the well known effects of prenatal exposure to endocrine disrupting agents such as diethylstilbesterol(24) or ionising radiation(25) on subsequent cancer risk, the precise environmental exposure which leads to cancer is often difficult to define and epidemiological studies are dependent upon testing the strength of associations with proxy measures such as birth weight. Since cancer encompasses a highly heterogeneous group of diseases, the strength of any association between markers of the prenatal environment and subsequent disease risk might be expected to be weak. Nevertheless, associations have been demonstrated between proxy markers of the intra-uterine environment and risk of specific cancers including breast cancer, and leukaemia and some other non-reproductive cancers. To allow comparison between markers of the prenatal environment, and risk of cancer and risk of metabolic and cardiovascular disease, the following discussion will focus on associations with birth weight. However, there are studies which report associations based on other markers of the intra-uterine environment such as length at birth. Many of these are cited in the references listed below.
Breast cancer
The findings of studies which have investigated the association between breast cancer and birth weight are summarised in Table 1. These studies differ markedly in design, the extent to which other variables were included in the analysis, the number of cases studied and whether pre and post -menopausal women were analysed together or separately. The possible impact of such differences in study design on the reported outcomes have been discussed in detail elsewhere(26,27). Where associations between birth weight and risk of breast cancer were found they suggest that, in contrast to cardiovascular or metabolic disease, lower birthweight tended to be associated with lower risk of breast cancer, and that cancer risk increased in a graded manner with increasing birth weight (see references in table 1). While it has been reported that the association between birth weight and cancer risk is strongest in women who develop the disease before their menopause(1), not all studies support this (Table 1). The extent of association between breast cancer risk and maternal age, parity and maternal smoking are less clear as studies have reported conflicting findings(32,34,39,53) and some have reported J-shaped associations(34). While in some studies the highest birth weights are above 4.5kg (Table 1), the majority fall below this level. Thus any effect of pathological changes associated with macrosomy cannot account entirely for the positive association between birth weight and risk of breast cancer. A recent meta-analysis supports the overall conclusion that risk of breast cancer is increased in individuals with higher birth weight(54). Higher birth weight was associated with 12% increase in relative risk of breast cancer, while higher birth length was associated with 28% increased risk of disease. Overall, these studies support the suggestion that the intra-uterine environment exerts a persistent effect on the risk of women developing breast cancer.
Table 1.
Associations between breast cancer and birthweight
| Reference | Number of cases | Direction of trend and menopausal status |
|---|---|---|
| 28 * | 153 | None; pre-menopausal |
| 29 | 458 | Positive; pre-menopausal & postmenopausal |
| 30 | 582 | Positive; pre-menopausal & postmenopausal |
| 31 | 746 and 401 | Positive, pre-menopausal; none, postmenopausal |
| 32 | 1068 | None; pre-menopausal & postmenopausal |
| 33 * | 57 | None, menopausal status not disclosed |
| 34 * | 484 | Positive; pre-menopausal |
| 35 | 37 | Positive; pre-menopausal & postmenopausal |
| 36 | 90 | Positive; pre-menopausal & postmenopausal |
| 37 | 62 | None; pre-menopausal & postmenopausal |
| 38 | 177 | None; postmenopausal |
| 39 | 288 | None; pre-menopausal |
| 40 * | 1716 | Positive; postmenopausal |
| 41 | 373 | Positive; pre-menopausal & postmenopausal |
| 42 * | 2334 | Positive; pre-menopausal & postmenopausal |
| 43 | 359 | Positive; pre-menopausal & postmenopausal |
| 44 | 127 | Positive; pre-menopausal & postmenopausal |
| 45 | 881 | Positive; pre-menopausal |
| 46 | 59 | Positive; pre-menopausal & postmenopausal |
| 47 | 2074 | Positive; menopausal status not disclosed |
| 48 | 196 | None; pre-menopausal & postmenopausal |
| 49 | 89 | Positive; postmenopausal |
| 50 | 367 | Positive pre-menopausal, none postmenopausal |
| 51 | 312 | Positive; pre-menopausal & postmenopausal |
| 52 | 828 and 2312 | Positive pre-menopausal, none postmenopausal |
Studies in which high birth weight was defined as ≥ 4500g.
Two recent studies have investigated the effect of maternal diet on mammary tumorigenesis in their offspring. De Assis et al.(55) found that the offspring of rats fed high fat diet had structural changes to mammary tissue, lower oestrogen receptor (ER)-α expression and increased levels of activated MAP kinase. These offspring showed altered mammary gland structure, increased ERα, insulin receptor and IGF-1 receptor expression and increased sensitivity to tumour induction with 7,12-dimethylbenz[a]anthracene. Fernandez-Twinn et al.(56) reported that the offspring of rats fed a PR diet during pregnancy and lactation had a 2-fold increase sensitivity to mammary tumour induction by nitrosomethylurea. Thus, although these studies used opposing maternal dietary insults, the overall outcome was to increase the sensitivity of mammary tissue to tumorigenic agents. An assessment of maternal folic acid intake in mice on tumour burden in the adult offspring genetically predisposed to intestinal tumours failed to show an effect of prenatal folate exposure or an interaction between prenatal and post-weaning folate exposure(57). However, since all mice produced tumours and the effects of varying maternal folic acid intake were not assessed in wild type mice, it is possible that any effects of prenatal folate supply on tumorigenesis were masked by the genetic defect.
Leukaemia and hepatoblastoma in childhood
A number of studies have reported a positive association between birth weight and risk of both acute myeloid and lymphoblastic leukaemia in childhood (Table 2). As with the reports that show a positive association between birth weight and breast cancer, the majority of studies report associations within the normal range of birth weights. In contrast, risk of hepatoblastoma has been reported to be increased in children born below the normal range of birth weights(75). This association is strongest in children born below <2,500g, particularly in those born <1500g(76-79). It is difficult to explain the opposing relationship between the intra-uterine environment and hepatoblastoma, compared to other cancers. One possible explanation is that the children at risk of hepatoblastoma were born at the lower extreme of birth weight which suggests intra-uterine constraint outside of the normal range which influences patterns of development in the fetus.
Table 2.
Associations between childhood leukaemia and birthweight
| Reference | Number of cases | Disease | Direction of trend |
|---|---|---|---|
| 58 | 1,323 | All leukaemias | Positive |
| 59 | 802 | All leukaemias | Positive |
| 60 | 72 | All leukaemias | Positive |
| 61 | 681 | All leukaemias | Positive, but only less than 2 years of age |
| 62 | 255 | All leukaemias | No association |
| 63 | 1,304 | All leukaemias | No association |
| 64 | 309 | ALL & AML | Positive, but only less than 6 years of age |
| 65 | 337 | ALL | Positive |
| 66 | 71 | ALL | Positive, but only above 4 years of age |
| 67 * | 613 | ALL | Positive |
| 68 * | 98 | AML | No association |
| 69 | 303 | ALL & AML | Positive |
| 70 | 1,687 | All leukaemias | Positive, only studied children less than 2 years of age |
| 71 * | 828 | ALL & AML | Positive |
| 72 * | 268 | ALL | Positive, only studied children less than 5 years |
| 73 | 65 | ALL & AML | Positive |
| 74 | 2,204 | ALL & AML | Positive |
ALL, acute lymphoblastic leukaemia; AML, acute myeloid leukaemia.
Studies in which high birth weight was defined as ≥ 4500g.
Other cancers
McCormack et al.(80) reported detailed statistical analysis of the associations between a number of different cancers and birth weight. When adjusted for birth order and socioeconomic factors at birth and in adulthood, the hazard ratio for each standard deviation increase in birth weight adjusted for gestational age (HRad) for endometrial cancer was 0.79, while the HRad for ovarian cancer was not significantly related to birth weight. There was no significant association between birthweight and prostate cancer in men. This study also described the effect of birth weight on risk of non-reproductive cancers. The HRad for colorectal cancer was 1.16, and lymphatic and haematopoietic tissues 1.19. However, there were no significant associations between birth weight and stomach, liver, pancreatic, respiratory, urinary, neurological, skin and endocrinological cancers. One study has shown an increased odds ratio (OR) for osteosarcoma in adults born ≥4000g compared to those born between 3000 to 3500 g(81). Also, the OR for all brain tumours in children was 1.05 in those born ≥4000g compared to those born between 2500g and 3990g, but was higher (OR 1.40) when astrocytomas were considered separately.
For all cancers, the HRad was 1.23 in women less than 50 years, but not related to birth weight in older women, while the HRad for men of all ages was 1.08(80).
Summary
There is a growing body of evidence which supports the suggestion that the intra-uterine environment modifies risk of specific cancers in children and adults. Despite the wide variability of the disease processes including histological origin, time of onset of disease, sex and age of the patients and the means of data collection there is an overall trend towards an increase in risk of specific cancers associated with higher birth weight, particularly at the upper end of the normal range.
Epigenetics and the developmental origins of metabolic disease
Prenatal environment, phenotype induction and gene transcription
The induction of changes to the phenotype of the offspring in response to the prenatal environment that persist throughout the lifespan implies stable changes to gene transcription resulting in altered activities of metabolic pathways and homeostatic control processes, and in differences in the structure of tissues. There are several studies which have investigated the effect of maternal nutrition during pregnancy and/ or lactation on gene expression in the offspring in animal models. For example, feeding a PR diet to pregnant rats induced changes to the expression of genes involved in energy balance and glucocorticoid activity in the adult offspring (Table 3). Although the number of genes studied so far is limited, these demonstrate stable effects of nutrient restriction on transcription. Importantly, some of the genes which show altered expression following prenatal under-nutrition are transcription factors which affect multiple pathways in development and nutrient homeostasis; for example peroxisomal proliferator-activated receptors (PPAR) and the glucocorticoid receptor (GR) (Table 3). Thus modifying the regulation of expression of a few key transcription factors may alter the activities of a large number of metabolic and developmental pathways.
Table 3.
The effects of maternal dietary protein restriction during pregnancy, or pregnancy and lactation in the rat on the expression of genes associated with energy balance in the adult offspring
| Gene | Tissue | Direction of change compared to control | Reference | |||
|---|---|---|---|---|---|---|
| Glucocorticoid receptor | Liver | Increased | 82-85 | |||
| Lung | Increased | 82 | ||||
| Kidney | Increased | |||||
| Brain | Increased | |||||
| 11βhydroxysteroid dehydrogenase | Liver | Decreased | 82 | |||
| Lung | Decreased | |||||
| Kidney | Decreased | |||||
| Brain | Decreased | |||||
| Phosphoenolpyruvate carboxykinase | Liver | Increased | 83-85 | |||
| Glucokinase | Liver | Increased | 86 | |||
| Acetyl-CoA carboxylase | Liver | Increased | 87 | |||
| Fatty acid synthase | Liver | Increased | ||||
| Peroxisomal proliferator activated receptor-α | Liver | Increased | 82-85,88 | |||
| Peroxisomal proliferator activated receptor-γ1 | Liver | Unchanged | 83,88 | |||
| Peroxisomal proliferator activated receptor-γ2 | Adipose tissue | Decreased | 88 | |||
| Acyl-CoA oxidase | Liver | Increased | 83 |
Epigenetic regulation of transcription
The methylation of CpG dinucleotides, which are clustered at the 5′ promoter regions of genes, confers stable silencing of transcription(89). Methylation patterns are largely established during embryogenesis or in early postnatal life. Following fertilisation, maternal and paternal genomes undergo extensive demethylation. This is followed by de novo methylation just prior to implantation(89,90). About 70% of CpGs are methylated, mainly in repressive heterochromatin regions and in repetitive sequences such as retrotransposable elements(91). Promoter methylation is important for asymmetrical silencing of imprinted genes(92) and retrotransposons(93,94). DNA methylation also plays a key role in cell differentiation by silencing the expression of specific genes during the development and differentiation of individual tissues, and thus the timing of gene methylation is tissue and gene -specific(95,96). For some genes, for example δ-crystallin II and PEPCK, there also appear to be gradations of promoter demethylation associated with developmental changes in role of the gene product(97,98).
DNA methylation can induce transcriptional silencing by blocking the binding of transcription factors and/ or through promoting the binding of the methyl CpG binding protein (MeCP2). The latter binds to methylated cytosines and, in turn, recruits histone modifying complexes composed of deacetylases (HDACs) and histone methyl transferases (HMTs) to the DNA resulting in a closed chromatin structure and transcriptional silencing(99,100). The precise effect on transcription depends on the nature of the covalent modification and which N-terminal lysine residue is altered(101-105).
Early life environment and epigenetic regulation of transcription in the offspring
Since epigenetic regulation of gene promoters is established during development and is responsible for patterns of transcriptional expression and silencing in adults, perturbations to this process represent a candidate molecular mechanism for induction of persistent alterations in phenotype by the environment experience in early life. In an elegant study of the effect of maternal behaviour during suckling on the development of stress response in the offspring, Weaver et al.(106) showed that pups raised by rat dams which showed poorer nurturing had an increased stress response. The effect was due to hypermethylation of specific CpG dinucleotides within the promoter of the GR gene in the hippocampus of the offspring which were reversed in the adult offspring by intra-cranial administration of Trichostatin A and L-methionine(106-108). Uterine artery ligation in the rat decreases p53 expression in the kidney of the offspring, which was associated with increased apoptosis and reduced nephron number(109).
Embryo culture and epigenetic regulation of transcription
Nutrition in early life has been shown to alter the epigenetic regulation of transposable elements and of imprinted genes, including insulin-like growth factor (IGF) -2. These will be summarised here as they are described in detail elsewhere(110). The composition of the culture medium used to grow mouse embryos alters the expression of IGF-2 and H19 genes by changing the methylation status of their respective promoters(111,112). In humans, in vitro fertilisation using the intracytoplasmic sperm injection technique is associated with increased risk of Angelman’s syndrome(113,114) and Beckwith-Weidemann syndrome(115) due to loss of methylation of regulatory regions of the UBE3A, and H19 and IGF-2 genes, respectively(113,115). While such alterations to the epigenetic regulation of imprinted genes produce dramatic alterations to the phenotype of the offspring which are evident in early life, these contrast with the phenotypes induced by variations in maternal nutrition during pregnancy which are more subtle and only become clinically apparent after the neonatal period in childhood or adulthood.
The agouti mouse model
Differences in maternal intake of nutrients involved in 1-carbon metabolism, betaine, choline, folic acid and vitamin B12, during pregnancy in the agouti mouse changed the offsprings’ coat colour from yellow (agouti) to brown (pseudo-agouti)(116). This shift is due to increased methylation of seven CpG islands 600 bp downstream of the Avy intracisternal-A particle insertion site which acts as a cryptic promoter directing the expression of the agouti gene(110). Differential methylation of the seven CpG dinucleotides was associated with a change in the proportions of mice with the agouti or pseudo-agouti coat colour.
Epigenetic regulation of genes in the offspring of the rat maternal dietary protein restriction model
Feeding a PR diet to rats during pregnancy induces hypomethylation of the PPARα and GR promoters and increased expression of the GR and PPARα in the liver of the recently-weaned offspring(83). This shows that stable changes to the epigenetic regulation of the expression of transcription factors, and hence a phenotype, can be induced in the offspring by modest changes to maternal intake of a macronutrient during pregnancy. However, there is evidence which shows that nutrients involved in 1-carbon metabolism play an important role in this process (see below). The expression of the PPARα and GR target genes, acyl-CoA oxidase and PEPCK, was also increased which supports the suggestion that such altered epigenetic regulation of transcription factors modifies the activities of important metabolic pathways(83,84). Sequence analysis of the PPARα promoter showed that the methylation status of only a few CpG dinucleotides was altered by the PR diet(117). This suggests that the process of induced epigenetic change is targeted and that the resulting change in transcription may reflect changes in the interaction of the gene with relatively few transcription factors, thus inducing specific changes in the regulation of gene function and hence response to environmental cues. Methylation of the GR and PPARα promoters was also reduced in the heart and the PPARα promoter was hypomethylated in the whole umbilical cord(88). Hypomethylation of the GR promoter was associated with an increase in histone modifications which facilitate transcription while those that suppress gene expression were reduced or unchanged(84). While this may be primarily the result of reduced binding of the methyl CpG binding protein (MeCP) -2 to the GR promoter because of the reduced level of DNA methylation, reduced MeCP2 expression may also have contributed to higher levels of histone acetylation.
Induction of vascular dysfunction in the offspring of rats fed PR diet during pregnancy was prevented by supplementation of the PR diet with glycine or folic acid(18-20). Hypomethylation of the hepatic GR and PPARα promoters was also prevented by addition of 5-fold more folic acid to the PR diet(83). Thus 1-carbon metabolism plays a central role in the induction of an altered phenotype by maternal dietary restriction as it does in the Agouti mouse(110).
DNA methyltransferases and 1-carbon metabolism
Methylation of CpG dinucleotides de novo is catalysed by DNA methyltransferases (Dnmt) 3a and 3b, and is maintained through mitosis by gene-specific methylation of hemimethylated DNA by Dnmt1(89) (Figure 1). Overexpression of Dnmt1 results in hypermethylation of DNA and embryonic lethality(118), while transient depletion of xDnmt1 in Xenopus embryos induces DNA hypomethylation producing an altered phenotype and sustained depletion causes apoptosis(119,120). Thus variations in Dnmt1 expression alter the phenotype of the embryo. Dnmt1 activity is inhibited by homocysteine (Hcyst)(121), and Dnmt1 and 3a expression is modulated by folic acid intake in adult rats(122). Furthermore, the Dnmt1 promoter contains a GR response element(123) which may induce negative regulation of Dnmt1 expression(124). This suggests how administration of glucocorticoids during pregnancy may induce stable changes to the expression of gluconeogenic enzymes in the offspring as a result of increasing GR activity(125). Thus Dnmt activity may be altered by increased glucocorticoid exposure or as a result of changes to 1-carbon metabolism, and so represent one candidate mechanism for the induction of altered epigenetic regulation of genes in response to the intra-uterine environment. Feeding a PR diet to rats during pregnancy induced a reduction in Dnmt1 expression and in binding of Dnmt1 at the GR promoter(84), but not the expression of Dnmt3a, Dnmt3b or the putative DNA demethylase methyl binding domain-2(126), and the binding of Dnmt3a at the GR promoter were unaltered. This suggests that hypomethylation of the GR promoter in the liver of the offspring, and probably other genes including PPARα, is induced by the maternal diet as a result of lower capacity to maintain patterns of cytosine methylation during mitosis. Down-regulation of Dnmt1 expression may result from increased exposure to Hcyst(19,127) and / or corticosteroids(128,129) which may act directly or by deceasing folate bioavailability(130). The central role of 1-carbon metabolism is highlighted by the prevention of reduced Dnmt1 expression by increasing the folic acid content of the PR diet(84). Since Dnmt1 activity appears to be targeted to a subset of gene promoters(131-133), altered Dnmt1 expression provides a mechanism for induction of gene-specific promoter hypomethylation. Since Dnmt1 activity is also required for progression through mitosis(134,135), reduced Dnmt1 activity could also account for the reduction in embryo cell mass(136).
Figure 1.
Gene silencing by DNA methylation. Gene expression is silenced in the early embryo by the activities of DNA methyltransferases (Dnmt) 3a and 3b which catalyse methylation of CpG dinucleotides do novo. This recruits methyl CpG binding protein (MeCP)-2 which in turn recruits the histone deacetylase (HDAC) / histone methyltransferase (HMT) complex which induce condensation of chromatin at the promoter. Methylation of CpG dinucleotides, and hence gene silencing, is maintained through mitotic cycles by Dnmt1 activity.
A model for the induction of an altered metabolic phenotype in the offspring by prenatal under-nutrition
Based upon current data, we have suggested a mechanism for the induction of an altered phenotype in the offspring by nutrient constraint during pregnancy in which promoter methylation is lost in a gene-specific manner during mitosis due to decreased Dnmt1 expression and activity(84,85). This is accompanied by reduced binding of the MeCP2/HADC/HMT complex leading to persistence of histone modifications that permit transcription.
Epigenetics and cancer
A change in the epigenetic regulation of genes has been implicated as a causal mechanism in specific cancers including lung, prostate and breast cancer(137), colon cancer(138) and haemopoietic cancers(139). Specifically, increased cancer risk is associated with global hypomethylation of the genome with concurrent hypermethylation or hypomethylation of the promoters of specific genes. The mechanism by which global hypomethylation is induced is unclear, but may reflect the global decline in DNA methylation associated with increasing age(137). The age-related decline in global methylation is related to a reduction in Dnmt1 activity(140) which, in turn, may induce expression of oncogenes such as c-Myc and c-N-ras(140). Thus it appears that modulation of Dnmt1 activity is a key regulatory step in both fetal programming and in the induction of the tumourigenesis. This may be accompanied by methylation de novo of tumour suppressor genes(141) by increased Dnmt3a activity leading to aberrant activation of genes involved in cell proliferation and cell differentiation(142). Together these changes represent a shift in the regulation of gene control which in turn, may predispose the genome to further changes in methylation which result ultimately in neoplasia(143). The mechanism leading to gene-specific hypermethylation in cancer is unclear, although it is possible that, as in fetal programming, the balance of nutrients involved in 1-carbon metabolism, including folate, vitamin B6 and B12, may modulate the activities of Dnmts(143). Hypermethylation of specific genes in cancer appears to be similar to the effect of increasing the folic acid content of the PR diet fed to pregnant rats on the methylation status of CpGs in the liver PPARα promoter in the offspring. While the methylation of CpGs which were hypomethylated in the offspring of dams fed a PR diet was normalised by increasing the folic acid content of the maternal PR diet, hypermethylation was also induced in other specific CpG dinucleotides(117). The observation that nutrition in early life can induce both hypomethylation and hypermethylation of specific CpG dinucleotides may suggest a mechanism by which differences in the direction of the association between size at birth and risk of metabolic disease or cancer. Prenatal under-nutrition results in hypomethylation of specific CpG dinucleotides in individual gene promoters which would tend to increase binding of regulatory proteins. It is possible therefore that higher nutrient availability, such as increased folic acid intake, may induce hypermethylation of other CpGs which would result in different DNA - protein interactions and so induce a shift in regulation. One key example of the role of epigenetics in modulating gene activity by shifting the balance between agonist and suppressor proteins is the induction of tumourigenesis by activation of telomerase in differentiated cells. Telomerase activity is down-regulated in most cells during terminal differentiation in embryogenesis as a result of methylation of the GC-rich promoter region, but is often active in cancer cells. It has been proposed that activation of telomerase in preneoplastic cells is due to a shift in regulation between the activator c-Myc and the suppressor WT1 by changes in the methylation status of specific CpGs within the binding domains of these transcription factors in the promoter of the catalytic subunit with confers reverse transcriptase activity (hTERT)(144). One consequence of hTERT activation is to increase Dnmt1 activity(145) leading to copying of aberrant patterns of cytosine methylation. This suggests a synergistic role for hTERT and Dnmt1 in controlling cell proliferation and the methylation status of the genome.
Summary
The findings of epidemiological studies of the relationship between prenatal growth and risk specific cancers, and metabolic and cardiovascular disease suggest that the early life environment is a causal component of the aetiology of these conditions. This is further implied by the common role for altered epigenetic regulation of specific genes and of altered DNA methyltransferase activity. Thus, risk of what may generally be considered to be very different disease entities may reflect a continuum of developmental changes which operate via the same enzymes and pathways which induce alterations to the epigenetic regulation of specific genes. Risk of specific diseases may reflect the nature and / or the magnitude of the environmental exposure during early life. It is not known how these environmental cues may be targeted in a manner which induces altered epigenetic regulation of specific genes or of individual CpG dinucleotides and so lead to increased risk of different disease processes. However, such specificity is implied by emerging evidence that the magnitude of the maternal nutritional challenge and the relative amount of specific nutrients in the maternal diet induce directionally opposite changes in the physiology and epigenotype of the offspring (21,83,146).
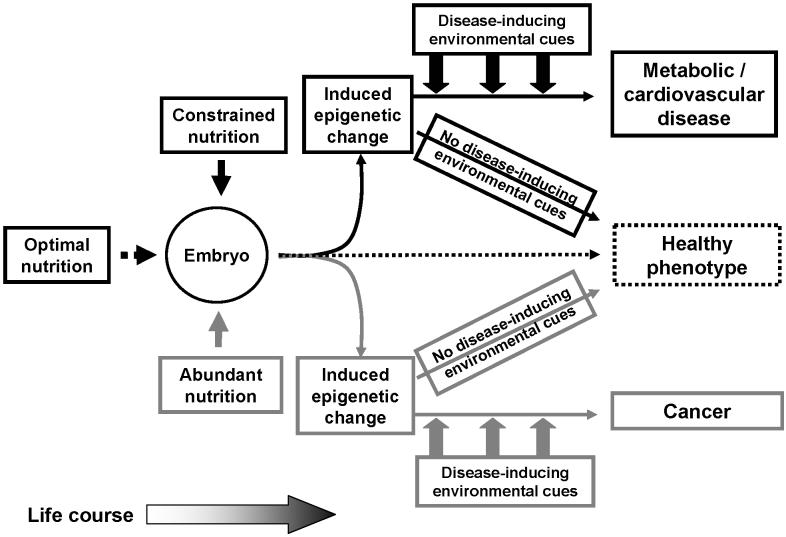
Overall, these findings support the concept that a range of prenatal nutritional environments from constraint to abundance may induce risk of ultimately different pathological processes (Figure 2). The induced epigenetic changes are likely to be permissive for altered gene expression and hence determine the interaction between an organism and its environment over the life course and, in turn, determine whether increased risk due to the early life environment becomes disease in later life. However, this is an emerging field of research and a substantial number of studies will be required to demonstrate directly a causal association between variations in early life nutrition, induced epigenetic change and differential disease risk, to characterise and understand the underlying mechanisms and to develop prognostic markers in order translate the research findings into clinical tools.
Figure 2.
A model for induction of increased risk of cardiovascular / metabolic disease or cancer by different nutritional exposures acting on the same genome during development. Optimal nutrition during development facilitates establishment of an epigenotype which is expressed as a healthy phenotype. Nutritional constraint induces altered epigenetic regulation in genes associated with increased risk of cardiovascular / metabolic disease. Conversely, nutrient abundance during development induces epigenetic changes in genes associated with increased risk of cancer. However, for both altered epigenotypes, the disease phenotype is only manifest when the organism is exposed to appropriate environmental signals, such as poor diet, during the life course. If these later environmental cues are avoided, possibly by lifestyle choice, then a healthy phenotype is maintained.
Acknowledgement
GCB receives salary support from the British Heart Foundation.
Footnotes
Author contributions and conflict of interest statement
GCB wrote the manuscript with substantial contributions from KAL and AAJ. All authors declare no conflict of interest.
References
- 1.World Cancer research fund. American Institute for Cancer Research . Food, nutrition, physical activity and the prevention of cancer: a global perspective. Washington DC: American Institute for Cancer Research; 2007. [Google Scholar]
- 2.Vucic EA, Brown CJ, Lam WL. Epigenetics of cancer progression. Pharmacogenomics. 2008;9:215–234. doi: 10.2217/14622416.9.2.215. [DOI] [PubMed] [Google Scholar]
- 3.Jackson AA. Integrating the ideas of life course across cellular, individual, and population levels in cancer causation. J Nutr. 2005;135(12 Suppl):2927S–2933S. doi: 10.1093/jn/135.12.2927S. [DOI] [PubMed] [Google Scholar]
- 4.Barker DJP. Mothers, babies and health in later life. 2nd Ed. Edinburgh: Churchill Livingstone; 1998. [Google Scholar]
- 5.Godfrey KM, Barker DJ. Fetal programming and adult health. Public Health Nutr. 2001;4:611–624. doi: 10.1079/phn2001145. [DOI] [PubMed] [Google Scholar]
- 6.Gluckman PD, Hanson MA, editors. Developmental Origins of Disease. Cambridge, New York, Melbourne, Madrid, Cape Town, Singapore, San Paulo: Cambridge University Press; 2006. [Google Scholar]
- 7.Roseboom TJ, van der Meulen JH, Ravelli AC, Osmond C, Barker DJ, Bleker OP. Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Mol Cell Endocrinol. 2001;185:93–98. doi: 10.1016/s0303-7207(01)00721-3. [DOI] [PubMed] [Google Scholar]
- 8.Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 9.Jasienska G, Thune I, Ellison PT. Fatness at birth predicts adult susceptibility to ovarian suppression: an empirical test of the Predictive Adaptive Response hypothesis. Proc Natl Acad Sci USA. 2006;103:12759–12762. doi: 10.1073/pnas.0605488103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gluckman PD, Hanson MA, Beedle AS. Non-genomic transgenerational inheritance of disease risk. Bioessays. 2007;29:145–154. doi: 10.1002/bies.20522. [DOI] [PubMed] [Google Scholar]
- 11.Kaati G, Bygren LO, Edvinsson S. Cardiovascular and diabetes mortality determined by nutrition during parents’ and grandparents’ slow growth period. Eur J Hum Genet. 2002;10:682–688. doi: 10.1038/sj.ejhg.5200859. [DOI] [PubMed] [Google Scholar]
- 12.Stein AD, Lumey LH. The relationship between maternal and offspring birth weights after maternal prenatal famine exposure: the Dutch Famine Birth Cohort Study. Hum Biol. 2000;72:641–54. [PubMed] [Google Scholar]
- 13.Armitage JA, Khan IY, Taylor PD, Nathanielsz PW, Poston L. Developmental programming of the metabolic syndrome by maternal nutritional imbalance: how strong is the evidence from experimental models in mammals? J Physiol. 2004;561:355–377. doi: 10.1113/jphysiol.2004.072009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertram CE, Hanson MA. Animal models and programming of the metabolic syndrome. Br Med Bull. 2001;60:103–121. doi: 10.1093/bmb/60.1.103. [DOI] [PubMed] [Google Scholar]
- 15.Langley SC, Jackson AA. Increased systolic blood pressure in adult rats induced by fetal exposure to maternal low protein diets. Clin Sci (Lond) 1994;86:217–222. doi: 10.1042/cs0860217. [DOI] [PubMed] [Google Scholar]
- 16.Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, Breier BH, Harris M. Neonatal leptin treatment reverses developmental programming. Endocrinol. 2005;146:4211–4216. doi: 10.1210/en.2005-0581. [DOI] [PubMed] [Google Scholar]
- 17.Khan IY, Dekou V, Douglas G, Jensen R, Hanson MA, Poston L, Taylor PD. A high-fat diet during rat pregnancy or suckling induces cardiovascular dysfunction in adult offspring. Am J Physiol Regul Integr Comp Physiol. 2005;288:R127–R133. doi: 10.1152/ajpregu.00354.2004. [DOI] [PubMed] [Google Scholar]
- 18.Jackson AA, Dunn RL, Marchand MC, Langley-Evans SC. Increased systolic blood pressure in rats induced by a maternal low-protein diet is reversed by dietary supplementation with glycine. Clin Sci (Lond) 2002;103:633–639. doi: 10.1042/cs1030633. [DOI] [PubMed] [Google Scholar]
- 19.Brawley L, Torrens C, Anthony FW, Itoh S, Wheeler T, Jackson AA, Clough GF, Poston L, Hanson MA. Glycine rectifies vascular dysfunction induced by dietary protein imbalance during pregnancy. J Physiol. 2004;554:497–504. doi: 10.1113/jphysiol.2003.052068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torrens C, Brawley L, Anthony FW, Dance CS, Dunn R, Jackson AA, Poston L, Hanson MA. Folate supplementation during pregnancy improves offspring cardiovascular dysfunction induced by protein restriction. Hypertension. 2006;47:982–987. doi: 10.1161/01.HYP.0000215580.43711.d1. [DOI] [PubMed] [Google Scholar]
- 21.Dunn RL, Burdge GC, Jackson AA. Folic acid reduces blood pressure in rat offspring from maternal low protein diet but increases blood pressure in offspring of the maternal control diet. Ped Res. 2003;53(Suppl):2A. [Google Scholar]
- 22.Burdge GC, Lillycrop KA, Jackson AA, Gluckman PD, Hanson MA. The nature of the growth pattern and of the metabolic response to fasting in the rat are dependent upon the dietary protein and folic acid intakes of their pregnant dams and post-weaning fat consumption. Br J Nutr. 2008;99:540–549. doi: 10.1017/S0007114507815819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waterland RA, Travisano M, Tahiliani KG, Rached MT, Mirza S. Methyl donor supplementation prevents transgenerational amplification of obesity. Int J Obes (Lond) 2008 doi: 10.1038/ijo.2008.100. doi:10.1038/ijo.2008.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenwald P, Barlow JJ, Nasca PC, Burnett WS. Vaginal cancer after maternal treatment with synthetic estrogens. N Engl J Med. 1971;285:390–392. doi: 10.1056/NEJM197108122850707. [DOI] [PubMed] [Google Scholar]
- 25.Kato H, Yoshimoto Y, Schull WJ. Risk of cancer among children exposed to atomic bomb radiation in utero: a review. IARC Sci Publ. 1989;96:365–74. [PubMed] [Google Scholar]
- 26.Ekbom . The developmental environment and the early origins of cancer. In: Gluckman PD, Hanson MA, editors. Developmental Origins of Disease. Cambridge, New York, Melbourne, Madrid, Cape Town, Singapore, San Paulo: Cambridge University Press; 2006. pp. 415–425. 2006. [Google Scholar]
- 27.Michels KB, Xue F. Role of birthweight in the etiology of breast cancer. Int J Cancer. 2006;119:2007–2025. doi: 10.1002/ijc.22004. [DOI] [PubMed] [Google Scholar]
- 28.Le Marchand L, Kolonel LN, Myers BC, Mi MP. Birth characteristics of premenopausal women with breast cancer. Br J Cancer. 1988;57:437–439. doi: 10.1038/bjc.1988.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ekbom A, Trichopoulos D, Adami HO, Hsieh CC, Lan SJ. Evidence of prenatal influences on breast cancer risk. Lancet. 1992;340:1015–1018. doi: 10.1016/0140-6736(92)93019-j. [DOI] [PubMed] [Google Scholar]
- 30.Michels KB, Trichopoulos D, Robins JM, Rosner BA, Manson JE, Hunter DJ, Colditz GA, Hankinson SE, Speizer FE, Willett WC. Birthweight as a risk factor for breast cancer. Lancet. 1996;348:1542–1546. doi: 10.1016/S0140-6736(96)03102-9. [DOI] [PubMed] [Google Scholar]
- 31.Sanderson M, Williams MA, Malone KE, Stanford JL, Emanuel I, White E, Daling JR. Perinatal factors and risk of breast cancer. Epidemiol. 1996;7:34–37. doi: 10.1097/00001648-199601000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Ekbom A, Hsieh CC, Lipworth L, Adami HQ, Trichopoulos D. Intrauterine environment and breast cancer risk in women: a population-based study. J Natl Cancer Inst. 1997;89:71–76. doi: 10.1093/jnci/89.1.71. [DOI] [PubMed] [Google Scholar]
- 33.Mogren I, Damber L, Tavelin B, Hogberg U. Characteristics of pregnancy and birth and malignancy in the offspring (Sweden) Cancer Causes Control. 1999;10:85–94. doi: 10.1023/a:1008813701634. [DOI] [PubMed] [Google Scholar]
- 34.Innes K, Byers T, Schymura M. Birth characteristics and subsequent risk for breast cancer in very young women. Am J Epidemiol. 2000;152:1121–1128. doi: 10.1093/aje/152.12.1121. [DOI] [PubMed] [Google Scholar]
- 35.De Stavola BL, Hardy R, Kuh D, Silva IS, Wadsworth M, Swerdlow AJ. Birthweight, childhood growth and risk of breast cancer in a British cohort. Br J Cancer. 2000;83:964–968. doi: 10.1054/bjoc.2000.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaijser M, Lichtenstein P, Granath F, Erlandsson G, Cnattingius S, Ekbom A. In utero exposures and breast cancer: a study of opposite-sexed twins. J Natl Cancer Inst. 2001;93:60–62. doi: 10.1093/jnci/93.1.60. [DOI] [PubMed] [Google Scholar]
- 37.Andersson SW, Bengtsson C, Hallberg L, Lapidus L, Niklasson A, Wallgren A, Hulthen L. Cancer risk in Swedish women: the relation to size at birth. Br J Cancer. 2001;84:1193–1198. doi: 10.1054/bjoc.2000.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hilakivi-Clarke L, Forsen T, Eriksson JG, Luoto R, Tuomilehto J, Osmond C, Barker DJ. Tallness and overweight during childhood have opposing effects on breast cancer risk. Br J Cancer. 2001;85:1680–1684. doi: 10.1054/bjoc.2001.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanderson M, Shu XO, Jin F, Dai Q, Ruan Z, Gao YT, Zheng W. Weight at birth and adolescence and premenopausal breast cancer risk in a low-risk population. Br J Cancer. 2002;86:84–88. doi: 10.1038/sj.bjc.6600009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Titus-Ernstoff L, Egan KM, Newcomb PA, Ding J, Trentham-Dietz A, Greenberg ER, Baron JA, Trichopoulos D, Willett WC. Early life factors in relation to breast cancer risk in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2002;11:207–210. [PubMed] [Google Scholar]
- 41.Vatten LJ, Maehle BO, Lund Nilsen TI, Tretli S, Hsieh CC, Trichopoulos D, Stuver SO. Birth weight as a predictor of breast cancer: a case-control study in Norway. Br J Cancer. 2002;86:89–91. doi: 10.1038/sj.bjc.6600011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahlgren M, Sorensen T, Wohlfahrt J, Haflidadottir A, Holst C, Melbye M. Birth weight and risk of breast cancer in a cohort of 106,504 women. Int J Cancer. 2003;107:997–1000. doi: 10.1002/ijc.11481. [DOI] [PubMed] [Google Scholar]
- 43.McCormack VA, dos Santos Silva I, De Stavola BL, Mohsen R, Leon DA, Lithell HO. Fetal growth and subsequent risk of breast cancer: results from long term follow up of Swedish cohort. BMJ. 2003;326:248. doi: 10.1136/bmj.326.7383.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaijser M, Akre O, Cnattingius S, Ekbom A. Preterm birth, birth weight, and subsequent risk of female breast cancer. Br J Cancer. 2003;89:1664–1666. doi: 10.1038/sj.bjc.6601357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mellemkjaer L, Olsen ML, Sorensen HT, Thulstrup AM, Olsen J, Olsen JH. Birth weight and risk of early-onset breast cancer (Denmark) Cancer Causes Control. 2003;14:61–64. doi: 10.1023/a:1022570305704. [DOI] [PubMed] [Google Scholar]
- 46.dos Santos Silva I, De Stavola BL, Hardy RJ, Kuh DJ, McCormack VA, Wadsworth ME. Is the association of birth weight with premenopausal breast cancer risk mediated through childhood growth? Br J Cancer. 2004;91:519–524. doi: 10.1038/sj.bjc.6601972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahlgren M, Melbye M, Wohlfahrt J, Sorensen TI. Growth patterns and the risk of breast cancer in women. N Engl J Med. 2004;351:1619–1626. doi: 10.1056/NEJMoa040576. [DOI] [PubMed] [Google Scholar]
- 48.Hodgson ME, Newman B, Millikan RC. Birth weight, parental age, birth order and breast cancer risk in African-American and white women: a population-based case-control study. Breast Cancer Res. 2004;6:R656–R667. doi: 10.1186/bcr931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lahmann PH, Gullberg B, Olsson H, Boeing H, Berglund G, Lissner L. Birth weight is associated with postmenopausal breast cancer risk in Swedish women. Br J Cancer. 2004;91:666–668. doi: 10.1038/sj.bjc.6602203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCormack VA, dos Santos Silva I, Koupil I, Leon DA, Lithell HO. Birth characteristics and adult cancer incidence: Swedish cohort of over 11,000 men and women. Int J Cancer. 2005;115:611–617. doi: 10.1002/ijc.20915. [DOI] [PubMed] [Google Scholar]
- 51.Vatten LJ, Nilsen TI, Tretli S, Trichopoulos D, Romundstad PR. Size at birth and risk of breast cancer: prospective population-based study. Int J Cancer. 2005;114:461–464. doi: 10.1002/ijc.20726. [DOI] [PubMed] [Google Scholar]
- 52.Michels KB, Xue F, Terry KL, Willett WC. Longitudinal study of birthweight and the incidence of breast cancer in adulthood. Carcinogenesis. 2006;27:2464–2468. doi: 10.1093/carcin/bgl105. [DOI] [PubMed] [Google Scholar]
- 53.Okasha M, McCarron P, Gunnell D, Smith GD. Exposures in childhood, adolescence and early adulthood and breast cancer risk: a systematic review of the literature. Breast Cancer Res Treat. 2003;78:223–276. doi: 10.1023/a:1022988918755. [DOI] [PubMed] [Google Scholar]
- 54.Xue F, Michels KB. Intrauterine factors and risk of breast cancer: a systematic review and meta-analysis of current evidence. Lancet Oncol. 2007;8:1088–1100. doi: 10.1016/S1470-2045(07)70377-7. [DOI] [PubMed] [Google Scholar]
- 55.de Assis S, Khan G, Hilakivi-Clarke L. High birth weight increases mammary tumorigenesis in rats. Int J Cancer. 2006;119:1537–1546. doi: 10.1002/ijc.21936. [DOI] [PubMed] [Google Scholar]
- 56.Fernandez-Twinn DS, Ekizoglou S, Gusterson BA, Luan J, Ozanne SE. Compensatory mammary growth following protein restriction during pregnancy and lactation increases early-onset mammary tumor incidence in rats. Carcinogenesis. 2007;28:545–552. doi: 10.1093/carcin/bgl166. [DOI] [PubMed] [Google Scholar]
- 57.McKay JA, Williams EA, Mathers JC. Gender-specific modulation of tumorigenesis by folic acid supply in the Apc mouse during early neonatal life. Br J Nutr. 2008;99:550–558. doi: 10.1017/S0007114507819131. [DOI] [PubMed] [Google Scholar]
- 58.MacMahon B, Newill VA. Birth characteristics of children dying of malignant neoplasms. J Natl Cancer Inst. 1962;28:231–244. [PubMed] [Google Scholar]
- 59.Fasal E, Jackson EW, Klauber MR. Birth characteristics and leukemia in childhood. J Natl Cancer Inst. 1971;47:501–509. [PubMed] [Google Scholar]
- 60.Wertelecki W, Mantel N. Increased birth weight in leukemia. Pediatr Res. 1973;7:132–138. doi: 10.1203/00006450-197303000-00005. [DOI] [PubMed] [Google Scholar]
- 61.Daling JR, Starzyk P, Olshan AF, Weiss NS. Birth weight and the incidence of childhood cancer. J Natl Cancer Inst. 1984;72:1039–1041. [PubMed] [Google Scholar]
- 62.Shaw G, Lavey R, Jackson R, Austin D. Association of childhood leukemia with maternal age, birth order, and paternal occupation. A case-control study. Am J Epidemiol. 1984;119:788–795. doi: 10.1093/oxfordjournals.aje.a113799. [DOI] [PubMed] [Google Scholar]
- 63.Eisenberg DE, Sorahan T. Birth weight and childhood cancer deaths. J Natl Cancer Inst. 1987;78:1095–1100. [PubMed] [Google Scholar]
- 64.Shu XO, Gao YT, Brinton LA, Linet MS, Tu JT, Zheng W, Fraumeni JF. A population-based case-control study of childhood leukemia in Shanghai. Cancer. 1988;62:635–644. doi: 10.1002/1097-0142(19880801)62:3<635::aid-cncr2820620332>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 65.Kaye SA, Robison LL, Smithson WA, Gunderson P, King FL, Neglia JP. Maternal reproductive history and birth characteristics in childhood acute lymphoblastic leukemia. Cancer. 1991;68:1351–1355. doi: 10.1002/1097-0142(19910915)68:6<1351::aid-cncr2820680627>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 66.Savitz DA, Ananth CV. Birth characteristics of childhood cancer cases, controls, and their siblings. Pediatr Hematol Oncol. 1994;11:587–599. doi: 10.3109/08880019409141806. [DOI] [PubMed] [Google Scholar]
- 67.Cnattingius S, Zack MM, Ekbom A, Gunnarskog J, Kreuger A, Linet M, Adami HO. Prenatal and neonatal risk factors for childhood lymphatic leukemia. J Natl Cancer Inst. 1995;87:908–914. doi: 10.1093/jnci/87.12.908. [DOI] [PubMed] [Google Scholar]
- 68.Cnattingius S, Zack M, Ekbom A, Gunnarskog J, Linet M, Adami HO. Prenatal and neonatal risk factors for childhood myeloid leukemia. Cancer Epidemiol Biomarkers Prev. 1995;4:441–445. [PubMed] [Google Scholar]
- 69.Ross JA, Potter JD, Shu XO, Reaman GH, Lampkin B, Robison LL. Evaluating the relationships among maternal reproductive history, birth characteristics, and infant leukemia: a report from the Children’s Cancer Group. Ann Epidemiol. 1997;7:172–179. doi: 10.1016/s1047-2797(97)00012-4. [DOI] [PubMed] [Google Scholar]
- 70.Yeazel MW, Ross JA, Buckley JD, Woods WG, Ruccione K, Robison LL. High birth weight and risk of specific childhood cancers: a report from the Children’s Cancer Group. J Pediatr. 1997;131:671–677. doi: 10.1016/s0022-3476(97)70091-x. [DOI] [PubMed] [Google Scholar]
- 71.Westergaard T, Andersen PK, Pedersen JB, Olsen JH, Frisch M, Sorensen HT, Wohlfahrt J, Melbye M. Birth characteristics, sibling patterns, and acute leukemia risk in childhood: a population-based cohort study. J Natl Cancer Inst. 1997;89:939–947. doi: 10.1093/jnci/89.13.939. [DOI] [PubMed] [Google Scholar]
- 72.Okcu MF, Goodman KJ, Carozza SE, Weiss NS, Burau KD, Bleyer WA, Cooper SP. Birth weight, ethnicity, and occurrence of cancer in children: a population-based, incident case-control study in the State of Texas, USA. Cancer Causes Control. 2002;13:595–602. doi: 10.1023/a:1019555912243. [DOI] [PubMed] [Google Scholar]
- 73.Paltiel O, Harlap S, Deutsch L, Knaanie A, Massalha S, Tiram E, Barchana M, Friedlander Y. Birth weight and other risk factors for acute leukemia in the Jerusalem Perinatal Study cohort. Cancer Epidemiol Biomarkers Prev. 2004;13:1057–1064. [PubMed] [Google Scholar]
- 74.Hjalgrim LL, Rostgaard K, Hjalgrim H, Westergaard T, Thomassen H, Forestier E, Gustafsson G, Kristinsson J, Melbye M, Schmiegelow K. Birth weight and risk for childhood leukemia in Denmark, Sweden, Norway, and Iceland. J Natl Cancer Inst. 2004;96:1549–1556. doi: 10.1093/jnci/djh287. [DOI] [PubMed] [Google Scholar]
- 75.Slovis TL, Roebuck DJ. Hepatoblastoma: why so many low-birth-weight infants? Pediatr Radiol. 2006;36:173–174. doi: 10.1007/s00247-006-0128-z. [DOI] [PubMed] [Google Scholar]
- 76.Reynolds P, Urayama KY, Von Behren J, Feusner J. Birth characteristics and hepatoblastoma risk in young children. Cancer. 2004;100:1070–1076. doi: 10.1002/cncr.20061. [DOI] [PubMed] [Google Scholar]
- 77.Feusner J, Buckley J, Robison L, Ross J, Van Tornout J. Prematurity and hepatoblastoma: more than just an association? J Pediatr. 1998;133:585–586. doi: 10.1016/s0022-3476(98)70084-8. [DOI] [PubMed] [Google Scholar]
- 78.Tanimura M, Matsui I, Abe J, Ikeda H, Kobayashi N, Ohira M, Yokoyama M, Kaneko M. Increased risk of hepatoblastoma among immature children with a lower birth weight. Cancer Res. 1998;58:3032–3035. [PubMed] [Google Scholar]
- 79.Feusner J, Plaschkes J. Hepatoblastoma and low birth weight: a trend or chance observation? Med Pediatr Oncol. 2002;39:508–509. doi: 10.1002/mpo.10176. [DOI] [PubMed] [Google Scholar]
- 80.McCormack VA, dos Santos Silva I, Koupil I, Leon DA, Lithell HO. Birth characteristics and adult cancer incidence: Swedish cohort of over 11,000 men and women. Int J Cancer. 2005;115:611–617. doi: 10.1002/ijc.20915. [DOI] [PubMed] [Google Scholar]
- 81.Troisi R, Masters MN, Joshipura K, Douglass C, Cole BF, Hoover RN. Perinatal factors, growth and development, and osteosarcoma risk. Br J Cancer. 2006;95:1603–1607. doi: 10.1038/sj.bjc.6603474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bertram C, Trowern AR, Copin N, Jackson AA, Whorwood CB. The maternal diet during pregnancy programs altered expression of the glucocorticoid receptor and type 2 11beta-hydroxysteroid dehydrogenase: potential molecular mechanisms underlying the programming of hypertension in utero. Endocrinol. 2001;142:2841–2853. doi: 10.1210/endo.142.7.8238. [DOI] [PubMed] [Google Scholar]
- 83.Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr. 2005;135:1382–1386. doi: 10.1093/jn/135.6.1382. [DOI] [PubMed] [Google Scholar]
- 84.Lillycrop KA, Slater-Jefferies JL, Hanson MA, Godfrey KM, Jackson AA, Burdge GC. Induction of altered epigenetic regulation of the hepatic glucocorticoid receptor in the offspring of rats fed a protein-restricted diet during pregnancy suggests that reduced DNA methyltransferase-1 expression is involved in impaired DNA methylation and changes in histone modifications. Br J Nutr. 2007;97:1064–1073. doi: 10.1017/S000711450769196X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Burdge GC, Hanson MA, Slater-Jefferies JL, Lillycrop KA. Epigenetic regulation of transcription: A mechanism for inducing variations in phenotype (fetal programming) by differences in nutrition during early life? Br J Nutr. 2007;97:1036–1046. doi: 10.1017/S0007114507682920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bogdarina I, Murphy HC, Burns SP, Clark AJ. Investigation of the role of epigenetic modification of the rat glucokinase gene in fetal programming. Life Sci. 2004;74:1407–1415. doi: 10.1016/j.lfs.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 87.Maloney CA, Gosby AK, Phuyal JL, Denyer GS, Bryson JM, Caterson ID. Site-specific changes in the expression of fat-partitioning genes in weanling rats exposed to a low-protein diet in utero. Obes Res. 2003;11:461–468. doi: 10.1038/oby.2003.63. [DOI] [PubMed] [Google Scholar]
- 88.Burdge GC, Phillips ES, Dunn RL, Jackson AA, Lillycrop KA. Effect of reduced maternal protein consumption during pregnancy in the rat on plasma lipid concentrations and expression of peroxisomal proliferator-activated receptors in the liver and adipose tissue of the offspring. Nutr Res. 2004;24:639–646. [Google Scholar]
- 89.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2001;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 90.Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293:1089–1093. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- 91.Yoder JA, Soman NS, Verdine GL, Bestor TH. DNA (cytosine-5)-methyltransferases in mouse cells and tissues. Studies with a mechanism-based probe. J Mol Biol. 1997;270:385–395. doi: 10.1006/jmbi.1997.1125. [DOI] [PubMed] [Google Scholar]
- 92.Li E, Beard C, Jaenisch R. Role for DNA methylation in genomic imprinting. Nature. 1993;366:362–365. doi: 10.1038/366362a0. [DOI] [PubMed] [Google Scholar]
- 93.Walsh CP, Chaillet JR, Bestor TH. Transcription of IAP endogenous retroviruses is constrained by cytosine methylation. Nature Genet. 1998;20:116–117. doi: 10.1038/2413. [DOI] [PubMed] [Google Scholar]
- 94.Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23:5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gidekel S, Bergman Y. A unique developmental pattern of Oct-3/4 DNA methylation is controlled by a cis-demodification element. J Biol Chem. 2002;277:34521–34530. doi: 10.1074/jbc.M203338200. [DOI] [PubMed] [Google Scholar]
- 96.Hershko AY, Kafri T, Fainsod A, Razin A. Methylation of HoxA5 and HoxB5 and its relevance to expression during mouse development. Gene. 2003;302:65–72. doi: 10.1016/s0378111902010910. [DOI] [PubMed] [Google Scholar]
- 97.Grainger RM, Hazard-Leonards RM, Samaha F, Hougan LM, Lesk MR, Thomsen GH. Is hypomethylation linked to activation of delta-crystallin genes during lens development? Nature. 1983;306:88–91. doi: 10.1038/306088a0. [DOI] [PubMed] [Google Scholar]
- 98.Benvenisty N, Mencher D, Meyuhas O, Razin A, Reshef L. Sequential changes in DNA methylation patterns of the rat phosphoenolpyruvate carboxykinase gene during development. Proc Nat. Acad Sci USA. 1985;82:267–271. doi: 10.1073/pnas.82.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fuks F, Hurd PJ, Wolf D, Nan X, Bird AP, Kouzarides T. The methyl-CpG-binding protein MeCP2 links DNA methylation to histone methylation. J Biol Chem. 2003;278:4035–4040. doi: 10.1074/jbc.M210256200. [DOI] [PubMed] [Google Scholar]
- 100.Turner BM. Histone acetylation and an epigenetic code. Bioessays. 2000;22:836–845. doi: 10.1002/1521-1878(200009)22:9<836::AID-BIES9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 101.Strahl BD, Ohba R, Cook RG, Allis CD. Methylation of histone H3 at lysine 4 is highly conserved and correlates with transcriptionally active nuclei in Tetrahymena. Proc Natl Acad Sci USA. 1999;96:14967–14972. doi: 10.1073/pnas.96.26.14967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lachner M, O’Carroll D, Rea S, Mechtler K, Jenuwein T. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature. 2001;410:116–120. doi: 10.1038/35065132. [DOI] [PubMed] [Google Scholar]
- 103.Zegerman P, Canas B, Pappin D, Kouzarides T. Histone H3 lysine 4 methylation disrupts binding of nucleosome remodeling and deacetylase (NuRD) repressor complex. J Biol Chem. 2002;277:11621–11624. doi: 10.1074/jbc.C200045200. [DOI] [PubMed] [Google Scholar]
- 104.Litt MD, Simpson M, Gaszner M, Allis CD, Felsenfeld G. Correlation between histone lysine methylation and developmental changes at the chicken beta-globin locus. Science. 2001;293:2453–2455. doi: 10.1126/science.1064413. [DOI] [PubMed] [Google Scholar]
- 105.Nakayama J, Rice JC, Strahl BD, Allis CD, Grewal SI. Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science. 2001;292:110–113. doi: 10.1126/science.1060118. [DOI] [PubMed] [Google Scholar]
- 106.Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nature Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 107.Weaver IC, Champagne FA, Brown SE, Dymov S, Sharma S, Meaney MJ, Szyf M. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J Neurosci. 2005;25:11045–11054. doi: 10.1523/JNEUROSCI.3652-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Weaver IC, Meaney MJ, Szyf M. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc Natl Acad Sci USA. 2006;103:3480–3485. doi: 10.1073/pnas.0507526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pham TD, MacLennan NK, Chiu CT, Laksana GS, Hsu JL, Lane RH. Uteroplacental insufficiency increases apoptosis and alters p53 gene methylation in the full-term IUGR rat kidney. Am J Physiol Regul Integr Comp Physiol. 2003;285:R962–R970. doi: 10.1152/ajpregu.00201.2003. [DOI] [PubMed] [Google Scholar]
- 110.Waterland RA, Jirtle RL. Early nutrition, epigenetic changes at transposons and imprinted genes, and enhanced susceptibility to adult chronic diseases. Nutrition. 2004;20:63–68. doi: 10.1016/j.nut.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 111.Doherty AS, Mann MR, Tremblay KD, Bartolomei MS, Schultz RM. Differential effects of culture on imprinted H19 expression in the preimplantation mouse embryo. Biol Reprod. 2000;62:1526–1535. doi: 10.1095/biolreprod62.6.1526. [DOI] [PubMed] [Google Scholar]
- 112.Khosla S, Dean W, Brown D, Reik W, Feil R. Culture of preimplantation mouse embryos affects fetal development and the expression of imprinted genes. Biol Reprod. 2001;64:918–926. doi: 10.1095/biolreprod64.3.918. [DOI] [PubMed] [Google Scholar]
- 113.Cox GF, Burger J, Lip V, Mau UA, Sperling K, Wu BL, Horsthemke B. Intracytoplasmic sperm injection may increase the risk of imprinting defects. Am J Hum Genet. 2002;71:162–164. doi: 10.1086/341096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Orstavik KH, Eiklid K, van der Hagen CB, Spetalen S, Kierulf K, Skjeldal O, Buiting K. Another case of imprinting defect in a girl with Angelman syndrome who was conceived by intracytoplasmic semen injection. Am J Hum Genet. 2003;72:218–219. doi: 10.1086/346030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.DeBaun MR, Niemitz EL, Feinberg AP. Association of in vitro fertilization with Beckwith-Wiedemann syndrome and epigenetic alterations of LIT1 and H19. Am J Hum Genet. 2003;72:156–160. doi: 10.1086/346031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wolff GL, Kodell RL, Moore SR, Cooney CA. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J. 12:949–957. [PubMed] [Google Scholar]
- 117.Lillycrop KA, Phillips ES, Torrens C, Hanson MA, Jackson AA, Burdge GC. Feeding pregnant rats a protein-restricted diet persistently alters the methylation of specific cytosines in the hepatic PPARα promoter of the offspring. Br J Nutr. 2008;100:278–282. doi: 10.1017/S0007114507894438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Biniszkiewicz D, Gribnau J, Ramsahoye B, Gaudet F, Eggan K, Humpherys D, Mastrangelo MA, Jun Z, Walter J, Jaenisch R. Dnmt1 overexpression causes genomic hypermethylation, loss of imprinting, and embryonic lethality. Mol Cell Biol. 2002;22:2124–2135. doi: 10.1128/MCB.22.7.2124-2135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stancheva I, Meehan RR. Transient depletion of xDnmt1 leads to premature gene activation in Xenopus embryos. Genes Dev. 2000;14:313–327. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 120.Stancheva I, Hensey C, Meehan RR. Loss of the maintenance methyltransferase, xDnmt1, induces apoptosis in Xenopus embryos. EMBO J. 2001;20:1963–1973. doi: 10.1093/emboj/20.8.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.James SJ, Melnyk S, Pogribna M, Pogribny IP, Caudill MA. Elevation in S-adenosylhomocysteine and DNA hypomethylation: potential epigenetic mechanism for homocysteine-related pathology. J Nutr. 2002;132(Suppl):2361S–2366S. doi: 10.1093/jn/132.8.2361S. [DOI] [PubMed] [Google Scholar]
- 122.Ghoshal K, Li X, Datta J, Bai S, Pogribny I, Pogribny M, Huang Y, Young D, Jacob ST. A folate- and methyl-deficient diet alters the expression of DNA methyltransferases and methyl CpG binding proteins involved in epigenetic gene silencing in livers of F344 rats. J Nutr. 2006;136:1522–1527. doi: 10.1093/jn/136.6.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rouleau J, Tanigawa G, Szyf M. The mouse DNA methyltransferase 5′-region. A unique housekeeping gene promoter. J Biol Chem. 1992;267:7368–7377. [PubMed] [Google Scholar]
- 124.Ichinose M, Miki K, Tatematsu M, Furihata C, Matsushima M, Ichihara Y, Tanji M, Konishi T, Obara M, Inoue H, Kurokawa K, Takahashi T, Kageyama T, Takahashi K. Hydrocortisone-induced enhancement of expression and changes in methylation of pepsinogen genes in stomach mucosa of the developing rat. Biochem Biophys Res Commun. 1990;172:1086–1093. doi: 10.1016/0006-291x(90)91558-a. [DOI] [PubMed] [Google Scholar]
- 125.Nyirenda MJ, Lindsay RS, Kenyon CJ, Burchell A, Seckl JR. Glucocorticoid exposure in late gestation permanently programs rat hepatic phosphoenolpyruvate carboxykinase and glucocorticoid receptor expression and causes glucose intolerance in adult offspring. J Clin Invest. 1998;101:2174–2181. doi: 10.1172/JCI1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Detich N, Theberge J, Szyf M. Promoter-specific activation and demethylation by MBD2/demethylase. J Biol Chem. 2002;277:35791–35794. doi: 10.1074/jbc.C200408200. [DOI] [PubMed] [Google Scholar]
- 127.Petrie L, Duthie SJ, Rees WD, McConnell JM. Serum concentrations of homocysteine are elevated during early pregnancy in rodent models of fetal programming. Br J Nutr. 2002;88:471–477. doi: 10.1079/BJN2002695. [DOI] [PubMed] [Google Scholar]
- 128.Langley-Evans SC, Gardner DS, Jackson AA. Maternal protein restriction influences the programming of the rat hypothalamic-pituitary-adrenal axis. J Nutr. 1996;126:1578–1585. doi: 10.1093/jn/126.6.1578. [DOI] [PubMed] [Google Scholar]
- 129.Langley-Evans SC. Hypertension induced by foetal exposure to a maternal low-protein diet, in the rat, is prevented by pharmacological blockade of maternal glucocorticoid synthesis. J Hypertens. 1997;15:537–544. doi: 10.1097/00004872-199715050-00010. [DOI] [PubMed] [Google Scholar]
- 130.Terzolo M, Allasino B, Bosio S, Brusa E, Daffara F, Ventura M, Aroasio E, Sacchetto G, Reimondo G, Angeli A, Camaschella C. Hyperhomocysteinemia in patients with Cushing’s syndrome. J Clin Endocrinol Metab. 2004;89:3745–3751. doi: 10.1210/jc.2004-0079. [DOI] [PubMed] [Google Scholar]
- 131.Rhee I, Jair KW, Yen RW, Lengauer C, Herman JG, Kinzler KW, Vogelstein B, Baylin SB, Schuebel KE. CpG methylation is maintained in human cancer cells lacking DNMT1. Nature. 2000;404:1003–1007. doi: 10.1038/35010000. [DOI] [PubMed] [Google Scholar]
- 132.Jackson-Grusby L, Beard C, Possemato R, Tudor M, Fambrough D, Csankovszki G, Dausman J, Lee P, Wilson C, Lander E, Jaenisch R. Loss of genomic methylation causes p53-dependent apoptosis and epigenetic deregulation. Nature Genet. 2001;27:31–39. doi: 10.1038/83730. [DOI] [PubMed] [Google Scholar]
- 133.Robertson KD, Ait-Si-Ali S, Yokochi T, Wade PA, Jones PL, Wolffe AP. Dnmt1 forms a complex with Rb, E2F1 and HDAC1 and represses transcription from E2F-responsive promoters. Nature Genet. 2000;25:338–342. doi: 10.1038/77124. [DOI] [PubMed] [Google Scholar]
- 134.Milutinovic S, Zhuang Q, Niveleau A, Szyf M. Epigenomic stress response. Knockdown of DNA methyltransferase 1 triggers an intra-S-phase arrest of DNA replication and induction of stress response genes. J Biol Chem. 2003;278:14985–14995. doi: 10.1074/jbc.M213219200. [DOI] [PubMed] [Google Scholar]
- 135.Suetake L, Shi L, Watanabe D, Nakamura M, Tajima S. Proliferation stage-dependent expression of DNA methyltransferase (Dnmt1) in mouse small intestine. Cell Struct Funct. 2001;26:79–86. doi: 10.1247/csf.26.79. [DOI] [PubMed] [Google Scholar]
- 136.Kwong WY, Wild AE, Roberts P, Willis AC, Fleming TP. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development. 2000;127:4195–4202. doi: 10.1242/dev.127.19.4195. [DOI] [PubMed] [Google Scholar]
- 137.Liu L, Wylie RC, Andrews LG, Tollefsbol TO. Aging, cancer and nutrition: the DNA methylation connection. Mech Ageing Dev. 2004;124:989–998. doi: 10.1016/j.mad.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 138.Zhu J. DNA methylation and hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2006;13:265–273. doi: 10.1007/s00534-005-1054-4. [DOI] [PubMed] [Google Scholar]
- 139.Galm O, Herman JG, Baylin SB. The fundamental role of epigenetics in hematopoietic malignancies. Blood Rev. 2006;20:1–13. doi: 10.1016/j.blre.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 140.Lopatina N, Haskell JF, Andrews LG, Poole JC, Saldanha S, Tollefsbol T. Differential maintenance and de novo methylating activity by three DNA methyltransferases in aging and immortalized fibroblasts. J Cell Biochem. 2002;84:324–334. doi: 10.1002/jcb.10015. [DOI] [PubMed] [Google Scholar]
- 141.Lengauer C. Cancer. An unstable liaison. Science. 2003;300:442–443. doi: 10.1126/science.1084468. [DOI] [PubMed] [Google Scholar]
- 142.Strathdee G, Appleton K, Illand M, Millan DW, Sargent J, Paul J, Brown R. Primary ovarian carcinomas display multiple methylator phenotypes involving known tumor suppressor genes. Am J Pathol. 2001;158:1121–1127. doi: 10.1016/S0002-9440(10)64059-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Issa JP. Aging, DNA methylation and cancer. Crit Rev Oncol Hematol. 1999;32:31–43. doi: 10.1016/s1040-8428(99)00019-0. [DOI] [PubMed] [Google Scholar]
- 144.Tollefsbol TO, Andrews LG. Mechanisms for telomerase gene control in aging cells and tumorigenesis. Med Hypotheses. 2001;56:630–637. doi: 10.1054/mehy.2000.1241. [DOI] [PubMed] [Google Scholar]
- 145.Young JI, Sedivy JM, Smith JR. Telomerase expression in normal human fibroblasts stabilizes DNA 5-methylcytosine transferase I. J Biol Chem. 2003;278:19904–19908. doi: 10.1074/jbc.M301685200. [DOI] [PubMed] [Google Scholar]
- 146.Gluckman PD, Lillycrop KA, Vickers MH, Pleasants AB, Phillips ES, Beedle AS, Burdge GC, Hanson MA. Proc Natl Acad Sci USA. 2007;104:12796–12800. doi: 10.1073/pnas.0705667104. [DOI] [PMC free article] [PubMed] [Google Scholar]